Abstract
Priming of mice with intact, heat-killed cells of Gram-negative Neisseria meningitidis, capsular serogroup C (MenC) or Gram-positive group B Streptococcus, capsular type III (GBS-III) bacteria resulted in augmented serum polysaccharide (PS)-specific IgG titers following booster immunization. Induction of memory required CD4+ T cells during primary immunization. We determined whether PS-specific memory for IgG production was contained within the B cell and/or T cell populations, and whether augmented IgG responses following booster immunization were also dependent on CD4+ T cells. Adoptive transfer of purified B cells from MenC- or GBS-III–primed, but not naive mice resulted in augmented PS-specific IgG responses following booster immunization. Similar responses were observed when cotransferred CD4+ T cells were from primed or naive mice. Similarly, primary immunization with unencapsulated MenC or GBS-III, to potentially prime CD4+ T cells, failed to enhance PS-specific IgG responses following booster immunization with their encapsulated isogenic partners. Furthermore, in contrast to GBS-III, depletion of CD4+ T cells during secondary immunization with MenC or another Gram-negative bacteria, Acinetobacter baumannii, did not inhibit augmented PS-specific IgG booster responses of mice primed with heat-killed cells. Also, in contrast with GBS-III, booster immunization of MenC-primed mice with isolated MenC-PS, a TI Ag, or a conjugate of MenC-PS and tetanus toxoid elicited an augmented PS-specific IgG response similar to booster immunization with intact MenC. These data demonstrate that memory for augmented PS-specific IgG booster responses to Gram-negative and Gram-positive bacteria is contained solely within the B cell compartment, with a differential requirement for CD4+ T cells for augmented IgG responses following booster immunization.
Infections with extracellular, polysaccharide (PS)-encapsulated bacteria are major sources of global morbidity and mortality among infants, the elderly, and the immunosuppressed, causing sepsis, pneumonia, and meningitis (1-4). Capsular PS serves as a major virulence factor by impeding phagocytosis and masking opsonins bound to underlying surface bacterial Ags (5). Isolated PS, with the exception of those that are zwitterionic (6), elicit Abs in a T cell–independent (TI) fashion (7, 8) due to their inability to associate with MHC-II on APCs for presentation to cognate CD4+ T cells (9, 10). However, immunization with a soluble conjugate vaccine formed by covalent attachment of PS with an immunogenic carrier protein facilitates recruitment of CD4+ T cell help for PS-specific IgG responses (11, 12). Thus, unlike isolated PS, conjugate vaccines can elicit robust germinal center reactions, class switching, somatic hypermutation, and immunologic memory (13).
The presentation of PS expressed by intact bacteria differs from the isolated PS or conjugate vaccines as they are coexpressed, noncovalently with proteins within a particulate framework, along with multiple TLR, NOD-like receptors, and scavenger receptor ligands (14). In this regard, we previously demonstrated that representative intact, heat-inactivated Gram-positive (GP) and Gram-negative (GN) extracellular bacteria elicit augmented and more rapid IgG responses following booster immunization of primed mice, similar to conjugate vaccines (14, 15). Induction of PS-specific B cell memory is dependent on CD4+ T cell interactions with APC through costimulatory molecules, such as B7 and ICOS-L during the primary response. PS-specific IgG responses to intact bacteria are most likely dependent on coexpressed bacterial proteins recognized by CD4+ T cells (16). These data further suggested a general dichotomy between GP and GN bacteria, in which GP bacteria (i.e., Streptococcus pneumoniae and group B Streptococcus) elicited relatively rapid (peak day 7) CD4+ T cell–dependent primary PS-specific IgG responses, whereas GN bacteria (i.e., Neisseria meningitidis and Acinetobacter baumannii) developed primary PS-specific IgG responses that developed more slowly (peak day 21) and were independent of T cell help (15). Immunity induced by intact bacteria also showed distinct response differences from soluble conjugate vaccines. For example, systemic immunization with intact S. pneumoniae induced PS-specific IgG that derived from splenic marginal zone B cells and expressed a distinct idiotype, whereas a pneumococcal conjugate vaccine of the same PS serotype (type 14) induced PS-specific IgG largely lacking this idiotype and derived from splenic follicular B cells (17-19).
The general consensus regarding elicitation of memory IgG responses is that the memory B cells generated in the presence of CD4+ T cell help during the primary response proliferate robustly upon re-exposure to Ag and generate plasma cells expressing high-affinity IgG that is dependent on help from cognate memory CD4+ T cells (20). However, most of these conclusions have been derived from studies carried out with isolated proteins that left unresolved whether PS-specific memory for IgG in response to intact GP and GN bacteria was similarly contained within both the B cell and CD4+ T cell compartments. Additionally, it was not determined whether the augmented PS-specific IgG responses following booster immunization with intact bacteria were also dependent on the presence of CD4+ T cells.
To begin addressing these questions, we demonstrated that mice primed with intact group B Streptococcus, capsular type III (GBS-III) elicited an augmented PS-specific IgG response only if CD4+ T cells were present during both the primary and booster immunizations (21). This augmented response in GBS-III–primed mice did not occur following booster immunization with the corresponding isolated PS. In this study, we have extended these initial observations utilizing intact GBS-III, N. meningitidis, capsular serogroup C (MenC), and A. baumannii expressing the surface PS, poly-N-acetylglucosamine (PNAG) to determine the cellular nature of PS-specific memory for IgG and whether CD4+ T cells are uniformly required for augmented IgG responses following booster immunization. In contrast to what has been previously observed for isolated proteins, which depend on both memory B cells and memory CD4+ T cells for augmented anti-protein IgG responses, we now show that PS-specific memory for GP and GN bacteria is contained solely within the B cell compartment. Furthermore, CD4+ T cells are required during booster immunization for augmented PS-specific IgG responses for GP, but not for GN bacteria. For this reason, PS-specific IgG responses to GN-primed, but not GP-primed mice can be augmented using only isolated PS from the priming bacteria. These observations may have relevance for future vaccine design.
Materials and Methods
Mice
Athymic nude (BALB/c background), SCID/NCr (BALB/c background; strain code. 561) mice and BALB/c mice were purchased from the National Cancer Institute (Frederick, MD). Mice used were between 7 and 10 wk of age. These studies were conducted in accordance with the principles set forth in the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, revised 1996), and were approved by the Uniformed Services University of the Health Sciences Institutional Animal Care and Use Committee.
Bacteria
GBS-III (strain M781; ATCC BAA-22) and MenC (strain M1883; ATCC 53414) were obtained from American Type Culture Collection (Manassas, VA). COH1, a different strain of GBS-III expressing the same capsular type III PS, and COH1-13, an isogenic COH1 mutant lacking a capsule, were used in this study. The COH strains were a gift of C. Rubens of Children’s Orthopedic Hospital (Seattle, WA). The encapsulated and unencapsulated MenC strains, FAM18 C+ and FAM18 C−, respectively, were used (22). Both M1883 and FAM18 C+ were similarly O-acetylated. Lyophilized or frozen stocks of bacteria were grown overnight on BBL blood agar plates (VWR International, Bridgeport, NJ). Isolated colonies of MenC or GBS-III on blood agar were grown to midlog phase in brain–heart infusion media or Todd-Hewitt broth media (BD Biosciences, San Jose, CA), respectively. Harvested cells were heat killed by incubation at 65°C for 2 h. Sterility was confirmed by subculture on blood agar plates. After extensive washings, the bacteria were suspended in PBS and adjusted to give an absorbance reading at 650 nm of 0.6, which corresponded to 109 CFU/ml. Bacteria were then aliquoted at 1010 CFU/ml and frozen at −20°C until their use for mouse immunizations. A. baumannii strain S1Δpga-c was constructed, as described (23), by first deleting the chromosome pga locus (Δpga) encoding PNAG biosynthetic proteins and then complementing the deletion in trans (Δpga-c) to achieve over-expression of PNAG on the cell surface. The frozen stocks of bacteria were grown overnight on lysogeny broth (LB) agar plates. Isolated colonies were grown in LB media to mid-log phase, collected, and heat killed by incubation at 65°C for 2 h. After thorough washings, bacteria were suspended in PBS and adjusted to give an absorbance reading of 0.4 at 600 nm, which corresponded to 108 CFU/ml. Bacteria were aliquoted at 109 CFU/ml and frozen at −20°C.
Reagents
Purified serogroup C capsular PS of N. meningitidis (MCPS) was a gift of A. Lees (Fina BioSolutions, Rockville, MD). A covalent conjugate of MCPS and tetanus toxoid (MCPS-TT) was prepared, as previously described (24). Purified type 14 capsular PS of S. pneumoniae (PPS14), which is structurally related to GBS-III PS but lacks the terminal sialic acid component (25), was purchased from American Type Culture Collection. Rat IgG2b anti-mouse CD4 mAb (clone GK1.5) was purchased from BioXcell (West Lebanon, NH). Purified polyclonal rat IgG was purchased from Sigma-Aldrich (St. Louis, MO). Alum (Allhydrogel 2%) was obtained from Brenntag Biosector (Frederikssund, Denmark). A stimulatory 30-mer CpG-containing oligodeoxynucleotide (CpG-ODN) was synthesized, as previously described (26). B and T cell isolation kits were purchased from Miltenyi Biotec (Auburn, CA).
