Abstract
Oncoretrovirus vectors pseudotyped with the feline endogenous retrovirus (RD114) envelope protein produced by the FLYRD18 packaging cell line have previously been shown to transduce human hematopoietic progenitor cells with a greater efficiency than similar amphotropic envelope-pseudotyped vectors. In this report, we describe the production and efficient concentration of RD114-pseudotyped murine leukemia virus (MLV)-based vectors. Following a single round of centrifugation, vector supernatants were concentrated approximately 200-fold with a 50 to 70% yield. Concentrated vector stocks transduced prestimulated human CD34+ (hCD34+) cells with approximately 69% efficiency (n = 7, standard deviation = 4.4%) using a single addition of vector at a low multiplicity of infection (MOI = 5). Introduction of transduced hCD34+ cells into irradiated NOD/SCID recipients resulted in multilineage engraftment with long-term transgene expression. These data demonstrate that RD114-pseudotyped MLV-based vectors can be efficiently concentrated to high titers and that hCD34+ cells transduced with concentrated vector stocks retain in vivo repopulating potential. These results highlight the potential of RD114-pseudotyped oncoretrovirus vectors for future clinical implementation in hematopoietic stem cell gene transfer.
Oncoretrovirus-based vectors remain the most widely used transfer vector for clinical gene therapy trials. In principle, in vitro stimulation of cell division should allow for efficient transduction using murine leukemia virus (MLV)-based vectors. However, amphotropic, vesicular stomatitis virus G protein (VSV-G)- and, to some extent, gibbon ape leukemia virus-pseudotyped MLV-based vectors have had limited effectiveness at transducing human CD34+ (hCD34+) cells with in vivo repopulating potential (14, 15, 23, 29). In a recent report, MLV-based vectors pseudotyped with the feline endogenous retrovirus (RD114) envelope protein were shown to efficiently transduce prestimulated hCD34+ cells (14). However, because of an unknown component of the culture medium conditioned by the human fibrosarcoma-derived (HT1080) FLYRD18 packaging cell line, CD34+ cells transduced with vector supernatants failed to engraft in irradiated NOD/SCID recipients (14). To avoid this problem, vector supernatants were panned over Retronectin-coated plates prior to addition of cells. CD34+ cells transduced in this manner were able to reconstitute NOD/SCID mice. In this manuscript, we describe the efficient concentration of RD114-pseudotyped MLV-based vectors as an alternative to panning or the use of unconcentrated vector supernatants. Furthermore, we show that concentrated vector stocks efficiently transduce hCD34+ cells and that, following transduction, these cells retain in vivo repopulating potential and multilineage transgene expression.
Efficient concentration of feline endogenous retrovirus RD114-pseudotyped vectors.
As an alternative to vector panning, centrifugation was used to concentrate virus preparations and remove conditioned-medium components. Vector particles pseudotyped with the feline endogenous retrovirus (RD114) envelope protein were made by generating a producer cell line from the packaging cell line FLYRD18 (5) by introducing a vector genome that encodes the enhanced green fluorescence protein (EGFP) expressed from the mouse stem cell virus (MSCV) promoter. This vector, designated MSCV-EGFP, was derived from MGirL22Y through the deletion of the internal ribosome entry site and the sequences encoding the drug-resistant variant of human dihydrofolate reductase (L22Y) (2). The vector genome was introduced into FLYRD18 cells in the form of VSV-G-pseudotyped retroviral particles produced by transient transfection of 293T cells (6). Individual clones were obtained by limiting dilution, and two clones, designated RD114/MSCV-EGFP c9 and c13, were used for further analyses. Both clones produced vector preparations with unconcentrated-supernatant titers of 2 × 105 to 5 × 105 infectious units (i.u.) per ml when HeLa cells were used as targets and flow cytometry was performed to detect transduced cells expressing EGFP.
