Abstract
Memory is a hallmark of immunity. Memory carried by antibodies is largely responsible for protection against reinfection with most known acutely lethal infectious agents and is the basis for most clinically successful vaccines. However, the nature of long-term B cell and antibody memory is still unclear. B cell memory was studied here after infection of mice with the rabies-like cytopathic vesicular stomatitis virus, the noncytopathic lymphocytic choriomeningitis virus (Armstrong and WE), and after immunization with various inert viral antigens inducing naive B cells to differentiate either to plasma cells or memory B cells in germinal centers of secondary lymphoid organs. The results show that in contrast to very low background levels against internal viral antigens, no significant neutralizing antibody memory was observed in the absence of antigen and suggest that memory B cells (i) are long-lived in the absence of antigen, nondividing, and relatively resistant to irradiation, and (ii) must be stimulated by antigen to differentiate to short-lived antibody-secreting plasma cells, a process that is also efficient in the bone marrow and always depends on radiosensitive, specific T help. Therefore, for vaccines to induce long-term protective antibody titers, they need to repeatedly provide, or continuously maintain, antigen in minimal quantities over a prolonged time period in secondary lymphoid organs or the bone marrow for sufficient numbers of long-lived memory B cells to mature to short-lived plasma cells.
B cell memory is characterized by (i) the persistence of elevated titers of specific antibodies, and (ii) the persistence of “memory” B cells (1, 2). Although protection against reinfection with hematogenically spreading pathogens correlates with increased frequencies of B or T helper cells, it critically depends on preexistent elevated titers of neutralizing antibodies (3, 4).
Because the half-life of serum Ig is less than 3 weeks (5, 6), continuous antibody production is necessary to maintain IgG antibody titers over a prolonged period. The following models have been proposed to explain the persistence of long-term antibody titers. They can be grouped into antigen-independent and -dependent mechanisms. Antigen independence is assumed for (i) “memory” B cells with special “memory” qualities that need fewer signals to mature to plasma cells (1, 7), and (ii) for plasma cells producing antibodies that are long-lived in the bone marrow in the absence of antigen (8–12). Alternatively, antigen dependence is postulated to be necessary for memory B cells to differentiate to antibody secreting, relatively short-lived plasma cells maintaining elevated memory antibody titers as follows: (i) persisting antigen on follicular dendritic cells of germinal centers in secondary lymphoid organs stimulates memory B cells (2, 13–15); (ii) repetitive stimulation of B cells occurs via a chronic low-grade infection of persisting (sometimes crippled) agents or by repeated exposure to external virus, bacteria, or toxins (16); or although unlikely, (iii) self-antigens may trigger crossreactive antibodies or antiidiotypic antibodies mimicking the antigen may maintain antibody titers in a hypothetical idiotypic-antiidiotypic network (17, 18). Protection against a primary infection or reinfection with an acute cytopathic virus usually is mediated by neutralizing antibodies (of at least 108 M−1 affinity; ref. 19) against specific surface determinants that are the sole determinants accessible to B cells on the intact virus (20, 21); such serotype-specific neutralizing antibodies are noncrossprotective and noncrossreactive. Neutralizing antibodies are readily studied with vesicular stomatitis virus (VSV), but less easily with lymphocytic choriomeningitis virus (LCMV), where immunopathology delays and impairs neutralizing but not antinucleoprotein-binding antibodies (22). In addition, antibodies against internal proteins measured by ELISA (affinities usually as for other proteins from 106 to >108 M−1) also are mounted. However, their role in host protection is questioned because they cannot recognize the intact infectious particle and usually are not protective in passive immunization protocols. In the context of the present study, it must be emphasized that in contrast to protective neutralizing antibodies that are monospecific, i.e., specific for one determinant and of >108 M−1 affinity (19), antibodies binding to isolated proteins are polyspecific and may bind to an unknown (great ?) number of different sites of variable affinity >106 M−1 (23). We analyzed critically protective neutralizing antibodies against VSV or nonprotective (ELISA binding) antibody memory by immunizing mice with replicating live nonpersisting VSV, potentially persisting LCMV, or nonpersisting abortively replicating UV-inactivated VSV; alternatively, to avoid persistence of viral genes different inert viral proteins were used as vaccines.
Materials and Methods
Mice, virus, antigens, antibody detection, and ELISPOT assays have been used as described (2, 3, 24, 25).
Results
Long-Term Antibody Memory Depends on Antigen Dose and Persistence.