Preparation of PNAG
PNAG was prepared as previously described (23). Briefly, A. baumannii strain S1Δpga-c was grown in LB media containing 1% glucose. Bacterial cells were treated with lysozyme, followed by DNase I and RNase A, and the cells were then removed by centrifugation, with the resulting supernatant precipitated with ethanol. The ethanol-insoluble material was collected by centrifugation, suspended in water, dialyzed against water, and freeze dried until use. Before use, PNAG was dissolved at a concentration of 5 mg/ml in 5 M HCl. An equal volume of NaOH was added to neutralize the solution by keeping the vial on ice.
Immunizations
Groups of seven mice each were immunized i.p. with 2 × 108 CFU intact, heat-killed MenC in PBS; 2 × 109 CFU intact, heat-killed GBS-III in PBS; 1 × 108 CFU intact, heat-killed A. baumannii in PBS; 5 μg purified MCPS; or 1 μg MCPS-TT, both adsorbed on 13 μg alum mixed with 25 μg CpG-ODN. Serum samples were prepared at different time points from blood obtained through the tail vein.
Adoptive transfer of B and T lymphocytes into scid mice
BALB/c mice were immunized i.p. with MenC or GBS-III, as above. A separate control group was injected with PBS only. At day 21 postimmunization, spleen cells were obtained from immunized and naive mice. B and T cells were separately isolated by magnetic sorting using B and T cell isolation kits, respectively (Miltenyi Biotec). Isolated cells were analyzed by flow cytometry and determined to be 96–98% pure, with no detectable cross-contamination of B and T cells. The purified cells were adoptively transferred into scid mice at 2 × 107 B cells/mouse and 1 × 107 T cells /mouse in four different combinations, as follows: 1) naive (n)B + nT, 2) nB + primed (p)T, 3) pB + nT, and 4) pB + pT. The recipient scid mice were immunized with MenC or GBS-III, as above, a day later (d0), and sera were obtained on day 7.
ELISA
For measurement of serum titers of PPS14-specific IgG, Immulon 4 ELISA plates were coated overnight at 4°C with purified PPS14 (5 μg/ml, 100 ml/well) in PBS. For measurement of serum titers of MCPS-specific or PNAG-specific IgG, Immulon 4 ELISA plates were precoated with poly-L-lysine (Sigma-Aldrich) (5 μg/ml, 100 μl/well) in PBS for 1 h at 37°C. The plates were then washed three times with PBS plus 0.1% Tween 20 and then coated overnight at 4°C with purified MCPS (10 μg/ml, 100 μl/well) or PNAG (3 μg/ml 100 μl/well) in PBS. Plates were then washed three times with PBS plus 0.1% Tween 20 and were blocked with PBS plus 1.0% BSA for 1 h at 37°C. Three-fold dilutions of serum samples, starting at a 1/50 serum dilution, in PBS plus 1.0% BSA, were incubated overnight at 4°C, and plates were then washed three times with PBS plus 0.1% Tween 20. Alkaline phosphatase-conjugated polyclonal goat anti-mouse IgG Abs (200 ng/ml) in PBS + 1.0% BSA were then added, and plates were incubated at 37°C for 1 h. Plates were washed three times with PBS plus 0.1% Tween 20. Substrate (p-nitrophenyl phosphate, disodium; Sigma-Aldrich) at 1 mg/ml in 1 M Tris plus 0.3 mM MgCl2 (pH 9.8) was then added for color development. Color was read at an absorbance of 405 nm on a Multiskan Ascent ELISA reader (Labsystems). Serum titers were determined as described previously (27).
Statistics
Serum titers of Ag-specific IgG were expressed as the geometric means ± SEM of the individual serum IgG titers. Significance was determined by performing comparative analysis of data using a one-way ANOVA test, followed by Tukey’s multiple comparisons (Fig. 1), and repeated-measures two-way ANOVA, followed by Tukey’s multiple comparisons (Figs. 3, 5, 6) or Šídák multiple comparisons (Figs. 2, 4). The p values ≤0.05 were considered statistically significant. All statistical tests were performed using GraphPad Prism 6.0 software. All experiments were performed at least two times.
FIGURE 1.
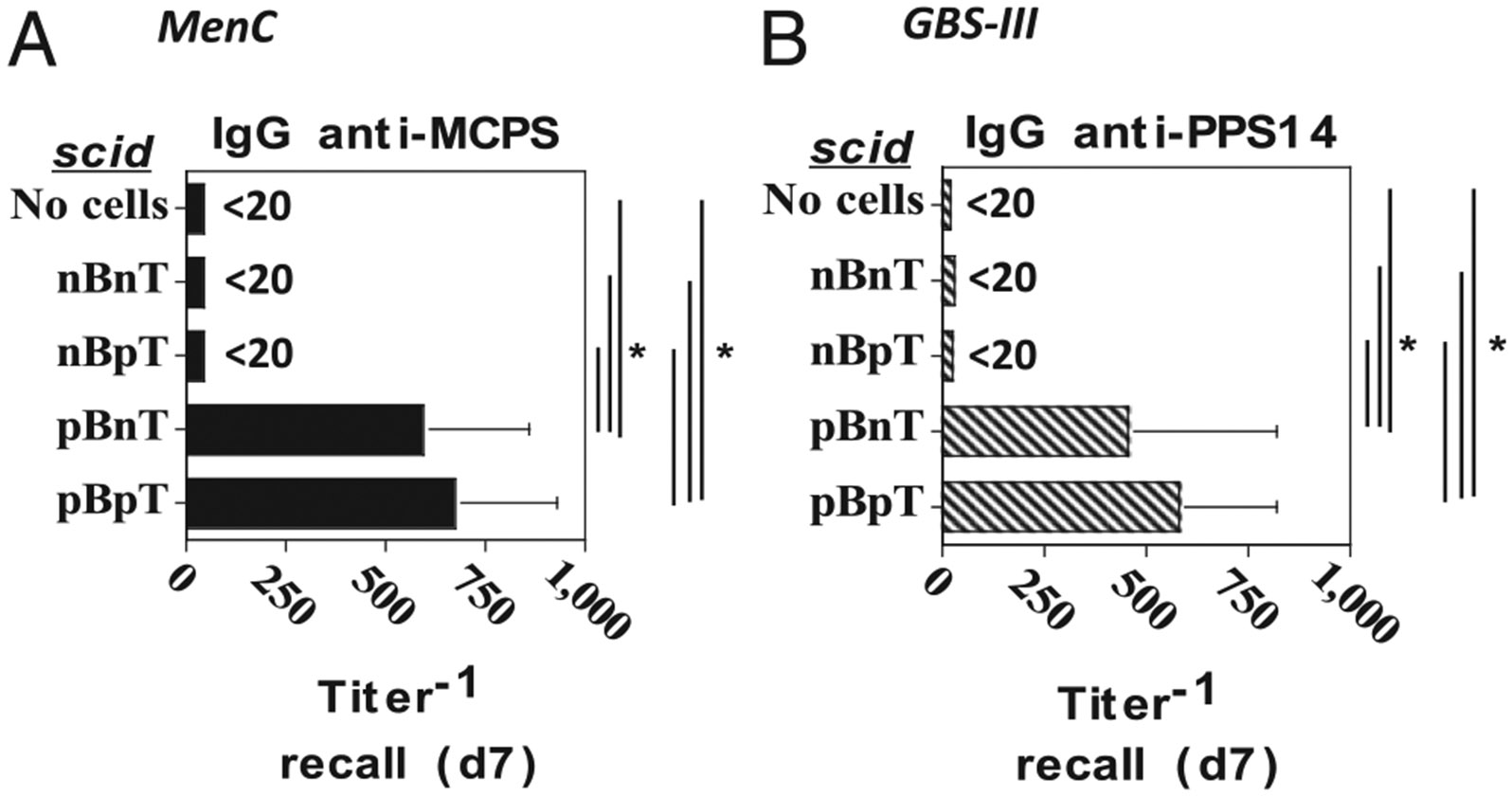
Adoptive transfer of B cells and CD4+ T cells from naive and/or MenC- or GBS-III–primed mice into scid recipients for induction of PS-specific IgG responses. BALB/c mice were immunized with (A) 2 × 108 CFU/mouse of intact heat-inactivated MenC or (B) 2 × 109 CFU/mouse of intact heat-inactivated GBS-III. At day 21 postimmunization, spleen cells were obtained from immunized and unimmunized (naive) mice, and B and CD4+ T cells were isolated by magnetic sorting and then adoptively transferred into scid mice (7 per group), as follows: 1) naive (n) B and naive (n) T cells, 2) nB and primed (p) T cells, 3) pB and nT, and 4) pB and pT. One day following adoptive transfer, scid recipients were boosted with (A) MenC (2 × 108 CFU/mouse) or (B) GBS-III (2 × 109 CFU/mouse), respectively. Serum titers of (A) MCPS-specific or (B) PPS14-specific IgG were determined by ELISA. *p < 0.05.
FIGURE 3.
