Abstract
The accumulation of aggregated forms of the α-Synuclein (α-Syn) is associated with the pathogenesis of Parkinson’s disease (PD), and the efficient clearance of aggregated α-Syn represents a potential approach in PD therapy. Astrocytes are the most numerous glia cells in the brain and play an essential role in supporting brain functions in PD state. In the present study, we demonstrated that cultured primary astrocytes engulfed and degraded extracellular aggregated recombinant human α-Syn. Meanwhile, we observed that the clearance of α-Syn by astrocytes was abolished by proteasome inhibitor MG132 and autophagy inhibitor 3-methyladenine (3MA). We further showed that intracellular α-Syn was reduced after ginkgolide B (GB) and bilobalide (BB) treatment, and the decrease was reversed by MG132 and 3MA. More importantly, GB and BB reduced indirect neurotoxicity to neurons induced by α-Syn-stimulated astrocytic conditioned medium. Together, we firstly find that astrocytes can engulf and degrade α-Syn aggregates via the proteasome and autophagy pathways, and further show that GB and BB enhance astrocytic clearance of α-Syn, which gives us an insight into the novel therapy for PD in future.
Keywords: α-Synuclein, Astrocytes, Autophagy, Ginkgolide B, Bilobalide
Introduction
Alpha-Synuclein (α-Syn), a 14 kDa protein (140 amino acids) physiologically found in presynaptic terminals of neurons, is the major fibrillary protein in Lewy bodies (LBs) and Lewy neuritis (LNs) (Vekrellis et al. 2011). Alpha-Syn has been suggested to play important roles in brain lipid metabolism, modulation of synaptic transmission, and synaptic vesicle biogenesis (Kim and Lee 2008). However, mounting evidence has indicated that α-Syn malfunction, especially the accumulation of misfolded α-Syn aggregates and genetic mutations, can also contribute to synucleinopathies such as dementia with LBs (DLB) and sporadic and inherited Parkinson’s disease (PD) (Kalia and Lang 2015; Spillantini et al. 1998).
PD is primarily characterized by selective loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and abrogation of dopaminergic tone along the nigrostriatal pathway (Dauer and Przedborski 2003; Obeso et al. 2017). However, previous studies have indicated that the progressive neuronal accumulation of aggregated α-Syn and the formation of LBs might trigger neuroinflammation, neurotoxicity, and synaptic dysfunction in PD (Gao et al. 2011; Ma et al. 2014; Volpicelli-Daley et al. 2011). Notably, the release of α-Syn aggregates from neuronal cells was significantly increased under PD condition (Jang et al. 2010), and extracellular α-Syn aggregates may cause paracrine effects on neighboring cells. Transmission of α-Syn aggregates from neuron-to-neuron (Tyson et al. 2017) and neuron-to-glia has been suggested as the underlying mechanism of neuroinflammation and neurotoxicity in PD (Choi et al. 2018). Hence, an efficient clearance of this protein might represent a potential approach in PD therapy.
Astrocytes are the most numerous and multifunctional type of glia cells in the central nervous system (CNS). Astrocytes are crucial to the neuronal microenvironment due to its abilities to maintain tissue structure, provide nutrition and energy to neurons, and regulate synaptic development and neurotransmitter uptake. Previously studies have shown that extracellular α-Syn acted on neuronal Toll-like receptor 2 (TLR2) might result in neurodegeneration by inhibition of autophagy via AKT/mTOR signaling (Kim et al. 2013), while extracellular α-Syn activated microglia via NF-κB and p38 MAPK pathway, thereby resulted in neuroinflammatory factors production (Kim et al. 2015). While extracellular α-Syn aggregates can be taken up by neurons (Lee et al. 2008a, b) and glial cells (Lee et al. 2008a, b) by endocytosis, it is not clear whether astrocytes are responsible for α-Syn clearance in PD. A recent study reported that Polo-like kinase 2 (PLK2) enhanced α-Syn autophagic degradation, and overexpression of PLK2 reduced intra-neuronal human α-Syn accumulation, and suppressed dopaminergic neurodegeneration induced by α-Syn overexpression in a rat genetic model of PD (Oueslati et al. 2013). Moreover, clearance of α-Syn monomers and aggregates occurs via direct proteolysis by matrix metalloprotease 9 (MMP-9) (Iwata et al. 2003) or binding to molecular chaperones such as the proteasome (McNaught et al. 2002) and autophagy (Oueslati et al. 2013).
We have previously shown that aggregated α-Syn induced cell apoptosis, and Ginkgo biloba extract (GBE), including ginkgolide B (GB) and bilobalide (BB), attenuated aggregated α-Syn-induced cell apoptosis (Hua et al. 2017). In the present study, we showed that astrocytes internalized and eliminated α-Syn aggregates in vitro, and GB and BB promoted the degradation of intercellular α-Syn aggregates via the autophagy and proteasome pathways. Moreover, GB and BB reduced the indirect neurotoxicity of α-Syn to neurons in vitro. Herein, we elucidate that the promotion of astrocytic clearance of α-Syn may be a potential approach in PD therapy.
Materials and Methods
Experimental Animals and Drug Administration
All study protocols were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (IACUC). C57BL/6 mice were provided by Model Animal Resource Center (MARC, Nanjing, China). All mice were maintained on a 12/12 h light/dark cycle with free access to food and water.
GB and BB (purity > 99%, supplied by the National Institutes for Food and Drug Control, China) were dissolved in dimethyl sulfoxide (DMSO) as stock solutions for in vitro studies, respectively.
Cell Culture and Treatment
Primary astrocytes were prepared from brains of 1- to 2-day-old C57BL/6 mice, as previously described (Zeng et al. 2007). Briefly, neonates (P1–2) were killed by rapid decapitation. The midbrain was removed, and then the tissue was dissociated with 0.25% tryptase (VWR Life Science, USA) at 37 °C and terminated by Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco Lab., USA) supplemented with 10% Fetal Bovine Serum (FBS, Gibco Lab., USA), and 100 mg/mL streptomycin (Sigma-Aldrich Biotechnology, USA) and 100 U/mL penicillin (Gibco Lab., USA). After centrifugation at 1500 rpm for 5 min, the cell pellets were re-suspended and plated on a poly-lysine-treated flask (Sigma, St Louis, MO). The cultures were maintained at 37 °C in a humidified 5% CO2–95% air atmosphere. Culture media were changed 24 h later to complete medium and subsequently twice a week. The purity of astrocyte was > 95% as determined with GFAP immunocytochemistry. Before experimental treatments, astrocytic cultures were passaged once.