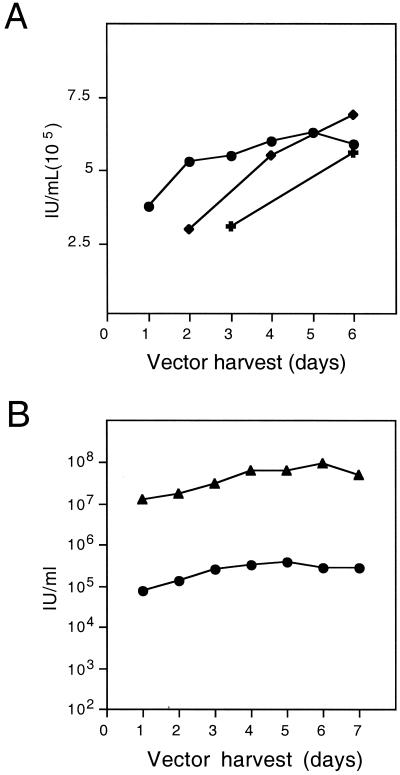
RD114/MSCV-EGFP (c9) producer cells were grown in 10-cm-diameter dishes, and supernatant collection was initiated 48 h after the cells had reached confluency. To determine the optimal harvest interval, supernatants were collected from three different sets of multiple plates in parallel, with serial collections performed every 24, 48, or 72 h over a period of 6 days (Fig. 1A). No significant differences in the titers of the supernatants collected at the different time intervals were noted. Interestingly, lengthening the time between harvests did not increase the titer in a fixed volume of medium. Moreover, no decrease in the production of vector was noted during 6 days of daily harvests from a given set of plates (Fig. 1A).
FIG. 1.
Production and concentration of feline endogenous virus (RD114) envelope protein-pseudotyped MLV vector particles. (A) FLYRD18/MSCV-EGFP cells were plated in triplicate, supernatants were harvested, and a titer (in i.u. per milliliter) was determined for unconcentrated supernatant collected every 24 h (circles), 48 h (diamonds), or 72 h (crosses). (B) For vector concentration, supernatants from FLYRD18/MSCV-EGFP cells were collected every 24 h, concentrated by centrifugation, and stored at −70°C. The titer (in i.u. per milliliter) of each preparation of vector was determined. Titers of unconcentrated supernatants (circles) and concentrated supernatants (triangles) were determined on HeLa cells by flow cytometry for EGFP expression.
We therefore chose to produce vector from 24 individual 10-cm dishes by harvesting and concentrating supernatants daily for 7 days. Supernatants were collected, pooled, and concentrated by a single centrifugation step (100,000 × g, 90 min) each day. Viral pellets were resuspended in 0.5 to 0.8 ml of serum-free medium and stored frozen in aliquots. For each harvest, a titer was determined for both concentrated and unconcentrated supernatants (Fig. 1B). Over the course of 7 days, titers of unconcentrated vectors ranged from 1 × 105 to 5 × 105 i.u./ml, while concentrated-vector titers ranged from 1 × 107 to 9 × 107 i.u./ml with approximately a 50 to 70% yield. This represents an approximately 200-fold increase in titer following concentration. These results demonstrate that RD114-pseudotyped MLV vectors can be efficiently and reproducibly concentrated to high titers with reasonable yields. Furthermore, large quantities of vector can be produced by serial harvesting and concentration of conditioned medium over a period of at least 7 days.
Transduction of hCD34+ cells with concentrated RD114-pseudotyped MLV-based vectors expressing EGFP.