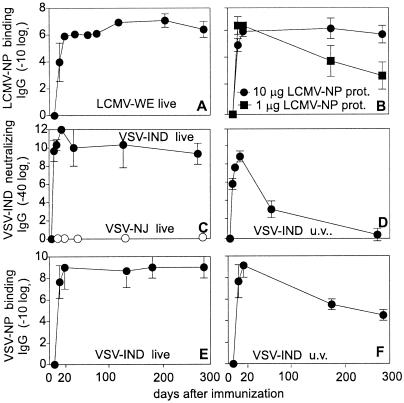
Antibody titers were followed after infection with replicating LCMV [200 plaque-forming units (pfu); Fig. 1A] and replicating VSV Indiana serotype (VSV-IND) (2 × 106 pfu; Fig. 1 C and E). Binding antibodies to the internal nucleoprotein (NP) of LCMV (LCMV-NP) and VSV (VSV-NP) were assessed by ELISA. Neutralizing antibodies directed against VSV-IND glycoprotein were measured in a neutralization assay. Immunization with live virus elicited high titers of both VSV-IND-neutralizing and -binding antibodies that were maintained up to day 300 after infection (Fig. 1 C and E), whereas anti-VSV serotype New Jersey (VSV-NJ) antibodies failed to neutralize VSV-IND (Fig. 1C). Immunization i.v. with 1 or 10 μg of Baculo-LCMV-NP without adjuvant induced comparable antibody titers by day 10 (Fig. 1B). However, whereas titers remained stable after immunization with 10 μg, after 1 μg, titers dropped by a factor of about 100 by day 300 (Fig. 1B). Similarly, immunization with a high dose of nonreplicating UV-inactivated VSV-IND particles (and therefore only replicating abortively in the initially infected cell) induced elevated VSV-IND-neutralizing and VSV-NP-binding antibodies at day 12–20 after immunization, but thereafter, neutralizing antibody titers dropped and became undetectable by day 300 (Fig. 1D). In contrast, VSV-NP-specific binding antibodies were still detectable on day 300 after immunization (Fig. 1F). Thus, long-term antibody production depends on initial antigen dose and/or antigen persistence or reexposure in vivo.
Figure 1.
B cell and antibody memory after immunization with live virus vs. viral proteins. C57BL/6 mice were infected with 200 pfu of LCMV-WE (A) or 2 × 106 pfu of VSV-IND (●) or –NJ (○) (C and E) i.v. or immunized with 10 or 1 μg of Baculo-derived LCMV-NP protein (B) or UV-inactivated VSV-IND (2 × 108 pfu; D and F) and LCMV-NP binding (A and B), VSV-NP-binding (E and F) and VSV-IND-neutralizing (C and D) antibody titers were assessed. Results are shown as means ± SD of 3–4 mice per group. The experiment was repeated twice with similar results.
Long-Lived Memory B Cells and Short-Lived Plasma Cells.
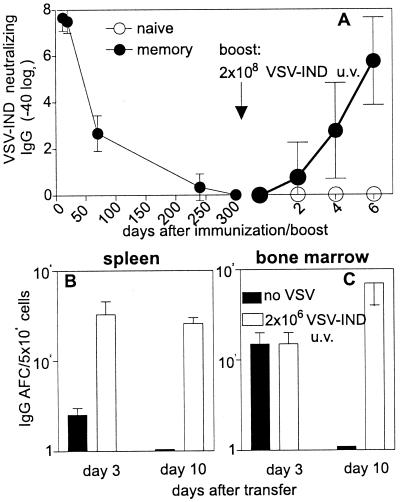
To analyze the life span of memory B cells, we analyzed mice that had been immunized with 2 × 108 pfu of UV-inactivated (to avoid virus persistence) VSV-IND 300 days previously and that did not possess a detectable neutralizing antibody titer at this time point (Fig. 2A). A booster immunization with 2 × 108 pfu of UV-inactivated VSV-IND led to a classical memory antibody response with early and high titers of neutralizing IgG compared with a primary immunization that initially induces IgM with unmeasurable IgG titers in naive mice at day 6.
Figure 2.
Life span of memory B cells and plasma cells. (A) C57BL/6 mice were immunized with UV-inactivated VSV-IND (2 × 108 pfu). At day 300, these mice (●) and a group of three naive C57BL/6 mice (○) were boosted with UV-inactivated VSV-IND (2 × 108 pfu) and the presence of neutralizing IgG antibodies was analyzed until day 6. (B and C) C57BL/6 mice were immunized with 2 × 106 pfu of VSV-IND. Thirty days later, 107 splenocytes + 107 bone marrow cells were injected into nonirradiated C57BL/6 recipient mice. Half of these mice received UV-inactivated VSV-IND (open bars; 2 × 106 pfu, yielding no IgG in naive mice) 20 min after the transfer of the cell suspension; 3 and 10 days later, spleen (B) and bone marrow (C) cells were prepared from recipient mice and IgG antibody-forming cells (AFC) were quantitatively assessed by ELISPOT assays against purified VSV-IND virions assessing largely neutralizing antibody-secreting cells. Bars indicate the means ± SD of four recipient mice and one of two similar experiments is shown.
The role of antigen in maintaining B or T cell memory in the absence of stimulating antigen usually is tested by transfer of memory cells into naive recipients (1, 11, 26). Spleen or bone marrow cells of mice that had been immunized with VSV-IND 30 days previously (Fig. 2 B and C) were transferred into naive nonirradiated recipient mice. In one group of mice, antigen (106 pfu of UV-inactivated VSV-IND i.v.) was added 20 min after adoptive transfer. Antibody-forming cells were quantitatively assessed by an ELISPOT assay 3 and 10 days after the transfer on plates coated with purified VSV-IND to assess largely neutralizing antibody responses (2, 21). In the presence of antigen, high numbers of antibody-producing cells were maintained, whereas the initially transferred plasma cells in the bone marrow and the spleen disappeared rapidly by day 10 in the absence of antigen (Fig. 2 B and C). Neither spleen nor bone marrow cells of naive mice immunized with the same antigen dose exhibited detectable numbers of IgG-secreting plasma cells (not shown) (10). Thus, memory B cells are long-lived in the absence of antigen. However, protective neutralizing antibody titers are maintained only by continuous antigenic stimulation of memory B cells and their subsequent differentiation to plasma cells that are short-lived (and end differentiated; ref. 27) in adoptive transfer experiments.