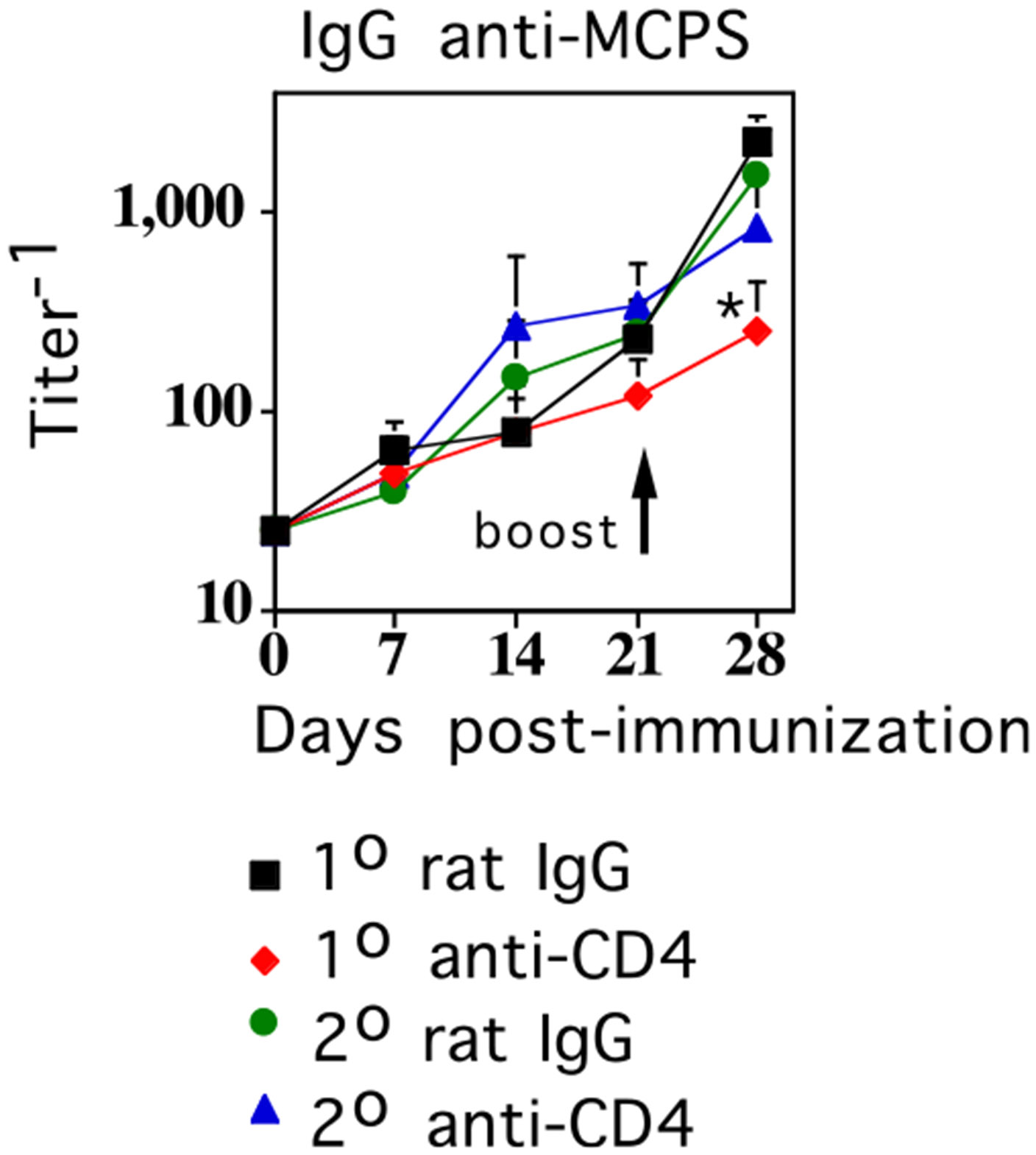
CD4+ T cells are not required during booster immunization with MenC for augmented MCPS-specific IgG responses. BALB/c mice (7 per group) were immunized i.p. with 2 × 108 CFU/mouse of intact heat-inactivated MenC (day 0) and boosted i.p. with the same dose of MenC on day 21. Depleting anti-CD4 mAb (clone GK1.5, 0.5 mg/mouse) or control rat IgG (0.5 mg/mouse) were injected on either day −1 or day 20. Serum titers of MCPS-specific IgG were determined by ELISA (*p ≤ 0.05) (significance between secondary titers of rat IgG-injected versus anti-CD4 mAb-injected groups).
FIGURE 5.
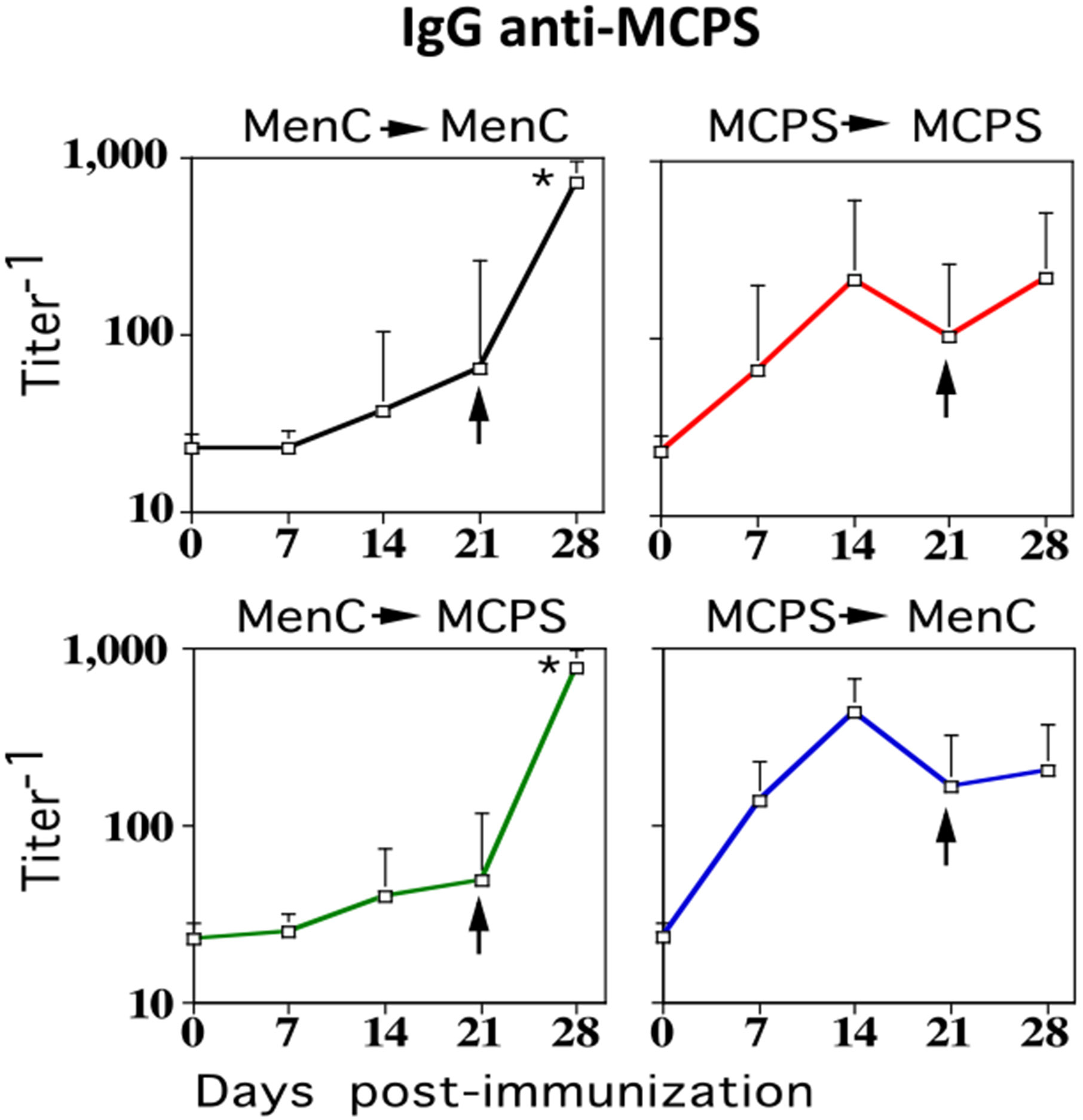
Mice primed with MenC elicit augmented MCPS-specific IgG responses following booster immunization with isolated MCPS. BALB/c mice (7 per group) were immunized i.p. with 2 × 108 CFU/mouse of intact heat-inactivated MenC or 5 μg MCPS adsorbed on 13 μg alum mixed with 25 μg CpG-ODN and were boosted i.p. with the same dose of MenC or MCPS, on day 21. Serum titers of MCPS-specific IgG were determined by ELISA (*p ≤ 0.05) (significance between serum titer on day 21 [preboost] versus day 28 [postboost]).
FIGURE 6.