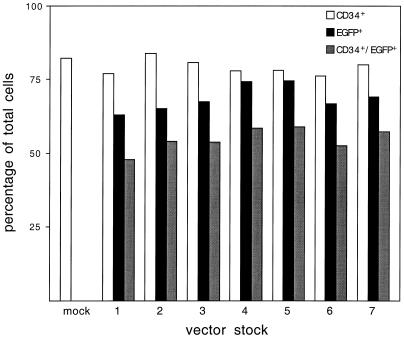
hCD34+ cells from cord blood were enriched to >85% by positive immunomagnetic selection, and isolated cells were analyzed by flow cytometry. Cells were preactivated in Iscove's modified Dulbecco's medium with 1% bovine serum albumin for 48 h in the presence of cytokines (5 μg of human insulin/ml, 100 μg of human transferrin/ml, 10 μg of low-density lipoprotein/ml, 0.1 mM β-mercaptoethanol, 300 ng of stem cell factor/ml, 300 ng of Flt-3 ligand/ml, 10 ng of interleukin-3/ml, and 10 ng of interleukin-6/ml [R&D Systems, San Jose, Calif.]) to induce cell cycling prior to addition of vector. Cells were then transduced in the presence of Retronectin (CH-296; Takara Shuzo, Otsu, Japan) by a single addition of concentrated vector (multiplicity of infection [MOI] = 5) from each of the seven vector stocks shown in Fig. 1B. Forty-eight hours after transduction, samples of transduced cells and a mock-transduced sample were analyzed by flow cytometry to determine transduction efficiency. As shown in Fig. 2, each vector stock efficiently transduced bulk hCD34+ cells (mean ± standard deviation, 68.7% ± 4.4%; n = 7). Similarly, the total cell populations contained on average 54.7% ± 3.9% hCD34+ EGFP+ cells. In addition, exposure to concentrated vector had no apparent adverse effects on the percentage of cells expressing hCD34. The population of mock-transduced (untransduced) cells contained 82.3% hCD34+ cells, compared with a mean of 79.2% ± 2.6% (n = 7) hCD34+ cells in the samples exposed to vector supernatants. These data indicate that the concentrated RD114 vectors can efficiently transduce human hematopoietic progenitor cells without having quantitative effects on surface hCD34 expression.
FIG. 2.
Transduction of hCD34+ cells by concentrated RD114-pseudotyped MLV vectors. hCD34+ cells were isolated from umbilical cord blood and transduced (MOI = 5) following 48 h of cytokine prestimulation. Flow-cytometric analysis was performed 48 h after transduction. The y axis represents the percentage of the total cell population. The x axis indicates the seven preparations of vector that were serially collected from a single set of plates and concentrated prior to addition to cells. Following transduction, cells were analyzed for hCD34 and EGFP expression by flow cytometry.
Engraftment of NOD/SCID mice with CD34+ cells transduced with concentrated RD114-pseudotyped vectors.
To determine the in vivo repopulating potential of hematopoietic cells following exposure to concentrated vector supernatants, 2 × 105 transduced or mock-transduced CD34+ cells were transplanted into sublethally irradiated NOD/SCID recipients. At the time of injection, transduced CD34+ cells were determined to be 44% EGFP+ by flow cytometry, and methyl cellulose cultures yielded 39.5% EGFP+ CFU (166 of 220) by visual analysis and 42% EGFP+ CFU (5 of 12) by PCR analysis of random colonies for EGFP sequences.
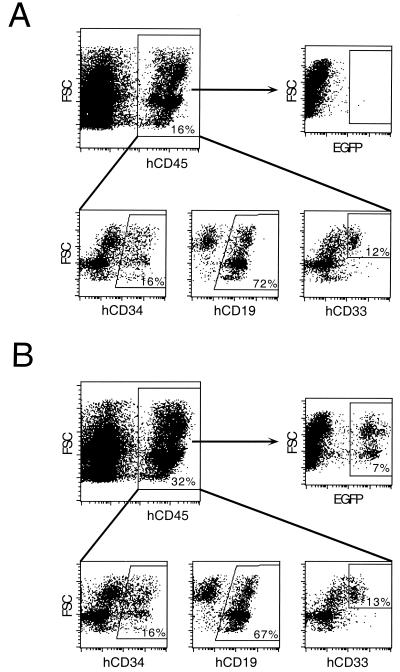
Fifteen weeks posttransplant, bone marrow was harvested and analyzed for reconstitution with human cells. All mice receiving transduced cells showed long-term engraftment in the bone marrow as determined by flow-cytometric analysis for hCD45 expression (mean, 10.8%; range, 0.3 to 32%; n = 11) (Fig. 3). One mouse that received mock-transduced (control) hCD34+ cells had 16.2% hCD45+ bone marrow cells. Data for a control and an experimental mouse are shown in Fig. 3. Bone marrow samples from mice that received transduced CD34+ cells (n = 11) contained on average 15.6% hCD34+ cells (range, 9.4 to 23.3), 56.7% hCD19+ cells (range, 42 to 76.6%), and 26.4% hCD33+ cells (range, 13.2 to 38.8%). The control mouse that received mock-transduced cells had 15.8% hCD34+ cells, 72.1% hCD19+ cells, and 11.9% hCD33+ cells in its bone marrow. As previously reported, no T lymphocytes were detected, presumably because of the lack of a functional thymus in the NOD/SCID mouse (27). These data indicate that exposure to concentrated vector supernatants did not negatively affect the multilineage engraftment potential of CD34+ cells following transduction.
