Abstract
Background
The change in symptoms necessary to be clinically relevant in obsessive-compulsive disorder (OCD) is currently unknown. In this study, we aimed to create an empirically validated threshold for clinical significance or minimal important difference (MID).
Methods
We analyzed individual participant data from short-term, double-blind, placebo-controlled registration trials of selective serotonin reuptake inhibitors in adult OCD patients. Data were collected from baseline to week 12. We used equipercentile linking to equate changes in the Clinical Global Impression (CGI) scale to changes in the Yale-Brown Obsessive-Compulsive Scale (YBOCS). We defined the MID as the YBOCS change linked to a CGI improvement of 3 (defined as “minimal improvement”).
Results
We included 7 trials with a total of 1216 patients. The CGI-scores and YBOCS were moderately to highly correlated. The MID corresponded to 4.9 YBOCS points (95% CI 4.4–5.4) for the full sample, or a 24% YBOCS-decrease compared to baseline. The MID varied with baseline severity, being lower in the group with mild symptoms and higher in the group with severe symptoms.
Conclusions
By linking the YBOCS to the CGI-I, this is the first study to propose an MID in OCD trials. Having a clearly defined MID can guide future clinical research and help interpretation of efficacy of existing interventions. Our results are clinician-based; however, there is further need for patient-reported outcomes as anchor to the YBOCS.
Keywords: Obsessive-compulsive disorder, Double-blind randomized controlled trials, Pharmacotherapy
Introduction
Obsessive-compulsive disorder (OCD) is characterized by persistent thoughts (obsessions) and repetitive behaviors (compulsions). The global lifetime prevalence rate is 2% [1]. OCD is associated with an increased mortality risk, and without treatment, it may profoundly impair quality of life and social functioning [2]. Selective serotonin reuptake inhibitors (SSRIs) are currently the recommended pharmacological treatment for management of OCD [3, 4]. The gold standard for measuring OCD symptom severity is the Yale-Brown Obsessive-Compulsive Scale (YBOCS) [5]. This is a 10-item scale (symptom score range 0–40) with good validity and inter-rater reliability, which has assured its widespread utilization, in both clinical trials and clinical practice [6]. Currently, the magnitude of changes necessary to be relevant to patients or clinicians is unclear.
In addition to disease-specific scales, an overall impression of the patient’s well-being, as assessed by the clinician, is used in order to evaluate illness. The scales often used in clinical trials are the Clinical Global Impression Severity and Improvement scales (respectively, CGI-S and CGI-I) [7]. The CGI-S is a 7-point scale that ranks the assessor’s impression of illness severity, and the CGI-I is a 7-point scale that captures global improvement during treatment. CGI-scales rely on the subjective impression of the assessor-clinician and take into account all available, illness-specific information. Both CGI-S and CGI-I are valid, intuitive, and simple to use [8, 9].
In psychiatric research, the concept minimal important difference (MID) can be employed to evaluate the clinical effects of treatment. The MID refers to the change in symptoms necessary to bring about a relevant improvement for patients and/or clinicians, and was originally defined as the smallest difference in measured health status that signifies an important, rather than a trivial difference in patient symptoms [10]. There are multiple approaches to calculating the MID, one of which is anchoring a symptom-specific scale to the CGI by using equipercentile linking, facilitating deduction of the necessary change on a symptom scale in order to bring about one point improvement on the CGI [11, 12]. Equipercentile linking has been employed in multiple psychiatric illnesses, such as schizophrenia, bipolar disorder, and major depressive disorder [11, 13–15]. While definitions of “response” in OCD research (i.e., a YBOCS reduction of at least 35%), and a CGI-I score of 1 (“very much improved”) or 2 (“much improved”) are mainly consensus-based, equipercentile linking can be employed to add to an empirical foundation for these response definitions [16]. To the best of our knowledge, no study has yet linked the YBOCS to the CGI-S and CGI-I, which might give new perspectives on the clinical efficacy of OCD treatments.
In this study, we set out to link the CGI to the YBOCS using short-term double-blind, placebo controlled trials of SSRIs in patients with OCD. SSRIs are commonly used for OCD, with a small (to medium) effect size when tested in double-blind randomized controlled trials [17, 18]. Our primary goal was to find the MID, which we defined as three points on the CGI-I (“minimally improved”). As a secondary outcome, we report the YBOCS change linked to a CGI-I score of 2 (“much improved”). Furthermore, we linked the YBOCS scores to the CGI-S at baseline, as well as to the change in CGI-S during treatment.