Protective Antibody Titers Are Best Maintained by Antigenic Stimulation of Splenocytes.
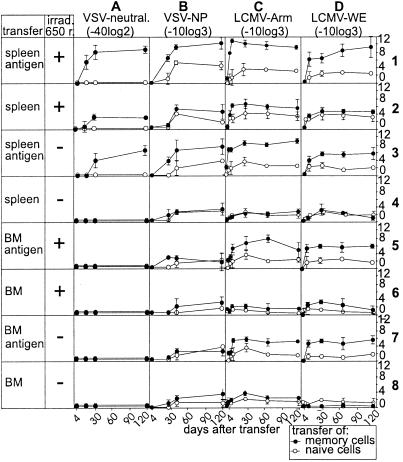
Recently, two studies with protein antigens and ELISA assays suggested a role of long-lived plasma cells in the maintenance of long-term antibody titers (10, 11). Therefore, we evaluated the relative contribution of adoptively transferred splenocytes or bone marrow cells in the absence or presence of stimulating antigen to the maintenance of long-term neutralizing antibody titers after i.v. infection with VSV-IND (2 × 106 pfu) and against protein antigens after infection with LCMV serotype Armstrong (LCMV-Armstrong; 200 pfu) or LCMV serotype WE (LCMV-WE) in irradiated and nonirradiated recipient mice (Fig. 3). Antibody titers were best maintained after transfer of 107 splenocytes together with stimulating antigen. Importantly, in the absence of a low dose of added antigen [nonreplicating VSV-IND (2 × 106 pfu of UV inactivated VSV, that by itself could not induce an IgG response)], no neutralizing VSV-IND-specific antibody titers could be mounted after transfer of VSV-IND-memory bone marrow cells [107 cells Fig. 3 A5–A8 or 8 × 107 cells (not shown)] into recipient mice. Low titers of binding VSV-NP- or LCMV-NP-specific antibodies were detected after transfer of memory bone marrow cells plus or minus additional transfer of antigen [in the form of 2 × 106 pfu of UV-inactivated VSV-IND (Fig. 3B) or 0.01 μg of Baculo-derived LCMV-NP (Fig. 3 C and D)]. However, the transfer of naive spleen cells or bone marrow cells into irradiated or nonirradiated recipient mice also caused marginally elevated antibody titers. These low titers of NP-specific antibodies reached 1:100 for LCMV-NP and VSV-NP after transfer of bone marrow cells and were quite comparable here and in other earlier experiments (12). This indicates that antibody titers maintained without stimulating antigen are around 1:100 and often only slightly [i.e., usually nonsignificantly 1 (−2) titer steps] above background level. Surprisingly, after transfer of bone marrow cells from LCMV memory mice (Armstrong or WE), antibody titers were drastically increased after the additional transfer of antigen when compared with recipient mice not given antigen (i.e., by a factor of 30–240). In general, irradiation of recipient mice lead to higher antibody titers, indicating that a nonspecific inflammatory stimulus also may induce or improve the differentiation of already primed B cells to plasma cells, perhaps via undefined crossreactive self-antigens released after irradiation. Irradiation caused a low level of neutralizing antibody titer of 1/80 compared with 1/10,000 if antigen was added (Fig. 3 A1 vs. A2).
Figure 3.
Adoptive transfer experiments of memory bone marrow or spleen cells. Single-cell suspensions of spleen and bone marrow cells of C57BL/6 mice were prepared 60–80 days after i.v. infection with 2 × 106 pfu of VSV-IND (A and B), 200 pfu of LCMV-Armstrong (C), or 200 pfu of LCMV-WE (D) and 107 spleen cells (rows 1–4) or bone marrow cells (rows 5–8) were adoptively transferred into mice irradiated with 650 rad (irrad.) or nonirradiated recipient mice. Where indicated, antigen was additionally injected 20 min after the adoptive transfer [2 × 106 pfu of UV-inactivated VSV-IND (A and B); and 0.01 μg of Baculo-derived LCMV-NP (C and D)]. VSV-IND-neutralizing, VSV-NP-binding, and LCMV-NP-binding antibodies were assessed up to 120 days after the adoptive transfer. Symbols indicate the means ± SD of four mice per group. The experiment was repeated twice with comparable results.
Taken together, many experimental [and perhaps not very physiological (adoptive transfer, irradiation)] manipulations may lead to the production of low levels of binding antibodies in the absence of antigen and may therefore wrongly suggest long-term antibody titers in the absence of antigen. However, highly specific, and for the protection of the host, relevant neutralizing antibody titers, of at least five titer steps (28) appear to strictly depend on the presence of antigen.
Memory B Cells Are Present in the Spleen and Bone Marrow and Their Differentiation to Plasma Cells Depends on CD4+ T Helper Cells.
The higher ELISA antibody titers in recipient mice receiving LCMV memory bone marrow cells plus stimulating antigen when compared with recipient mice given bone marrow cells without antigen suggested the presence of memory B cells in the bone marrow (e.g., Fig. 3 C5, C7, D5, and D7 vs. C6, C8, D6, and D8). Several previous studies failed to detect memory B cells that are inducible by antigen in the bone marrow (11, 29, 30). However, recent experiments (31) and also our own similar observation (not shown) detected B cells with a memory phenotype (surface IgG positive) in the bone marrow. One possible explanation for this apparent discrepancy may be that memory B cells only differentiate to plasma cells in the presence of T help.