FIG. 3.
Bone marrow engraftment of NOD/SCID recipients following transplantation of hCD34+ cells transduced with concentrated RD114-pseudotyped MLV vectors. Mononuclear cells were isolated from bone marrow 15 weeks posttransplant from animals receiving mock-transduced (A) or vector-transduced (B) cells. Isolated cells were stained with anti-hCD45 antibody conjugated to allophycocyanin (APC), and flow-cytometric analysis was performed. The upper left panels represent total live cells. The upper right and lower panels are gated for hCD45+ cells from the same sample. The y axis represents forward scatter (FSC), and the x axis represents staining for human cells (anti-hCD45–APC) (upper left panels), EGFP expression (upper right panels), anti-hCD34–PE (progenitor cells), anti-hCD19–PE (B cells), or hCD33–PE (myeloid cells) (lower panels). Values indicate the percentage of cells in each region compared to that of an unmanipulated mouse (that did not receive human cells) (upper left panel) or a mouse that received mock-transduced human cells (upper right panel).
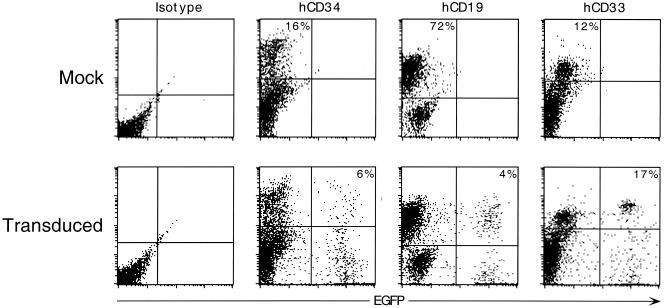
In the cohort of mice that received transduced cells, PCR analysis for the presence of transgene within bone marrow cells indicated that 7 of 11 mice exhibited reconstitution with transduced cells (data not shown). Transgene expression was determined by flow-cytometric analysis of gated human (hCD45+) cells. Data for a control and an experimental mouse are shown in Fig. 4. No EGFP expression was observed in a mouse that received mock-transduced cells (upper panels) or in mice that received tranduced cells but were negative for transgene DNA by PCR. In the bone marrow of the animals positive for transgene by PCR, up to 70% of the human cells expressed EGFP (n = 7; mean, 24.5%; range, 7.2 to 70.1%). EGFP was expressed in both lymphoid (hCD19+) (mean, 21.6%; range, 3.1 to 67.6%) and myeloid (hCD33+) (mean, 18.5%; range, 4.2 to 48.5%) compartments as well as in hematopoietic progenitor cells (hCD34+) (mean, 16.7%; range, 6.8 to 37.9%). These results are indicative of multilineage engraftment with sustained long-term transgene expression and reveal that concentrated RD114-pseudotyped vectors can transduce human SCID repopulating cells.
FIG. 4.
In vivo transgene expression following hematopoietic reconstitution. The results of flow-cytometric analyses of gated hCD45+ cells from one animal that received mock-transduced cells (upper panels) and one that received transduced cells (lower panels) are presented. The values in the upper panels indicate the percentage of total hCD45+ cells expressing the designated surface marker. The values in the lower panels indicate the percentage of cells expressing the designated surface marker that are EGFP+. Bone marrow mononuclear cells were isolated and stained with anti-hCD45–APC (human cells), anti-hCD34–PE (progenitor cells), anti-hCD19–PE (B cells), or hCD33–PE (myeloid cells). The y axis represents lineage-specific staining, and the x axis represents EGFP expression.