Methods
For this study, we used individual participant data obtained from short-term, randomized, placebo-controlled efficacy trials with SSRI’s. These studies were submitted to the Dutch Regulatory Authority (the Medicines Evaluation Board, MEB) in order to obtain marketing authorization. Trial data were shared with the MEB under the condition that names of the compounds would not be released and original publication would not be cited. We only included studies in adult patients (18 years and older) using both YBOCS and CGI measures. We used outcomes from the active and placebo treatment arms.
The YBOCS is a 10-item psychometric test, with five items specifically on obsessions and five items specifically on compulsions. Each item can be rated from 0 to 4 points (4 being worst), which means that assigned scores range from 0 to 40. The YBOCS is used to score baseline severity and improvement (or deterioration) during treatment. The CGI-S captures the assessor’s interpretation of the patient’s current illness with the following scores: 1 = normal (not at all ill), 2 = borderline mentally ill, 3 = mildly ill, 4 = moderately ill, 5 = markedly ill, 6 = severely ill, and 7 = extremely ill. The CGI-I captures the assessor’s impression of change (positive or negative), with the following scores: 1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change, 5 = minimally worse, 6 = much worse, and 7 = very much worse. We used biweekly measurements of the CGI-I from weeks 2 to 12, and we predefined a CGI-I score of 3 as a measure for MID, as this equals “minimally improved” [19–21]. In addition to the CGI-I, we used biweekly measurements of the CGI-S and YBOCS from weeks 0 to 12.
Our primary outcome was defined as the YBOCS change score (YBOCS score at follow-up minus YBOCS at baseline) that was linked to a CGI-I score of 3. Our secondary outcomes were the CGI-I 2 (“much improved”) and CGI-S scores linked to the YBOCS at baseline, as well as the CGI-S change score (CGI-S at follow-up minus CGI-S at baseline) linked to the YBOCS change score.
We first tested our hypothesis that the CGI and YBOCS were correlated by calculating the Spearman correlation coefficients for each time point, and we predefined a correlation of 0.5 or higher as sufficient to perform equipercentile linking.
In order to find the MID, we searched for the corresponding psychometric points on the YBOCS and CGI-I/-S by equipercentile linking [12]. We calculated percentile rank functions for the CGI and YBOCS variables and performed equipercentile linking with pre-smoothing using the log-linear smoothing method to address the potential issues of sparse data in extreme score ranges. To evaluate the stability and variability of our linking results, we applied a bootstrapping approach with 500 resamples [22, 23]. This process allowed us to generate confidence intervals. We then visualized our results for our primary and secondary analyses using a plot depicting the relationship between the linked scores.
Because OCD symptom change depends on baseline severity in SSRI RCTs, it is likely that the clinical relevance of symptom reduction does, as well [24]. Therefore, we included percentage YBOCS reduction in our analysis. Furthermore, to account for this dependence, we employed a post hoc analysis linking the CGI-I to patients from different severity groups using established severity benchmarks (YBOCS 14–21 for mild symptoms, 22–29 for moderate symptoms, and 30–40 for severe symptoms) [25]. We did so as absolute point reduction is more easily clinically interpretable compared to percentage change.
The analysis was conducted using the R programming language, specifically employing the equate package for equipercentile linking [26, 27]. We preregistered our analysis plan at the Open Science Forum (https://doi.org/10.17605/OSF.IO/JEM7V).
Results
We included 7 trials with a total of 1216 patients with CGI-S and YBOCS scores at baseline. Then, 635 patients were prescribed an SSRI and 510 a placebo, and 122 patients were included for randomization but did not receive medication due to early attrition. They were included in our baseline measures and excluded from the follow-up measures. Mean YBOCS severity at baseline was 24.5 (±4.9 SD), which is indicative of severe OCD; mean age was 37.9 (±12 SD) [28]. A total of 46% of the included patients were female. We found no baseline differences between the treatment and placebo groups. For an account of baseline variables, see Table 1. During follow-up, a decline in the number of patients is visible, with the most sudden drop between weeks 10 and 12, as not all studies had a 12-week follow-up (week 2 N = 1224, week 4 N = 1160, week 6 N = 1110, week 8 N = 1054, week 10 N = 984, and week 12 N = 650; see Supplementary Table S1).
Table 1.