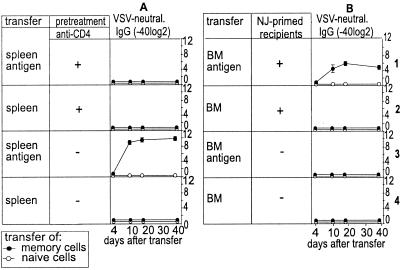
We therefore repeated the adoptive transfer experiments described above but depleted donor and recipient mice of CD4+ T cells before the transfer (Fig. 4). No antibody titers were mounted and for the production of both VSV-IND-neutralizing and VSV-NP-binding antibodies, the presence of T help was crucial (Fig. 4 A1and A2 vs. A3 and A4). To provide primed VSV-specific T help to adoptively transferred bone marrow cells, we took advantage of the fact that, in contrast to the strictly noncrossreactive neutralizing antibodies (32), VSV-NJ- and VSV-IND-specific T help is completely shared. Therefore, infection of recipient mice with 2 × 106 pfu of VSV-NJ provided specific T help to VSV-IND-primed bone marrow B cells without interfering with the anti-VSV-IND-neutralizing antibody readout. Interestingly, the adoptive transfer of VSV-IND memory bone marrow cells plus antigen in combination with specific T help yielded elevated anti-VSV-IND-neutralizing antibody titers, whereas in the absence of antigen, no antibody titer above background was detectable (Fig. 4 B1 vs. B2). In the absence of VSV-NJ-primed crossreactive T help, no antibody titers were elicited after transfer of bone marrow plus or minus antigen (Fig. 4B3,4).
Figure 4.
Role of CD4+ T cells in maintaining long-term antibody titers. Single-cell suspensions of spleen or bone marrow cells were prepared 60–80 days after infection of C57BL/6 mice with 2 × 106 pfu of VSV-IND i.v. and were adoptively transferred (107 per recipient) into nonirradiated naive recipient mice. Donor mice and recipients were CD4+ T cell-depleted where indicated by i.p. injection of 1 mg of monoclonal anti-CD4 antibody YTS191.1 on days −3 and −1 before sacrifice or adoptive transfer, respectively. Bone marrow cells were either transferred into naive C57BL/6 recipient mice or VSV-NJ immune recipient mice (immunized with 2 × 106 pfu of VSV-NJ 12 days earlier). Where indicated, antigen (2 × 106 pfu of UV-inactivated VSV-IND) was additionally injected 20 min after the adoptive transfer. VSV-IND-neutralizing antibodies were measured up to 40 days after the transfer. Symbols indicate the means ± SD of four mice per group. The experiment was repeated twice with comparable results.
These results suggested that (i) specific T helper cells are localized and stimulated mainly in lymphoid organs, whereas (ii) memory B cells are localized both in the spleen and in the bone marrow, and (iii) the differentiation from memory B cells to plasma cells strictly depends on both viral antigen and CD4+ T helper cells.
Reduction of CD4+ Helper Cells But Less So of Long-Lived Memory B Cells by Irradiation.
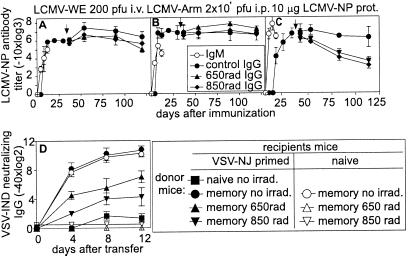
Irradiation of memory mice has been used in several studies to eliminate memory B cells and analyze the role of plasma cells in the maintenance of long-term antibody titers (8, 11, 33). In those studies, irradiation did not impair long-term antibody memory titers and therefore it was concluded that long-lived plasma cells are responsible for the memory antibody titer. We repeated these experiments in mice infected with 200 pfu of LCMV-WE i.v. (Fig. 5A). or with 106 pfu of LCMV-Armstrong i.p. (Fig. 5B). or in mice immunized i.v. with 10 μg of Baculo LCMV-NP without adjuvant (Fig. 5C) 30 days previously. Irradiation was either performed with sublethal 650 rad or alternatively with lethal 850 rad with additional cell reconstitution 1 day after irradiation. As shown in previous studies (11), irradiation of LCMV-WE (Fig. 5A) or LCMV-Armstrong immune mice (Fig. 5B) did not change long-term antibody titers. However, irradiation of Baculo LCMV-NP-immune mice (without adjuvant) caused a 20- to 100-fold reduction in antibody titers (Fig. 5C). An important difference between infection with live LCMV and exposure to LCMV-NP protein is their different in vivo persistence; LCMV, at least LCMV-WE (34) [but as suspected also for LCMV-Armstrong (35)], has been shown to persist up to at least 60–90 days. Therefore, at the time point of the irradiation, replicative LCMV virus must still have been present (34) although at very low levels and restimulated antibody responses.
Figure 5.