Significant advances in hematopoietic stem cell gene transfer have been due in part to the development of novel retrovirus envelope pseudotypes and the flexibility they afford. Several reports have demonstrated that the feline endogenous retrovirus (RD114) envelope protein is tropic for human hematopoietic cells (14, 20, 22). Here we report that RD114-pseudotyped MLV-based vectors can be efficiently concentrated by a single round of ultracentrifugation. We also demonstrate that concentrated vector stocks can efficiently transduce hCD34+ cells and that transduced cells repopulate NOD/SCID mice with an efficiency similar to that of mock-transduced cells. Furthermore, animals that exhibited reconstitution with transduced cells expressed transgene in all hematopoietic lineages examined.
The production of high-titer, concentrated retrovirus vector stocks provides significant advantages for both basic and clinical gene transfer applications. Concentrated vector preparations are better suited than unconcentrated ones for the in vivo study of gene transfer in animal models because larger numbers of effective transducing units can be administered using relatively low volumes. Furthermore, following concentration, vector preparations can be resuspended in a medium of specified composition and afford higher MOIs, thus maximizing overall transduction efficiency and transgene expression. In the case of FLYRD18-conditioned supernatants, concentration provides an important preparative step that can effectively separate vector particles from potentially harmful components found in the culture medium (14).
Analysis of human engraftment levels in transplanted mice yielded several interesting observations that highlight the potential of these vectors for clinical use and some of the challenges that have yet to be overcome. The fact that the majority of transplanted mice showed long-term hematopoietic reconstitution with transduced cells demonstrates the utility of RD114 pseudotypes. Similarly, transgene expression was observed in all hematopoietic lineages examined, further indicating that an MSCV promoter-driven vector can sustain multilineage expression in vivo. These results compare favorably with those recently published by two other groups using different vectors, transduction conditions, and sources of CD34+ cells (3, 26). Of particular interest is the fact that some animals that received transduced CD34+ cells showed human cell reconstitution without detectable transgene expression. PCR analysis of peripheral blood and bone marrow genomic-DNA samples from these animals failed to detect vector sequences. These results emphasize the fact that although RD114-pseudotyped oncoretrovirus vectors can transduce bulk CD34+ cells, they transduce cells with in vivo repopulating potential with limited efficiency. With further improvements in the transduction of quiescent hematopoietic cells, sustained transgene expression in the majority of transplanted cells may be attained.
In light of these highly encouraging results with the RD114 envelope pseudotype, one exciting possibility to be considered is the incorporation of the RD114 envelope protein into lentivirus vectors. Presently, most lentivirus-based vectors are pseudotyped with the VSV-G envelope (1, 4, 6, 8, 10, 16, 17, 24, 28). Lentivirus vectors of this pseudotype can be concentrated to high titers and efficiently transduce unstimulated hCD34+ cells with in vivo repopulating potential (9, 11, 16, 21). However, a major problem with this pseudotype is the cellular toxicity associated with VSV-G expression (7, 18). The generation of VSV-G-expressing producer cell lines for large-scale vector production requires the use of inducible systems to regulate VSV-G expression (12, 18). This adds a significant level of complexity to vector production that is further compounded by the requirement that packaging functions also be expressed inducibly (12, 13, 19, 25, 30). Given the apparent lack of toxicity associated with concentrated RD114 envelope-pseudotyped vector supernatants, this pseudotype may represent an excellent alternative envelope protein for use in the establishment of future lentivirus vector producer lines.
Acknowledgments
We thank B. Fredericksen-McIver, R. Gerard, and R. Munford for critical review of the manuscript; S. Brandon for collection of clinical samples; M. Bennett and T. George for expert assistance with animal procedures; Jay T. Evans and D. Todd for the construction of the MSCV-EGFP vector; and A. Mobley for assistance with flow cytometry. We especially thank and acknowledge A. W. Nienhuis and B. Sorrentino for providing the plasmid pMGirL22Y, R. G. Hawley for the use of the MSCV vector, and Y. Takeuchi for the FLY-RD18 packaging cell line (obtained via the European Tissue Culture Collection). We thank D. Foster for continued support of this work.
