Abstract
Cohesins promote proper chromosome segregation, gene transcription, genomic architecture, DNA condensation, and DNA damage repair. Mutations in either cohesin subunits or regulatory genes can give rise to severe developmental abnormalities (such as Robert Syndrome and Cornelia de Lange Syndrome) and also are highly correlated with cancer. Despite this, little is known about cohesin regulation. Eco1 (ESCO2/EFO2 in humans) and Rad61 (WAPL in humans) represent two such regulators but perform opposing roles. Eco1 acetylation of cohesin during S phase, for instance, stabilizes cohesin-DNA binding to promote sister chromatid cohesion. On the other hand, Rad61 promotes the dissociation of cohesin from DNA. While Eco1 is essential, ECO1 and RAD61 co-deletion results in yeast cell viability, but only within a limited temperature range. Here, we report that eco1rad61 cell lethality is due to reduced levels of the cohesin subunit Mcd1. Results from a suppressor screen further reveals that FDO1 deletion rescues the temperature-sensitive (ts) growth defects exhibited by eco1rad61 double mutant cells by increasing Mcd1 levels. Regulation of MCD1 expression, however, appears more complex. Elevated expression of MBP1, which encodes a subunit of the MBF transcription complex, also rescues eco1rad61 cell growth defects. Elevated expression of SWI6, however, which encodes the Mbp1-binding partner of MBF, exacerbates eco1rad61 cell growth and also abrogates the Mpb1-dependent rescue. Finally, we identify two additional transcription factors, Fkh1 and Fkh2, that impact MCD1 expression. In combination, these findings provide new insights into the nuanced and multi-faceted transcriptional pathways that impact MCD1 expression.
Keywords: Roberts Syndrome (RBS), Cornelia de Lange Syndrome (CdLS), cohesins, ECO1/ESCO2, Rad61/WAPL, Mcd1/Scc1/RAD21, MBF (Mbp1 and Swi6), Fdo1, Fkh1, Fkh2
Introduction
The biological functions of cohesins include DNA loop extrusion, sister chromatid tethering, chromosome compaction, and DNA damage repair (Guacci et al. 1997; Michaelis et al. 1997; Sjögren and Nasmyth 2001; Jessberger 2002; Kim et al. 2002; de Wit et al. 2015; Davidson et al. 2016). These chromatin conformations, however, vary extensively over the cell cycle. During G1 phase, for instance, cohesins produce both megabase sized epigenetically-defined topologically associating domains (TADs) and also smaller loops that alter the registration of regulatory elements (enhancer, promotors, insulator, etc.) (Rollins et al. 1999; Dorsett et al. 2005; Wendt et al. 2008; Degner et al. 2009, 2011; Chien et al. 2011; Seitan et al. 2011; de Wit et al. 2015; Davidson et al. 2016, 2019; Gassler et al. 2017; Haarhuis et al. 2017; Rao et al. 2017; Schwarzer et al. 2017; Wutz et al. 2017; Cuadrado et al. 2019; Kim et al. 2019; Zhang et al. 2019; Li et al. 2020; Xiang and Koshland 2021; Hsieh et al. 2022; Valton et al. 2022; Liang et al. 2023; Sept et al. 2023). Cohesin-based DNA loops, and loop resolution, are highly dynamic which could allow cells to efficiently respond to changes in external cues (nutrients, hormones, temperature, etc.) (Giorgetti et al. 2014; Hansen et al. 2017; Gabriele et al. 2022; Mach et al. 2022). During S phase, however, cohesins perform very different functions in that they tether together sister chromatids. This tethering is non-dynamic such that cohesins are stably bound until anaphase, when cohesion between sisters is inactivated to allow sister chromatids to segregate into the newly forming daughter cells (Guacci et al. 1997; Michaelis et al. 1997; Uhlmann and Nasmyth 1998; Skibbens et al. 1999).
The mechanisms that underlie cohesin activities remain hotly debated, although many of the fundamental aspects of cohesin complex assembly are known. For instance, budding yeast cohesin core components consist of Smc1, Smc3, and Mcd1 (Scc1). Smc1 and Smc3 each fold to create two extended intramolecular antiparallel coiled coils that bind to one another at their hinge-like domains (Haering et al. 2002; Gruber et al. 2003). Distal to the hinges, globular domains of Smc1 and Smc3 form ATPases (Haering et al. 2002; Arumugam et al. 2003). These ATPase head domains are capped by Mcd1 (Gligoris et al. 2014). The Mcd1 cap of this presumed core complex in turn recruits Irr1 (Scc3), Pds5, and Rad61 (Wpl1) (Hartman et al. 2000; Panizza et al. 2000; Losada et al. 2005; Rowland et al. 2009; Sutani et al. 2009; Gause et al. 2010; Kulemzina et al. 2012; Zhang et al. 2013; Gligoris et al. 2014; Roig et al. 2014; Tong and Skibbens 2014; Orgil et al. 2015; Muir et al. 2016). The mechanisms through which cohesins tether together sister chromatids (as one, two, or a cluster of cohesins associated with each sister) or extrude DNA (so that both strands are simultaneously extruded into a growing loop) remain largely unknown (Haering et al. 2002; Zhang et al. 2008).Cohesin functions across the cell cycle are regulated both by auxiliary factors as well as by modifications of cohesin subunits. Relevant to the current study, Eco1/Ctf7 in budding yeast (Eso1 in fission yeast, Eco/Deco in Drosophila, CTF7 in Arabidopsis, and EFO1/ESCO1 and EFO2/ESCO2 paralogs in vertebrates) comprises a highly conserved family of essential acetyltransferases that regulate cohesins throughout the cell cycle (Skibbens et al. 1999; Toth et al. 1999; Tanaka et al. 2000; Williams et al. 2003; Vega et al. 2005; Seitan et al. 2006; Kawauchi et al. 2009; Mönnich et al. 2011; Whelan et al. 2012). During DNA replication, Eco1 is recruited to the replication fork by PCNA to acetylate Smc3 (Skibbens et al. 1999; Moldovan et al. 2006; Rowland et al. 2009; J. Zhang et al. 2017; W. Zhang et al. 2017; Bender et al. 2020). This modification converts the cohesin complex to a stable form. During G1, however, Eco1/ESCO family members help regulate DNA loop lengths (Alomer et al. 2017; Wutz et al. 2020; Van Ruiten et al. 2022). Rad61 (human WAPL), a cohesin-associated component, promotes the dissociation of unacetylated cohesin from DNA (Gandhi et al. 2006; Kueng et al. 2006; Rowland et al. 2009; Sutani et al. 2009). In this respect, Rad61 DNA-dissociating activity works in opposition to Eco1-based stabilization of cohesin binding to DNA such that yeast co-deleted for both remain viable (Ben-Shahar et al. 2008; Sutani et al. 2009; Maradeo and Skibbens 2010; Guacci and Koshland 2012). Cohesins also are regulated through one of the core subunits—Mcd1. Mcd1 is unique among cohesin subunits in that it is largely degraded at anaphase onset and must be newly transcribed each and every cell cycle (Guacci et al. 1997; Uhlmann et al. 1999).
Mutation of cohesin pathway genes can give rise to Robert Syndrome (RBS) and Cornelia de Lange Syndrome (CdLS). Individuals with RBS and CdLS manifest a multitude of overlapping developmental abnormalities that, when severe, result in premature mortality and terminal miscarriages (Krantz et al. 2004; Tonkin et al. 2004; Schüle et al. 2005; Vega et al. 2005; Musio et al. 2006; Deardorff et al. 2007; Gordillo et al. 2008). Defects in cohesin regulation are also tightly correlated with several forms of cancer (Antony et al. 2021; Di Nardo et al. 2022; Pati 2024). Despite the importance of cohesin functions, little is known regarding the regulation of cohesins early during the cell cycle (pre-S phase). Recent findings, however, suggest that MCD1 expression may be a critical component of cohesin regulation throughout the cell cycle (Buskirk and Skibbens 2022; Choudhary et al. 2022). In this study, we identify FDO1 as a novel regulator of MCD1 expression. We further report that two additional transcriptional factors in the Forkhead box (Fkh1 and Fkh2 in yeast) family, as well as the Swi6–Mbp1 MBF transcription complex, play key roles in MCD1 regulation and therefore likely impact all cohesin activities.
Materials and methods
Yeast strains, media, and growth conditions
All strains (Supplementary Table 1) were grown on YPD-rich media unless placed on selective medium to facilitate plasmid transformations or spore identification (Rose et al. 1990).
Phenotypic analysis was assessed as previously described with minor modifications (Buskirk and Skibbens 2022). Briefly, log phase cultures grown at the permissive temperature (30°C unless otherwise indicated) were normalized based on OD600, serially 10-fold dilutions plated in duplicates on either YPD agar plates or selective medium plates, and then maintained across a range of temperatures (typically comparing growth at 30°C vs 37°C).
Strain generation
The primers utilized to delete or partially delete genes, and then verify deletions, are listed in Supplementary Table 2. DNA products used to replace FDO1, FKH1 and FKH2 ORFs were obtained using PCR and a kanMX6 template as previously described (Longtine et al. 1998).
Plasmid generation
Overexpression of selected genes was generated following a strategy previously described. In brief, DNA oligos (Supplementary Table 2) were designed to produce complete FKH1, FKH2, FDO1, SWI6, and MBP1 ORFs (including roughly 300 base pairs upstream of the starting codons). PCR products and pRS424 (2μ TRP) or pRS425 (2μ LEU) plasmids were digested and ligated to generate the following plasmids: pGS7, pGS8 (FKH1 and pRS424 digested with SacII-XhoI), pGS6 (FKH2 and pRS424 digested with SacII/Xho1fragment), pGS11, pGS12, pGS13 (FDO1 and pRS 424 digested with SacII/Xho1), pGS14, pGS15, pGS16 (SWI6 and pRS 424 digested with SacII/XmaI), pGS31, pGS32, pGS33 (MBP1 and pRS 425 digested with XhoI/SacII). The MCD1 (2μ TRP and 2μ LEU) plasmids were generated by digesting (with ClaI for the 2μ TRP and XhoI—SacII for the 2μ LEU plasmid) the MCD1 coding sequence along with its endogenous promotor from pVG185 (2μ URA, kindly provided by Dr. Vincent Guacci) and ligating it in pRS424. All generated plasmids were verified by restriction digest. The specific isolates of the plasmid transformed in the parent or wildtype cells are contained in the Supplementary Table 1 and the primers used to create the plasmids are enclosed in Supplementary Table 2.
Genomic sequencing
Genemomic sequencing of the revertant strains was performed as previously described (Buskirk and Skibbens 2022).
Western blot protein extraction and quantification
Cell numbers for each strain within an experiment were normalized by OD600. Cells were then washed prior to suspending in 1.0 Sorbitol and exposed to Zymolyase (100T) (USBiological Life Sciences) and β-mercaptoethanol (1/50th of total volume) for 30 min at 37°C. Cell wall digestion was confirmed by assessing cell lysis microscopically, upon exposure to 0.5% SDS. Cell extracts were resolved by SDS PAGE, then transferred to a PVDF membrane. Post blocking (5% NFDM, 0.1% BSA, 1X PBS), proteins were detected using primary antibodies PGK (mouse) at 1:1,000 K, Invitrogen catalog number 459250; Mcd1 (Rabbit) at 1:20 K—kindly provided by Dr Vincent Guacci- followed by secondary antibodies Goat anti-Rabbit HRP at 1:10 K, BIO-RAD catalog number 170-6515; goat anti-Mouse HRP at 1:10 K, BIO-RAD catalog number 170-6516 and ECL Prime (Amersham, RPN2232) and Xray film (Denville Scientific, HyBlot ES™, catalog number E3218) development (Xomat) using the Konica Minolta SRX-101A film processor. Protein band intensities, obtained by film scanning (EPSONPERFECTION V300 PHOTO) were quantified using Image J. Significance was determined by a two-tailed test (P-value less than 0.05).