Effect of irradiation on B cell and antibody memory. C57BL/6 mice were infected with (A) 200 pfu of LCMV-WE i.v.; (B) 2 × 106 pfu of LCMV-Armstrong i.p.; or immunized with (C) 10 μg of Baculo-derived LCMV-NP protein. Thirty days after the infection/immunization, mice were irradiated with 650 or 850 rad or left nonirradiated as indicated by the arrow. Mice irradiated with 850 rad were substituted with 2 × 107 naive bone marrow and 2 × 107 naive spleen cells. LCMV-NP-binding antibodies were assessed up to day 125 after infection. (D) As in C but mice were immunized with 2 × 106 pfu of VSV-IND i.v. and irradiated 60 days later. Ten days later, 2 × 107 splenocytes isolated from the above irradiated and nonirradiated donor mice were adoptively transferred into nonirradiated recipient mice. Half of the recipient mice were also infected with 2 × 106 pfu of VSV-NJ 12 days earlier (closed symbols); 2 × 106 pfu of UV-inactivated VSV-IND was injected into all mice (+20 min) and neutralizing IgG antibody titers were analyzed (▪ are negative controls, and ●, ○ are positive controls). Results are shown as means ± SD of 3–4 mice per group. Each experiment was repeated 2–3 times with comparable results.
Because, as shown in Fig. 4, the differentiation of memory B cells to plasma cells is CD4+ T cell dependent, we checked the effects of irradiation separately on memory B and memory CD4+ T cells. VSV-IND memory mice were irradiated with 650 or 850 rad (the latter were substituted with naive bone marrow and splenocytes). Ten days later, their spleen cells were transferred into mice that had been infected with 2 × 106 pfu of VSV-NJ 12 days earlier (exhibiting primed specific T help) or into naive control mice possessing no primed T help. All recipient mice were given 2 × 106 pfu of UV-inactivated VSV-IND as a source of antigen that is not sufficient to induce an IgG response in naive mice (36). Adoptive transfer of 107 irradiated VSV-IND-primed B cells to VSV-NJ (T help)-primed—but not in naive—recipients generated neutralizing antibody titers (Fig. 5D).
Therefore, we conclude that irradiation very efficiently eliminated primed and activated CD4+ T cells that seem necessary for the differentiation of memory B cells to plasma cells. In contrast, memory B cells seemed relatively more resistant to irradiation.
The Role of Lymphoid Organs in Maintaining Protective Long-Term Antibody Titers.
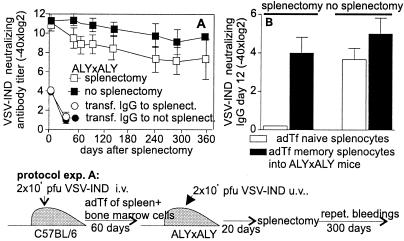
As pointed out above, we observed the presence of memory B cells outside of classical secondary lymphoid organs, i.e., in the bone marrow compatible with earlier results (31). We assessed long-term antibody titers in lymph node- and Peyer‘s patches-deficient ALY/ALY mice (24) that were additionally splenectomized (Fig. 6 A and B); splenectomy did not change the overall kinetics of memory antibody titers (Fig. 6A).
Figure 6.
The role of secondary lymphoid organs in the maintenance of B cell and antibody memory. (A) Splenocytes (107) plus 107 bone marrow cells from VSV-IND-primed (2 × 106 pfu 60 days earlier) C57BL/6 mice were adoptively transferred into ALY × ALY mice. Twenty minutes later, 2 × 108 pfu of UV-inactivated VSV-IND were injected into recipient mice. Day 20 after the adoptive transfer, half of the recipient ALY × ALY mice were splenectomized. Neutralizing antibody titers were followed up to 360 days after the adoptive transfer. Antibody titers in splenectomized and nonsplenectomized ALY × ALY mice were also followed after transfer of 500 μl of VSV-IND immune serum (pooled of VSV-IND memory mice infected 60 days previously with 2 × 106 pfu of VSV-IND). (B) C57BL/6 mice were infected with 2 × 106 pfu of VSV-IND i.v. Sixty days later, 2 × 107 splenocytes from these mice or naive C57BL/6 mice were transferred into ALY × ALY recipient mice. At the same time, 2 × 107 splenocytes of VSV-NJ-primed (2 × 106 pfu i.v., 14 days earlier) mice were transferred into the same recipient mice as a source of primed T help. Four days later, half of the ALY × ALY recipient mice were splenectomized. All mice were boosted with 2 × 108 pfu of UV-inactivated VSV-IND 7 days after splenectomy and VSV-IND-neutralizing antibody titers were determined 22 days later. Results are shown as means ± SD of 3–4 mice per group. The experiments were repeated twice with comparable results.
Adoptive transfer of naive B cells failed to produce neutralizing anti-VSV-IND antibodies in splenectomized ALY/ALY mice boosted with 2 × 108 pfu of UV-inactivated VSV-IND even if VSV-NJ-primed crossreactive T help was additionally transferred (107 splenocytes of day 12 VSV-NJ-immune mice; Fig. 6B). In contrast, memory B cells transferred into splenectomized ALY × ALY recipients were readily activated by specific antigen if primed T help was added, even in the absence of secondary lymphoid organs. Long-term antibody memory also was studied after transfer of 107 splenocytes plus bone marrow cells from day 60 VSV-IND immune mice into ALY/ALY mice (Fig. 6A). On the same day of cell transfer, recipient mice were also given 2 × 108 pfu of UV-inactivated VSV-IND i.v. Thirty days after the adoptive transfer, half of the ALY/ALY recipient mice were splenectomized. Although splenectomized ALY/ALY mice had slightly reduced neutralizing antibody titers (factor 2–4), these titers were maintained comparable to controls. Splenectomy of ALY × ALY mice did not change the antibody half-life as controlled by transfer of VSV-IND hyperimmune serum (Fig. 6A).