Patient characteristics at baseline
| Placebo | Active treatment | Test for between-group differences | |
|---|---|---|---|
| N | 510 | 706 | |
| N Gender F (%) | 223 (44) | 335 (48) | χ2 = 1.5 (p 0.22) |
| N Gender M (%) | 287 (56) | 371 (52) | |
| Mean age (SD) | 37.7 (12) | 38.0 (12) | t = −0.38 (p 0.70) |
| Mean YBOCS (SD) | 24.6 (5.0) | 24.4 (4.8) | t = −0.57 (p 0.57) |
| Mild N | 141 | 182 | |
| Moderate N | 294 | 429 | |
| Severe N | 74 | 95 | |
| Median CGI–S (IQR) | 5 (1) | 5 (1) | W = 150908 (p 0.51) |
Note: p = probability value after t-test for numerical values (age, YBOCS), χ2 for dichotomous outcomes (gender) and Mann–Whitney U test (W-statistic) for categorical variables (CGI-S). N = number, SD = standard deviation, YBOCS = Yale-Brown Obsessive-Compulsive Scale, CGI-S = Clinical Global Impression – Severity. “Mild” = YBOCS 14–21, “Moderate” = YBOCS 22–29, “Severe” = YBOCS 30–40, according to empirical benchmarks by Cervin et al. [25].
CGI-I for each week was correlated with YBOCS change scores (Spearman’s correlation ranging between 0.64 and 0.82 with an increase every week, for a full table of correlation scores; see Supplementary Table S2). Equipercentile linking showed that a YBOCS reduction of 4.9 points (95% CI −5.5 to −4.4, corresponding to a 24% YBOCS reduction) was linked to a CGI improvement score of 3 (“minimally improved”). After splitting the sample according to severity, the “mild” group had an MID of – 3.1 (95% CI −4.2 to −2.1), the “moderate” group had an MID of 5.1 (95% CI −5.8 to −4.4) and the “severe” group had an MID of 7.2 (−9.1 to −5.3) (see Supplementary Table S5).
Furthermore, a CGI-I score of 2 (“much improved”) corresponded to a 46% YBOCS reduction compared to baseline (95% CI −56 to −36), or an average 10 point reduction (95% CI (−11 to –9.4). Table 2 and Figure 1 show the linked results of the remaining CGI-I and YBOCS scores. Since the weekly number of patients with a CGI-I score of 6 or 7 was low, we did not calculate linking scores for these values (see Supplementary Table S1 for number of patient per CGI per week).
Table 2.
Change in YBOCS linked to change in CGI-I
| CGI-I | YBOCS change (95% CI) | YBOCS % change (95% CI) |
|---|---|---|
| 1 | −17 (−19 to −16) | −83 (−97 to −70) |
| 2 | −10 (−11 to −9.4) | −46 (−56 to −36) |
| 3 | −4.9 (−5.4 to −4.4) | −24 (−42 to 6.5) |
| 4 | −0.26 (−0.66 to 0.13) | −1.1 (−9.3 to 7.0) |
| 5 | 4.5 (3.8–5.3) | 21 (12–29) |
Note: Mean scores for weeks 2, 4, 6, 8, 10, and 12. YBOCS change in absolute point difference and in percentage change. 95% CI = 95% confidence interval (upper – lower), CGI-I = Clinical Global Impression Scale – Improvement.
Figure 1.
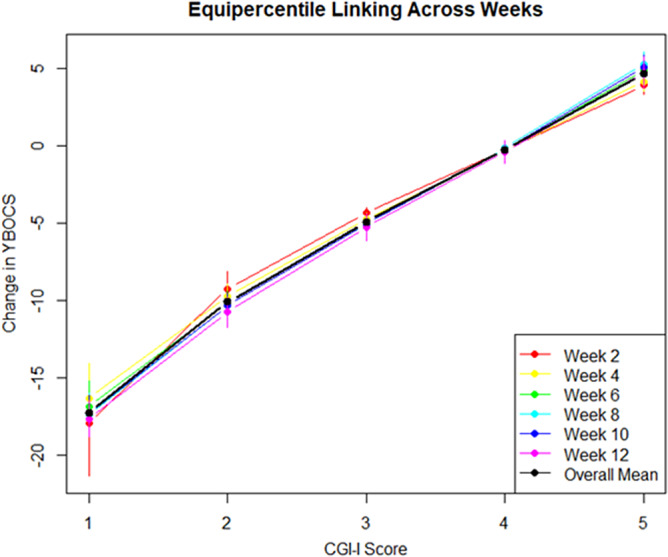
Equipercentile linking of CGI-I and change in YBOCS score, per week. Black line = mean linking for each week. CGI-I = Clinical global impression scale - improvement, YBOCS = Yale-Brown Obsessive Compulsive Scale
At baseline, YBOCS and CGI-S were moderately correlated with a Spearman correlation coefficient of 0.62 (see Supplementary Table S2). After equipercentile linking, a CGI-S score of 4 (moderately ill) corresponded with a YBOCS of 21 (95% CI 20–22) (Table 3, Figure 2). Patients who were scored markedly ill at baseline (CGI-S of 5) had an equated baseline YBOCS of 26 (95% CI 26–27). CGI-S 6 and CGI-S 7 were equated with, respectively, a YBOCS of 31 and 38 (95% CI 31–32, 95% CI 36–39). A baseline of CGI-S 3 was linked to 10 points on the YBOCS (95% CI 7.8–13).