Flow cytometry and cell cycle progression
Prior to generating cell extracts, all strains within an experiment were maintained in log growth over a two day regimen as previously described (Buskirk and Skibbens 2022). Briefly, log phase cultures were then normalized (OD600, typically to achieve 1.0 OD of cells) and then synchronized in early S phase by the addition of hydroxyurea (SIGMA, H8627) at final concentration of 0.2 M. Log phase growth and cell cycle arrest in S phase was confirmed by flow cytometry (BD FACScan) as previously described (Maradeo and Skibbens 2010; Tong and Skibbens 2015).
Results
Reduced Mcd1 levels are responsible for eco1Δ rad61Δ double mutant yeast cell temperature-sensitive inviability
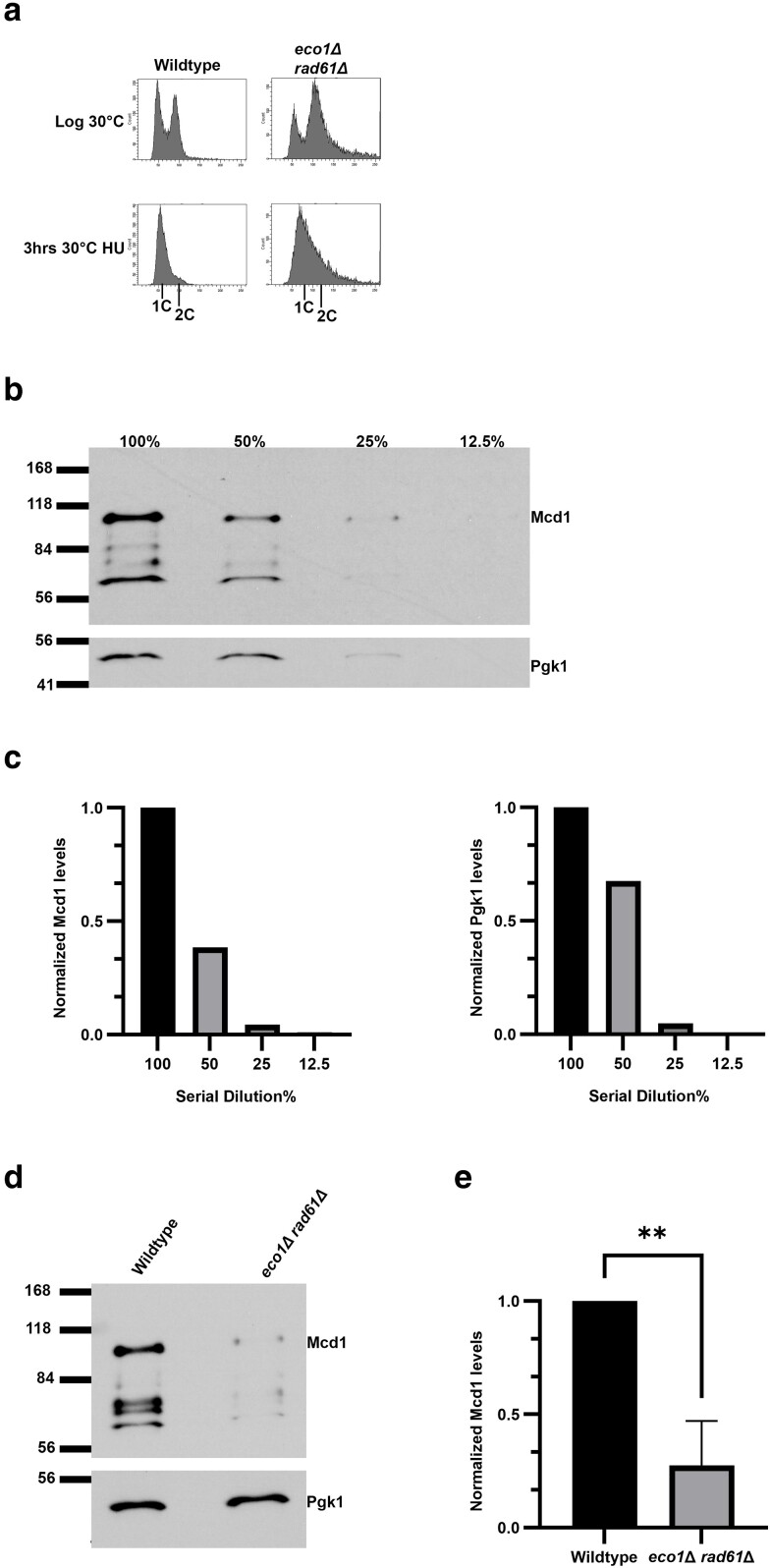
Eco1 (which is essential only during S phase) and Pds5 (an auxiliary cohesin subunit required for cohesion from S phase to anaphase onset) support cohesin functions through independent mechanisms (Skibbens et al. 1999; Toth et al. 1999; Hartman et al. 2000; Panizza et al. 2000). In a remarkable convergence of studies, however, deletion of CLN2 was found to promote the viability of both eco1Δ rad61Δ at 37°C and pds5Δ elg1Δ cells (Buskirk and Skibbens 2022; Choudhary et al. 2022). The latter study provided evidence that CLN2 deletion increased Mcd1 levels, such that simply elevating Mcd1 protein levels were sufficient to render viable pds5Δ elg1Δ double mutant cells (Choudhary et al. 2022). Given that CLN2 is common to both eco1Δ rad61Δ and pds5Δ elg1Δ cells as a bypass suppressor (Buskirk and Skibbens 2022; Choudhary et al. 2022), it became important to test whether Mcd1 protein levels are similarly reduced in eco1Δ rad61Δ cells, even at permissive temperatures. To test this possibility, Mcd1 levels from wildtype and eco1Δ rad61Δ cells were quantified by Western Blots. Given that Mcd1 levels rise during S phase and fall precipitously during anaphase (Guacci et al. 1997), log phase cultures were arrested in early S phase (hydroxy urea, HU) and samples harvested to assess DNA content (flow cytometry) and Mcd1 levels (Western blot using Mcd1-directed antibody—a generous gift from Dr. Vincent Guacci) (Guacci et al. 1997). As expected, all log phase cultures contained both 1C and 2C DNA peaks that collapsed to an early stage of DNA replication in response to HU (Fig. 1a). To facilitate quantification by Western blot, we first performed a dilutions series to identify a linear range of detection for both Mcd1 and Pgk1 (loading control) across each of our three biological replicates (Fig. 1b,c). Appropriately diluted extracts were then assessed. As expected, wildtype cells arrested in the S phase contained high levels of Mcd1 (Fig. 1d), relative to other portions of the cell cycle (data not shown). In contrast, Mcd1 levels were significantly decreased (P = 0.0031) in S phase-arrested eco1Δ rad61Δ cells, compared to wildtype cells (Fig. 1e). Mcd1 protein levels fell to nearly undetectable levels upon shifting to 37°C (data not shown), at which point eco1Δ rad61Δ cells are inviable.
Fig. 1.
eco1 Δ rad61Δ double mutant cells contain reduced Mcd1 levels. a) Flow cytometry data of DNA contents for wildtype (YBS255) and eco1Δ rad61Δ double mutant cells (YMM828). b) Representative Western Blot of Mcd1 (top panel) and Pgk1 (lower panel) protein levels obtained from serially diluted (100%, 50%, 25%, 12.5%) extracts of HU-synchronized wildtype cells indicated in (A). c) Quantification of Mcd1 (left panel) and Pgk1 (right panel) of the serially diluted (100%, 50%, 25%, 12.5%) sample in (B). d) Representative Western Blot of Mcd1 (top panel) and Pgk1 (lower panel) protein levels of 50% diluted wildtype and eco1rad61 null cell extracts obtained from HU-synchronized cells indicated in (A). e) Quantification of Mcd1, normalized to Pgk1 loading controls. Statistical analysis was performed using a two-tailed t-test. Statistical differences (**) are based on a P < 0.01 obtained across three experiments (n = 3). P = 0.0031 for eco1Δ rad61Δ compared to wildtype cells. Error bars indicate the standard deviation.
If the reduction of Mcd1 protein is the sole basis for eco1Δ rad61Δ cell inviability during thermal stress, then exogenously elevating Mcd1 levels should rescue eco1Δ rad61Δ cell temperature-sensitive (ts) growth. To test whether the increase in Mcd1 protein alone is sufficient to rescue eco1Δ rad61Δ cell temperature sensitivity, we generated an MCD1 high-copy (2µ TRP1) plasmid. We validated this reagent by confirming that it indeed rescued the ts-growth of mcd1-1 mutated cells (Fig. 2a). Log phase wildtype and eco1Δ rad61Δ cells, transformed with vector alone or vector overexpressing MCD1, were serially diluted, plated onto selective media, and then incubated at either 30°C or 37°C. Cells are apparently unaffected by elevated Mcd1 levels in that both wildtype and eco1Δ rad61Δ cells exhibited robust growth at 30°C (Fig. 2b). As expected, eco1Δ rad61Δ cells that contained vector alone exhibited severe growth defects at 37°C. eco1Δ rad61Δ cells that harbored the MCD1 overexpression vector, however, exhibited robust growth at 37°C (Fig. 2b). These findings reveal that eco1Δ rad61Δ cells contain reduced Mcd1 protein, even at permissive temperatures, and that elevating Mcd1 protein levels is solely sufficient to rescue eco1Δ rad61Δ cell lethality at otherwise non-permissive temperatures.
Fig. 2.
MCD1 overexpression rescues eco1rad61 double mutant cell ts-growth. a) Streak test of three independent isolates of an mcd1-1 temperature-sensitive strain (YMM396) transformed with vector alone, compared to three independent isolates of mcd1-1 ts cells transformed with vector overexpressing MCD1. Temperature growth conditions are indicated. b) Growth of 10-fold serial dilutions of wildtype cells overexpressing vector alone (YGS26) or overexpressing MCD1 (YGS27), and eco1Δ rad61Δ double mutant cells overexpressing vector alone (YGS28), or overexpressing MCD1 (2 isolates shown; YGS29, YGS30). Temperatures and days of growth are indicated.
Identification of Fdo1 as a novel regulator of cohesin
Previously, a genome-wide suppressor screen identified the G1 cyclin Cln2 as a novel regulator of eco1Δ rad61Δ mutant cells (Buskirk and Skibbens 2022). To further identify novel genes that rescue eco1Δ rad61Δ temperature sensitivity, a second revertant (YBS1638) was back-crossed 2 times to wildtype cells and multiple non-ts eco1Δ rad61Δ segregants pooled together prior to whole genome sequencing as previously described (Buskirk and Skibbens 2022). Mutations that were common across all segregants (present at a frequency of 1.0) were prioritized as revertant gene candidates that rescued the temperature sensitivity otherwise exhibited by eco1Δ rad61Δ cells. In contrast, non-revertant mutations were expected to occur at frequencies below 1.0. Only one gene, FDO1, was mutated (encoding only the first 82 amino acids, herein fdo11–82Δ) in all segregants, although mutation of RPL31A was also present at a high frequency (Table 1). Other gene mutations (MSH5, ARN1, and EAF1) were present at significantly reduced frequencies, eliminating them as likely reverting candidate genes. We decided to delete FDO1 de novo from parent eco1Δ rad61Δ cells to test for the rescue of ts-growth defects in the absence of other mutations. We generated multiple isolates of eco1Δ rad61Δ cells either deleted for the complete FDO1 coding sequence, or the partial deletion that arose in the revertant spontaneous suppressor screen (fdo11–82Δ). Log phase cultures of the wildtype, parental eco1Δ rad61Δ cells, and three independent eco1Δ rad61Δ fdo1Δ triple null cells were serially diluted, plated onto YPD agar, and incubated at either 30°C or 37°C. As expected, all strains exhibited robust growth at 30°C while eco1Δ rad61Δ cells were inviable at 37°C (Fig. 3a). In contrast, all three isolates of eco1Δ rad61Δ fdo1Δ triple null cells were viable at 37°C (Fig. 3a). A similar rescue of ts-growth was obtained for isolates (two shown) of eco1Δ rad61Δ cells that harbored truncated Fdo1 protein (fdo11–82Δ) (Fig. 3b). These results confirm that FDO1 loss-of-function rescues the ts-growth defects otherwise present in eco1Δ rad61Δ double mutant cells.