Taken together, these results suggest that the induction of naive B cells requires secondary lymphoid organs whereas “antigen-experienced” memory B cells may be stimulated by antigen outside classical secondary lymphoid organs, such as the bone marrow to differentiate to antibody-secreting plasma cells. Importantly, in all cases, this process is primed T help-dependent.
Discussion
This study confirms that B cell memory is characterized by a pool of antigen-independent long-lived B cells with higher frequencies than found in unprimed mice (1, 37). Our results establish that memory B cells require additional encounter with specific antigen to differentiate to antibody-secreting plasma cells but do so only if appropriate T help is available. We failed to find antigen-independent long-lived persisting antibody levels.
The half-life of plasma cells was shown here to be between 3 and 10 days compatible with earlier estimates of 1–3 days (38–41). Therefore, to maintain long-term antibody titers, a continuous differentiation of B cells to plasma cells apparently must take place. Earlier studies using CD4+ T cell depletion by antibodies (ref. 42 and unpublished observations for LCMV and VSV-IND) suggested that long-term antibody titers were maintained independently of T help. However, primed CD4+ T cells have been shown to be more resistant to depletion by anti-CD4 antibody treatment than naïve T cells and therefore elimination of primed T helper cells may be incomplete (43). Therefore, it is likely that primed CD4+ T cells were still present in these experiments or were regenerated when antigen in adjuvant or low-level infection persisted in the original host (34). Why should our results particularly with neutralizing anti-VSV antibodies differ from recent studies describing the presence of long-lived antigen-independent plasma cells specific for LCMV-Armstrong internal antigens (11) or ovalbumin (10)? Whereas both these previous studies analyzed B-cell memory of binding antibodies, we studied, in addition to ELISA-binding antibodies, also protective neutralizing antibody responses (21, 44). We find here that in nonirradiated recipients, neutralizing antibody titers strictly depended on antigen stimulation. In contrast, LCMV-NP ELISA-binding antibodies were detectable after adoptive transfer of LCMV memory bone marrow or spleen cells to naive recipients (in the absence of antigen), but these titers were usually only a factor of 3 (one titer step) above the background of mice receiving naive bone marrow or spleen. This discrepancy may reflect differences either in avidities of measured antibodies that probably include lower values for ELISA (>106 M−1) in contrast to higher ones for neutralization (>108 M−1) (28) and/or differences in the numbers of antigenic sites assessed (i.e., one for neutralizing and many for protein-binding antibodies). This number is large, although undetermined, for protein-binding antibodies and small, defined by the one antigenic site on the intact virus (19).
The longevity of plasma cells has been deduced from irradiation experiments which largely disregard the role of T help and assume that B cells are radiosensitive whereas plasma cells are radioresistant (8, 11, 45). However, our experiments here show that, in contrast to T help, the pool of memory B cells is reduced but not completely eliminated after irradiation with 650 or 850 rad. Often cited indications for the radiosensitivity of memory B cells have been derived mainly from experiments where the role of CD4+ T cells was not assessed (11, 45) or from experiments with haptens that are based on high precursor frequencies of hapten (e.g., 2,4-dinitrophenyl)-specific B cells and where, therefore, T help is usually limiting (8). Our results indicate that irradiation of an LCMV-immune host may mainly eliminate specific CD4+ T helper cells rather than primed B cells and therefore cannot be used to analyze longevity of plasma cells. As has been shown previously, LCMV may persist over a prolonged time period at very low levels (i.e., >80 days for LCMV-WE and possibly also for LCMV-Armstrong; refs. 34, 35, and 46) and may therefore induce CD4+ T cells after irradiation. Similarly, antigen in adjuvant may persist for a long time and induce new T cells.
Memory B cells are present within secondary lymphoid organs but also outside of classical secondary lymphoid organs, in the bone marrow. It is widely accepted that antigen may persist on follicular dendritic cells as antigen–antibody complexes that restimulate B cells (14, 37, 47, 48). Surprising was the finding that classical secondary lymphoid organs were not required at all for the T help-dependent maintenance of memory antibody titers in splenectomized ALY/ALY mice (24). We therefore may conclude that antigen-experienced memory B cells that have already switched to IgG can be restimulated to differentiate to plasma cells outside of germinal centers and lymphoid organs, as shown here apparently in the bone marrow, an organ that exhibits excellent cytokine conditions for plasma cell/plasmocytoma differentiation (29, 31, 49–51). In an earlier study analyzing affinity maturation and antibody memory to a hapten antigen, it was observed that antigen-driven affinity maturation of bone marrow plasma cells still was detectable after disruption of germinal centers by injection of an antibody specific for CD154 (52). Because there are no indications that plasma cells can be stimulated by antigen and plasma cells possess no (27, 53) or at most only very low (questionable ?) levels (10) of surface IgG, the most likely explanation of those findings is that memory B cells can differentiate to plasma cells in the absence of germinal centers.
It is possible that dependent on the definition of memory and the assay method used, immunological memory may not necessarily correlate with protective immunity (16, 54). Whereas highly frequent memory B cells are long-lived (1) and independent of antigen, they do not produce antibodies and therefore cannot mediate the type of immediate protection necessary against acutely cytopathic infectious agents (3, 4). Therefore, maintenance of protective antibody titers depends on a continuous or repetitive stimulation by antigen from within or outside of the host. This suggests that rather than memory B cells, effector B cells, i.e. plasma cells secreting neutralizing antibodies, are the bearer of protective immunity. Therefore, for improved antibody-protection by B cell vaccination, antigen persistence in some form over a prolonged time period in the host, for example, as antigen–antibody complexes as low-level persistent infection or crippled virus (e.g., measles), persistence of antigen or repetitive exposure from the outside (e.g., polio virus) seems essential.