This work was supported by NIH grant CA82055 (to J.V.G.).
REFERENCES
- 1.Akkina R K, Walton R M, Chen M L, Li Q-X, Planelles V, Chen I S Y. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J Virol. 1996;70:2581–2585. doi: 10.1128/jvi.70.4.2581-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allay J A, Persons D A, Galipeau J, Riberdy J M, Ashmun R A, Blakley R L, Sorrentino B P. In vivo selection of retrovirally transduced hematopoietic stem cells. Nat Med. 1998;4:1136–1143. doi: 10.1038/2632. [DOI] [PubMed] [Google Scholar]
- 3.Barquinero J, Segovia J C, Ramirez M, Limon A, Guenechea G, Puig T, Briones J, Garcia J, Bueren J A. Efficient transduction of human hematopoietic repopulating cells generating stable engraftment of transgene-expressing cells in NOD/SCID mice. Blood. 2000;95:3085–3093. [PubMed] [Google Scholar]
- 4.Blömer U, Naldini L, Kafri T, Trono D, Verma I M, Gage F H. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosset F-L, Takeuchi Y, Battini J-L, Weiss R A, Collins M K L. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas J, Kelly P, Evans J T, Garcia J V. Efficient transduction of human lymphocytes and CD34+ cells via human immunodeficiency virus-based gene transfer vectors. Hum Gene Ther. 1999;10:935–945. doi: 10.1089/10430349950018337. [DOI] [PubMed] [Google Scholar]
- 7.Eidelman O, Schlegel R, Tralka T S, Blumenthal R. pH-dependent fusion induced by vesicular stomatitis virus glycoprotein reconstituted into phospholipid vesicles. J Biol Chem. 1984;259:4622–4628. [PubMed] [Google Scholar]
- 8.Evans J T, Kelly P F, O'Neill E, Garcia J V. Human cord blood CD34+ CD38− cell transduction via lentivirus-based gene transfer vectors. Hum Gene Ther. 1999;10:1479–1489. doi: 10.1089/10430349950017815. [DOI] [PubMed] [Google Scholar]
- 9.Follenzi A, Ailles L E, Bakovic S, Geuna M, Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- 10.Gatlin J, Douglas J, Evans J T, Collins R H, Wendel G D, Garcia J V. In vitro selection of lentivirus vector-transduced human CD34+ cells. Hum Gene Ther. 2000;11:1949–1957. doi: 10.1089/10430340050129558. [DOI] [PubMed] [Google Scholar]
- 11.Guenechea G, Gan O I, Inamitsu T, Dorrell C, Pereira D S, Kelly M, Naldini L, Dick J E. Transduction of human CD34+ CD38− bone marrow and cord blood-derived SCID-repopulating cells with third-generation lentiviral vectors. Mol Ther. 2000;1:566–573. doi: 10.1006/mthe.2000.0077. [DOI] [PubMed] [Google Scholar]
- 12.Kafri T, van Praag H, Ouyang L, Gage F H, Verma I M. A packaging cell line for lentivirus vectors. J Virol. 1999;73:576–584. doi: 10.1128/jvi.73.1.576-584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan A H, Swanstrom R. The HIV-1 gag precursor is processed via two pathways: implications for cytotoxicity. Biomed Biochim Acta. 1991;50:647–653. [PubMed] [Google Scholar]
- 14.Kelly P F, Vandergriff J, Nathwani A, Nienhuis A W, Vanin E F. Highly efficient gene transfer into cord blood nonobese diabetic/severe combined immunodeficiency repopulating cells by oncoretroviral vector particles pseudotyped with the feline endogenous retrovirus (RD114) envelope protein. Blood. 2000;96:1206–1214. [PubMed] [Google Scholar]
- 15.Marandin A, Dubart A, Pflumio F, Cosset F-L, Cordette V, Chapel-Fernandes S, Coulombel L, Vainchenker W, Louache F. Retrovirus-mediated gene transfer into human CD34+38low primitive cells capable of reconstituting long-term cultures in vitro and nonobese diabetic-severe combined immunodeficiency mice in vivo. Hum Gene Ther. 1998;9:1497–1511. doi: 10.1089/hum.1998.9.10-1497. [DOI] [PubMed] [Google Scholar]