Human neuroblastoma SH-SY5Y cells were purchased from American Type Culture Collection (ATCC, USA). Cells were cultured in DMEM (Gibco Lab., USA) containing 10% FBS (Gibco Lab., USA), 100 mg/mL streptomycin (Sigma-Aldrich Biotechnology, USA), and 100 U/mL penicillin (Gibco Lab., USA). The cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. Before the experiments, cells were inoculated into 6-well plate or 96-well plate, and cultured in DMEM containing 10% FBS for 24 h. Then the medium was replaced with serum-free medium with α-Syn (1 μM) or GB (10 μM)/BB (10 μM) or MG132 (20 μM)/3MA (5 mM) in different experimental parts.
Preparation of Recombinant α-Syn Aggregates
The wild-type human α-Syn was purified as previously described (Hua et al. 2017). Briefly, α-Syn gene was obtained by digesting the plasmid pcDNA3.1(−)-α-Syn using restriction enzymes NcoI and XhoI. PCR fragments of pcDNA3.1(−)-α-Syn were obtained by gel electrophoresis, respectively, and the accuracy of pET-28b(+)-α-Syn was verified in NCBI. The purified α-Syn was inserted into NcoI/XhoI sites of pET-28b(+) to form a recombinant expression plasmid pET-28b(+)-α-Syn, and then the construct was transformed into E. coli JM109 competent cells. The fusion protein pET-28b(+)-α-Syn was expressed by addition of IPTG and purified by ammonium sulfate, ammonium acetate, and anhydrous ethanol according to its acid stability. After analyzed by MALDI analysis, the purified α-Syn protein was lyophilized and stored at − 80 °C until further use.
The forms of α-Syn samples (1 mg/mL) were prepared by dissolving the α-Syn stock in Tris buffer (50 mM Tris, 150 mM NaCl, 10 mM EDTA, pH 8.0). Aggregated α-Syn was generated by incubating the samples at 37 °C for 7 days (with shaking). Electron microscope and Thioflavin T (ThT) were used to identify α-Syn morphologies before subsequent experiments.
The Thioflavin T (ThT) Assay
ThT binding assay is widely used to observe the formation of aggregates. During the preparation of α-Syn aggregates, aliquots of 10 μL α-Syn were removed from the incubated solution for ThT binding experiments. 1 mg ThT was dissolved in 1 mL methanol, and stored at in the dark 4 °C. Then, 5 μM freshly prepared ThT (dissolved in 50 mM glycine, pH 8.5) and 5 μL α-Syn sample were added to the 96-well plate to a final volume of 200 μL. Phosphate buffered saline (PBS) was as a blank control. The reaction was carried out at room temperature for 1 min, and the absorbance value was read by a full-wavelength microplate reader. The excitation light is 446 nm and the absorption light is 482 nm. ThT binding (unit a.u.) = Absorbance of Sample − Absorbance of PBS blank control.
FITC-labeled α-Syn Aggregates
The α-Syn aggregates were placed in 4-mL ultrafiltration tubes (30 kDa, Millipore, UFC803008) and centrifuged at 7500 g for 20 min, following replaced PBS with a freshly prepared cross-linked reaction solution (7.56 g NaHCO3, 1.06 g Na2CO3, 7.36 g NaCl, pH 9.0, plus ddH2O fixed volume to 1 L). Freshly prepared 1 mg/mL fluorescein isothiocyanate (FITC, Sigma, # 3326-32-7) was dissolved in DMSO against the light. 50 μL FITC was added to 1 mL protein solution, gently shaking to mix well with the protein, then, keeping in dark at 4 °C for 8 h. Afterwards, 5 M NH4Cl was added to protein solution at a final concentration of 50 mM and the reaction was stopped at 4 °C for 2 h. The production was transferred to ultrafiltration tubes (4 mL), centrifuged at 7500 g for 20 min, and the buffer was replaced with PBS. Labeled FITC-α-Syn aggregates were protected at 4 °C, and detected with anti-α-Syn antibody by immunofluorescence.
In Vitro α-Syn Internalization Analysis
Primary astrocytes were seeded in a 24-well dish (2 × 104 cells/well) and cultured for 24 h. Astrocytes were treated with α-Syn monomers (1 μM) or aggregates (1 μM) for different time-point (0.5, 2, 6, 12, and 24 h), the content of α-Syn in cell supernatant and cytoplasm was detected by western blotting assay.
In Vitro α-Syn Degradation Analysis
Primary astrocytes were seeded in a 24-well dish (2 × 104 cells/well) and cultured for 24 h. Astrocytes were pretreated with α-Syn aggregates (1 μM) for 1 h, then the α-Syn-containing culture medium was removed, and cells were washed with PBS for three times, followed by incubation with normal culture medium, medium with MG132 and/or 3MA, medium with GB or BB, or medium with MG132 and/or 3MA in the presence of GB or BB. The content of α-Syn in cell cytoplasm was detected by western blotting assay and immunofluorescence.
Immunofluorescence
Primary astrocytes were seeded in a 24-well dish (2 × 104 cells/well) and cultured for 24 h. The cells were then incubated with α-Syn and GB/BB according to study design. The control group was treated only with the same amount of solvent for 24 h. Cells were washed three times with 0.01 M PBS (5 min/wash), blocked with 5% BSA (Bovine Serum Albumin) at room temperature for 30 min, and incubated with primary antibody against α-Syn (1:200, #610787, BD-Bioscience) and GFAP (Glial fibrillary acidic protein, 1:500, #MAB360, Millipore) overnight at 4 °C. In the following day, cells were incubated with the secondary antibody A488 goat anti-mice IgG (1:1000, #A11001, Invitrogen) or A555 (1:1000, #A21422, Invitrogen) for 2 h at room temperature, washed three times with 0.01 M PBS (15 min/wash), and incubated with 100 ng/mL DAPI or Hoechst for 3 min. Images were captured using a fluorescence microscope (Nikon, Japan) equipped with 20× objectives. Images were collected using sequential scanning with the 405-, 488-, and 594-nm laser lines to produce three color overlays.
Conditioned Medium Transfer Experiments
Cultured primary astrocytes were incubated with GB (10 μM) or BB (10 μM) for 24 h before α-Syn aggregate treatment for another 24 h. Then, the culture mediums were collected and centrifuge at 4000×g for 5 min at 20 °C to remove debris. Checking a small aliquot of CM at the microscope for the presence of debris, and the medium was used as conditioned medium to stimulate SH-SY5Y cells.