The parallels to T cell memory as documented, e.g., by tuberculosis (55) or against peripheral sarcomas or carcinomas (56) are striking. The persisting tuberculosis granuloma, but not the disappearing bacillus Calmette–Guérin vaccine, maintain protective “infectious immunity” (55). Memory T cells are usually long-lived in the absence of antigen (26, 57), but they are not necessarily protective (58, 59). Their capacity to protect against a reinfection of peripheral tissues depends on antigenic stimulation for T cells to become activated to express effector function. This includes the capacity to emigrate rapidly into solid peripheral tissues and mediate effector function (56, 59–61). Therefore, for improved T and/or B cell-based vaccination, antigen persistence over a prolonged time period in the host or repetitive exposure from the outside seems necessary.
Acknowledgments
We thank K. McCoy for critically reading the manuscript. Additional aspects are discussed at www.pathol.unizh.ch/experimentelle-immunologie/additions.html (see number 1).
Abbreviations
- LCMV
lymphocytic choriomeningitis virus
- LCMV-WE
LCMV serotype WE
- LCMV-Armstrong
LCMV serotype Armstrong
- LCMV-NP
LCMV nucleoprotein
- VSV
vesicular stomatitis virus
- VSV-IND
VSV Indiana serotype
- VSV-NJ
VSV serotype New Jersey
- pfu
plaque-forming units
- NP
nucleoprotein
- VSV-NP
VSV nucleoprotein
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230417497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230417497
References
- 1.Schittek B, Rajewsky K. Nature (London) 1990;346:749–751. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann M F, Odermatt B, Hengartner H, Zinkernagel R M. J Exp Med. 1996;183:2259–2269. doi: 10.1084/jem.183.5.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinhoff U, Müller U, Schertler A, Hengartner H, Aguet M, Zinkernagel R M. J Virol. 1995;69:2153–2158. doi: 10.1128/jvi.69.4.2153-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mims C A. The Pathogenesis of Infectious Disease. London: Academic; 1997. pp. 254–269. [Google Scholar]
- 5.Talbot P J, Buchmeier M J. Immunology. 1987;60:485–489. [PMC free article] [PubMed] [Google Scholar]
- 6.Vieira P, Rajewsky K. Eur J Immunol. 1988;18:313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- 7.Sprent J, Tough D F. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 8.Okudaira H, Ishizaka K. Cell Immunol. 1981;58:188–201. doi: 10.1016/0008-8749(81)90160-x. [DOI] [PubMed] [Google Scholar]
- 9.Ho F, Lortan J E, MacLennan I C, Khan M. Eur J Immunol. 1986;16:1297–1301. doi: 10.1002/eji.1830161018. [DOI] [PubMed] [Google Scholar]
- 10.Manz R A, Thiel A, Radbruch A. Nature (London) 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 11.Slifka M K, Antia R, Whitmire J K, Ahmed R. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 12.Slifka M K, Ahmed R. Curr Opin Immunol. 1998;10:252–258. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 13.Mandel T E, Phipps R P, Tew J G. Immunol Rev. 1980;53:29–59. doi: 10.1111/j.1600-065x.1980.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 14.Szakal A K, Kosco M H, Tew J G. Annu Rev Immunol. 1989;7:91–109. doi: 10.1146/annurev.iy.07.040189.000515. [DOI] [PubMed] [Google Scholar]
- 15.Tew J G, Phipps R P, Mandel T E. Immunol Rev. 1980;53:175–201. doi: 10.1111/j.1600-065x.1980.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 16.Zinkernagel R M, Bachmann M F, Kündig T M, Oehen S, Pircher H, Hengartner H. Annu Rev Immunol. 1996;14:333–367. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]
- 17.Shoenfeld Y, George J. Ann NY Acad Sci. 1997;815:342–349. doi: 10.1111/j.1749-6632.1997.tb52080.x. [DOI] [PubMed] [Google Scholar]
- 18.Grandien A, Andersson J, Portnoi D, Coutinho A. Eur J Immunol. 1997;27:1808–1815. doi: 10.1002/eji.1830270732. [DOI] [PubMed] [Google Scholar]
- 19.Roost H-P, Bachmann M F, Haag A, Kalinke U, Pliska V, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1995;92:1257–1261. doi: 10.1073/pnas.92.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith C A, Barnett B C, Thomas D B, Temoltzin-Palacios F. J Exp Med. 1991;173:953–959. doi: 10.1084/jem.173.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachmann M F, Zinkernagel R M. Annu Rev Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- 22.Planz O, Ehl S, Furrer E, Horvath E, Brundler M A, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1997;94:6874–6879. doi: 10.1073/pnas.94.13.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldbaum F A, Cauerhff A, Velikovsky C A, Llera A S, Riottot M M, Poljak R J. J Immunol. 1999;162:6040–6045. [PubMed] [Google Scholar]
- 24.Miyawaki S, Nakamura Y, Suzuka H, Koba M, Yasumizu R, Ikehara S, Shibata Y. Eur J Immunol. 1994;24:429–434. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- 25.Fehr T, Rickert R C, Odermatt B, Roes J, Rajewsky K, Hengartner H, Zinkernagel R M. J Exp Med. 1998;188:145–155. doi: 10.1084/jem.188.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau L L, Jamieson B D, Somasundaram T, Ahmed R. Nature (London) 1994;369:648–652. [Google Scholar]