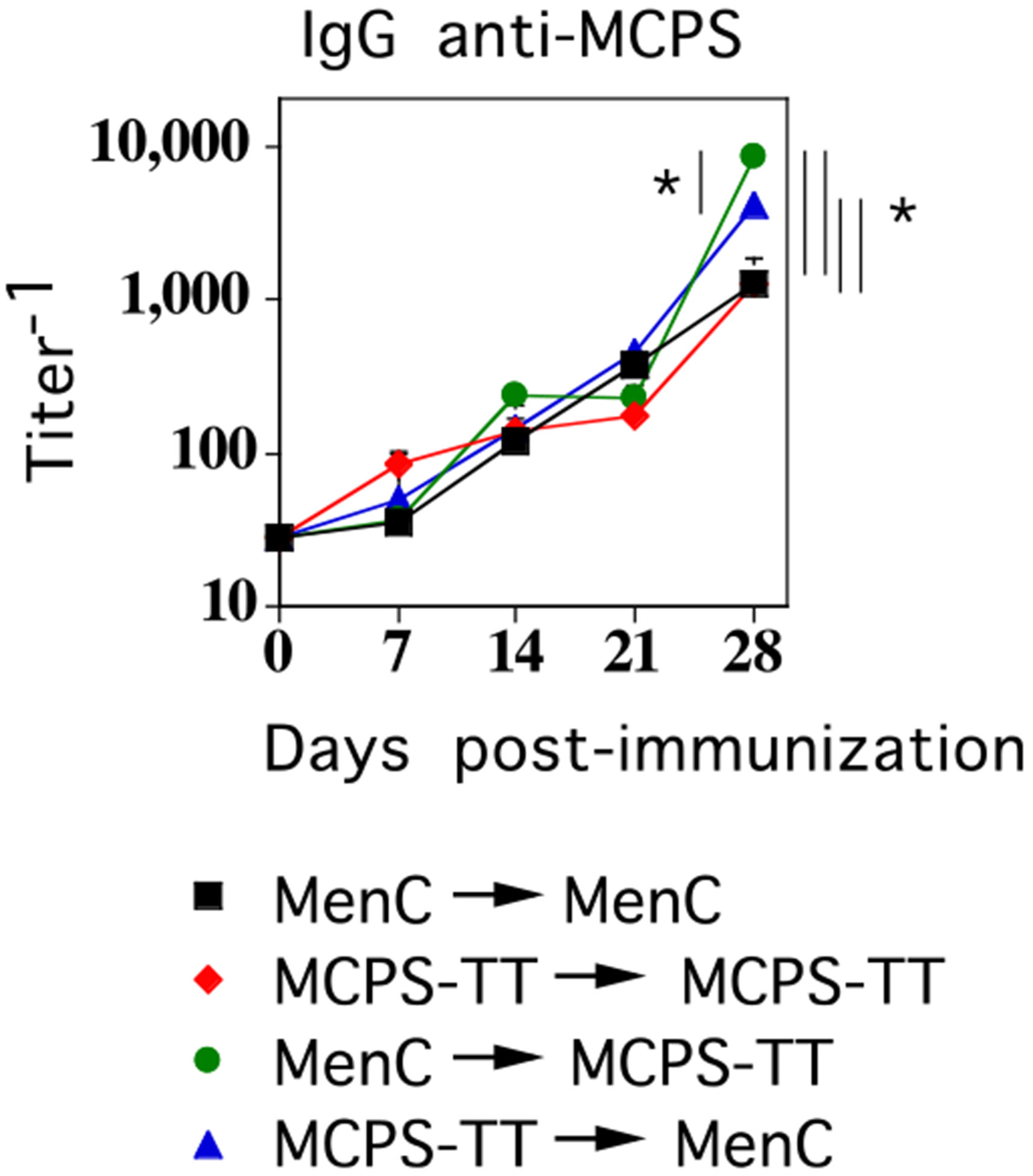
Mice primed with either MenC or MCPS-TT elicit augmented MCPS-specific IgG responses following booster immunization with either MenC or MCPS-TT. BALB/c mice (7 per group) were immunized i.p. with 2 × 108 CFU/mouse of intact, heat-inactivated MenC or 1 μg MCPS-TT adsorbed on 13 μg alum mixed with 25 μg CpG-ODN, and then boosted i.p. with the same dose of MenC or MCPS-TT on day 21. Serum titers of MCPS-specific IgG were determined by ELISA (*p < 0.05) (significant differences in secondary serum titers of MCPS-specific IgG between the indicated groups).
FIGURE 2.
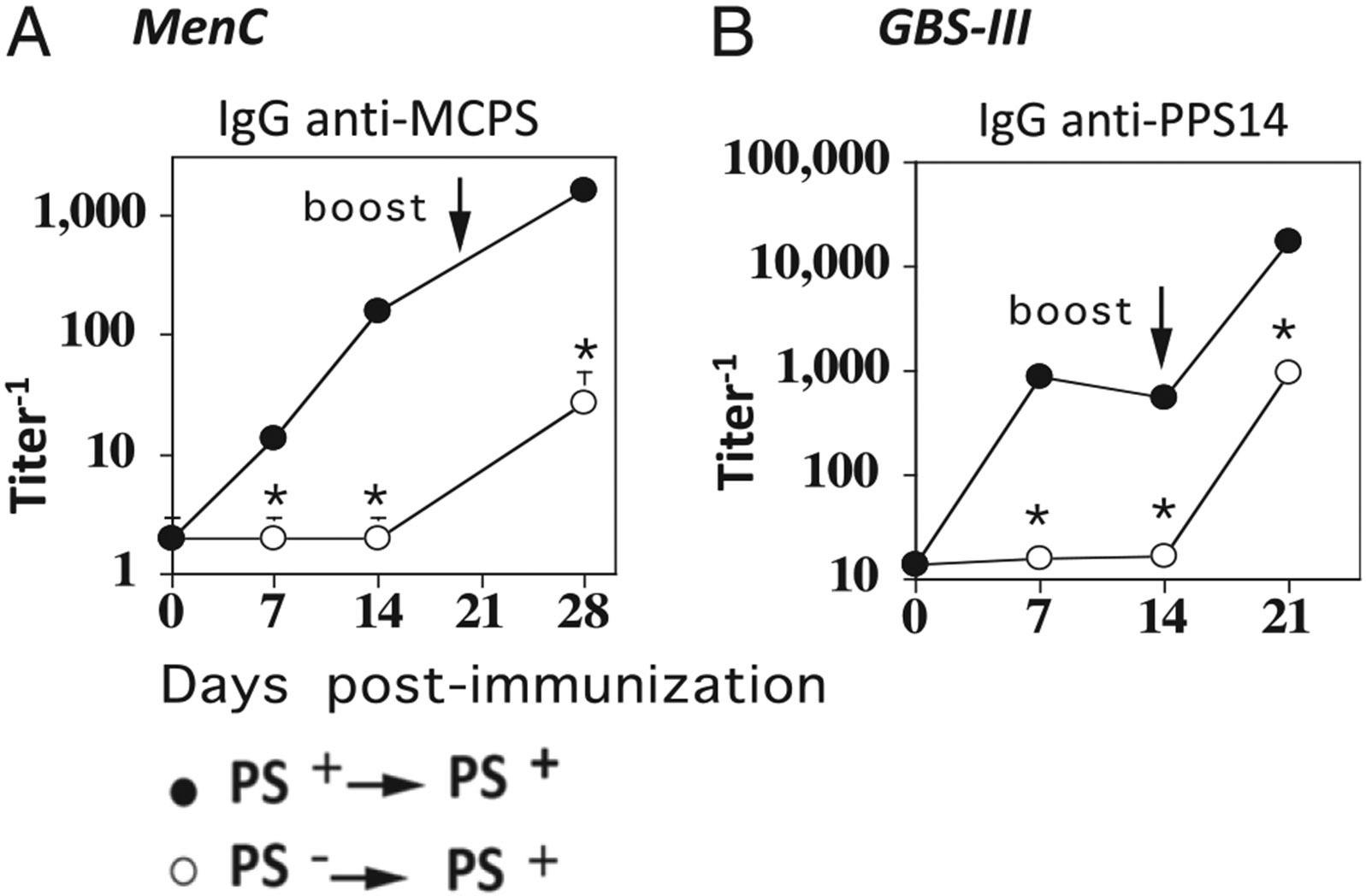
Primary immunization with unencapsulated isogenic mutants of MenC or GBS-III does not augment PS-specific IgG responses following booster immunization with the corresponding PS-encapsulated strains. BALB/c mice (7 per group) were immunized i.p. with (A) 2 × 108 CFU/mouse intact, heat-inactivated PS+ (strain FAM18C+) MenC or PS− (strain FAM18C−) and boosted on day 14 with the same dose of PS+ MenC, or (B) with 2 × 109 CFU/mouse PS+ (strain COH1) GBS-III or PS− (strain COH1-13) GBS-III and boosted on day 14 with the same dose of COH1. Serum titers of (A) MCPS-specific or (B) PPS14-specific IgG were determined by ELISA. *p < 0.05 (significance between primary and secondary titers of PS+ versus PS− primary groups.
FIGURE 4.