MTT Analysis
Cell viability was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay. Cells were treated with conditioned medium from treated astrocytes and incubated at 37 °C and 5% CO2 for 24 h, and then the cell supernatant was collected for LDH assay. After discarding serum-free DMEM medium in 96-well plate and adding 150 μL 0.5 mg/mL MTT (Sigma-Aldrich Biotechnology, USA) dissolved in PBS solution, the cells were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air for 4 h. Subsequently, cell supernatant was removed followed by incubation with 200 μL DMSO (Aladdin Industrial Corporation, Shanghai, China) at room temperature for 20 min. The absorbance of each hole was detected at 450 nm wave length.
LDH Analysis
Lactate dehydrogenase (LDH) activity-based cytotoxicity assay LDH activity measurements were performed using a commercially available assay (Jiancheng, Nanjing, China) according to the manufacturer’s instructions. The ratio of LDH activity in the supernatant to the total LDH activity was taken as the percentage of cell death. For each experiment, four wells per concentration were averaged and each experiment was performed in triplicate.
Purification of Cell Culture Supernatant Protein
The cell culture supernatant was collected, centrifuged to remove dead cells, and the supernatant medium was transferred into new tubes. Then, 500 μL methanol and 125 μL chloroform were added to precipitate medium, vortexed, and centrifuged for 5 min at 16,000 g. The upper phase was discarded without touching the proteins disk, and 500 μL methanol was added and centrifuged for 5 min at 16,000 g for cleaning. Then, the medium was removed, and the pellet was dried at 37 °C for 5 min. Finally, 50 μL 2.5× loading buffer was added with DTT and vortexed.
Western Blotting Analysis
Detergent solubility was performed by adding Triton X-100 to total cell lysates (final concentration 1%) and incubating for 30 min on ice followed by centrifugation (15,000 g, 60 min, 4 °C). The supernatant was designated Triton X-100 soluble fraction, and the pellet was re-dissolved in 2% SDS-containing lysis buffer and sonicated for 10 s (Triton X-100 insoluble fraction). Protein concentration was determined using a Bicinchoninic acid (BCA) protein assay. α-Syn monomer existed in Triton X-100 soluble and insoluble fraction, while aggregates only existed in Triton X-100 insoluble fraction. After stimulation, other protein samples were extracted by the protein extraction kit (Nanjing Keygen, China).
Proteins were separated by SDS-PAGE electrophoresis and transferred to PVDF membranes with the electrophoretic transfer system (Bio-Rad, USA). Non-specific binding sites were blocked with 10% non-fat dry milk in Tris-Hcl Buffer Saline containing 0.1% Tween 20 (TBS-T) for 1 h at room temperature. The membranes were then incubated with primary antibody against α-Syn (1:500, #610787, BD-Bioscience), LC3 (Microtubule-associated protein 1A/1B-light chain 3, 1:1000, #2775, CST), p62 (1:1000, #5114, CST), Beclin-1 (1:1000, #3495, CST), and β-actin (1:1000, Sigma-Aldrich,) overnight at 4 °C. Then, the membranes were incubated with corresponding secondary antibody at room temperature for 1 h. Finally, immunoblots were scanned and determined by Omega16IC (Ultra-Lum, Claremont, CA, USA).
Statistical Analysis
All statistical analysis were done using GraphPad Prism version 5.0 (GraphPad software, Inc., CA, USA) and Adobe Illustrator CS5 software. Values were presented as mean ± standard error of the mean (S.E.M.) and analyzed using one-way analysis of variance (ANOVA) followed by Tukey post hoc test. The significance level was set at p < 0.05.
Results
Astrocytes Engulfed Recombinant α-Syn Aggregates In Vitro
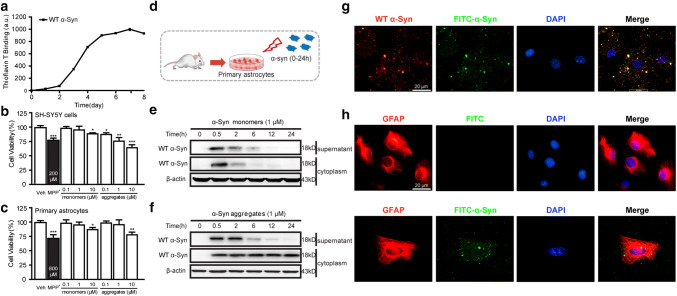
We first constructed and purified recombinant human α-Syn protein in vitro as previously described (Hua et al. 2017). The ThT assay for α-Syn protein showed increased binding force during 3–5 days, reaching a plateau level after 6 day (Fig. 1a). To observe the biological function of recombinant α-Syn aggregates, both SH-SY5Y cells and primary astrocytes were exposed to various concentrations (0.1, 1, and 10 μM) of α-Syn monomers and aggregates for 24 h. The viability of SH-SY5Y cells was decreased after incubation with α-Syn monomers at dose of 10 μM and aggregates at dose of 0.1, 1, and 10 μM (Fig. 1b). Meanwhile, the viability of primary astrocytes was decreased after incubation with monomers and aggregates only at dose of 10 μM (Fig. 1c). These results suggested the success of α-Syn aggregate preparation, and the dose of 1 μM was chosen for the following experiments.
Fig. 1.
Internalization of α-Syn aggregates in astrocytes. a Biophysical characterization of the aggregation of α-Syn proteins monitored by ThT fluorescence. b, c MTT assay was used to identify cell viability of SH-SY5Y cell and primary astrocytes after treated with MPP+, α-Syn monomers, and aggregates. Data are representative of three independent experiments (mean ± S.E.M). *p < 0.05, **p < 0.01, and ***p < 0.01 compared with the Vehicle (Veh) group. d Primary astrocytes were prepared and treated with 1 μM of FITC-α-Syn protein for several time-points (0.5, 2, 6, 12, and 24 h). e, f Western blotting analysis of α-Syn monomers (1 μM) and aggregates (1 μM) in cell supernatant and cytoplasm. g Immunocytofluorescence imaging of anti-α-Syn antibody (red) and FITC-α-Syn (green) in primary astrocytes. h Immunocytofluorescence imaging of GFAP (red) and FITC (green) in astrocytes treated with FITC (upper line). Co-location of GFAP and FITC-α-Syn in α-Syn-treated astrocytes (lower line). The cells covered with 4,6-linked amidine-2-phenylindole (DAPI, blue) for nucleus staining. Scale bar: 20 μm
To determine whether astrocyte-mediated phagocytosis was triggered in response to recombinant α-Syn, cultured primary astrocytes were incubated with 1 μM α-Syn monomers and aggregates for different times (Fig. 1d). As shown in Fig. 1e and f, we observed a decreased content of both α-Syn monomers and aggregates in the cell supernatant after treatment for 6 h. However, compared to rapidly clearance of monomers in cytoplasm, increased content of α-Syn aggregates in cytoplasm was observed during 24 h after incubation. We further investigated whether α-Syn aggregates were engulfed by astrocytes by immunofluorescence staining. A totally co-localization of FITC-labeled α-Syn and anti-α-Syn protein is shown in Fig. 1g. In addition, FITC-labeled α-Syn was majorly expressed in astrocyte, shown as co-localization of α-Syn and GFAP (Fig. 1h). These results indicated that astrocyte may engulf extracellular recombinant α-Syn protein.