Table 1.
Identification of spontaneous revertant mutations.
| Chromosome | Position | DNA change | Protein change | Coding impact | Gene | Description from SGDa | Frequency |
|---|---|---|---|---|---|---|---|
| chrXIII | 553608 | 247C>T | Nonsense at Q83 | nonsense | FDO1 (YMR144W) | Protein involved in directionality of mating-type switching | 1.00 |
| chrIV | 322263 | 38C>T | T13I | missense | RPL31A (YDL075W) | Ribosomal 60S subunit protein L31A | 0.91 |
| chrIV | 178744 | 411G>T | W137C | missense | MSH5 (YDL154W) | Protein of the MutS family | 0.59 |
| chrVIII | 19897 | 1075G>C | A359P | missense | ARN1 (YHL040C) | ARN family transporter for siderophore-iron chelates | 0.73 |
| chrIV | 1193539 | 1346T>C | I449T | missense | EAF1 (YDR359C) | Component of the NuA4 histone acetyltransferase complex | 0.24 |
a Description of gene from SGD.
Fig. 3.
Identification of FDO1 deletion as a suppressor of eco1Δ rad61Δ cell temperature-sensitive growth defects. a) Growth of 10-fold serial dilutions of wildtype (YBS255), eco1Δ rad61Δ double mutant cells (YMM828) and three independent isolates of eco1Δ rad61Δ fdo1Δ triple mutant cells (YGS4, YGS36, YGS37). Temperatures and days of growth are indicated. b) Growth of 10-fold serial dilutions of eco1Δ rad61Δ double mutant cells (YMM828), two independent isolates of eco1Δ rad61Δ fdo1Δ triple mutant cells (YGS3, YGS1), and two independent isolates of eco1Δ rad61Δ fdo11–82Δ (YGS5, YGS6) triple mutant cells. Temperature and days of growth are indicated.
Fdo1 regulates MCD1 expression in eco1Δ rad61Δ double mutant
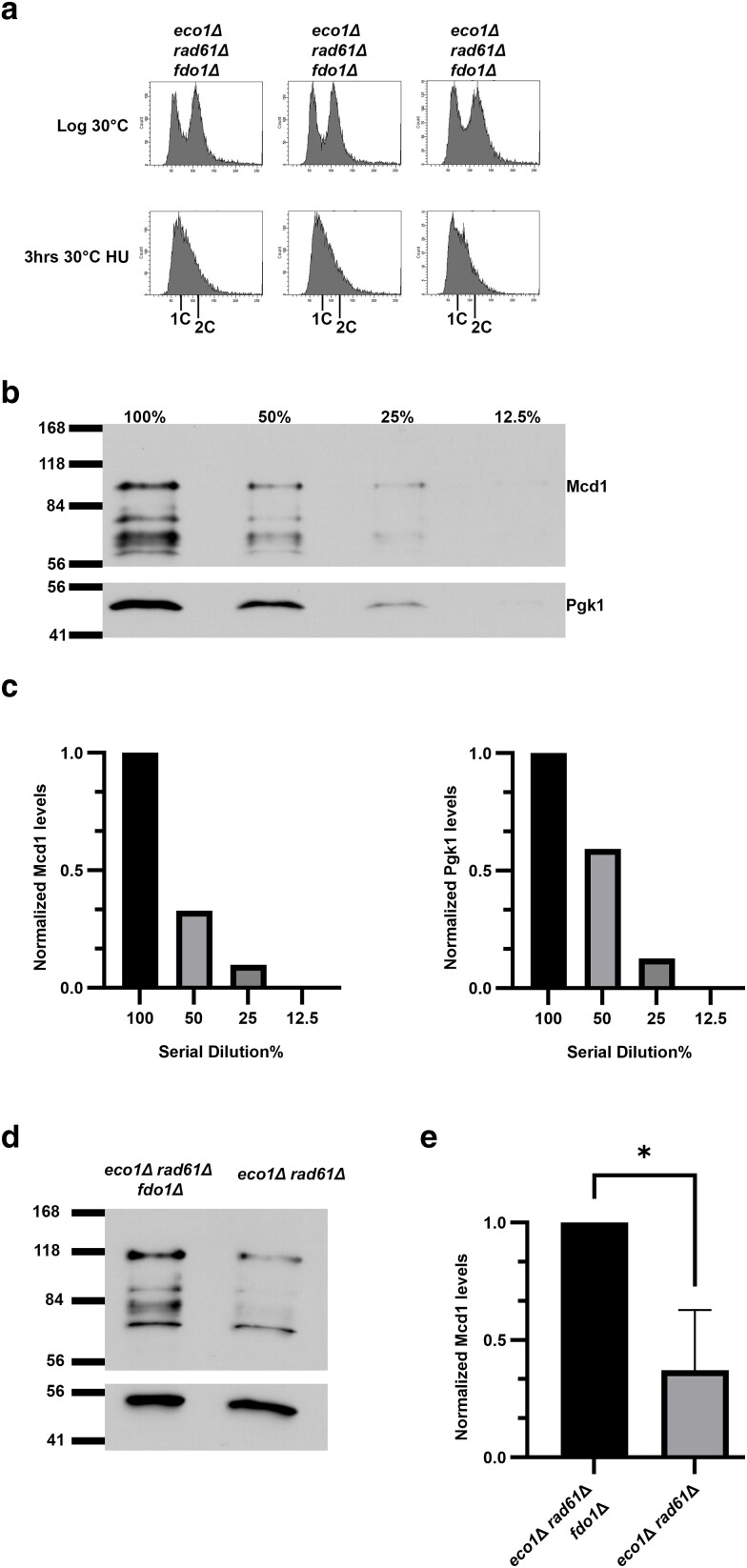
Deletion of FDO1 rescues eco1Δ rad61Δ cell ts-growth defects (Fig. 2). It thus became important to test whether deletion of FDO1 increased Mcd1 protein levels in eco1Δ rad61Δ cells. Whole cell extracts from eco1Δ rad61Δ fdo1Δ triple mutant cells, obtained from each of the three biological replicates arrested in early S phase (Fig. 4a), were diluted and probed by Western blot to obtain Mcd1 and Pgk1 signals that fell within a linear range (Fig. 4b, c). Appropriately diluted extracts from eco1Δ rad61Δ fdo1Δ cells were then compared to extracts obtained from eco1Δ rad61Δ cells (Fig. 4d). The results reveal that Mcd1 levels are increased significantly (P = 0.0257) across the eco1Δ rad61Δ fdo1Δ triple mutant isolates, compared to eco1Δ rad61Δ cells (Fig. 4d, e). In combination, these findings document that eco1Δ rad61Δ cells contain low levels of Mcd1 and that FDO1 deletion rescues the temperature sensitivity of the eco1Δ rad61Δ double mutant cells at least in part by increasing Mcd1 levels.
Fig. 4.
Deletion of FDO1 elevates Mcd1 levels in eco1Δ rad61Δ double mutant cells. a) Flow cytometry data of DNA content for three independent isolates of eco1Δ rad61Δ fdo1Δ triple mutant cells (YGS3, YGS4, YGS31). Log phase and early S phase synchronized (HU, hydroxyurea) DNA profiles are shown for each strain. b) Representative Western Blot of Mcd1 (top panel) and Pgk1 (lower panel) protein levels of the serially diluted (100%, 50%, 25%, 12.5%) eco1Δ rad61Δ fdo1Δ triple mutant cell extracts obtained from HU-synchronized cells indicated in (a). c) Quantifications of Mcd1 (left panel) and Pgk1 (right panel) of the serially diluted (100%, 50%, 25%, 12.5%) sample in (b). d) Representative Western Blot of Mcd1 (top panel) and Pgk1 (lower panel) protein levels of a 50% diluted eco1Δ rad61Δ fdo1Δ triple mutant and eco1Δ rad61Δ fdo1Δ double mutant cell extracts obtained from HU-synchronized cells indicated in (a). e) Quantification of Mcd1, normalized to Pgk1 loading controls. Statistical analysis was performed using a two-tailed t-test. Statistical differences (*) are based on a P < 0.05 obtained across three experiments (n = 3). P = 0.0133 for eco1Δ rad61Δ fdo1Δ compared to eco1Δ rad61Δ cells. Error bars indicate the standard deviation.
If FDO1 deletion rescues eco1Δ rad61Δ cell ts-growth defects by increasing Mcd1 protein levels, we reasoned that elevated Fdo1 levels might produce adverse growth defects. To test this possibility, FDO1 was inserted into a high-copy plasmid (2µ TRP1) and transformed into wildtype and eco1Δ rad61Δ cells. Log phase cultures of the resulting strains were serially diluted onto selective media plates and incubated at either 30°C or 37°C. While elevated FDO1 had no effect on wildtype cells, eco1Δ rad61Δ cells that harbor exogenous FDO1 constructs were largely inviable even at 30°C (Fig. 5a). While these results provide strong evidence that Fdo1 acts as a repressor of MCD1 expression, we realized that elevated Fdo1 levels likely alters the transcription of genes beyond MCD1, which could account for the observed cell lethality. If the lethal effect produced by elevated Fdo1 is independent of MCD1 expression in eco1Δ rad61Δ cells, then co-expressing high-copy constructs of both FDO1 and MCD1 should fail to rescue that cell lethality. To test this possibility, we transformed eco1Δ rad61Δ cells with a combination of vectors alone and vectors driving elevated expression of either FDO1, MCD1, or both FDO1 and MCD1. Log phase cultures of the resulting strains were serially diluted onto selective media plates and incubated at either 30°C or 37°C. As expected, elevated expression of MCD1 alone rescued eco1Δ rad61Δ cell ts-growth while elevated expression of FDO1 greatly exacerbated the ts-growth defect (Fig. 5b). Notably, eco1Δ rad61Δ cells that harbored both MCD1 and FDO1 high-copy plasmids exhibited a partial rescue of the cell ts-growth defects (Fig. 5b). This somewhat attenuated rescue likely reflects a level of inhibition exerted by elevated Fdo1 levels on the native MCD1 promotor present on the high-copy plasmid. In combination, these and the preceding results reveal that Fdo1 acts to repress MCD1 expression and to a level that is lethal in eco1Δ rad61Δ cells during thermic stress.
Fig. 5.