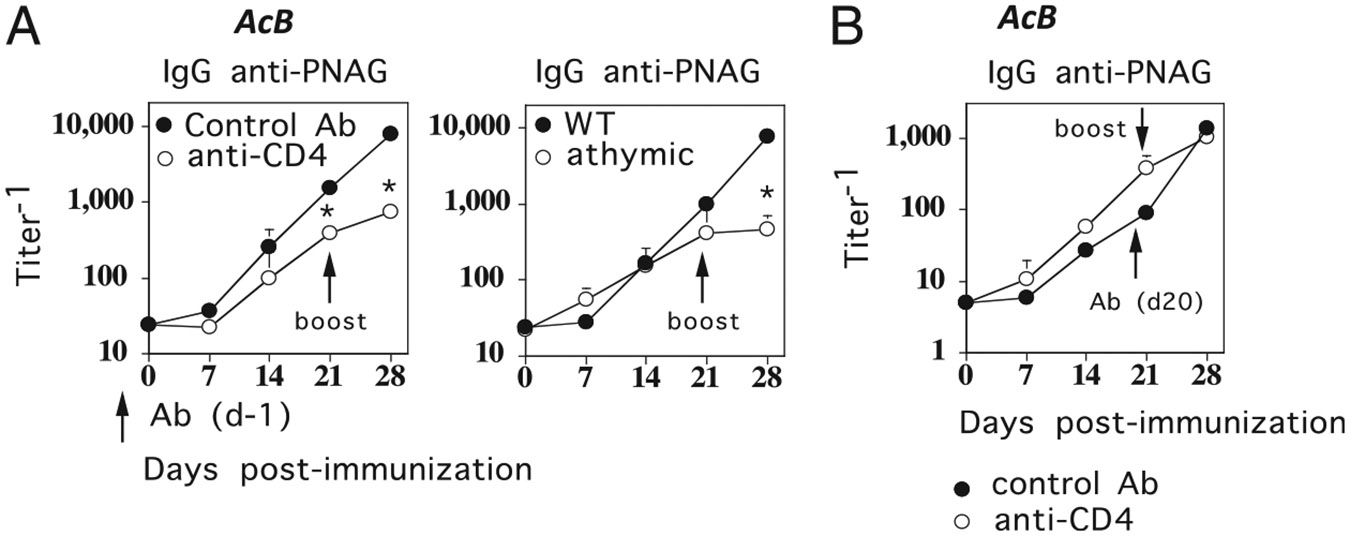
The requirement for CD4+ T cells during primary and booster immunization for elicitation of PS-specific IgG responses to A. baumannii, another GN bacteria, is similar to that for MenC. (A) BALB/c (left) or athymic mice (right) (7 per group) were immunized i.p. (day 0) with 1 × 108 CFU/mouse of intact, heat-inactivated A. baumannii and boosted i.p. with the same dose of A. baumannii on day 21. For BALB/c mice (left), anti-CD4 mAb (clone GK1.5, 0.5 mg/mouse) or control rat IgG (0.5 mg/mouse) were injected on day −1. (B) BALB/c mice (7 per group) were immunized as in (A), except that anti-CD4 mAb and rat IgG were injected on day 20. Serum titers of PNAG-specific IgG were determined by ELISA (*p < 0.05) (significance between rat IgG-injected versus anti-CD4 mAb-injected groups or between wild type [WT] versus athymic groups). Acb, A. baumannii.
Results
Adoptive transfer of B cells and CD4+ T cells from naive and/or MenC- or GBS-III–primed mice into scid recipients for induction of PS-specific IgG responses
Intact, heat-killed MenC (28) and GBS-III (21) both elicit augmented PS-specific IgG responses following booster immunization of primed mice, which required CD4+ T cells during the primary immunization. These data demonstrated that both MenC and GBS-III induce a state of CD4+ T cell–dependent memory for PS-specific IgG responses, but left unresolved whether the specific memory was contained within the B cell and/or CD4+ T cell populations. To determine this, we purified B cells or CD4+ T cells 21 d after immunization of BALB/c mice with MenC or GBS-III. B cells or CD4+ T cells were also purified from unprimed (naive) mice for control purposes. Four separate combinations of B cells plus T cells were injected into B cell/T cell–deficient BALB/c-scid mice, as follows: 1) naive (n)B plus nT, 2) nB plus primed(p)T, 3) pB plus nT, and 4) pB plus pT, followed by corresponding immunization with MenC or GBS-III. The number of B and CD4+ T cells adoptively transferred per mouse was based on an earlier study on T cell–dependent IgG responses in reconstituted B cell/T cell–deficient mice (29). A separate group of scid mice received neither B or T cells as a negative control. Sera were obtained 7 d postimmunization to measure PS-specific IgG titers to determine whether a memory PS-specific IgG response occurred. The MenC PS is the capsular PS referred to as MCPS, whereas the GBS-III PS used is actually the core component of this Ag lacking a terminal sialic acid rendering the Ag structurally identical to the pneumococcal serotype 14 PS (PPS14) (30) (Fig. 1). As expected, scid mice receiving neither B cells nor CD4+ T cells failed to elicit a detectable MCPS-specific IgG response following immunization with MenC cells (Fig. 1A) nor a PPS14-specific IgG response following immunization with GBS-III cells (Fig. 1B). Scid mice receiving B cells from naive BALB/c mice also failed to elicit a detectable MCPS- or PPS14-specific IgG response on day 7 following either MenC (Fig. 1A) or GBS-III priming (Fig. 1B). In contrast, transfer of B cells, but not CD4+ T cells, from primed mice was sufficient to elicit a substantial MCPS-specific IgG response or PPS14-specific IgG response on day 7 following immunization with MenC (Fig. 1A) or to GBS-III (Fig. 1B). Thus, these data strongly suggest that priming of B cells, but not CD4+ T cells, is critical for generating an augmented PS-specific IgG response following a booster immunization with intact bacteria.
Primary immunization with unencapsulated isogenic mutants of MenC or GBS-III does not augment PS-specific IgG responses following booster immunization with the corresponding PS-encapsulated strains
In the next experiment, we wished to further test the hypothesis that CD4+ T cells from primed mice are no more effective than naive CD4+ T cells in promoting a boosted, PS-specific IgG response to secondary bacterial challenge (see Fig. 1). Thus, we immunized mice with unencapsulated isogenic mutants of MenC (MCPS−) or GBS-III (GBS-III/PPS14−) to activate protein-specific CD4+ T cells, without priming PS-specific B cells. Mice were then boosted 14 d later with encapsulated (MCPS+) MenC or (GBS-III/PPS14+) GBS-III, respectively. In this regard, we previously provided evidence that CD4+ T cells specific for bacterial protein can provide help for PS-specific and protein-specific IgG responses to intact bacteria (16). Positive control mice received both a primary and booster immunization with MCPS+ MenC or GBS-III/PPS14+ GBS-III. As expected, mice primed with MCPS− MenC (Fig. 2A) or GBS-III/PPS14− GBS-III (Fig. 2B) made no detectable primary MCPS- or PPS14-specific IgG responses on either day 7 or day 14 postimmunization, whereas substantial primary MCPS- and PPS14-specific IgG responses were induced by the encapsulated bacteria. Upon booster immunization with MCPS+ MenC (Fig. 2A) or GBS-III/PPS14+ GBS-III (Fig. 2B), mice primed with MCPS− MenC or PPS14− GBS-III, respectively, elicited MCPS- and PPS14-specific IgG responses that were not significantly different from the primary responses induced by the corresponding encapsulated bacteria. In contrast, mice primed and rechallenged with MCPS+ MenC or PPS14+ GBS-III elicited highly augmented MCPS- and PPS14-specific IgG responses following booster immunization. These data complement the observations shown in Fig. 1, by demonstrating that CD4+ T cells following primary immunization by themselves do not enhance subsequent PS-specific IgG responses following an initial immunization with an intact bacterium.
CD4+ T cells are not required during booster immunization with MenC for augmented MCPS-specific IgG responses
We previously demonstrated that depletion of CD4+ T cells during booster immunization with GBS-III completely inhibits the augmented PPS14-specific IgG response in GBS-III–primed mice (21). In this regard, we wished to determine whether there was a similar requirement for CD4+ T cells during secondary immunization with MenC for boosted MCPS-specific IgG responses in MenC-primed mice. To determine this, we injected a depleting anti-CD4 mAb or negative control rat IgG, 1 d prior to secondary immunization with MenC, into BALB/c mice that received MenC 21 d earlier, in the absence of any additional Ab treatments. As a control, separate groups of mice received anti-CD4 mAb or rat IgG, 1 d prior to primary immunization with MenC, followed by boosting with MenC, 21 d later in the absence of Ab. These latter two groups were included to confirm functionally that anti-CD4 mAb was effective in depleting CD4+ T cells by blocking the development of MCPS-specific memory for IgG, as previously described (28). Injection of anti-CD4 mAb depleted CD4+ T cells by >95%, as determined 24 h later by flow cytometry, whereas rat IgG had no effect (data not shown). As illustrated in Fig. 3, injection of anti-CD4 mAb prior to secondary immunization with MenC had no effect on the boosted MCPS-specific IgG response, whereas, as expected, injection of anti-CD4 mAb prior to primary immunization with MenC completely inhibited the boosted MCPS-specific IgG response following secondary challenge. Thus, MenC differs from GBS-III (21) in exhibiting no apparent requirement for CD4+ T cells during secondary challenge, to elicit boosted PS-specific IgG responses.
The requirement for CD4+ T cells during primary and booster immunization for elicitation of PS-specific IgG responses to A. baumannii, another GN bacteria, is similar to that for MenC
We next wished to determine whether the dichotomy between GBS-III (GP bacterium) and MenC (GN bacterium) for CD4+ T cell help during primary and secondary PS-specific IgG responses might reflect a more general distinction between GP and GN bacteria. Thus, we used a strain of A. baumannii, a GN bacterium that was engineered to overexpress the PS PNAG on its surface. In a first set of experiments, BALB/c mice were immunized with intact, heat-killed A. baumannii in the presence of either depleting anti-CD4 mAb or negative control rat IgG and then boosted 21 d later with A. baumannii in the absence of additional Ab treatments. Complementary studies were performed in which BALB/c or athymic nude (BALB/c background) mice, which lack T cells, were immunized with A. baumannii and boosted 21 d later. As shown in Fig. 4A, depletion of CD4+ T cells prior to primary immunization with A. baumannii had only a minimal effect on primary PNAG-specific IgG response, similar to MenC (Fig. 3), and in contrast to GBS-III (21). However, similar to both MenC (Fig. 3) and GBS-III (21), CD4+ T cells were required during primary immunization for induction of an augmented PNAG-specific IgG response following booster immunization (day 28 > day 21, p ≤ 0.05). Immunization of athymic nude mice with A. baumannii confirmed the essentially TI nature of the primary PNAG-specific IgG response, but the requirement for T cells to elicit a boosted PNAG-specific IgG response following secondary challenge (Fig. 4A). In a second set of experiments, BALB/c mice were immunized with A. baumannii alone, and then boosted 21 d later with A. baumannii 1 d following injection of depleting anti-CD4 mAb or negative control rat IgG. As indicated in Fig. 4B, anti-CD4 mAb injected prior to a booster immunization had no effect on the augmented PNAG-specific IgG response, similar to MenC and in contrast to GBS-III. These data thus lend support to the notion of a potential, general dichotomy in the role of CD4+ T cells during primary and secondary PS-specific IgG responses to GP versus GN bacteria.