Astrocytes Eliminated α-Syn Aggregates In Vitro
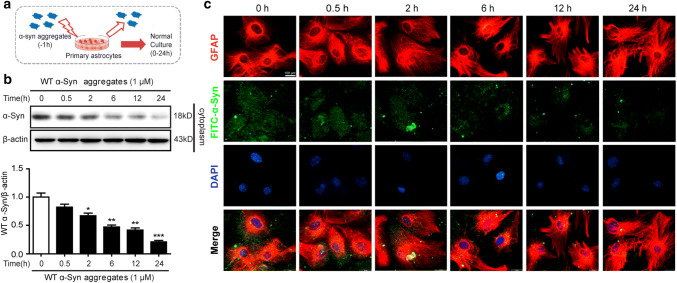
Since phagocytosis and degradation was a dynamic process, we verified the cellular presence of α-Syn for different times (0, 0.5, 2, 6, 12, 24 h) after 1-h treatment (Fig. 2a). We found that the intracellular α-Syn aggregates were decreased in a time-dependent manner after treatment (Fig. 2b). To further confirm the elimination of α-Syn aggregates in astrocyte, immunocytochemistry was used to identify FITC-labeled α-Syn aggregates in astrocytes. Notably, FITC-labeled α-Syn was co-expressed with GFAP positive astrocyte, and content of α-Syn was decreased over time (Fig. 2c). These data demonstrated that astrocyte eliminated intracellular recombinant α-Syn protein.
Fig. 2.
Clearance of α-Syn aggregates in astrocytes. a Primary astrocytes were treated with 1 μM of α-Syn aggregates for 1 h and washed twice with fresh medium, followed by incubation in fresh medium for the indicated times. b Triton X-100-insoluble protein extracts were analyzed using western blotting. Data are representative of three independent experiments (mean ± S.E.M). *p < 0.05, **p < 0.01, ***p < 0.001 compared with Con (0 h) group. c Immunocytofluorescence imaging of GFAP (red) and FITC-α-Syn aggregates (green) in astrocytes. DAPI (blue) for nucleus staining. Scale bar: 20 μm
Both the Ubiquitin–Proteasome and Autophagy–Lysosome Pathways were Involved in the Astrocytic Clearance of α-Syn Aggregates
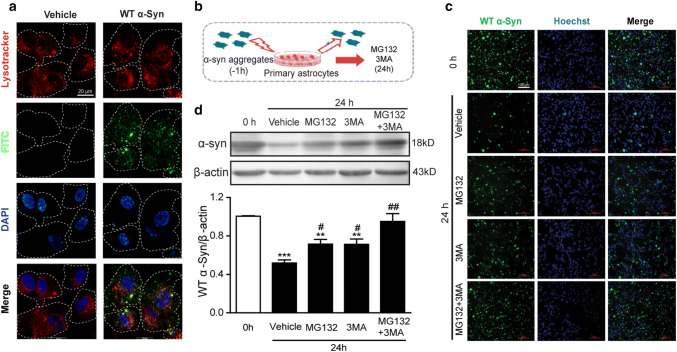
It has been reported that α-Syn can be degraded by the ubiquitin proteasome system (UPS) and autophagy–lysosome pathways (ALP) (Webb et al. 2003). In order to identify whether astrocytes clear α-Syn through the lysosomal pathway, astrocytes were incubated with LysoTracker and FITC- α-Syn aggregates for 1 h. Immunofluorescence microscopy showed the presence of α-Syn and lysosomes in astrocytes was co-located (Fig. 3a), suggesting that astrocytes may clear the α-Syn aggregates through the lysosomal pathway.
Fig. 3.
Astrocytes degraded α-Syn aggregates through the proteasome system and autophagy–lysosome pathway. a Co-location of LysoTracker and FITC-α-syn aggregates in astrocytes. Fluorescence imaging of astrocytes after incubating with LysoTracker (red) and FITC- α-syn aggregates (green) for 1 h. DAPI (blue) for nucleus staining. Scale bar: 20 μm. b Astrocytes were treated with 1 μM of α-Syn aggregates for 1 h and washed twice with fresh medium, followed by the treatment of MG132 (20 μM) or/and 3MA (5 mM) for 24 h. c Western blotting analysis of Triton X-100-insoluble protein extracts in astrocytes treated with α-Syn aggregates and MG132 or/and 3MA. **p < 0.01, ***p < 0.001 compared with Con (0 h) group, #p < 0.05, ##p < 0.01 compared with Vehicle group. Data are representative of three independent experiments (mean ± S.E.M). d Immunocytofluorescence imaging of α-Syn (green) of astrocytes treated with aggregated α-Syn. Hoechst (blue) for nucleus staining. Scale bar: 100 μm
To further investigate whether recombinant α-Syn protein can be degraded by UPS or ALP, cultured primary astrocytes were pretreated with 1 μM α-Syn aggregates for 1 h. Afterwards, α-Syn-containing medium was removed and cells were incubated with MG132 (20 μM) and 3MA (5 mM)-containing medium for 24 h (Fig. 3b). Western blotting (Fig. 3c) and immunocytochemistry assay (Fig. 3d) showed that α-Syn was significantly increased after MG132 and 3MA treatment. These results may prove that astrocytes engulf and degrade α-Syn aggregates via both the proteasome and autophagy pathways.
Ginkgolides Enhanced Astrocytic Uptake and Degradation of α-Syn Aggregates
We previously have found that Ginkgolides, including GB and BB, could attenuate aggregated α-Syn-induced cell apoptosis in SH-SY5Y cells (Hua et al. 2017). To further evaluate the effect of astrocytic clearance on aggregated α-Syn-induced cell apoptosis, we firstly investigated the effects of GB and BB on the phagocytosis α-Syn protein in astrocytes. The cultured primary astrocytes were treated with GB or BB for 2 h prior to α-Syn aggregate (1 μM) incubation for 24 h (Fig. 4a). As a result, the contents of the α-Syn in cytoplasm were decreased after GB or BB treatment by Western blotting analysis (Fig. 4b), and total content of α-Syn protein was detected decreased by immunocytochemistry (Fig. 4c). These results showed that GB and BB reduced α-Syn aggregates in cytoplasm, indicating an enhancement of astrocytic clearance of α-Syn aggregates by GB and BB.