Table 3.
Change in YBOCS linked to one point CGI-S change at baseline
| CGI severity | YBOCS score | 95% CI lower | 95% CI upper |
|---|---|---|---|
| 3 | 10 | 7.8 | 13 |
| 4 | 21 | 21 | 22 |
| 5 | 26 | 26 | 27 |
| 6 | 31 | 31 | 32 |
| 7 | 38 | 36 | 39 |
Figure 2.
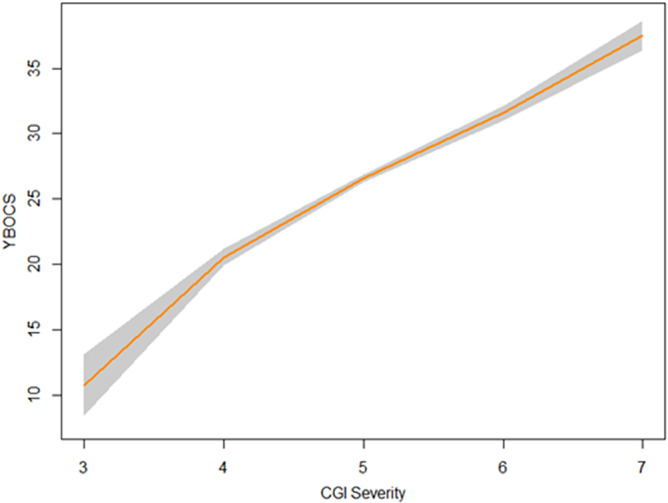
Equipercentile linking of CGI-S and YBOCS at baseline. CGI - S = Clinical global impression scale - severity, YBOCS = Yale-Brown Obessive Compulsive Scale.
Equipercentile linking of the change in CGI-S scores compared to baseline showed a difference of 5.3–6.4 points on the YBOCS per CGI-S point (see Table 4 and Figure 3). Correlation coefficients between CGI-S change scores and YBOCS were moderate to strong from week 4, increasing from 0.58 to 0.78 (see Supplementary Table S1).
Table 4.
Change in YBOCS linked to one point difference in CGI-S Change
| CGI-S change | YBOCS change | 95% CI upper | 95% CI lower |
|---|---|---|---|
| −4 to −3 | 5.3 | 2.4 | 8.0 |
| −3 to −2 | 5.8 | 3.3 | 8.4 |
| −2 to −1 | 6.0 | 4.5 | 7.5 |
| −1 to 0 | 5.8 | 5.0 | 6.6 |
| 0–1 | 6.1 | 5.0 | 7.0 |
| 1–2 | 6.4 | 4.1 | 8.7 |
Note: Mean scores for week 2, 4, 6, 8, 10, and 12. A negative CGI-S change score means an apparent clinical improvement compared to baseline.
Figure 3.
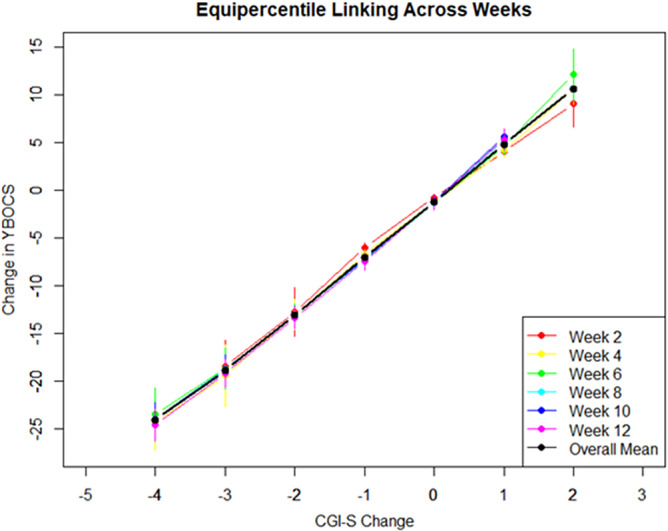
Equipercentile linking of CGI-S change and YBOCS change, per week. Black line = mean linking for each week. CGI - S = Clinical global impression scale - severity, YBOCS = Yale-Brown Obessive Compulsive Scale.