Increased Mcd1 levels partially rescue the growth defect caused by elevated Fdo1 levels. a) Top panel: Growth of 10-fold serial dilutions of wildtype cells overexpressing vector alone (YGS26) and three independent isolates of wildtype cells overexpressing FDO1 (YGS98, YGS100, YGS102). Temperatures and days of growth are indicated. Bottom panel: Growth of 10-fold serial dilutions of wildtype cells overexpressing vector alone (YGS26), eco1Δ rad61Δ double mutant cells overexpressing vector alone (YGS28), and three independent isolates of eco1Δ rad61Δ double mutant cells overexpressing FDO1 (YGS99, YGS101, and YGS103). Temperatures and days of growth are indicated. b) Growth of 10-fold serial dilutions of eco1Δ rad61Δ double mutant cells (YMM828) overexpressing MCD1 (YGS179), overexpressing FDO1 (YGS193), and two independent isolates of eco1Δ rad61Δ co-overexpressing both FDO1 and MCD1 (YGS195, YGS196). Temperature and days of growth are indicated.
Forkhead proteins, Fkh1 and Fkh2, play additional roles in MCD1 regulation
FDO1 was originally identified as prohibiting misdirected mating-type switching (Dummer et al. 2016). Biochemical studies further revealed that Fdo1 interacts with the FOX-type transcription factor Forkhead 1 (Fkh1) (Ho et al. 2002; Dummer et al. 2016), which similarly inhibits misdirected mating-type switching and also negatively regulates transcriptional control of cell cycle progression (Dummer et al. 2016; Aref et al. 2021). It thus became important to test whether deletion of FKH1 would rescue the temperature sensitivity of eco1Δ rad61Δ cells, similar to the deletion of FDO1. The entire FKH1 ORF was deleted from eco1Δ rad61Δ cells and confirmed following established protocols (Longtine et al. 1998). Parental eco1Δ rad61Δ cells, and two independent eco1Δ rad61Δ fkh1Δ triple null cells were serially diluted, plated onto YPD agar, and incubated at either 30°C or 37°C. Surprisingly, the deletion of FKH1 failed to revert the temperature sensitivity otherwise exhibited by eco1Δ rad61Δ cells (Fig. 6a). The budding yeast genome contains a paralog of Fkh1, termed Fkh2, that exhibits redundant transcriptional roles in cell cycle control (Zhou and Shi 2022). Thus, we considered the possibility that Fdo1 impacts cohesin function through Fkh2 instead of Fkh1. Similar to the above strategy, the entire FKH2 ORF was deleted from eco1Δ rad61Δ cells. Serial dilution analyses, however, revealed that FKH2 deletion provides no growth benefit to eco1Δ rad61Δ cells at 37°C. In fact, all independent isolates of eco1Δ rad61Δ fkh2Δ triple mutant cells exhibited significantly exacerbated ts-growth defects at 37°C (Fig. 6b). In combination, these findings differentiate the role of Fdo1 from that of Fkh paralogs and further reveals a novel negative genetic interaction that further differentiates Fkh2 from Fkh1.
Fig. 6.
FKH2 deletion exacerbates eco1Δ rad61Δ cell ts-growth. a) Growth of 10-fold serial dilutions of eco1Δ rad61Δ double mutant cells (YMM828) and two independent isolates of eco1Δ rad61Δ fkh1Δ triple mutant cells (YGS 11, YGS12). Temperatures and days of growth are indicated. b) Growth of 10-fold serial dilutions of eco1Δ rad61Δ double mutant cells (YMM828) and two independent isolates of eco1Δ rad61Δ fkh2Δ triple mutant cells (YGS13, YGS14). Temperatures and days of growth are indicated.
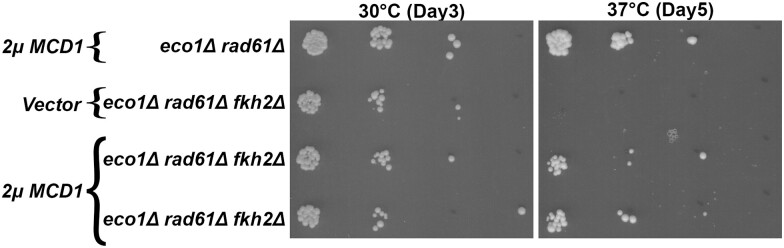
The negative effect observed by the deletion of FKH2, but not the close paralog encoded by FKH1, was surprising. To further investigate this link, we wondered whether FKH2 deletion might exacerbate eco1Δ rad61Δ cell viability specifically by reducing Mcd1 levels. If correct, then the increased ts-growth defects should be rescued by exogenous MCD1 overexpression. To test this prediction, eco1Δ rad61Δ fkh2Δ cells were transformed with vector alone or vector driving elevated expression of MCD1. Log phase cultures of the resulting transformants were serially diluted and plated onto selective media. Elevated expression of MCD1 had no effect on eco1Δ rad61Δ fkh2Δ cells held at the permissive temperature of 30°C (Fig. 7). As expected, eco1Δ rad61Δ fkh2Δ cells transformed with vector alone exhibited severe growth defects at the restrictive temperature of 37°C. In contrast, MCD1 overexpression partially rescued the ts-growth defect otherwise exhibited by eco1Δ rad61Δ fkh2Δ cells (Fig. 7). In parallel, we transformed eco1Δ rad61Δ fkh1Δ cells with vector alone and vector driving elevated expression of MCD1. Here, we expected a full rescue since FKH1 deletion does not overtly impact eco1Δ rad61Δ growth at 37°C. Indeed, MCD1 overexpression fully rescued the ts-growth defects otherwise exhibited by eco1Δ rad61Δ (Supplementary Fig. 1). In combination, these findings reveal that Fkh2 plays a key role, and unique from that of Fkh1, in MCD1 regulation during thermal stress.
Fig. 7.
MCD1 overexpression rescues the deleterious effect of FKH2 deletion from eco1rad61 null cells. Growth of 10-fold serial dilutions of eco1Δ rad61Δ doble mutant cells (YMM828) overexpressing MCD1 (YGS29), eco1Δ rad61Δ fkh2Δ triple mutant cells overexpressing vector alone (YGS176), and two independent isolates of eco1Δ rad61Δ fkh2Δ overexpressing MCD1 (YGS177, YGS178). Temperature and days of growth are indicated.
Despite the adverse effect of deleting FKH2, we considered the possibility that co-deletion of both FKH1 and FKH2 might be required to rescue the ts-growth defects exhibited by eco1Δ rad61Δ cells. Diploid cells obtained by direct mating of eco1Δ rad61Δ fkh1Δ (YGS11) to eco1Δ rad61Δ fkh2Δ (YGS14), however, produced few tetrads upon sporulation. To circumvent this effect, we generated a diploid (YBS4450) homozygous for ECO1 and RAD61 deletions, heterozygous for FKH1 and FKH2 deletions, but that also harbored ECO1 (linked to LEU2 and integrated into chromosome III, independent of the native ECO1 locus on chromosome VI). Since both FKH1 and FKH2 are replaced by the kanMX6 selectable marker (Longtine et al. 1998), the final dispositions of FKH deletions were performed using PCR. Subsequent sporulations/dissections of 43 tetrads produced 19 eco1Δ rad61Δ KAN+ spores, 6 of which were excluded from further analyses since fkh1Δ and fkh2Δ clearly segregated independent of one another across the four-spore tetrads. Of the remaining 13 spores, PCR confirmed that none of the eco1Δ rad61Δ cells contained both fkh1Δ and fkh2Δ (Supplementary Fig. 2). In contrast, we recovered roughly equal numbers of eco1Δ rad61Δ fkh1Δ (7) and eco1Δ rad61Δ fkh2Δ (6) spores. These results provide strong evidence that the combined deletion of both FHK1 and FKH2 not only fails to rescue ts-growth defects, but instead may be lethal in eco1Δ rad61Δ cells.
To further test the conclusion that co-deletions of FKH genes may be lethal in eco1Δ rad61Δ cells, we turned to eco1Δ rad61Δ KAN+ECO1:LEU2 isolates obtained from the above dissections. Did retention of ECO1 allow for the recovery of eco1Δ rad61Δ fkh1Δ fkh2Δ mutant cells? As before, we excluded from further analyses tetrads in which fkh1Δ and fkh2Δ segregated independent of one another across four-spore tetrads. We then performed PCR on 20 of the remaining 53 spores. The results reveal that 8 of the 20 eco1Δ rad61Δ spores harbor deletions of both FKH1 and FKH2 (Supplementary Fig. 3), obviating concerns that the above analyses failed to identify quadruple deletions for technical reasons. Importantly, all eight spores were LEU+, providing additional support for the model that the fkh1Δ fkh2Δ combination is indeed lethal in eco1Δ rad61Δ cells.
The results provided throughout this study predict that the synthetic lethality observed in eco1Δ rad61Δ fkh1Δ fkh2Δ cells might be due to decreased levels of Mcd1. If correct, then elevated levels of MCD1 should allow for the recovery of eco1Δ rad61Δ fkh1Δ fkh2Δ. To test this, the above diploid (YBS4450 harboring eco1Δ rad61Δ fkh1Δ fkh2Δ ECO1:LEU2) was transformed with the high-copy vector (2µ TRP1) that contains MCD1. The resulting strain (YBS4535) was sporulated and the ability to recover eco1Δ rad61Δ fkh1Δ fkh2Δ 2µm-TRP1-MCD1 (without ECO1:LEU2) assessed. Surprisingly, we recovered few TRP1+ spores (10 of 42 viable spores), two of which were devoid of ECO1 (LEU−, HIS+) but retained kanMX6+. The first of these (YBS4547) exhibited normal growth at 30°C while the second (YB4548) required several additional days to form even a small colony (not shown). PCR analyses revealed that the first eco1Δ rad61Δ 2µm-TRP1-MCD1 spore (YBS4547) retained only fhk2Δ. The second eco1Δ rad61Δ 2µm-TRP1-MCD1 spore (YBS4548), however, retained both fkh1Δ and fhk2Δ (Supplementary Fig. 4). These results support the model that deletion of both FKH1 and FKH2 are lethal in eco1Δ rad61Δ due, in part, to reduced MCD1 expression.
Forkhead factors 1 and 2 both perform independent roles in cohesin regulation
Given that fkh2Δ alone produces adverse effects on eco1Δ rad61Δ cell growth, but that fkh1Δ fkh2Δ is lethal in eco1Δ rad61Δ cells, we considered the possibility that Fkh2 and Fkh1 might both promote MCD1 expression. To test this, we generated high-copy (2µ TRP1) plasmids that contain either FKH2 or FKH1. Log phase wildtype and eco1Δ rad61Δ cells, each transformed with vector alone or vector containing either FKH2 or FKH1, were serially diluted and plated onto selective media. While elevated expression of FKH2 had no effect on wildtype cells, elevated FKH2 expression rendered eco1Δ rad61Δ cells largely inviable even at 30°C (Fig. 8a). FKH1 elevated expression also produced eco1Δ rad61Δ cell growth defects at 30°C, but less severe than cells with elevated FKH2 expression (Fig. 8b). In combination, these findings suggest that elevated levels of Fkh1 and Fkh2 each negatively impact cohesin pathways in an undetermined fashion.
Fig. 8.
Overexpression of FKH1 and FKH2 each are detrimental to the growth of the eco1Δ rad61Δ double mutant cells. a) Left panel: Growth of 10-fold serial dilutions of wildtype cells overexpressing vector alone (YGS26), and two independent isolates of wildtype cells overexpressing FKH2 (YGS126, YGS127). Temperatures and days of growth are indicated. Right panel: Growth of 10-fold serial dilutions of eco1Δ rad61Δ double mutant cells overexpressing vector alone (YGS28), and two independent isolates of eco1Δ rad61Δ double mutant cells overexpressing FKH2 (YGS53, YGS55). Temperatures and days of growth are indicated. b) Left panel: Growth of 10-fold serial dilutions of wildtype cells overexpressing vector alone (YGS26), and two independent isolates of wildtype cells overexpressing FKH1 (YGS79, YGS83). Temperatures and days of growth are indicated. Right panel: Growth of 10-fold serial dilutions of wildtype cells overexpressing vector alone (YGS26), eco1Δ rad61Δ double mutant cells overexpressing vector alone (YGS28), and two independent isolates of eco1Δ rad61Δ double mutant cells overexpressing FKH1 (YGS80, YGS84). Temperatures and days of growth are indicated.