Fig. 4.
Ginkgolide B and bilobalide accelerated the clearance of α-Syn in astrocytes. a After the pretreatment with GB or BB for 2 h, astrocytes were incubated with aggregated α-Syn for 24 h. b Triton X-100-insoluble protein extracts of astrocytes were analyzed by western blotting. Data are representative of three independent experiments (mean ± S.E.M). ***p < 0.001 compared with Vehicle group, ##p < 0.01, ###p < 0.001 compared with Vehicle group. c Immunocytofluorescence imaging of α-Syn of astrocytes treated with α-Syn aggregates and GB or BB. Scale bar: 100 μm. d Astrocytes were treated with 1 μM of α-Syn aggregates for 1 h and washed twice with fresh medium, followed by incubation with GB or BB for 24 h. e, f α-Syn content was analyzed using western blotting and immunocytofluorescence. Scale bar: 100 μm. Data are representative of three independent experiments (mean ± S.E.M). **p < 0.01 compared with Con (0 h) group, #p < 0.05, ##p < 0.01 compared with Vehicle group
To further confirm the effect of GB and BB on the degradation of α-Syn aggregates internalized in astrocytes, cultured primary astrocytes were pretreated with 1 μM α-Syn aggregates to let α-Syn transfer into cells. After 1-h incubation, α-Syn-containing medium was removed, and cells were incubated with GB or BB-containing medium for 24 h (Fig. 4d). As shown in Fig. 4e, the content of the α-Syn in cell cytoplasm was reduced after GB or BB treatment, and intracellular α-Syn was detected decreased by immunocytochemistry (Fig. 4f). Collectedly, our results showed that GB and BB can enhance astrocytic phagocytosis and degradation of α-Syn aggregates.
Ginkgolides Promoted the Astrocytic Elimination of α-Syn Mainly via Regulating Autophagy
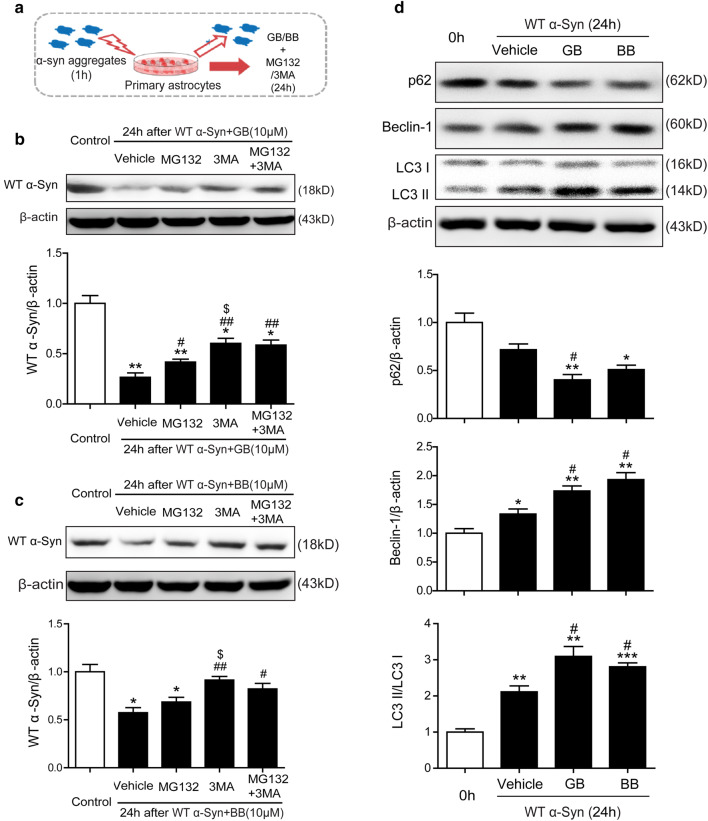
To further determine whether GB and BB can accelerate elimination via promoting the UPS or ALP pathway, primary astrocytes were pretreated with 1 μM α-Syn aggregates for 1 h. Then, α-Syn-containing medium was removed and GB/BB-containing medium mixed with MG132 (20 μM) and 3MA (5 mM) were added for 24 h (Fig. 5a). As showed in Fig. 5b, and Fig. 5c, the content of the α-Syn was significant increased with MG132 (20 μM) and/or 3MA (5 mM) decreased after GB (10 μM) or BB (10 μM) treatment. Interestingly, 3MA inhibited the decrease of α-Syn more significant than MG132, whereas no distinguishable change of intracellular α-Syn was observed between 3MA-treated group and MG132 + 3MA combination.
Fig. 5.
GB and BB accelerated the clearance of α-Syn aggregates in astrocytes through an enhanced autophagy pathway. a Astrocytes were treated with 1 μM of α-Syn aggregates for 1 h and washed twice with fresh medium, followed by incubation with MG132 (20 μM) or/and 3MA (5 mM) in the presence of GB or BB for 24 h. b, c Triton X-100-insoluble α-Syn content was analyzed using western blotting in GB and BB groups. d Expression of p62, Beclin-1, and LC3II/I was analyzed using western blotting in astrocytes treated with GB and BB for 24 h. Data are representative of three independent experiments (mean ± S.E.M). *p < 0.05, **p < 0.01 compared with Con group, #p < 0.05, ##p < 0.01 compared with Vehicle group, $p < 0.05 compared with MG132 group
Several lines of evidence have suggested that autophagy enhancers can promote the clearance of aggregate-prone proteins (Casarejos et al. 2011). During autophagy initiation and autophagosome formation, Beclin-1 binds microtubule-associated protein-1 light chain 3 (LC3-I) that is converted to LC3-II (the membrane bound form) and interacts with the ubiquitin-binding protein p62 (Park et al. 2013). As showed in Fig. 5d, western blot analysis showed that the treatment of GB and BB significantly reduced the expression of p62 after 24-h incubation. Meanwhile, protein expression of autophagy-related genes Beclin-1 and the ratio of LC3-II/LC3-I increased after GB or BB treatment compared with Vehicle group. As showed in Fig. 5d, western blot analysis showed that the treatment of GB and BB significantly reduced the expression of p62 after 24-h incubation. Meanwhile, the level of Beclin-1 and the ratio of LC3-II/LC3-I increased after GB or BB treatment compared with Vehicle group. Taken together, GB and BB reduced astrocytic expression of α-Syn via proteasome and autophagy, and autophagy may be more efficiently than proteasome in astrocytes.