Mice primed with MenC elicit augmented MCPS-specific IgG responses following booster immunization with isolated MCPS
The lack of a requirement for CD4+ T cells following booster immunization with MenC for induction of boosted PS-specific IgG suggested the possibility that a boosted PS-specific IgG response could be elicited in MenC-primed mice, by isolated MCPS, a TI Ag. To determine this, mice were primed with MenC and boosted with either MCPS or MenC as a positive control. Because MCPS is a TI Ag that does not by itself elicit memory MCPS-specific IgG responses (28), as further controls, separate groups of mice were also primed with MCPS and then boosted with either MCPS or MenC. MCPS was injected with alum plus CpG-ODN. As depicted in Fig. 5, in MenC-primed, but not MCPS-primed mice, booster immunization with either MCPS or MenC elicited augmented MCPS-specific IgG responses. We previously reported that contaminating TLR ligands in preparations of isolated pneumococcal PS were critical for induction of PS-specific IgG in vivo (31). We also previously demonstrated that the preparation of MCPS used in this study elicited PS-specific IgG in vivo, when injected in saline only, thus suggesting the possibility of contaminating TLR ligands also playing a stimulatory role (28). To determine whether contaminating TLR ligands might be critical for the augmented PS-specific IgG response following booster immunization with isolated MCPS in MenC-primed mice, we used a second MCPS preparation that was unable to elicit MCPS-specific IgG responses in vivo (32) and was confirmed to lack innate stimulating activity on macrophages in vitro (data not shown). Using this MCPS preparation, which was injected only in saline for booster immunization, a similar level of augmentation in the MCPS-specific IgG response was observed in MenC-primed mice (data not shown), strongly arguing against a critical role for contaminating TLR ligands in eliciting an augmented booster IgG response from primed MCPS-specific B cells.
Mice primed with either MenC or a soluble conjugate of MCPS-TT elicit augmented MCPS-specific IgG responses following booster immunization with either MenC or MCPS-TT
Previous studies using intact heat-killed S. pneumoniae and a soluble pneumococcal conjugate vaccine strongly suggested that PS-specific IgG responses were derived from marginal zone and follicular B cells, respectively (18, 19), with elicitation of distinct idiotypes on the PS-specific (serotype 14) IgG (17). Indeed, mice primed with intact serotype 14 S. pneumoniae do not elicit an augmented PS-specific IgG response following booster immunization with a soluble type 14 pneumococcal conjugate vaccine, in which the carrier protein is pneumococcal surface protein A (33), but do elicit an augmented response following booster immunization with intact GBS-III expressing type 14 PS (21). These findings suggested that mice primed with a MCPS conjugate vaccine might not elicit an augmented MCPS-specific IgG response following booster immunization with MenC, and vice versa, because these responses might be derived from distinct B cell subsets. However, as illustrated in Fig. 6, augmented MCPS-specific IgG responses were observed regardless of whether MenC or MCPS-TT was used for priming and regardless of whether MenC or MCPS-TT was used for booster immunization. Of interest, of the four groups, mice primed with MenC and subsequently challenged with MCPS-TT or primed with MCPS-TT and subsequently challenged with MenC gave significantly higher secondary serum titers of MCPS-specific IgG compared with the other two groups. In addition, mice primed with MenC and subsequently challenged with MCPS-TT demonstrated a significantly higher MCPS-specific IgG titer compared with mice primed with MCPS-TT and challenged with MenC. Because boosted MCPS-specific IgG responses in primed mice do not require CD4+ T cells following booster immunization (see Fig. 3), the presence of TT in the MCPS-TT conjugate, but not in intact MenC, should not preclude augmented MCPS-specific IgG responses when using MenC and MCPS-TT for immunization in sequence. These data demonstrate further potential differences between GP and GN bacteria that may have important implications for future vaccine design.
Discussion
We previously demonstrated that capsular PS-specific IgG responses to intact extracellular bacteria in vivo are markedly influenced by the architecture and/or the composition of the underlying subcapsular domain (15, 21, 24, 28). Thus, the primary PS-specific IgG response to intact S. pneumoniae peaks relatively early (by day 7) is dependent on CD4+ T cells, and demonstrates no augmentation following booster immunization (24). In contrast, the primary PS-specific IgG response to intact MenC peaks relatively late (day 14–21) is independent of CD4+ T cells, and demonstrates substantial augmentation following booster challenge (28). Moreover, the primary PS-specific IgG response to GBS-III that expresses PS biochemically similar to PS expressed by S. pneumoniae (capsular type 14), in which there is a shared core structure, also peaks by day 7 and is dependent on CD4+ T cells, like S. pneumoniae, but in contrast demonstrates a highly augmented PPS14-specific IgG booster response (21). This demonstrates that structurally similar capsular PS expressed by two distinct bacteria may be associated with different PS-specific IgG responses to their shared epitopes, in vivo. The induction of memory for PS-specific IgG in response to GBS-III and MenC requires CD4+ T cells during the primary immunization. These data left unresolved whether memory for PS-specific IgG was contained within the B cell and/or memory T cell population, whether CD4+ T cells were required for boosted IgG responses during secondary challenge, and whether a more general dichotomy might exist in how induction of PS-specific IgG is regulated in response to GP versus GN bacteria.
In this study, we demonstrate that induction of memory for PS-specific IgG in response to either MenC or GBS-III is contained within the B cell, but not CD4+ T cell population. We demonstrated earlier that the augmented PPS14-specific IgG response to the GP bacterium, GBS-III expressing PPS14 as its core capsular component also requires CD4+ T cells during the booster immunization (21). This is consistent with the lack of an augmented PS-specific IgG response in GBS-III–primed mice following booster immunization with isolated PPS14, a TI Ag. By contrast, in this study, we show that the augmented MCPS-specific IgG response to MenC is independent of CD4+ T cells following the booster immunization. This is consistent with the augmented MCPS-specific IgG response observed in MenC-primed mice following booster immunization with isolated MCPS, also a TI Ag, or a soluble covalent conjugate of MCPS and TT. Results similar to that obtained with MenC are also observed using another GN bacteria, A. baumannii expressing PNAG, that is, a primary PNAG-specific IgG response that peaks relatively late (by day 14–21) and that is independent of CD4+ T cells, and an augmented PNAG-specific IgG response following booster immunization with A. baumannii that requires CD4+ T cells during the primary, but not during the booster immunization. These data further support the notion that the regulation of capsular PS-specific IgG responses to intact bacteria is dependent on the nature of the subcapsular domain, and suggest a potential, general dichotomy between GP and GN bacteria.
The distinct structural and compositional differences between GP and GN bacteria may play an important role in distinguishing anti-PS Ab responses among these two subgroups of pathogens. Thus, PS expressed by GP bacteria are covalently linked to a thick, underlying cell wall peptidoglycan, to which a number of proteins are also covalently attached (34, 35). PS expressed by GN bacteria, which express a thin peptidoglycan cell wall, is covalently attached to the acyl glycerol moiety of the outer membrane, which contains highly immunogenic proteins, including porins, and LPS, a potentially potent stimulator of the innate immune system depending on its biochemical composition (36, 37). Shedding of vesicles containing the outer membrane/PS complex is a unique property of GN bacteria that may have distinct immunologic consequences for the anti-PS response (38, 39). How these structural and biochemical differences between GP and GN bacteria lead to differences in the cellular regulation of PS-specific IgG responses remains to be determined. One possible difference may lie in the different TLR ligands expressed by these two classes of bacteria. In this regard, previous studies from our laboratory demonstrated a key role for endogenous TLR2 and TLR4 in augmenting IgG responses to S. pneumoniae (40) and N. meningitidis (28), respectively.
Early studies using isolated haptenated PS observed that booster immunization fails to augment specific serum Ab titers unless primed B cells are first adoptively transferred into a naive host (41, 42). The requirement for adoptive transfer to observe an augmented Ab response from TI-specific memory B cells was subsequently shown to be due to an inhibitory effect of circulating Ag-specific IgG induced during primary immunization (43). TI, in contrast to T cell dependent, memory B cells are derived from the B-1b subset, and memory is manifested by rapid induction of specific IgM, but not IgG following secondary immunization (44, 45). In the current study, the PS-specific B cells isolated following immunization with intact bacteria, in contrast to TI memory B cells, mediate augmented PS-specific IgG responses following booster immunization without the need for adoptive transfer to circumvent inhibitory Ag-specific IgG, and require CD4+ T cell help for their generation, thus strongly suggesting their derivation from a different mechanistic pathway.
Our current data in mice may have relevance for vaccination of humans with PS-based vaccines. IgG+CD27+ human memory B cells specific for MCPS have been demonstrated following vaccination with a MenC-TT vaccine. These memory B cells can be subsequently triggered by intact MenC to differentiate into plasma cells, similar to our data, although in this case requiring contact-dependent (in part through CD40) but noncognate help from bystander CD4+ T cells specific for MenC proteins (46). Meningococcal, Haemophilus influenzae type b, and pneumococcal conjugate vaccines also induce memory B cells that can be subsequently triggered for PS-specific IgG responses by the respective unconjugated PS, implying that only memory B cells, and not T cells, play a key role in these IgG responses (47-53). It has been proposed that natural priming of humans by exposure to PS-encapsulated bacteria may induce memory B cells that can be subsequently triggered in a TI manner, because vaccination of adults with capsular PS vaccines shows features of a secondary Ab (i.e., hypermutated IgG) (54-58).