Of the many scenarios that could explain the negative effect produced by elevated FHK proteins, we were intrigued by the possibility that reduced Mcd1 levels were involved. If this model is correct, then co-expression of MCD1 should partially rescue the deleterious effects on eco1Δ rad61Δ cells produced by high-copy FKH1 or FKH2 constructs. To test this prediction, eco1Δ rad61Δ cells were co-transformed with the various plasmid combinations, serially diluted, and plated onto selective media. As before, high-copy MCD1 alone rescued eco1Δ rad61Δ cell ts-growth defects while high-copy FHK1 and FKH2 constructs each exacerbated eco1Δ rad61Δ cell ts-growth defects (Fig. 9). In contrast, eco1Δ rad61Δ cells that co-overexpressed both MCD1 and either FKH1 or FKH2 exhibited improved growth, relative to either FKH alone, even at 37°C (Fig. 9). In combination, these results suggest that Fkh1 and FKh2 both regulate MCD1 expression in a manner that is highly sensitive to changes in FKH protein levels.
Fig. 9.
MCD1 overexpression rescues the deleterious growth effect that result from elevated FKH1 and FKH2 levels in eco1rad61 null cells. a) Growth of 10-fold serial dilutions of eco1Δ rad61Δ doble mutant cells (YMM828) overexpressing MCD1 (YGS179), eco1Δ rad61Δ double mutant cells overexpressing FKH1 (YGS180), and two independent isolates of eco1Δ rad61Δ co-overexpressing FKH1 and MCD1 (YGS181, YGS182). Temperature and days of growth are indicated. b) Growth of 10-fold serial dilutions of eco1Δ rad61Δ doble mutant cells (YMM828) overexpressing MCD1 (YGS179), eco1Δ rad61Δ double mutant cells overexpressing FKH2 (YGS183), and two independent isolates of eco1Δ rad61Δ co-overexpressing FKH2 and MCD1 (YGS184, YGS185). Temperature and days of growth are indicated.
Swi6 inhibits Mbp1-based transcription of MCD1
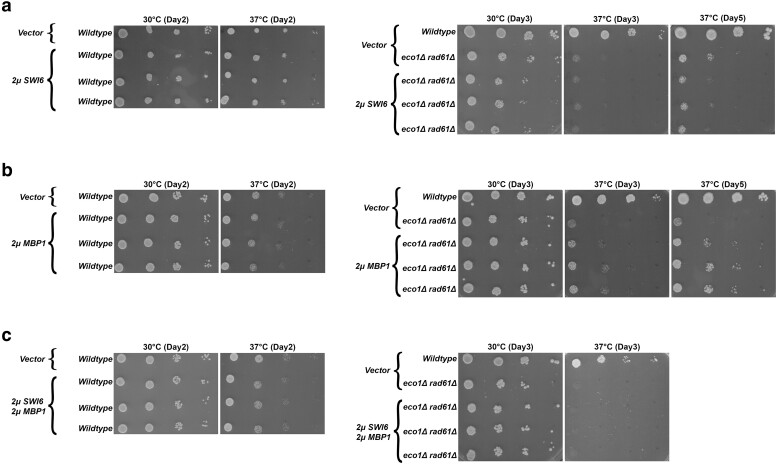
In yeast, as with most cells, G1 is largely defined as low CDK activity. The rise of G1-CDKs promotes START, a point at which cells commit to cell cycle progression -beginning with transitioning from G1 to S phase. A key facet of START in yeast is the deployment of transcriptional programs required, for instance, for the upregulation of bud growth and DNA synthesis genes (Horak et al. 2002). The Mlu I binding factor (MBF) transcription complex (Swi6 and Mbp1) is one component of START that relies on G1-CDK activity (Costanzo et al. 2004; De Bruin et al. 2004; Charvin et al. 2010). It is therefore intriguing that it is the deletion of the G1 cyclin, CLN2, that rescues both eco1Δ rad61Δ and pds5Δ elg1Δ cell growth defects (Choudhary et al. 2022). Previously, CLN2 deletion was posited to hyperactivate the MBF complex (Swi6, Mbp1) and that MBF may regulate MCD1 expression (Choudhary et al. 2022). These latter two mechanisms, however, remain untested. If these models are correct, then simply overexpressing Swi6 (an activating component of MBF) (Nasmyth and Dirick 1991; Lowndes et al. 1992; Morgan et al. 1996; Watanabe et al. 2011), should rescue the temperature sensitivity of eco1Δ rad61Δ cells. To test this possibility, SWI6 was inserted into a high copy (2µ TRP1) plasmid and transformed into both wildtype and eco1Δ rad61Δ cells. Log phase wildtype and eco1Δ rad61Δ double mutant cells, transformed with either vector alone or vector directing the elevated expression of SWI6, were serially diluted, plated onto selective media and incubated at either 30°C or 37°C. Unexpectedly, the overexpression of SWI6 not only failed to revert the temperature sensitivity of eco1Δ rad61Δ cells but, instead, slightly reduced growth at both 30°C and 37°C (Fig. 10a). This adverse effect appears cohesin-dependent given that elevated SWI6 expression produced no impact on wildtype cell growth (Fig. 10a).
Fig. 10.
SWI6 overexpression reduces eco1rad61 cell growth, whereas MBP1 overexpression partially restores eco1rad61 cell growth at 37°C. a) Left panel: Growth of 10-fold serial dilutions of wildtype cells overexpressing vector alone (YGS26), and three independent isolates of wildtype cells overexpressing SWI6 (YGS104, YGS106, YGS108). Temperatures and days of growth are indicated in this and all subsequent panels. Right panel: Growth of 10-fold serial dilutions (dilution series used in all subsequent panels) of wildtype cells overexpressing vector alone (YGS26), eco1Δ rad61Δ double mutant cells overexpressing vector alone (YGS28), and three independent isolates of eco1Δ rad61Δ double mutant cells overexpressing SWI6 (YGS105, YGS107, YGS109). b) Left panel: Serial dilutions of wildtype cells overexpressing vector alone (YGS158), and three independent isolates of wildtype cells overexpressing MBP1 (YGS162, YGS164, YGS166) are shown. Right panel: Growth of serial dilutions of wildtype cells overexpressing vector alone (YGS158), eco1Δ rad61Δ double mutant cells overexpressing vector alone (YGS160), and three independent isolates of eco1Δ rad61Δ double mutant cells overexpressing MBP1 (YGS163, YGS165, YGS167). Temperatures and days of growth are indicated. c) Left panel: Serial dilutions of wildtype cells overexpressing two empty vectors (YGS168), and three independent isolates of wildtype cells overexpressing SWI6 and MBP1 (YGS170, YGS171, YGS172) are shown. Right panel: Serial dilutions of wildtype cells overexpressing two empty vectors (YGS168), eco1Δ rad61Δ double mutant cells overexpressing two empty vectors (YGS130), and three independent isolates of eco1Δ rad61Δ double mutant cells overexpressing SWI6 and MBP1 (YGS134, YGS138, YGS142).
Swi6 typically is considered to promote Mbp1 activity—the sequence-specific DNA binding component of the MBF transcription complex (Primig et al. 1992; Koch et al. 1993; Breeden 1996). Other studies, however, suggest that MBF (via Swi6) has an inhibitory role in the transcription of some genes (Dirick et al. 1992; Lowndes et al. 1992; Sedgwick et al. 1998; Li et al. 2005; Travesa et al. 2013). Together, these findings raised the possibilities that either MCD1 expression occurs independent of MBF or that Swi6 inhibits a positive role for Mbp1 in MCD1 expression. To differentiate between these models, MBP1 was inserted into a high copy (2µ LEU2) plasmid. Wildtype and eco1Δ rad61Δ cells were then transformed with vector alone, vector driving MBP1 overexpression, or co-transformed with individual vectors driving SWI6 and MBP1 overexpression. MBP1 overexpression succeeded in partly suppressing the temperature-sensitive growth defects of the eco1Δ rad61Δ cells (Fig. 10b). Surprisingly, however, simultaneous overexpression of SWI6 and MBP1 not only failed to rescue eco1Δ rad61Δ cell ts-growth defects, but fully abrogated any positive growth effect observed by cells expressing MBP1 alone (Fig. 10c). These findings suggest that Swi6 is a negative regulator of MBF during Mbp1-mediated transcription of MCD1.
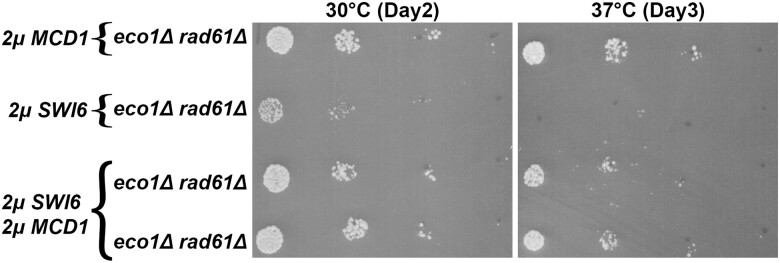
We considered the possibility that Swi6, in addition to inhibiting Mbp1-dependent expression of MCD1, could be deregulating other genes that exacerbate eco1Δ rad61Δ cell viability. If correct, this model predicts that co-expressing MCD1 along with SWI6 should fail to rescue eco1Δ rad61Δ cell ts-growth defects. In contrast, a partial rescue would indicate that the critical role of Swi6 centers on MCD1 repression. To differentiate between these predications, eco1Δ rad61Δ cells were transformed with vector driving overexpression of MCD1 and SWI6 individually, as well as co-transformed with both SWI6 and MCD1 vectors. The resulting transformants were serially diluted, plated onto selective media and incubated at either 30°C or 37°C. As expected, eco1Δ rad61Δ cells overexpressing SWI6 exhibited diminished growth while cells overexpressing MCD1 exhibited improved growth 30°C (Fig. 11). Importantly, the slow growth phenotype exhibited by eco1Δ rad61Δ cells that contained SWI6 alone was fully rescued by co-expression of MCD1 at 30°C (Fig. 11). Moreover, while eco1Δ rad61Δ cells overexpressing SWI6 were inviable at 37°C, co-expression of MCD1 largely rescued cell viability at 37°C. These findings provide strong evidence that Swi6 is a negative regulator of Mbp1-mediated transcription of MCD1.
Fig. 11.
Swi6 negatively regulates Mbp1-based transcription of MCD1. Growth of 10-fold serial dilutions of eco1Δ rad61Δ double mutant cells (YMM828) overexpressing MCD1 (YGS179), overexpressing SWI6 (YGS197), and two independent isolates co-overexpressing SWI6 and MCD1 (YGS199, YGS200). Temperature and days of growth are indicated.