Ginkgolides Indirectly Protected the SH-SY5Y Cells from the Neurotoxicity Induced by α-Syn-Stimulated Astrocytic Conditioned Medium
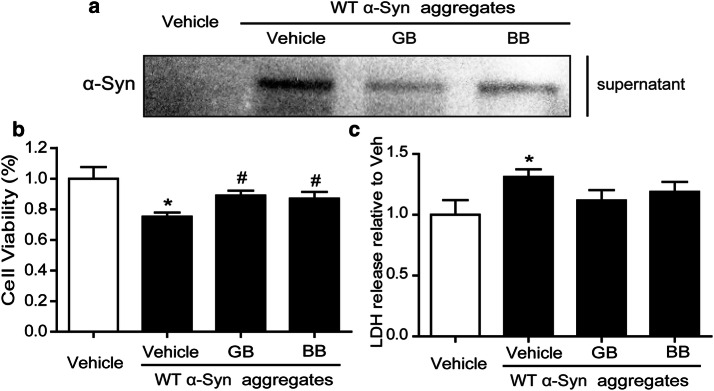
Since we found that GB and BB can accelerate the elimination of α-Syn aggregates in astrocyte, we next investigated whether it may protect neurons from the indirect neurotoxicity induced by α-Syn-stimulated astrocytic conditioned medium (CM). Primary astrocytes were pretreated with GB (10 μM) or BB (10 μM) 24 h. Then, GB/BB-containing medium was removed before α-Syn aggregates (1 μM) stimulation. Cellular culture supernatants (CM) were harvested, and protein levels of α-Syn were detected by western blot 24 h later. As shown in Fig. 6a, GB and BB treatment can both reduce the α-Syn content in cell culture supernatant. When human SH-SY5Y cells were treated with the CM, a significantly cell injury was found in α-Syn aggregate-treated group detected by MTT and LDH assay, and GB and BB can increase cell vitality and decrease cell LDH release (Fig. 6b and c).
Fig. 6.
GB and BB ameliorated astrocytic conditioned medium-induced SH-SY5Y injury. a Western blotting analysis of α-Syn aggregates in α-Syn aggregate-stimulated astrocytic conditioned medium treated with GB and BB. b, c MTT assay and LDH assay of SH-SY5Y treated with astrocytic conditioned medium. Data are representative of three independent experiments (mean ± S.E.M). *p < 0.05 compared with Vehicle group. #p < 0.05 compared with Vehicle+α-Syn group
Discussion
In the present study, we constructed and purified recombinant human α-Syn protein, and focused on astrocytic properties to clear extracellular recombinant α-Syn in vitro. Our results showed that astrocytes internalized and eliminated recombinant human α-Syn aggregates in vitro, and GB and BB promoted the degradation of intercellular α-Syn aggregates via the pathway of autophagy and proteasome. Moreover, GB and BB reduced the indirect neurotoxicity of α-Syn to neurons in vitro.
Synucleinopathies such as PD and DLB destroy life of aging population over 65 years, and approaches to interrupt α-Syn misfolding and aggregation of represent promising paradigms for therapeutic interventions (Lee et al. 2014). Currently, mounting evidence indicated that transmission of toxic α-Syn between neurons and glia cells is the major way causing neurodegeneration. Released α-Syn can promote formation of inclusion bodies and induce cell death in neurons and pro-inflammatory responses from astrocytes (Desplats et al. 2009). Recent studies have suggested LAG3 (lymphocyte-activation gene 3) might be a receptor that contributes to α-Syn transmission (Mao et al. 2016), but the details are not clear. In our study, we treated primary astrocytes with α-Syn aggregates, and intracellular α-Syn can be identified by western blotting and immunocytochemistry assay, indicating that extracellular α-Syn can be engulfed by astrocytes. Moreover, Changyoun Kim et al. have showed that α-Syn released from differentiated SH-SY5Y cells might interacted with TLR2 and induced activation of microglia (Kim et al. 2013), but the relationship between released α-Syn and astrocytes is still largely unknown.
Afterwards, we detected the content of α-Syn in astrocytes by western blotting and immunofluorescence assay after α-Syn incubation for 1 h. As expected, we found that the content of intracellular α-Syn aggregates was decreased in a time-dependent manner after treatment, indicating that astrocyte can also eliminate intracellular recombinant α-Syn protein after internalization. Through gene expression analysis, astrocytes were found to express a lot of genes that have been implicated in engulfment and phagocytosis (Cahoy et al. 2008). In addition, α-Syn can be detected in blood plasma and cerebrospinal fluid, and the levels of oligomeric α-Syn in these body fluids are elevated in PD patients (El-Agnaf et al. 2006; Foulds et al. 2011). Therefore, the clearance of released α-Syn may be beneficial to PD patients, and astrocytic intake and degradation may provide efficient probabilities in PD therapy.
It has been suggested that the clearance of α-Syn was determined by assembly state of the protein. α-Syn is degraded by both the UPS and ALP in healthy neurons, keeping axons and dendrites functional (Ebrahimi-Fakhari et al. 2012). Monomeric and dimeric α-Syn species are degraded by UPS system and chaperone-mediated autophagy (Mak et al. 2010), while autophagy/lysosomal pathway is involved in the clearance of oligomeric and fibrilar species of α-Syn (Sacino et al. 2017). When autophagic clearance is impaired in neurons, α-Syn accumulates in axons, starts forming oligomers, and further aggregates and becomes deposited in Lewy bodies (Plotegher and Civiero 2012). A nearly study indicated that the overexpression of α-Syn proteins promoted the decrease of LC3-II and the increase of p62 protein levels, suggesting the inhibition of autophagy (Erustes et al. 2018). Autophagy is a process that allows bulk degradation of cytoplasmic contents. It involves the formation of autophagosome, a double membrane structure, which fuses with lysosome to form autolysosome where the contents are degraded (Xie and Klionsky 2007; Yorimitsu and Klionsky 2005). In the present study, we found FITC-α-Syn protein and LysoTracker had a partly co-localization in immunofluorescence assay, indicating a localization of α-Syn in lysosome of astrocyte. We further treated primary astrocytes with proteasome inhibitor MG132 or/and autophagy inhibitor 3MA after α-Syn aggregate incubation, and Western blotting and immunocytochemistry assay showed that α-Syn protein was significantly increased after MG132 or/and 3MA treatment. These results proved that the inhibition of autophagy and the proteasome in astrocyte significantly decreased the elimination of α-Syn protein, and methods to enhance astrocytic autophagy may be ideal approaches in α-Syn clearance.