Our data using intact MenC are similar to a previous study on the CD4+ T cell requirements for eliciting a secondary, memory response to human CMV (HCMV) or tick-borne encephalitis virus. Thus, transfer of B cells from virus-primed mice into B cell/T cell–deficient RAG-1−/− recipients resulted in an Ag-specific memory response, including rapid IgG induction with long-term persistence, that was independent of CD4+ T cells during the secondary challenge (59). Furthermore, transfer of memory B cells into immunocompetent mice demonstrated no evidence of Th cell–mediated enhancement of the memory IgG response. Finally, similar to our study with MenC, secondary immunization with HCMV, of mice primed with a soluble HCMV-derived protein (i.e., glycoprotein B), elicited a gB-specific memory response. A more recent study also demonstrated that secondary IgG anti-Env responses to virus-like particles of SIV or adenoviral vectors expressing Env were TI (60). These data are in contrast to experimental models using soluble proteins and hapten-carrier conjugates, in which CD4+ T cells are required during secondary immunization to activate specific memory B cells for IgG production (61). In contrast, an earlier study using vesicular stomatitis virus or lymphocytic choriomeningitis virus (62) demonstrated a requirement for T cells for elicitation of the secondary response, although this may have reflected the brief time (20 min) after which virus was injected following adoptive transfer of memory B cells (59).
Acknowledgments
This work was supported by the Uniformed Services University of the Health Sciences Research and Education Endowment Fund (to C.M.S.).
Abbreviations:
- GBS-III
group B Streptococcus, capsular type III
- GN
Gram-negative
- GP
Gram-positive
- HCMV
human CMV
- LB
lysogeny broth
- MCPS
serogroup C capsular PS of N. meningitidis
- MenC
N. meningitidis, capsular serogroup C
- ODN
oligodeoxynucleotide
- PNAG
poly-N-acetylglucosamine
- PPS14
type 14 capsular polysaccharide of S. pneumoniae
- PS
polysaccharide
- TI
T cell independent
- TT
tetanus toxoid
Footnotes
The opinions expressed herein are those of the authors and are not necessarily representative of those of the Uniformed Services University of the Health Sciences, the Department of Defense, or the United States Army, Navy, or Air Force.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Edwards MS, and Baker CJ. 2005. Group B streptococcal infections in elderly adults. Clin. Infect. Dis 41: 839–847. [DOI] [PubMed] [Google Scholar]
- 2.Ferrieri P. 1990. Neonatal susceptibility and immunity to major bacterial pathogens. Rev. Infect. Dis 12(Suppl. 4): S394–S400. [DOI] [PubMed] [Google Scholar]
- 3.Klein Klouwenberg P, and Bont L. 2008. Neonatal and infantile immune responses to encapsulated bacteria and conjugate vaccines. Clin. Dev. Immunol 2008: 628963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tikhomirov E, Santamaria M, and Esteves K. 1997. Meningococcal disease: public health burden and control. World Health Stat. Q 50: 170–177. [PubMed] [Google Scholar]
- 5.AlonsoDeVelasco E, Verheul AF, Verhoef J, and Snippe H. 1995. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol. Rev 59: 591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avci FY, and Kasper DL. 2010. How bacterial carbohydrates influence the adaptive immune system. Annu. Rev. Immunol 28: 107–130. [DOI] [PubMed] [Google Scholar]
- 7.Mond JJ, Lees A, and Snapper CM. 1995. T cell-independent antigens type 2. Annu. Rev. Immunol 13: 655–692. [DOI] [PubMed] [Google Scholar]
- 8.Vos Q, Lees A, Wu ZQ, Snapper CM, and Mond JJ. 2000. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol. Rev 176: 154–170. [DOI] [PubMed] [Google Scholar]
- 9.Harding CV, Roof RW, Allen PM, and Unanue ER. 1991. Effects of pH and polysaccharides on peptide binding to class II major histocompatibility complex molecules. Proc. Natl. Acad. Sci. USA 88: 2740–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishioka GY, Lamont AG, Thomson D, Bulbow N, Gaeta FC, Sette A, and Grey HM. 1992. MHC interaction and T cell recognition of carbohydrates and glycopeptides. J. Immunol 148: 2446–2451. [PubMed] [Google Scholar]
- 11.Guttormsen HK, Wetzler LM, Finberg RW, and Kasper DL. 1998. Immunologic memory induced by a glycoconjugate vaccine in a murine adoptive lymphocyte transfer model. Infect. Immun 66: 2026–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guttormsen HK, Sharpe AH, Chandraker AK, Brigtsen AK, Sayegh MH, and Kasper DL. 1999. Cognate stimulatory B-cell-T-cell interactions are critical for T-cell help recruited by glycoconjugate vaccines. Infect. Immun 67: 6375–6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollard AJ, Perrett KP, and Beverley PC. 2009. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat. Rev. Immunol 9: 213–220. [DOI] [PubMed] [Google Scholar]
- 14.Snapper CM 2012. Mechanisms underlying in vivo polysaccharide-specific immunoglobulin responses to intact extracellular bacteria. Ann. N. Y. Acad. Sci 1253: 92–101. [DOI] [PubMed] [Google Scholar]
- 15.Snapper CM 2016. Differential regulation of polysaccharide-specific antibody responses to isolated polysaccharides, conjugate vaccines, and intact Gram-positive versus Gram-negative extracellular bacteria. Vaccine. In press. [DOI] [PubMed] [Google Scholar]
- 16.Colino J, Duke L, and Snapper CM. 2013. Noncovalent association of protein and capsular polysaccharide on bacteria-sized latex beads as a model for polysaccharide-specific humoral immunity to intact Gram-positive extracellular bacteria. J. Immunol 191: 3254–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colino J, Duke L, Arjunaraja S, Chen Q, Liu L, Lucas AH, and Snapper CM. 2012. Differential idiotype utilization for the in vivo type 14 capsular polysaccharide-specific Ig responses to intact Streptococcus pneumoniae versus a pneumococcal conjugate vaccine. J. Immunol 189: 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colino J, Chattopadhyay G, Sen G, Chen Q, Lees A, Canaday DH, Rubtsov A, Torres R, and Snapper CM. 2009. Parameters underlying distinct T cell-dependent polysaccharide-specific IgG responses to an intact Gram-positive bacterium versus a soluble conjugate vaccine. J. Immunol 183: 1551–1559. [DOI] [PubMed] [Google Scholar]
- 19.Chattopadhyay G, Khan AQ, Sen G, Colino J, DuBois W, Rubtsov A, Torres RM, Potter M, and Snapper CM. 2007. Transgenic expression of Bcl-xL or Bcl-2 by murine B cells enhances the in vivo antipolysaccharide, but not antiprotein, response to intact Streptococcus pneumoniae. J. Immunol 179: 7523–7534. [DOI] [PubMed] [Google Scholar]
- 20.McHeyzer-Williams M, Okitsu S, Wang N, and McHeyzer-Williams L. 2011. Molecular programming of B cell memory. Nat. Rev. Immunol 12: 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arjunaraja S, Paoletti LC, and Snapper CM. 2012. Structurally identical capsular polysaccharide expressed by intact group B streptococcus versus Streptococcus pneumoniae elicits distinct murine polysaccharide-specific IgG responses in vivo. J. Immunol 188: 5238–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kocabas C, Katsenelson N, Kanswal S, Kennedy MN, Cui X, Blake MS, Segal DM, and Akkoyunlu M. 2007. Neisseria meningitidis type C capsular polysaccharide inhibits lipooligosaccharide-induced cell activation by binding to CD14. Cell. Microbiol 9: 1297–1310. [DOI] [PubMed] [Google Scholar]
- 23.Choi AH, Slamti L, Avci FY, Pier GB, and Maira-Litrán T. 2009. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J. Bacteriol 191: 5953–5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan AQ, Lees A, and Snapper CM. 2004. Differential regulation of IgG anti-capsular polysaccharide and antiprotein responses to intact Streptococcus pneumoniae in the presence of cognate CD4+ T cell help. J. Immunol 172: 532–539. [DOI] [PubMed] [Google Scholar]
- 25.Brisson JR, Uhrinova S, Woods RJ, van der Zwan M, Jarrell HC, Paoletti LC, Kasper DL, and Jennings HJ. 1997. NMR and molecular dynamics studies of the conformational epitope of the type III group B Streptococcus capsular polysaccharide and derivatives. Biochemistry 36: 3278–3292. [DOI] [PubMed] [Google Scholar]
- 26.Sen G, Flora M, Chattopadhyay G, Klinman DM, Lees A, Mond JJ, and Snapper CM. 2004. The critical DNA flanking sequences of a CpG oligo-deoxynucleotide, but not the 6 base CpG motif, can be replaced with RNA without quantitative or qualitative changes in Toll-like receptor 9-mediated activity. Cell. Immunol 232: 64–74. [DOI] [PubMed] [Google Scholar]