Discussion
Conservatively, cohesins could be considered master regulators of DNA in that cohesin mutations impact replication origin firing, nuclear organization, gene transcription, sister chromatid segregation, chromosome condensation, and high-fidelity DNA repair (Guacci et al. 1997; Michaelis et al. 1997; Uhlmann and Nasmyth 1998; Skibbens et al. 1999; Zhang et al. 2008; Kulemzina et al. 2012; Tong and Skibbens 2014, 2015; Eng et al. 2015; Cattoglio et al. 2019). Often, factors and processes of such wide-ranging importance are placed under surveillance mechanisms (Vaddavalli and Schumacher 2022; Kalmykova 2023; Kornepati et al. 2023; Maiato and Silva 2023). While cohesins have been extensively studied, whether cells monitor cohesin integrity remains largely unknown. The first revelation of this study involves the potential emergence of a new class of checkpoint. Mcd1 must be degraded (to allow for sister chromatid segregation during mitosis) and then replenished (induced transcription at the G1/S transition in yeast cells) in every cell cycle. Here, we report that Mcd1 levels are precipitously reduced in both eco1Δ rad61Δ cells. Prior studies similarly note reductions in Mcd1 levels in pds5Δ elg1Δ cells as well as cells mutated for either SMC1 or SMC3 or RAD61 (Toth et al. 1999; Bloom et al. 2018; Choudhary et al. 2022; Boardman et al. 2023). We thus posit that regulating Mcd1 levels provide for a novel “protection” mechanism akin to apoptosis: that defects in cohesin structure promote elevated and/or prolonged Mcd1 degradation. However, this can only be half of the “checkpoint,” given that MCD1 is newly transcribed each and every cell cycle. Importantly, MCD1 levels apparently fail to rise in any of the mutated cells listed above. This suggests a feedback mechanism in which defects in cohesin structure also block MCD1 transcription. In this way, cohesin defects invoke transcriptional control over MCD1 (and possibly degradative control over Mcd1) to preclude the rise of an aneuploid population. It is tempting to speculate that cohesins of the prior cell cycle form a positive feedback loop by impacting the transcriptional networks required for subsequent MCD1 expression. Our discovery that elevating Mcd1 levels is solely sufficient to rescue eco1Δ rad61Δ cell inviability at 37°C, and similarly rescues other cohesin-mutated cell inviabilities (Bloom et al. 2018; Choudhary et al. 2022), suggests that an MCD1-dependent checkpoint pathway may monitor for a wide range of cohesin defects. Future efforts will be required to test the extent to which RAD21 (human homolog of Mcd1) culling may protect multicellular organisms from tumorigenesis and developmental abnormalities.
By extension, central questions now emerge regarding the fundamental mechanism(s) that regulate MCD1 expression—control of which likely underlies all subsequent cohesin functions. In this light, the second revelation of current study is the identification of multiple transcriptional pathways that appear to regulate MCD1 (Fig. 12). Fdo1 is the first such regulator of MCD1 expression in that FDO1 deletion rescues eco1Δ rad61Δ cell ts-growth and that MCD1 elevated expression rescues eco1Δ rad61Δ cell inviability that otherwise arises in response to FDO1 elevated expression. As a direct-binding transcription factor repressor, Fdo1 is unique compared to other gene deletions (RAD61, ELG1, and CLN2) that can rescue cohesin mutant cell inviability. Rad61 (an auxiliary cohesin subunit) and Elg1 (regulating PCNA-dependent recruitment of Eco1 to the DNA replication fork) directly impact cohesin function (Skibbens et al. 1999; Kueng et al. 2006; Moldovan et al. 2006; Ben-Shahar et al. 2008; Heidinger-Pauli et al. 2009; Maradeo and Skibbens 2009, 2010; Parnas et al. 2009; Rowland et al. 2009; Sutani et al. 2009; Bender et al. 2020; Zuilkoski and Skibbens 2020; Buskirk and Skibbens 2022; Choudhary et al. 2022). Cln2 (G1 cyclin component of CDK) appears to likely regulate MCD1 expression indirectly through post-translational modifications. FDO1 deletion is also unique to other allele-specific mutations that encode cohesin subunits (Irr1/Scc3 and Pds5) or cohesin modifiers (Eco1) that also can rescue eco1Δ cell inviability (Rowland et al. 2009; Sutani et al. 2009). This latter category is comprised of mutations in essential factors that normally are required for cohesin function and therefore are not negative regulators in the strictest sense in which full gene deletion rescues Eco1-deficient cell inviability.
Fig. 12.
Schematic highlights integrated mechanisms of MCD1 regulation. MBF complex (Swi6 and Mbp1) and Forkhead (Fkh1 and Fkh2) transcription factors bind non-overlapping DNA sequences upstream of MCD1. Swi6 and Mbp1 play antagonistic roles in MCD1 expression: Swi6 inhibits the positive role that Mpb1 otherwise performs in MCD1 transcription. Of the two forkhead transcription factors in yeast, Fkh2 appears to play a positive role in MCD1 expression, although elevated expression of either FKH1 or FKH2 (in bold with up arrow) is detrimental to eco1Δ rad61Δ cell growth. Fdo1, a co-repressor that binds Fkh1, inhibits MCD1 expression. Notably, elevated expression of MCD1 rescues (or partially suppresses) the negative effects produced by alterations in any one of these three (MBF, FKHs, and Fdo1) pathways. Further analysis is required to clarify the dual role of FKHs in regulating MCD1 expression, as well the molecular mechanisms through which Cln2-CDK may impact the transcriptional pathways defined here to regulate Mcd1 levels.
Related discoveries include the extended and complex roles of additional transcriptional networks that overlay MCD1 expression (Fig. 12). Fkh1 and the Fkh2 transcriptional factor paralogs were previously reported to regulate mating-type switching (silencing), replication origin firing (Casey et al. 2008), RNA Polymerase II transcription elongation (Morillon et al. 2003), and transcription of the CLB2-cluster genes (Koranda et al. 2000; Kumar et al. 2000). Here, we report that eco1Δ rad61Δ cells are highly sensitive to increased Fkh1 and Fkh2 levels and that the resulting exacerbated growth in each case is ameliorated by co-expression of MCD1. Fkh1 and Fkh2 differ however in that only deletion of FKH2 adversely impacts eco1Δ rad61Δ cell growth—an effect also reversed by elevated expression MCD1. Intriguingly, biochemical studies found that Fdo1 interacts with the FOX-type transcription factor Forkhead 1 (Fkh1) (Ho et al. 2002; Dummer et al. 2016). Given the antagonistic and dose-dependent effects reported here, future efforts may be challenging given that Forkhead transcription factors, which are widespread from yeast to humans, bind over 100 proteins (via forkhead binding-domains) that play critical roles in development and ciliogensis, metabolism, ageing and cancer (Jonsson and Peng 2005; Jin et al. 2020; Lewis and Stracker 2021).
The final revelation of the current study centers on our analyses of the MBF transcription complex—including the antagonistic activities of each MBF component on MCD1 expression (Fig. 12). A prior study inferred the role of MBF in promoting MCD1 expression, but this model remained untested (Choudhary et al. 2022). MBF is comprised of Swi6 and Mbp1. An analogous transcriptional regulator of START, SBF, is comprised of Swi6 and Swi4 (Primig et al. 1992; Moll et al. 1993; Dirick et al. 1995). In both cases, Swi6 is positioned as a positive regulator of Mbp1 and Swi4, the latter two factors providing DNA sequence-dependent promoter binding activities. Our findings suggest that Swi6 is a strong negative regulator of the positive role that Mbp1 performs in MCD1 expression, consistent with results from a high-through put study that SWI6 deletion promotes mcd1 mutant cell growth (128). These results also are consistent with prior studies that (1) MBF/SBF transcription continues in the absence of Swi6, although the expression of those genes are no longer correctly regulated in the cell cycle (Dirick et al. 1992; Lowndes et al. 1992; Li et al. 2005). In fact, prior studies revealed that SBF and MBF can act as transcriptional suppressors such that loss of SWI4 or MBF enhances the transcription of certain genes (Bean et al. 2005). Clearly, the roles of MBF and SBF are nuanced and results, such as these involving MCD1, must be viewed through the lens of specific gene expressions.
Supplementary Material
Acknowledgements
The authors thank past and present Skibbens lab members (Annie Sanchez, Grace Duke, Abbie Brown, Fiona Mensching, Niusha Banoukh, and MJ Schwab) for helpful discussions during the preparation of this paper. The authors gratefully acknowledge the generous gift of Mcd1-directed antibody by Dr. Vincent Guacci and expertise in genome sequence analyses performed by Prof. Sean Buskirk.
Contributor Information
Gurvir Singh, Department of Biological Sciences, Lehigh University, Bethlehem, PA 18015, USA.
Robert V Skibbens, Department of Biological Sciences, Lehigh University, Bethlehem, PA 18015, USA.
Data availability
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables with the following exceptions. Supplementary Table 1 contains yeast strains and genotypes used in this study. Supplementary Table 2 contains DNA oligo sequences used in this study. Supplementary Figs. 1–4 contain supplemental figures and figure legends. Illumina sequencing data was deposited into the short read archive (SRA): BioSample SAMN42761258 within the BioProject PRJNA836598.
Supplemental material available at GENETICS online.