Discussion
In this study, we for the first time developed an MID for adults with OCD in placebo-controlled RCT’s, in order to create an empirically validated threshold for clinical significance. By linking YBOCS change to minimal improvement according to the CGI-I, we found a general MID of 4.9 points on the YBOCS.
We further showed that the MID is dependent on baseline symptom severity. In patients with higher baseline symptom scores, a more substantial decrease in symptoms is necessary to bring about a noticeable change, relative to less severely ill patients. Using percentage symptom reduction, which incorporates differences in baseline severity, we found that a YBOCS decrease of about 25% corresponded with minimal improvement, which corresponds to what has been defined as a threshold for partial response for OCD [29].
Using data registries from the Medicines Evaluation Board, we were able to use a large sample of high-quality registration studies to develop an MID for obsessive-compulsive disorder. As most clinical OCD studies, SSRI trials use symptom reduction measured with the YBOCS as outcome, which is the primary efficacy measure recommended by regulatory agencies [30]. A statistically significant difference on a continuous outcome scale between active treatment and placebo is used to test clinical efficacy. Our study shows the smallest observable difference, or MID, for clinicians (4.9 YBOCS points) to be larger than the 3.5-point treatment effect found in short-term efficacy trials on SSRIs in OCD [31]. This discrepancy between the MID and the average improvement in OCD mirrors the literature on SSRIs for major depressive disorder, where multiple studies on a variety of scales found an MID that exceeds results from placebo-controlled trials [32].
Both at baseline and during follow-up, we also found that one point change in the CGI-S is consistent with five to six point changes in the YBOCS. Even though the CGI-S and CGI-S-change scores were not predefined as signifying MID, these results further illustrate that a minimum improvement of five YBOCS points is necessary in order to bring about a change in symptoms that is noticeable in the clinical setting.
Aside from an MID, we found that a CGI-I score of 2 points (“much improved”) was linked to a YBOCS reduction compared to baseline of 46%. This is higher than the commonly used definition of clinical response as 35% YBOCS reduction [33]. Consistent with our results, a study using a different methodological approach and different patient populations found that solely using the 35% YBOCS reduction criterion would lead to a overestimation of response by about 2%, compared to combining the YBOCS and CGI-I [29]. This is further supported by findings from a retrospective analysis showing that a YBOCS reduction between 40 and 50% optimally predicts CGI-I 1 or 2 after OCD treatment [34]. As recently stated in a consensus paper, an expert-based definition of response in OCD is operationalized as 35% clinical reduction plus a CGI-I score of 1 or 2 [16]. Our findings support this operationalization, indicating that in clinical practice, relying solely on a 35% cut-off score on the YBOCS for defining response may be too lenient, potentially resulting in the undertreatment of patients.
An MID might act as an empirically informed measure for the interpretation of judging overall effects that are found in a trials (placebo-controlled, head-to-head) or cohorts. However, as this pertains group-level outcomes, individual clinical response within trials varies widely and a group effect smaller than that of the MID does not exclude the possibility of patients having an adequate treatment effect. Regarding clinical research, the MID might be applied as a dichotomous outcome, similar to response or remission. This would identify the chance of minimal improvement after intervention compared to placebo and could be used in addition to the chances of response/remission. As such, it could also be employed for clinical decision-making, by informing patients regarding the effects of the intervention.
However, there are some caveats regarding interpretation of the MID. For instance, we have shown that the MID is dependent on baseline severity, and thus, the overall outcome cannot be assumed to be relevant for every patient. Other baseline criteria, such as gender or age, might also influence the size of the MID [35]. When linking the CGI to the YBOCS, we still rely only on symptom-related outcome measures. A recent transdiagnostic survey showed that when estimating the clinical status of the patients, CGI-S assessors rely more on symptom scales and clinical interviews, and less on staff observation or non-symptom-specific patient-perspective health outcomes [36]. None of the trials available used standardized questionnaires focusing on patient recovery and/or quality of life. To our knowledge, only one short-term double-blind SSRI trial has used such a scale as an outcome measure [37]. Non-illness-specific scales of disability of quality of life would be an important addition to future clinical trials on OCD pharmacotherapy. In fact, novel clinical trials focusing on interventions such as Deep Brain Stimulation have included these questionnaires [38]. Furthermore, the CGI was designed as a clinician-rated instrument; thus, linking the CGI with another clinician-rated instrument makes for an assessor-based MID. A patient-centered MID would require linking symptom scores to patient-reported outcome measures (PROMs) [39]. The individual participant data that were made available to us did not include these outcomes. Reevaluation of all double-blind RCT’s on SSRI’s included in a recent systematic review from our research group shows that only four studies used some form of patient-reported severity scale, namely the patient-rated global impression scale [31, 40–43]. Together, these findings emphasize the paucity of PROMs in double-blind SSRI trials in OCD.