- 27.Chen Q, Cannons JL, Paton JC, Akiba H, Schwartzberg PL, and Snapper CM. 2008. A novel ICOS-independent, but CD28- and SAP-dependent, pathway of T cell-dependent, polysaccharide-specific humoral immunity in response to intact Streptococcus pneumoniae versus pneumococcal conjugate vaccine. J. Immunol 181: 8258–8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arjunaraja S, Massari P, Wetzler LM, Lees A, Colino J, and Snapper CM. 2012. The nature of an in vivo anti-capsular polysaccharide response is markedly influenced by the composition and/or architecture of the bacterial subcapsular domain. J. Immunol 188: 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasilevsky S, Chattopadhyay G, Colino J, Yeh TJ, Chen Q, Sen G, and Snapper CM. 2008. B and CD4+ T-cell expression of TLR2 is critical for optimal induction of a T-cell-dependent humoral immune response to intact Streptococcus pneumoniae. Eur. J. Immunol 38: 3316–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guttormsen HK, Baker CJ, Nahm MH, Paoletti LC, Zughaier SM, Edwards MS, and Kasper DL. 2002. Type III group B streptococcal polysaccharide induces antibodies that cross-react with Streptococcus pneumoniae type 14. Infect. Immun 70: 1724–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sen G, Khan AQ, Chen Q, and Snapper CM. 2005. In vivo humoral immune responses to isolated pneumococcal polysaccharides are dependent on the presence of associated TLR ligands. J. Immunol 175: 3084–3091. [DOI] [PubMed] [Google Scholar]
- 32.Kanswal S, Katsenelson N, Allman W, Uslu K, Blake MS, and Akkoyunlu M. 2011. Suppressive effect of bacterial polysaccharides on BAFF system is responsible for their poor immunogenicity. J. Immunol 186: 2430–2443. [DOI] [PubMed] [Google Scholar]
- 33.Chattopadhyay G, Chen Q, Colino J, Lees A, and Snapper CM. 2009. Intact bacteria inhibit the induction of humoral immune responses to bacterial-derived and heterologous soluble T cell-dependent antigens. J. Immunol 182: 2011–2019. [DOI] [PubMed] [Google Scholar]
- 34.Sørensen UB, Henrichsen J, Chen HC, and Szu SC. 1990. Covalent linkage between the capsular polysaccharide and the cell wall peptidoglycan of Streptococcus pneumoniae revealed by immunochemical methods. Microb. Pathog 8: 325–334. [DOI] [PubMed] [Google Scholar]
- 35.Jedrzejas MJ 2004. Extracellular virulence factors of Streptococcus pneumoniae. Front. Biosci 9: 891–914. [DOI] [PubMed] [Google Scholar]
- 36.Whitfield C 2006. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem 75: 39–68. [DOI] [PubMed] [Google Scholar]
- 37.Rietschel ET, Brade H, Holst O, Brade L, Müller-Loennies S, Mamat U, Zähringer U, Beckmann F, Seydel U, Brandenburg K, et al. 1996. Bacterial endotoxin: chemical constitution, biological recognition, host response, and immunological detoxification. Curr. Top. Microbiol. Immunol 216: 39–81. [DOI] [PubMed] [Google Scholar]
- 38.Beuvery EC, Miedema F, van Delft RW, Haverkamp J, Leussink AB, te Pas BJ, Teppema KS, and Tiesjema RH. 1983. Preparation and physicochemical and immunological characterization of polysaccharide-outer membrane protein complexes of Neisseria meningitidis. Infect. Immun 40: 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Latz E, Franko J, Golenbock DT, and Schreiber JR. 2004. Haemophilus influenzae type b-outer membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human Toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J. Immunol 172: 2431–2438. [DOI] [PubMed] [Google Scholar]
- 40.Khan AQ, Chen Q, Wu ZQ, Paton JC, and Snapper CM. 2005. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on Toll-like receptor 2. Infect. Immun 73: 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hosokawa T, Tanaka Y, Aoike A, Kawai K, and Muramatsu S. 1984. Studies on B-cell memory. III. T-dependent aspect of B memory generation in mice immunized with T-independent type-2(TI-2) antigen. Immunology 53: 97–104. [PMC free article] [PubMed] [Google Scholar]
- 42.Le Moal MA, and Truffa-Bachi P. 1985. Immune memory expression to Tnp-Ficoll in CB.20 mice: evidence for a multigenic control. Cell. Immunol 95: 428–436. [DOI] [PubMed] [Google Scholar]
- 43.Obukhanych TV, and Nussenzweig MC. 2006. T-independent type II immune responses generate memory B cells. J. Exp. Med 203: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, and Gerstein RM. 2004. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21: 379–390. [DOI] [PubMed] [Google Scholar]
- 45.Haas KM, Poe JC, Steeber DA, and Tedder TF. 2005. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23: 7–18. [DOI] [PubMed] [Google Scholar]
- 46.Clarke ET, Williams NA, Findlow J, Borrow R, Heyderman RS, and Finn A. 2013. Polysaccharide-specific memory B cells generated by conjugate vaccines in humans conform to the CD27+IgG+ isotype-switched memory B cell phenotype and require contact-dependent signals from bystander T cells activated by bacterial proteins to differentiate into plasma cells. J. Immunol 191: 6071–6083. [DOI] [PubMed] [Google Scholar]
- 47.Borrow R, Fox AJ, Richmond PC, Clark S, Sadler F, Findlow J, Morris R, Begg NT, and Cartwright KA. 2000. Induction of immunological memory in UK infants by a meningococcal A/C conjugate vaccine. Epidemiol. Infect 124: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Granoff DM, Holmes SJ, Osterholm MT, McHugh JE, Lucas AH, Anderson EL, Belshe RB, Jacobs JL, Medley F, and Murphy TV. 1993. Induction of immunologic memory in infants primed with Haemophilus influenzae type b conjugate vaccines. J. Infect. Dis 168: 663–671. [DOI] [PubMed] [Google Scholar]
- 49.MacDonald NE, Halperin SA, Law BJ, Forrest B, Danzig LE, and Granoff DM. 1998. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers: a randomized controlled trial. JAMA 280: 1685–1689. [DOI] [PubMed] [Google Scholar]
- 50.Richmond P, Borrow R, Miller E, Clark S, Sadler F, Fox A, Begg N, Morris R, and Cartwright K. 1999. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J. Infect. Dis 179: 1569–1572. [DOI] [PubMed] [Google Scholar]
- 51.Weinberg GA, Einhorn MS, Lenoir AA, Granoff PD, and Granoff DM. 1987. Immunologic priming to capsular polysaccharide in infants immunized with Haemophilus influenzae type b polysaccharide-Neisseria meningitidis outer membrane protein conjugate vaccine. J. Pediatr 111: 22–27. [DOI] [PubMed] [Google Scholar]
- 52.Breukels MA, Rijkers GT, Voorhorst-Ogink MM, Zegers BJ, and Sanders LA. 1999. Pneumococcal conjugate vaccine primes for polysaccharide-inducible IgG2 antibody response in children with recurrent otitis media acuta. J. Infect. Dis 179: 1152–1156. [DOI] [PubMed] [Google Scholar]
- 53.Granoff DM, and Holmes SJ. 1991. Comparative immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugate vaccines. Vaccine 9: S30–S34; discussion S42-S43. [DOI] [PubMed] [Google Scholar]
- 54.Barington T, Hougs L, Juul L, Madsen HO, Ryder LP, Heilmann C, and Svejgaard A. 1996. The progeny of a single virgin B cell predominates the human recall B cell response to the capsular polysaccharide of Haemophilus influenzae type b. J. Immunol 157: 4016–4027. [PubMed] [Google Scholar]
- 55.Baxendale HE, Davis Z, White HN, Spellerberg MB, Stevenson FK, and Goldblatt D. 2000. Immunogenetic analysis of the immune response to pneumococcal polysaccharide. Eur. J. Immunol 30: 1214–1223. [DOI] [PubMed] [Google Scholar]
- 56.Hougs L, Juul L, Ditzel HJ, Heilmann C, Svejgaard A, and Barington T. 1999. The first dose of a Haemophilus influenzae type b conjugate vaccine reactivates memory B cells: evidence for extensive clonal selection, intraclonal affinity maturation, and multiple isotype switches to IgA2. J. Immunol 162: 224–237. [PubMed] [Google Scholar]
- 57.Lucas AH, and Granoff DM. 2001. Imperfect memory and the development of Haemophilus influenzae type B disease. Pediatr. Infect. Dis. J 20: 235–239. [DOI] [PubMed] [Google Scholar]
- 58.Zhou J, Lottenbach KR, Barenkamp SJ, Lucas AH, and Reason DC. 2002. Recurrent variable region gene usage and somatic mutation in the human antibody response to the capsular polysaccharide of Streptococcus pneumoniae type 23F. Infect. Immun 70: 4083–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hebeis BJ, Klenovsek K, Rohwer P, Ritter U, Schneider A, Mach M, and Winkler TH. 2004. Activation of virus-specific memory B cells in the absence of T cell help. J. Exp. Med 199: 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nabi G, Temchura V, Grossmann C, Kuate S, Tenbusch M, and Überla K. 2012. T cell independent secondary antibody responses to the envelope protein of simian immunodeficiency virus. Retrovirology 9: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vieira P, and Rajewsky K. 1990. Persistence of memory B cells in mice deprived of T cell help. Int. Immunol 2: 487–494. [DOI] [PubMed] [Google Scholar]
- 62.Ochsenbein AF, Pinschewer DD, Sierro S, Horvath E, Hengartner H, and Zinkernagel RM. 2000. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc. Natl. Acad. Sci. USA 97: 13263–13268. [DOI] [PMC free article] [PubMed] [Google Scholar]