A large number of studies have been conducted on the pharmacological activities of GBE and commercial available extract (EGb-761). GBE has been popular in many parts of the world for its multi-potential properties (Yoshitake et al. 2010). Studies have demonstrated the antioxidant (Guo et al. 2015), anticancer (Hoenerhoff et al. 2013), antidiabetic (Hirata et al. 2015), and cardio-protective (Mozet et al. 2009) activities of GB and BB. GB is one of the main active ingredients of GBE, and it is a strong and specific platelet activating factor antagonist and free radical scavenger. GB can inhibit the apoptosis of neurons induced by multiple factors and play a neuroprotective role (Lin et al. 2008; Shi et al. 2010; Xiao et al. 2010). Meanwhile, as the only sesquiterpene in GBE, BB is shown to play beneficial effect on PD (Di Matteo and Esposito 2003), which is closely related to anti-excitotoxicity and anti-apoptosis. In the present study, we further found that GB and BB accelerated elimination of α-Syn via enhancing autophagy in astrocyte. Previous studies reported that induction of autophagy reduces the levels of mutant huntingtin and protects against its toxicity in cells and in transgenic Drosophila and mouse models of Huntington’s Disease (Ravikumar et al. 2004; Sarkar et al. 2005). Therefore, the enhancement of astrocytic autophagy to clear α-Syn protein may protect neurons from extracellular α-Syn-mediated cytotoxicity under PD condition.
However, the role of autophagy in neurodegenerative disease is controversial, as excessive autophagy can also induce cell apoptosis (Mizushima and Komatsu 2011; Shintani and Klionsky 2004). We next investigated how did neurons response to α-Syn-stimulated astrocytic CM with or without GB/BB treatment. Our results demonstrated that GB or BB treatment reduce the neurotoxicity caused by α-Syn in astrocytic conditioned medium. Collectively, GB and BB may promote astrocytic engulfment and degradation by enhancing autophagy. These data also indicated that the timely and effective clearance of cerebral α-Syn is protective to PD patients. Further experiments are needed to examine how astrocytes engulf α-Syn and the selectivity between monomer and aggregate, and the underlying mechanisms of GB and BB regulate autophagy and the exact pathway.
Acknowledgements
We are thankful to Prof. Zhuohua Zhang for providing the plasmid pcDNA3.1(−)-α-Syn. This research was supported by the grants from the National Natural Science Foundation of China (Grant Nos. 81673408 and 81703485).
Abbreviations
- α-Syn
α-Synuclein
- ALP
Autophagy–lysosome pathways
- BB
Bilobalide
- BCA
Bicinchoninic acid
- CM
Conditioned medium
- DAPI
4,6-Linked amidine-2-phenylindole
- DLB
Dementia with LBs
- DMSO
Dimethyl sulfoxide
- DTT
DL-Dithiothreitol
- GB
Ginkgolide B
- GBE
Ginkgo biloba extract
- GFAP
Glial fibrillary acidic protein
- LBs
Lewy bodies
- LC3
Microtubule-associated protein 1A/1B-light chain 3
- LDH
Lactate dehydrogenase
- LNs
Lewy neuritis
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
- PBS
Phosphate buffered saline
- PD
Parkinson’s disease
- SNpc
Substantia Nigra pars compacta
- ThT
Thioflavin T
- 3MA
3-Methyladenine
- UPS
Ubiquitin proteasome system
Author contributions
YF and GH conceived the study and analyzed the data. JH and NY constructed lentiviral vectors and involved in manuscript preparation. JH and SX designed and performed animal experiment. NY and QC performed biochemical analysis. TT and JZ performed immunohistochemical and immunolabeling analysis. JH and JD performed in vitro cell culture experiments. NY and JD performed in vitro α-synuclein propagation assay. JH and YF wrote the manuscript. All authors reviewed and approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical Approval
All study protocols were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (IACUC).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jun Hua and Nuo Yin have contributed equally to this work.
Contributor Information
Yi Fan, Email: yfan@njmu.edu.cn.
Gang Hu, Email: ghu@njmu.edu.cn.
References
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS et al (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarejos MJ, Solano RM, Gomez A, Perucho J, de Yebenes JG, Mena MA (2011) The accumulation of neurotoxic proteins, induced by proteasome inhibition, is reverted by trehalose, an enhancer of autophagy, in human neuroblastoma cells. Neurochem Int 58:512–520 [DOI] [PubMed] [Google Scholar]
- Choi MG, Kim MJ, Kim DG, Yu R, Jang YN, Oh WJ (2018) Sequestration of synaptic proteins by alpha-synuclein aggregates leading to neurotoxicity is inhibited by small peptide. PLoS ONE 13:e195339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909 [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L et al (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA 106:13010–13015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Matteo V, Esposito E (2003) Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Curr Drug Targets CNS Neurol Disord 2:95–107 [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Wahlster L, McLean PJ (2012) Protein degradation pathways in Parkinson’s disease: curse or blessing. Acta Neuropathol 124:153–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA et al (2006) Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J 20:419–425 [DOI] [PubMed] [Google Scholar]
- Erustes AG, Stefani FY, Terashima JY, Stilhano RS, Monteforte PT, Da SPG et al (2018) Overexpression of alpha-synuclein in an astrocyte cell line promotes autophagy inhibition and apoptosis. J Neurosci Res 96:160–171 [DOI] [PubMed] [Google Scholar]
- Foulds PG, Mitchell JD, Parker A, Turner R, Green G, Diggle P et al (2011) Phosphorylated alpha-synuclein can be detected in blood plasma and is potentially a useful biomarker for Parkinson’s disease. FASEB J 25:4127–4137 [DOI] [PubMed] [Google Scholar]
- Gao HM, Zhang F, Zhou H, Kam W, Wilson B, Hong JS (2011) Neuroinflammation and alpha-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ Health Perspect 119:807–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo RZ, Liu XG, Gao W, Dong X, Fanali S, Li P et al (2015) A strategy for screening antioxidants in Ginkgo biloba extract by comprehensive two-dimensional ultra high performance liquid chromatography. J Chromatogr A 1422:147–154 [DOI] [PubMed] [Google Scholar]
- Hirata BK, Banin RM, Dornellas AP, de Andrade IS, Zemdegs JC, Caperuto LC et al (2015) Ginkgo biloba extract improves insulin signaling and attenuates inflammation in retroperitoneal adipose tissue depot of obese rats. Mediat Inflamm 2015:419106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenerhoff MJ, Pandiri AR, Snyder SA, Hong HH, Ton TV, Peddada S et al (2013) Hepatocellular carcinomas in B6C3F1 mice treated with Ginkgo biloba extract for two years differ from spontaneous liver tumors in cancer gene mutations and genomic pathways. Toxicol Pathol 41:826–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Yin N, Yang B, Zhang J, Ding J, Fan Y et al (2017) Ginkgolide B and bilobalide ameliorate neural cell apoptosis in alpha-synuclein aggregates. Biomed Pharmacother 96:792–797 [DOI] [PubMed] [Google Scholar]