Another important element of developing a patient-centered MID would be to directly involve mental health service users and/or lived experience groups [44]. For instance, a recent OCD psychotherapy trial has predefined their MID as five YBOCS points [45]. They did so after consulting a Lived Experience Advisory Panel, who determined that a mean 0.5 point reduction on all 10 YBOCS items might be considered clinically meaningful. Even though the authors did not use direct empirical analysis and the interventions differed, their proposed minimum for clinically significant change resembles our findings.
Our study has some shortcomings. We only included double-blind randomized controlled trials in which the included sample and clinical setting might have biased our results. For example, in the real-world clinical setting (as opposed to the RCT-setting), an assessor might have known the patient for a longer time or might have spoken with the patient’s family or spouse, which could influence how the CGI-I or CGI-S are scored. Since all included studies were SSRI trials, generalizability to other interventions, such as psychotherapy or neuromodulation might be limited. Furthermore, CGI-I and CGI-S measurements do not take into account side effects when determining improvement in health status, as they instruct assessors not to incorporate side effects [46]. Considering side effects with SSRI treatment for OCD are common, it is to be expected that MID’s would increase when also taking into account adverse drug reactions [47].
Notwithstanding the limitations of our study, using equipercentile linking of the CGI-I and YBOCS, we were able to propose an MID of 4.9 YBOCS points for the pharmacological treatment of OCD. This finding sheds new light on the efficacy of existing pharmacological interventions and can guide future clinical research. To better inform shared decision-making between patients and clinicians when managing OCD, future pharmacotherapy trials must prioritize the use of patient-reported outcomes and quality-of-life measures.
Supporting information
Cohen et al. supplementary material
Acknowledgments
No acknowledgments.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1192/j.eurpsy.2024.1768.
Funding
The authors declare that they did not receive specific grants from public, not-for-profit, or commercial agencies for this research.
Competing interest
The authors declare that they have no competing interest.
References
- 1.Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 2010;15(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorena Fernández de la C, Kayoko I, Paul L, Henrik L, K-H Ralf, Zheng C, et al. All cause and cause specific mortality in obsessive-compulsive disorder: nationwide matched cohort and sibling cohort study. BMJ 2024;384:e077564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Collaborating Centre for Mental Health (UK). Obsessive-compulsive disorder: core interventions in the treatment of obsessive-compulsive disorder and body dysmorphic disorder. Leicester (UK): British Psychological Society (UK); 2006. (NICE Clinical Guidelines, No. 31.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK56458/. [PubMed]
- 4.American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Arlington, VA: American Psychiatric Association, 2007. Available online at http//www.psych.org/psych_pract/treatg/pg/prac_guide.cfm. [PubMed]
- 5.Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown obsessive compulsive scale: I. Development, use, and reliability. Arch Gen Psychiatry 1989;46(11):1006–11. [DOI] [PubMed] [Google Scholar]
- 6.Rapp AM, Bergman RL, Piacentini J, McGuire JF. Evidence-based assessment of obsessive–compulsive disorder. J Cent Nerv Syst Dis 2016;8:JCNSD.S38359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- 8.Guy W. ECDEU assessment manual for psychopharmacology: National Institute of Mental Health, US Department of Health, Education, and Welfare, Public Health Service; 1976.