Literature cited
- Alomer RM, Da Silva EML, Chen J, Piekarz KM, McDonald K, Sansam CG, Sansam CL, Rankin S. 2017. Esco1 and Esco2 regulate distinct cohesin functions during cell cycle progression. Proc Natl Acad Sci. 114(37):9906–9911. doi: 10.1073/pnas.1708291114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony J, Chin CV, Horsfield JA. 2021. Cohesin mutations in cancer: emerging therapeutic targets. Int J Mol Sci. 22(13):6788. doi: 10.3390/ijms22136788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aref R, Sanad MNME, Schüller H-J. 2021. Forkhead transcription factor Fkh1: insights into functional regulatory domains crucial for recruitment of Sin3 histone deacetylase complex. Curr Genet. 67(3):487–499. doi: 10.1007/s00294-021-01158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam P, Gruber S, Tanaka K, Haering CH, Mechtler K, Nasmyth K. 2003. ATP hydrolysis is required for Cohesin's association with chromosomes. Curr Biol. 13(22):1941–1953. doi: 10.1016/j.cub.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Bean JM, Siggia ED, Cross FR. 2005. High functional overlap between mlui cell-cycle box binding factor and swi4/6 cell-cycle box binding factor in the G1/S transcriptional program in Saccharomyces cerevisiae. Genetics. 171(1):49–61. doi: 10.1534/genetics.105.044560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender D, Da Silva EML, Chen J, Poss A, Gawey L, Rulon Z, Rankin S. 2020. Multivalent interaction of ESCO2 with the replication machinery is required for sister chromatid cohesion in vertebrates. Proc Natl Acad Sci. 117(2):1081–1089. doi: 10.1073/pnas.1911936117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MS, Koshland D, Guacci V. 2018. Cohesin function in cohesion, condensation, and DNA repair is regulated by Wpl1p via a common mechanism in Saccharomyces cerevisiae. Genetics. 208(1):111–124. doi: 10.1534/genetics.117.300537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman K, Xiang S, Chatterjee F, Mbonu U, Guacci V, Koshland D. 2023. A model for Scc2p stimulation of cohesin's ATPase and its inhibition by acetylation of Smc3p. Genes Dev. 37(7–8):277–290. doi: 10.1101/gad.350278.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeden L. 1996. Start-specific transcription in yeast. In: Farnham PJ, editors. Transcriptional Control of Cell Growth. Vol. 208. Berlin, Heidelberg: Springer Berlin Heidelberg. p. 95–127. [DOI] [PubMed] [Google Scholar]
- Buskirk S, Skibbens RV. 2022. G1-Cyclin2 (Cln2) promotes chromosome hypercondensation in eco1/ctf7 rad61 null cells during hyperthermic stress in Saccharomyces cerevisiae. G3 (Bethesda) (Bethesda, Md.). 12(8):jkac157. doi: 10.1093/g3journal/jkac157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey L, Patterson EE, Müller U, Fox CA. 2008. Conversion of a replication origin to a silencer through a pathway shared by a forkhead transcription factor and an S phase cyclin. Mol Biol Cell. 19(2):608–622. doi: 10.1091/mbc.e07-04-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoglio C, Pustova I, Walther N, Ho JJ, Hantsche-Grininger M, Inouye CJ, Hossain MJ, Dailey GM, Ellenberg J, Darzacq X, et al. 2019. Determining cellular CTCF and cohesin abundances to constrain 3D genome models. eLife. 8:e40164. doi: 10.7554/eLife.40164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvin G, Oikonomou C, Siggia ED, Cross FR. 2010. Origin of irreversibility of cell cycle start in budding yeast. PLoS Biol. 8(1):e1000284. doi: 10.1371/journal.pbio.1000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien R, Zeng W, Kawauchi S, Bender MA, Santos R, Gregson HC, Schmiesing JA, Newkirk DA, Kong X, Ball AR, et al. 2011. Cohesin mediates chromatin interactions that regulate mammalian β-globin expression. J Biol Chem. 286(20):17870–17878. doi: 10.1074/jbc.M110.207365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary K, Itzkovich Z, Alonso-Perez E, Bishara H, Dunn B, Sherlock G, Kupiec M. 2022. S. cerevisiae cells can grow without the Pds5 cohesin subunit. mBio. 13(4):e01420-22. doi: 10.1128/mbio.01420-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M, Nishikawa JL, Tang X, Millman JS, Schub O, Breitkreuz K, Dewar D, Rupes I, Andrews B, Tyers M. 2004. CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast. Cell. 117(7):899–913. doi: 10.1016/j.cell.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Giménez-Llorente D, Kojic A, Rodríguez-Corsino M, Cuartero Y, Martín-Serrano G, Gómez-López G, Marti-Renom MA, Losada A. 2019. Specific contributions of cohesin-SA1 and cohesin-SA2 to TADs and polycomb domains in embryonic stem cells. Cell Rep. 27(12):3500–3510.e4. doi: 10.1016/j.celrep.2019.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson IF, Bauer B, Goetz D, Tang W, Wutz G, Peters J-M. 2019. DNA loop extrusion by human cohesin. Science. 366(6471):1338–1345. doi: 10.1126/science.aaz3418. [DOI] [PubMed] [Google Scholar]
- Davidson IF, Goetz D, Zaczek MP, Molodtsov MI, Huis In ‘T Veld PJ, Weissmann F, Litos G, Cisneros DA, Ocampo-Hafalla M, Ladurner R, et al. 2016. Rapid movement and transcriptional re-localization of human cohesin on DNA. EMBO J. 35(24):2671–2685. doi: 10.15252/embj.201695402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff MA, Kaur M, Yaeger D, Rampuria A, Korolev S, Pie J, Gil-Rodríguez C, Arnedo M, Loeys B, Kline AD, et al. 2007. Mutations in cohesin complex members SMC3 and SMC1A cause a mild variant of cornelia de lange syndrome with predominant mental retardation. Am J Hum Genet. 80(3):485–494. doi: 10.1086/511888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin RAM, McDonald WH, Kalashnikova TI, Yates J, Wittenberg C. 2004. Cln3 activates G1-specific transcription via phosphorylation of the SBF bound repressor Whi5. Cell. 117(7):887–898. doi: 10.1016/j.cell.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Degner SC, Verma-Gaur J, Wong TP, Bossen C, Iverson GM, Torkamani A, Vettermann C, Lin YC, Ju Z, Schulz D, et al. 2011. CCCTC-binding factor (CTCF) and cohesin influence the genomic architecture of the Igh locus and antisense transcription in pro-B cells. Proc Natl Acad Sci. 108(23):9566–9571. doi: 10.1073/pnas.1019391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degner SC, Wong TP, Jankevicius G, Feeney AJ. 2009. Cutting edge: developmental stage-specific recruitment of cohesin to CTCF sites throughout immunoglobulin loci during B lymphocyte development. J Immunol. 182(1):44–48. doi: 10.4049/jimmunol.182.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E, Vos ESM, Holwerda SJB, Valdes-Quezada C, Verstegen MJAM, Teunissen H, Splinter E, Wijchers PJ, Krijger PHL, de Laat W. 2015. CTCF binding polarity determines chromatin looping. Mol Cell. 60(4):676–684. doi: 10.1016/j.molcel.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Di Nardo M, Pallotta MM, Musio A. 2022. The multifaceted roles of cohesin in cancer. J Exp Clin Cancer Res. 41(1):96. doi: 10.1186/s13046-022-02321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirick L, Böhm T, Nasmyth K. 1995. Roles and regulation of Cln-Cdc28 kinases at the start of the cell cycle of Saccharomyces cerevisiae. EMBO J. 14(19):4803–4813. doi: 10.1002/j.1460-2075.1995.tb00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirick L, Moll T, Auer H, Nasmyth K. 1992. A central role for SWI6 in modulating cell cycle start-specific transcription in yeast. Nature. 357(6378):508–513. doi: 10.1038/357508a0. [DOI] [PubMed] [Google Scholar]
- Dorsett D, Eissenberg JC, Misulovin Z, Martens A, Redding B, McKim K. 2005. Effects of sister chromatid cohesion proteins on cut gene expression during wing development in Drosophila. Development. 132(21):4743–4753. doi: 10.1242/dev.02064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer AM, Su Z, Cherney R, Choi K, Denu J, Zhao X, Fox CA. 2016. Binding of the Fkh1 forkhead associated domain to a phosphopeptide within the Mph1 DNA helicase regulates mating-type switching in budding yeast. PLOS Genet. 12(6):e1006094. doi: 10.1371/journal.pgen.1006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng T, Guacci V, Koshland D. 2015. Interallelic complementation provides functional evidence for cohesin–cohesin interactions on DNA. Mol Biol Cell. 26(23):4224–4235. doi: 10.1091/mbc.e15-06-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriele M, Brandão HB, Grosse-Holz S, Jha A, Dailey GM, Cattoglio C, Hsieh T-HS, Mirny L, Zechner C, Hansen AS. 2022. Dynamics of CTCF- and cohesin-mediated chromatin looping revealed by live-cell imaging. Science. 376(6592):496–501. doi: 10.1126/science.abn6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi R, Gillespie PJ, Hirano T. 2006. Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr Biol. 16(24):2406–2417. doi: 10.1016/j.cub.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassler J, Brandão HB, Imakaev M, Flyamer IM, Ladstätter S, Bickmore WA, Peters J, Mirny LA, Tachibana K. 2017. A mechanism of cohesin-dependent loop extrusion organizes zygotic genome architecture. EMBO J. 36(24):3600–3618. doi: 10.15252/embj.201798083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gause M, Misulovin Z, Bilyeu A, Dorsett D. 2010. Dosage-Sensitive regulation of cohesin chromosome binding and dynamics by nipped-B, pds5, and wapl. Mol Cell Biol. 30(20):4940–4951. doi: 10.1128/MCB.00642-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti L, Galupa R, Nora EP, Piolot T, Lam F, Dekker J, Tiana G, Heard E. 2014. Predictive polymer modeling reveals coupled fluctuations in chromosome conformation and transcription. Cell. 157(4):950–963. doi: 10.1016/j.cell.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gligoris TG, Scheinost JC, Bürmann F, Petela N, Chan K-L, Uluocak P, Beckouët F, Gruber S, Nasmyth K, Löwe J. 2014. Closing the cohesin ring: structure and function of its Smc3-kleisin interface. Science. 346(6212):963–967. doi: 10.1126/science.1256917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordillo M, Vega H, Trainer AH, Hou F, Sakai N, Luque R, Kayserili H, Basaran S, Skovby F, Hennekam RCM, et al. 2008. The molecular mechanism underlying Roberts syndrome involves loss of ESCO2 acetyltransferase activity. Hum Mol Genet. 17(14):2172–2180. doi: 10.1093/hmg/ddn116. [DOI] [PubMed] [Google Scholar]
- Gruber S, Haering CH, Nasmyth K. 2003. Chromosomal cohesin forms a ring. Cell. 112(6):765–777. doi: 10.1016/S0092-8674(03)00162-4. [DOI] [PubMed] [Google Scholar]
- Guacci V, Koshland D. 2012. Cohesin-independent segregation of sister chromatids in budding yeast. Mol Biol Cell. 23(4):729–739. doi: 10.1091/mbc.e11-08-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guacci V, Koshland D, Strunnikov A. 1997. A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell. 91(1):47–57. doi: 10.1016/S0092-8674(01)80008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarhuis JHI, Van Der Weide RH, Blomen VA, Yáñez-Cuna JO, Amendola M, Van Ruiten MS, Krijger PHL, Teunissen H, Medema RH, Van Steensel B, et al. 2017. The cohesin release factor WAPL restricts chromatin loop extension. Cell. 169(4):693–707.e14. doi: 10.1016/j.cell.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haering CH, Löwe J, Hochwagen A, Nasmyth K. 2002. Molecular architecture of SMC proteins and the yeast cohesin Complex. Mol Cell. 9(4):773–788. doi: 10.1016/S1097-2765(02)00515-4. [DOI] [PubMed] [Google Scholar]
- Hansen AS, Pustova I, Cattoglio C, Tjian R, Darzacq X. 2017. CTCF and cohesin regulate chromatin loop stability with distinct dynamics. eLife. 6:e25776. doi: 10.7554/eLife.25776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman T, Stead K, Koshland D, Guacci V. 2000. Pds5p is an essential chromosomal protein required for both sister chromatid cohesion and condensation in Saccharomyces cerevisiae. J Cell Biol. 151(3):613–626. doi: 10.1083/jcb.151.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidinger-Pauli JM, Ünal E, Koshland D. 2009. Distinct targets of the Eco1 acetyltransferase modulate cohesion in S phase and in response to DNA damage. Mol Cell. 34(3):311–321. doi: 10.1016/j.molcel.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y, Gruhler A, Heilbut A, Bader GD, Moore L, Adams S-L, Millar A, Taylor P, Bennett K, Boutilier K, et al. 2002. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature. 415(6868):180–183. doi: 10.1038/415180a. [DOI] [PubMed] [Google Scholar]