- 16.Miyoshi H, Smith K A, Mosier D E, Verma I M, Torbett B E. Efficient transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science. 1999;284:682–686. doi: 10.1126/science.283.5402.682. [DOI] [PubMed] [Google Scholar]
- 17.Naldini L, Blömer U, Gage F H, Trono D, Verma I M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ory D S, Neugeboren B A, Mulligan R C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Planelles V, Bachelerie F, Jowett J B M, Haislip A, Xie Y, Banooni P, Masuda T, Chen I S Y. Fate of the human immunodeficiency virus type 1 provirus in infected cells: a role for vpr. J Virol. 1995;69:5883–5889. doi: 10.1128/jvi.69.9.5883-5889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter C D, Collins M K, Tailor C S, Parkar M H, Cosset F-L, Weiss R A, Takeuchi Y. Comparison of efficiency of infection of human gene therapy target cells via four different retroviral receptors. Hum Gene Ther. 1996;7:913–919. doi: 10.1089/hum.1996.7.8-913. [DOI] [PubMed] [Google Scholar]
- 21.Ramezani A, Hawley T S, Hawley R G. Lentiviral vectors for enhanced gene expression in human hematopoietic cells. Mol Ther. 2000;2:458–469. doi: 10.1006/mthe.2000.0190. [DOI] [PubMed] [Google Scholar]
- 22.Rasko J E, Battini J-L, Gottschalk R J, Mazo I, Miller A D. The RD114/simian type D retrovirus receptor is a neutral amino acid transporter. Proc Natl Acad Sci USA. 1999;96:2129–2134. doi: 10.1073/pnas.96.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rebel V I, Tanaka M, Lee J-S, Hartnett S, Pulsipher M, Nathan D G, Mulligan R C, Sieff C A. One-day ex vivo culture allows effective gene transfer into human nonobese diabetic/severe combined immune-deficient repopulating cells using high-titer vesicular stomatitis virus G protein pseudotyped retrovirus. Blood. 1999;93:2217–2224. [PubMed] [Google Scholar]
- 24.Reiser J, Harmison G, Kluepfel-Stahl S, Brady R O, Karlsson S, Schubert M. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc Natl Acad Sci USA. 1996;93:15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogel M E, Wu L I, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiedlmeier B, Kuhlcke K, Eckert H G, Baum C, Zeller W J, Fruehauf S. Quantitative assessment of retroviral transfer of the human multidrug resistance 1 gene to human mobilized peripheral blood progenitor cells engrafted in nonobese diabetic/severe combined immunodeficient mice. Blood. 2000;95:1237–1248. [PubMed] [Google Scholar]
- 27.Shultz L D, Schweitzer P A, Christianson S W, Gott B, Schweitzer I B, Tennent B, McKenna S, Mobraaten L, Rajan T V, Greiner D L, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- 28.Sutton R E, Wu H T M, Rigg R, Böhnlein E, Brown P O. Human immunodeficiency virus type 1 vectors efficiently transduce human hematopoietic stem cells. J Virol. 1998;72:5781–5788. doi: 10.1128/jvi.72.7.5781-5788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Hennik P B, Verstegen M M A, Bierhuizen M F A, Limon A, Wognum A W, Cancelas J A, Barquinero J, Ploemacher R E, Wagemaker G. Highly efficient transduction of the green fluorescent protein in human umbilical cord stem cells capable of cobblestone formation in long-term cultures and multilineage engraftment of immunodeficient mice. Blood. 1998;92:4013–4022. [PubMed] [Google Scholar]
- 30.Yu H, Rabson A B, Kaul M, Ron Y, Dougherty J P. Inducible human immunodeficiency virus type 1 packaging cell lines. J Virol. 1996;70:4530–4537. doi: 10.1128/jvi.70.7.4530-4537.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]