- 9.Berk M, Ng F, Dodd S, Callaly T, Campbell S, Bernardo M, Trauer T. The validity of the CGI severity and improvement scales as measures of clinical effectiveness suitable for routine clinical use. J Eval Clin Pract 2008;14(6):979–83. [DOI] [PubMed] [Google Scholar]
- 10.Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. J Clin Epidemiol 1996;49(11):1215–9. [DOI] [PubMed] [Google Scholar]
- 11.Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale scores. Br J Psychiatry 2005;187(4):366–71. [DOI] [PubMed] [Google Scholar]
- 12.Brennan K. Test equating, scaling, and linking. 3 ed. Linden FVd, editor. New York: Springer Media; 2014. 25 p. [Google Scholar]
- 13.Samara MT, Engel RR, Millier A, Kandenwein J, Toumi M, Leucht S. Equipercentile linking of scales measuring functioning and symptoms: examining the GAF, SOFAS, CGI-S, and PANSS. Eur Neuropsychopharmacol 2014;24(11):1767–72. [DOI] [PubMed] [Google Scholar]
- 14.Samara MT, Levine SZ, Leucht S. Linkage of Young Mania rating scale to clinical global impression scale to enhance utility in clinical practice and research trials. Pharmacopsychiatry 2023;56(1):18–24. [DOI] [PubMed] [Google Scholar]
- 15.Bobo WV, Angleró GC, Jenkins G, Hall-Flavin DK, Weinshilboum R, Biernacka JM. Validation of the 17-item Hamilton Depression Rating Scale definition of response for adults with major depressive disorder using equipercentile linking to Clinical Global Impression scale ratings: analysis of Pharmacogenomic Research Network Antidepressant Medication Pharmacogenomic Study (PGRN-AMPS) data. Hum Psychopharmacol Clin Exp 2016;31(3):185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mataix-Cols D, Fernández de la Cruz L, Nordsletten AE, Lenhard F, Isomura K, Simpson HB. Towards an international expert consensus for defining treatment response, remission, recovery and relapse in obsessive-compulsive disorder. World Psychiatry 2016;15(1):80–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotapati VP, Khan AM, Dar S, Begum G, Bachu R, Adnan M, et al. The effectiveness of selective serotonin reuptake inhibitors for treatment of obsessive-compulsive disorder in adolescents and children: a systematic review and meta-analysis. Front Psychiatry. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skapinakis P, Caldwell DM, Hollingworth W, Bryden P, Fineberg NA, Salkovskis P, et al. Pharmacological and psychotherapeutic interventions for management of obsessive-compulsive disorder in adults: a systematic review and network meta-analysis. Lancet Psychiatry 2016;3(8):730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masson SC, Tejani AM. Minimum clinically important differences identified for commonly used depression rating scales. J Clin Epidemiol 2013;66(7):805–7. [DOI] [PubMed] [Google Scholar]
- 20.Stefanovics EA, Rosenheck RA, Jones KM, Huang G, Krystal JH. Minimal Clinically Important Differences (MCID) in assessing outcomes of post-traumatic stress disorder. Psychiatr Q 2018;89(1):141–55. [DOI] [PubMed] [Google Scholar]
- 21.Czobor P, Sebe B, Acsai K, Barabássy Á, Laszlovszky I, Németh G, et al. What is the minimum clinically important change in negative symptoms of schizophrenia? PANSS based post-hoc analyses of a phase III clinical trial. Front Psychiatry. 2022;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Efron BT, R.J. An introduction to the bootstrap chapman and Hall/CRC; 1994.
- 23.Brennan K. Test equating, scaling, and linking. 3 edn. Linden Fvd, editor. New York: Springer Media; 2014. 19 p. [Google Scholar]
- 24.de Vries YA, de Jonge P, van den Heuvel E, Turner EH, Roest AM. Influence of baseline severity on antidepressant efficacy for anxiety disorders: meta-analysis and meta-regression. Br J Psychiatry 2016;208(6):515–21. [DOI] [PubMed] [Google Scholar]
- 25.Cervin M, Consortium OSB, Mataix-Cols D. Empirical severity benchmarks for obsessive-compulsive disorder across the lifespan. World Psychiatry 2022;21(2):315–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albano AD. equate: an R Package for Observed-Score Linking and Equating. J Stat Softw 2016;74(8):1–36. [Google Scholar]
- 27.R Core Team, R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. [Google Scholar]
- 28.Wootton BM, Tolin DF. Obsessive–compulsive disorder. In: Friedman HS, editor. Encyclopedia of mental health (2nd Edn). Oxford: Academic Press; 2016. p. 227–31. [Google Scholar]
- 29.Mataix-Cols D, Andersson E, Aspvall K, Boberg J, Crowley JJ, de Schipper E, et al. Operational definitions of treatment response and remission in obsessive-compulsive disorder capture meaningful improvements in everyday life. Psychother Psychosom 2022;91(6):424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Committee for Medicinal Products for Human Use (CHMP), Guideline on clinical investigation of medicinal products for the treatment of Obsessive Compulsive Disorder. The European Medicines Agency. CHMP/EWP/4279/022005.