- 27.Abney E R, Cooper M D, Kearney J F, Lawton A R, Parkhouse R M. J Immunol. 1978;120:2041–2049. [PubMed] [Google Scholar]
- 28.Bachmann M F, Kalinke U, Althage A, Freer G, Burkhart C, Roost H-P, Aguet M, Hengartner H, Zinkernagel R M. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 29.Koch G, Osmond D G, Julius M H, Benner R. J Immunol. 1981;126:1447–1451. [PubMed] [Google Scholar]
- 30.Shepherd D M, Noelle R J. Transplantation. 1991;52:97–100. doi: 10.1097/00007890-199107000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Paramithiotis E, Cooper M D. Proc Natl Acad Sci USA. 1997;94:208–212. doi: 10.1073/pnas.94.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta S C, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1986;83:2604–2608. doi: 10.1073/pnas.83.8.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz D H, Paul W E, Goidl E A, Benacerraf B. Science. 1970;170:462–464. doi: 10.1126/science.170.3956.462. [DOI] [PubMed] [Google Scholar]
- 34.Ciurea A, Klenermann P, Hunziker L, Horvath E, Odermatt B, Ochsenbein A F, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1999;96:11964–11969. doi: 10.1073/pnas.96.21.11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomsen A R, Johansen J, Marker O, Christensen J P. J Immunol. 1996;157:3074–3080. [PubMed] [Google Scholar]
- 36.Bachmann M F, Kündig T M, Kalberer C P, Hengartner H, Zinkernagel R M. J Virol. 1993;67:3917–3922. doi: 10.1128/jvi.67.7.3917-3922.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray D, Siepmann K, van Eden D, Poudrier J, Wykes M, Jainandunsing S, Bergthorsdottir S, Dullforce P. Immunol Rev. 1996;150:45–61. doi: 10.1111/j.1600-065x.1996.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 38.Schooley J C. J Immunol. 1961;86:331–337. [PubMed] [Google Scholar]
- 39.Cooper E H. Immunology. 1961;4:219–231. [PMC free article] [PubMed] [Google Scholar]
- 40.Nossal G J V, Makela O. J Exp Med. 1962;115:209–230. doi: 10.1084/jem.115.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller J J. J Immunol. 1964;92:673–681. [PubMed] [Google Scholar]
- 42.Vieira P, Rajewsky K. Int Immunol. 1990;2:487–494. doi: 10.1093/intimm/2.6.487. [DOI] [PubMed] [Google Scholar]
- 43.Chace J H, Cowdery J S, Field E H. J Immunol. 1994;152:405–412. [PubMed] [Google Scholar]
- 44.Christian A Y, Barna M, Bi Z, Reiss C S. Viral Immunol. 1996;9:195–205. doi: 10.1089/vim.1996.9.195. [DOI] [PubMed] [Google Scholar]
- 45.Makinodan T, Kastenbaum M A, Peterson W J. J Immunol. 1962;88:31–37. [PubMed] [Google Scholar]
- 46.Larsen J H. Immunology. 1969;16:15–23. [PMC free article] [PubMed] [Google Scholar]
- 47.Tew J G, Kosco M H, Burton G F, Szakal A K. Immunol Rev. 1990;117:185–211. doi: 10.1111/j.1600-065x.1990.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 48.MacLennan I C, Liu Y J, Johnson G D. Immunol Rev. 1992;126:143–161. doi: 10.1111/j.1600-065x.1992.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 49.Benner R, Hijmans W, Haaijman J J. Clin Exp Immunol. 1981;46:1–8. [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y J, Zhang J, Lane P J, Chan E Y, MacLennan I C. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 51.Tew J G, DiLosa R M, Burton G F, Kosco M H, Kupp L I, Masuda A, Szakal A K. Immunol Rev. 1992;126:99–112. doi: 10.1111/j.1600-065x.1992.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi Y, Dutta P R, Cerasoli D M, Kelsoe G. J Exp Med. 1998;187:885–895. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halper J, Fu S M, Wang C Y, Winchester R, Kunkel H G. J Immunol. 1978;120:1480–1484. [PubMed] [Google Scholar]
- 54.Ahmed R, Gray D. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 55.Mackaness G B. Infect Immun. 1964;9:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bachmann M F, Kündig T M, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1997;94:640–645. doi: 10.1073/pnas.94.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jamieson B D, Ahmed R. J Exp Med. 1989;169:1993–2005. doi: 10.1084/jem.169.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kündig T M, Bachmann M F, Ohashi P S, Pircher H, Hengartner H, Zinkernagel R M. Immunol Rev. 1996;150:63–90. doi: 10.1111/j.1600-065x.1996.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 59.Ochsenbein A F, Karrer U, Klenerman P, Althage A, Ciurea A, Shen H, Miller J F, Whitton J L, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1999;96:9293–9298. doi: 10.1073/pnas.96.16.9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gray D, Matzinger P. J Exp Med. 1991;174:969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kündig T M, Bachmann M F, Oehen S, Hoffmann U W, Simard J J L, Kalberer C P, Pircher H, Ohashi P S, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]