- Iwata A, Maruyama M, Akagi T, Hashikawa T, Kanazawa I, Tsuji S et al (2003) Alpha-synuclein degradation by serine protease neurosin: implication for pathogenesis of synucleinopathies. Hum Mol Genet 12:2625–2635 [DOI] [PubMed] [Google Scholar]
- Jang A, Lee HJ, Suk JE, Jung JW, Kim KP, Lee SJ (2010) Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem 113:1263–1274 [DOI] [PubMed] [Google Scholar]
- Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912 [DOI] [PubMed] [Google Scholar]
- Kim C, Lee SJ (2008) Controlling the mass action of alpha-synuclein in Parkinson’s disease. J Neurochem 107:303–316 [DOI] [PubMed] [Google Scholar]
- Kim C, Ho DH, Suk JE, You S, Michael S, Kang J et al (2013) Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun 4:1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Rockenstein E, Spencer B, Kim HK, Adame A, Trejo M et al (2015) Antagonizing neuronal toll-like receptor 2 prevents synucleinopathy by activating autophagy. Cell Rep 13:771–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ (2008a) Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol 40:1835–1849 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Bae EJ, Lee SJ (2008b) Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem Biophys Res Commun 372:423–428 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Bae EJ, Lee SJ (2014) Extracellular alpha-synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol 10:92–98 [DOI] [PubMed] [Google Scholar]
- Lin Y, Wang R, Wang X, He RR, Wu YM (2008) Effects of ginkgolide B on neuronal discharges in paraventricular nucleus of rat hypothalamic slices. Neurosci Bull 24:345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma KL, Song LK, Yuan YH, Zhang Y, Han N, Gao K et al (2014) The nuclear accumulation of alpha-synuclein is mediated by importin alpha and promotes neurotoxicity by accelerating the cell cycle. Neuropharmacology 82:132–142 [DOI] [PubMed] [Google Scholar]
- Mak SK, McCormack AL, Manning-Bog AB, Cuervo AM, Di Monte DA (2010) Lysosomal degradation of alpha-synuclein in vivo. J Biol Chem 285:13621–13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y et al (2016) Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 353:aah3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaught KS, Mytilineou C, Jnobaptiste R, Yabut J, Shashidharan P, Jennert P et al (2002) Impairment of the ubiquitin-proteasome system causes dopaminergic cell death and inclusion body formation in ventral mesencephalic cultures. J Neurochem 81:301–306 [DOI] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147:728–741 [DOI] [PubMed] [Google Scholar]
- Mozet C, Martin R, Welt K, Fitzl G (2009) Cardioprotective effect of EGb 761 on myocardial ultrastructure of young and old rat heart and antioxidant status during acute hypoxia. Aging Clin Exp Res 21:14–21 [DOI] [PubMed] [Google Scholar]
- Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D et al (2017) Past, present, and future of Parkinson’s disease: a special essay on the 200th anniversary of the shaking palsy. Mov Disord 32:1264–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oueslati A, Schneider BL, Aebischer P, Lashuel HA (2013) Polo-like kinase 2 regulates selective autophagic alpha-synuclein clearance and suppresses its toxicity in vivo. Proc Natl Acad Sci USA 110:E3945–E3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Huang S, Wu TT, Foster NR, Sinicrope FA (2013) Prognostic impact of Beclin 1, p62/sequestosome 1 and LC3 protein expression in colon carcinomas from patients receiving 5-fluorouracil as adjuvant chemotherapy. Cancer Biol Ther 14:100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotegher N, Civiero L (2012) Neuronal autophagy, alpha-synuclein clearance, and LRRK2 regulation: a lost equilibrium in parkinsonian brain. J Neurosci 32:14851–14853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG et al (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36:585–595 [DOI] [PubMed] [Google Scholar]
- Sacino AN, Brooks MM, Chakrabarty P, Saha K, Khoshbouei H, Golde TE et al (2017) Proteolysis of alpha-synuclein fibrils in the lysosomal pathway limits induction of inclusion pathology. J Neurochem 140:662–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M et al (2005) Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol 170:1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Wu F, Yew DT, Xu J, Zhu Y (2010) Bilobalide prevents apoptosis through activation of the PI3K/Akt pathway in SH-SY5Y cells. Apoptosis 15:715–727 [DOI] [PubMed] [Google Scholar]
- Shintani T, Klionsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306:990–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M (1998) alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA 95:6469–6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson T, Senchuk M, Cooper JF, George S, Van Raamsdonk JM, Brundin P (2017) Novel animal model defines genetic contributions for neuron-to-neuron transfer of alpha-synuclein. Sci Rep 7:7506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L (2011) Pathological roles of alpha-synuclein in neurological disorders. Lancet Neurol 10:1015–1025 [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A et al (2011) Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72:57–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC (2003) Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278:25009–25013 [DOI] [PubMed] [Google Scholar]
- Xiao Q, Wang C, Li J, Hou Q, Li J, Ma J et al (2010) Ginkgolide B protects hippocampal neurons from apoptosis induced by beta-amyloid 25–35 partly via up-regulation of brain-derived neurotrophic factor. Eur J Pharmacol 647:48–54 [DOI] [PubMed] [Google Scholar]
- Xie Z, Klionsky DJ (2007) Autophagosome formation: core machinery and adaptations. Nat Cell Biol 9:1102–1109 [DOI] [PubMed] [Google Scholar]
- Yorimitsu T, Klionsky DJ (2005) Autophagy: molecular machinery for self-eating. Cell Death Differ 12(Suppl 2):1542–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitake T, Yoshitake S, Kehr J (2010) The Ginkgo biloba extract EGb 761(R) and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex. Br J Pharmacol 159:659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XN, Sun XL, Gao L, Fan Y, Ding JH, Hu G (2007) Aquaporin-4 deficiency down-regulates glutamate uptake and GLT-1 expression in astrocytes. Mol Cell Neurosci 34:34–39 [DOI] [PubMed] [Google Scholar]