- 31.Cohen SE, Zantvoord JB, Storosum BWC, Mattila TK, Daams J, Wezenberg B, et al. Influence of study characteristics, methodological rigour and publication bias on efficacy of pharmacotherapy in obsessive-compulsive disorder: a systematic review and meta-analysis of randomised, placebo-controlled trials. BMJ Ment Health 2024;27(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hengartner MP, Plöderl M. Estimates of the minimal important difference to evaluate the clinical significance of antidepressants in the acute treatment of moderate-to-severe depression. BMJ Evid Based Med 2022;27(2):69–73. [DOI] [PubMed] [Google Scholar]
- 33.Farris SG, McLean CP, Van Meter PE, Simpson HB, Foa EB. Treatment response, symptom remission, and wellness in obsessive-compulsive disorder. J Clin Psychiatry 2013;74(7):685–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tolin DF, Abramowitz JS, Diefenbach GJ. Defining response in clinical trials for obsessive-compulsive disorder: a signal detection analysis of the Yale-Brown obsessive compulsive scale. J Clin Psychiatry 2005;66(12):1549–57 [DOI] [PubMed] [Google Scholar]
- 35.Fernandes-Taylor S, Zhao J, Francis DO. New directions for improving the minimal clinically important difference in patient-reported outcome measures for use in clinical care and research. JAMA Otolaryngol–Head Neck Surg 2023;149(3):276–7. [DOI] [PubMed] [Google Scholar]
- 36.Dunlop BW, Gray J, Rapaport MH. Transdiagnostic clinical global impression scoring for routine clinical settings. Behav Sci (Basel) 2017;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montgomery SA, Kasper S, Stein DJ, Bang Hedegaard K, Lemming OM. Citalopram 20 mg, 40 mg and 60 mg are all effective and well tolerated compared with placebo in obsessive-compulsive disorder. Int Clin Psychopharmacol 2001;16(2):75–86. [DOI] [PubMed] [Google Scholar]
- 38.Graat I, van Rooijen G, Prinsen J, Bergfeld I, Figee M, Denys D, Mocking R. Cyclic versus continuous deep brain stimulation in patients with obsessive compulsive disorder: a randomized controlled trial. Brain Stimul 2023;16(1):82–7. [DOI] [PubMed] [Google Scholar]
- 39.Subramaniam M, Soh P, Ong C, Esmond Seow LS, Picco L, Vaingankar JA, Chong SA. Patient-reported outcomes in obsessive-compulsive disorder. Dialogues Clin Neurosci 2014;16(2):239–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodman WK, Price LH, Rasmussen SA, Delgado PL, Heninger GR, Charney DS. Efficacy of fluvoxamine in obsessive-compulsive disorder. A double-blind comparison with placebo. Arch Gen Psychiatry 1989;46(1):36–44. [DOI] [PubMed] [Google Scholar]
- 41.Montgomery SA, McIntyre A, Osterheider M, Sarteschi P, Zitterl W, Zohar J, et al. A double-blind, placebo-controlled study of fluoxetine in patients with DSM-III-R obsessive-compulsive disorder. The Lilly European OCD Study Group. Eur Neuropsychopharmacol 1993;3(2):143–52. [DOI] [PubMed] [Google Scholar]
- 42.Tollefson GD, Rampey AH, Jr., Potvin JH, Jenike MA, Rush AJ, kominguez RA, et al. A multicenter investigation of fixed-dose fluoxetine in the treatment of obsessive-compulsive disorder. Arch Gen Psychiatry 1994;51(7):559–67. [DOI] [PubMed] [Google Scholar]
- 43.Zohar J, Judge R. Paroxetine versus clomipramine in the treatment of obsessive-compulsive disorder. OCD Paroxetine Study Investigators. Br J Psychiatry 1996;169(4):468–74. [DOI] [PubMed] [Google Scholar]
- 44.Roe D, Slade M, Jones N. The utility of patient-reported outcome measures in mental health. World Psychiatry 2022;21(1):56–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strauss C, Lea L, Hayward M, Forrester E, Leeuwerik T, Jones A-M, Rosten C. Mindfulness-based exposure and response prevention for obsessive compulsive disorder: findings from a pilot randomised controlled trial. J Anxiety Disord 2018;57:39–47. [DOI] [PubMed] [Google Scholar]
- 46.Busner J, Targum SD, Miller DS. The Clinical Global Impressions scale: errors in understanding and use. Compr Psychiatry 2009;50(3):257–62. [DOI] [PubMed] [Google Scholar]
- 47.Gosmann NP, Costa MdA, MdB Jaeger, Frozi J, Spanemberg L, Manfro GG, et al. Incidence of adverse events and comparative tolerability of selective serotonin reuptake inhibitors, and serotonin and norepinephrine reuptake inhibitors for the treatment of anxiety, obsessive-compulsive, and stress disorders: a systematic review and network meta-analysis. Psychol Med 2023;53(9):3783–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cohen et al. supplementary material