- Horak CE, Luscombe NM, Qian J, Bertone P, Piccirrillo S, Gerstein M, Snyder M. 2002. Complex transcriptional circuitry at the G1/S transition in Saccharomyces cerevisiae. Genes Dev. 16(23):3017–3033. doi: 10.1101/gad.1039602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T-HS, Cattoglio C, Slobodyanyuk E, Hansen AS, Darzacq X, Tjian R. 2022. Enhancer–promoter interactions and transcription are largely maintained upon acute loss of CTCF, cohesin, WAPL or YY1. Nat Genet. 54(12):1919–1932. doi: 10.1038/s41588-022-01223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger R. 2002. The many functions of smc proteins in chromosome dynamics. Nat Rev Mol Cell Biol. 3(10):767–778. doi: 10.1038/nrm930. [DOI] [PubMed] [Google Scholar]
- Jin Y, Liang Z, Lou H. 2020. The emerging roles of fox family transcription factors in chromosome replication, organization, and genome stability. Cells. 9(1):258. doi: 10.3390/cells9010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson H, Peng SL. 2005. Forkhead transcription factors in immunology. CMLS Cell Mol Life Sci. 62(4):397–409. doi: 10.1007/s00018-004-4365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmykova A. 2023. Telomere checkpoint in development and aging. Int J Mol Sci. 24(21):15979. doi: 10.3390/ijms242115979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S, Calof AL, Santos R, Lopez-Burks ME, Young CM, Hoang MP, Chua A, Lao T, Lechner MS, Daniel JA, et al. 2009. Multiple organ system defects and transcriptional dysregulation in the Nipbl+/− mouse, a model of cornelia de lange syndrome. PLoS Genet. 5(9):e1000650. doi: 10.1371/journal.pgen.1000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-S, Krasieva TB, LaMorte V, Taylor AMR, Yokomori K. 2002. Specific recruitment of human cohesin to Laser-induced DNA damage. J Biol Chem. 277(47):45149–45153. doi: 10.1074/jbc.M209123200. [DOI] [PubMed] [Google Scholar]
- Kim Y, Shi Z, Zhang H, Finkelstein IJ, Yu H. 2019. Human cohesin compacts DNA by loop extrusion. Science. 366(6471):1345–1349. doi: 10.1126/science.aaz4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, Moll T, Neuberg M, Ahorn H, Nasmyth K. 1993. A role for the transcription factors Mbp1 and Swi4 in progression from G1 to S phase. Science. 261(5128):1551–1557. doi: 10.1126/science.8372350. [DOI] [PubMed] [Google Scholar]
- Koranda M, Schleiffer A, Endler L, Ammerer G. 2000. Forkhead-like transcription factors recruit Ndd1 to the chromatin of G2/M-specific promoters. Nature. 406(6791):94–98. doi: 10.1038/35017589. [DOI] [PubMed] [Google Scholar]
- Kornepati AVR, Rogers CM, Sung P, Curiel TJ. 2023. The complementarity of DDR, nucleic acids and anti-tumour immunity. Nature. 619(7970):475–486. doi: 10.1038/s41586-023-06069-6. [DOI] [PubMed] [Google Scholar]
- Krantz ID, McCallum J, DeScipio C, Kaur M, Gillis LA, Yaeger D, Jukofsky L, Wasserman N, Bottani A, Morris CA, et al. 2004. Cornelia de lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster nipped-B. Nat Genet. 36(6):631–635. doi: 10.1038/ng1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueng S, Hegemann B, Peters BH, Lipp JJ, Schleiffer A, Mechtler K, Peters J-M. 2006. Wapl controls the dynamic association of cohesin with chromatin. Cell. 127(5):955–967. doi: 10.1016/j.cell.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Kulemzina I, Schumacher MR, Verma V, Reiter J, Metzler J, Failla AV, Lanz C, Sreedharan VT, Rätsch G, Ivanov D. 2012. Cohesin rings devoid of Scc3 and Pds5 maintain their stable association with the DNA. PLoS Genet. 8(8):e1002856. doi: 10.1371/journal.pgen.1002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Reynolds DM, Andrej S, Anna S, Goldstone SD, Dalton S. 2000. Forkhead transcription factors, Fkh1p and Fkh2p, collaborate with Mcm1p to control transcription required for M-phase. Curr Biol. 10(15):896–906. doi: 10.1016/S0960-9822(00)00618-7. [DOI] [PubMed] [Google Scholar]
- Lewis M, Stracker TH. 2021. Transcriptional regulation of multiciliated cell differentiation. Semin Cell Dev Biol. 110:51–60. doi: 10.1016/j.semcdb.2020.04.007. [DOI] [PubMed] [Google Scholar]
- Li Y, Haarhuis JHI, Sedeño Cacciatore Á, Oldenkamp R, Van Ruiten MS, Willems L, Teunissen H, Muir KW, De Wit E, Rowland BD, et al. 2020. The structural basis for cohesin–CTCF-anchored loops. Nature. 578(7795):472–476. doi: 10.1038/s41586-019-1910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Quinton T, Miles S, Breeden LL. 2005. Genetic interactions between mediator and the late G1-specific transcription factor Swi6 in Saccharomyces cerevisiae. Genetics. 171(2):477–488. doi: 10.1534/genetics.105.043893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Zhao L, Ye AY, Lin SG, Zhang Y, Guo C, Dai H-Q, Ba Z, Alt FW. 2023. Contribution of the IGCR1 regulatory element and the 3′ Igh CTCF-binding elements to regulation of Igh V(D)J recombination. Proc Natl Acad Sci. 120(26):e2306564120. doi: 10.1073/pnas.2306564120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, Mckenzie Iii A, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 14(10):953–961. doi:. [DOI] [PubMed] [Google Scholar]
- Losada A, Yokochi T, Hirano T. 2005. Functional contribution of Pds5 to cohesin-mediated cohesion in human cells and Xenopus egg extracts. J Cell Sci. 118(10):2133–2141. doi: 10.1242/jcs.02355. [DOI] [PubMed] [Google Scholar]
- Lowndes NF, Johnson AL, Breeden L, Johnston LH. 1992. SWI6 protein is required for transcription of the periodically expressed DNA synthesis genes in budding yeast. Nature. 357(6378):505–508. doi: 10.1038/357505a0. [DOI] [PubMed] [Google Scholar]
- Mach P, Kos PI, Zhan Y, Cramard J, Gaudin S, Tünnermann J, Marchi E, Eglinger J, Zuin J, Kryzhanovska M, et al. 2022. Cohesin and CTCF control the dynamics of chromosome folding. Nat Genet. 54(12):1907–1918. doi: 10.1038/s41588-022-01232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato H, Silva S. 2023. Double-checking chromosome segregation. J Cell Biol. 222(5):e202301106. doi: 10.1083/jcb.202301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maradeo ME, Skibbens RV. 2009. The Elg1-RFC clamp-loading complex performs a role in sister chromatid cohesion. PLoS One. 4(3):e4707. doi: 10.1371/journal.pone.0004707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maradeo ME, Skibbens RV. 2010. Replication factor C complexes play unique pro- and anti-establishment roles in sister chromatid cohesion. PLoS One. 5(10):e15381. doi: 10.1371/journal.pone.0015381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis C, Ciosk R, Nasmyth K. 1997. Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell. 91(1):35–45. doi: 10.1016/S0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]
- Moldovan G-L, Pfander B, Jentsch S. 2006. PCNA controls establishment of sister chromatid cohesion during S phase. Mol Cell. 23(5):723–732. doi: 10.1016/j.molcel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Moll T, Schwob E, Koch C, Moore A, Auer H, Nasmyth K. 1993. Transcription factors important for starting the cell cycle in yeast. Philos Trans R Soc Lond B Biol Sci. 340(1293):351–360. doi: 10.1098/rstb.1993.0078. [DOI] [PubMed] [Google Scholar]
- Mönnich M, Kuriger Z, Print CG, Horsfield JA. 2011. A zebrafish model of Roberts syndrome reveals that esco2 depletion interferes with development by disrupting the cell cycle. PLoS One. 6(5):e20051. doi: 10.1371/journal.pone.0020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan BA, Conlon FL, Manzanares M, Millar JB, Kanuga N, Sharpe J, Krumlauf R, Smith JC, Sedgwick SG. 1996. Transposon tools for recombinant DNA manipulation: characterization of transcriptional regulators from yeast. Xenopus, and mouse. Proc Natl Acad Sci. 93(7):2801–2806. doi: 10.1073/pnas.93.7.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillon A, O'Sullivan J, Azad A, Proudfoot N, Mellor J. 2003. Regulation of elongating RNA polymerase II by forkhead transcription factors in yeast. Science. 300(5618):492–495. doi: 10.1126/science.1081379. [DOI] [PubMed] [Google Scholar]
- Muir KW, Kschonsak M, Li Y, Metz J, Haering CH, Panne D. 2016. Structure of the Pds5-Scc1 complex and implications for cohesin function. Cell Rep. 14(9):2116–2126. doi: 10.1016/j.celrep.2016.01.078. [DOI] [PubMed] [Google Scholar]
- Musio A, Selicorni A, Focarelli ML, Gervasini C, Milani D, Russo S, Vezzoni P, Larizza L. 2006. X-linked cornelia de lange syndrome owing to SMC1L1 mutations. Nat Genet. 38(5):528–530. doi: 10.1038/ng1779. [DOI] [PubMed] [Google Scholar]
- Nasmyth K, Dirick L. 1991. The role of SWI4 and SWI6 in the activity of G1 cyclins in yeast. Cell. 66(5):995–1013. doi: 10.1016/0092-8674(91)90444-4. [DOI] [PubMed] [Google Scholar]
- Orgil O, Matityahu A, Eng T, Guacci V, Koshland D, Onn I. 2015. A conserved domain in the Scc3 subunit of cohesin mediates the interaction with both Mcd1 and the cohesin loader complex. PLOS Genet. 11(3):e1005036. doi: 10.1371/journal.pgen.1005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizza S, Tanaka T, Hochwagen A, Eisenhaber F, Nasmyth K. 2000. Pds5 cooperates with cohesin in maintaining sister chromatid cohesion. Curr Biol. 10(24):1557–1564. doi: 10.1016/S0960-9822(00)00854-X. [DOI] [PubMed] [Google Scholar]
- Parnas O, Zipin-Roitman A, Mazor Y, Liefshitz B, Ben-Aroya S, Kupiec M. 2009. The Elg1 clamp loader plays a role in sister chromatid cohesion. PLoS One. 4(5):e5497. doi: 10.1371/journal.pone.0005497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati D. 2024. Role of chromosomal cohesion and separation in aneuploidy and tumorigenesis. Cell Mol Life Sci CMLS. 81(1):100. doi: 10.1007/s00018-024-05122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primig M, Sockanathan S, Auer H, Nasmyth K. 1992. Anatomy of a transcription factor important for the start of the cell cycle in Saccharomyces cerevisiae. Nature. 358(6387):593–597. doi: 10.1038/358593a0. [DOI] [PubMed] [Google Scholar]
- Rao SSP, Huang S-C, Glenn St Hilaire B, Engreitz JM, Perez EM, Kieffer-Kwon K-R, Sanborn AL, Johnstone SE, Bascom GD, Bochkov ID, et al. 2017. Cohesin loss eliminates all loop domains. Cell. 171(2):305–320.e24. doi: 10.1016/j.cell.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig MB, Löwe J, Chan K-L, Beckouët F, Metson J, Nasmyth K. 2014. Structure and function of cohesin's scc3/SA regulatory subunit. FEBS Lett. 588(20):3692–3702. doi: 10.1016/j.febslet.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolef Ben-Shahar TR, Heeger S, Lehane C, East P, Flynn H, Skehel M, Uhlmann F. 2008. Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science. 321(5888):563–566. doi: 10.1126/science.1157774. [DOI] [PubMed] [Google Scholar]
- Rollins RA, Morcillo P, Dorsett D. 1999. Nipped-B, a Drosophila homologue of chromosomal adherins, participates in activation by remote enhancers in the cut and ultrabithorax genes. Genetics. 152(2):577–593. doi: 10.1093/genetics/152.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. 1990. Methods in Yeast Genetics. New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Rowland BD, Roig MB, Nishino T, Kurze A, Uluocak P, Mishra A, Beckouët F, Underwood P, Metson J, Imre R, et al. 2009. Building sister chromatid cohesion: Smc3 acetylation counteracts an antiestablishment activity. Mol Cell. 33(6):763–774. doi: 10.1016/j.molcel.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Schüle B, Oviedo A, Johnston K, Pai S, Francke U. 2005. Inactivating mutations in ESCO2 cause SC phocomelia and Roberts syndrome: no phenotype-genotype correlation. Am J Hum Genet. 77(6):1117–1128. doi: 10.1086/498695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer W, Abdennur N, Goloborodko A, Pekowska A, Fudenberg G, Loe-Mie Y, Fonseca NA, Huber W, Haering CH, Mirny L, et al. 2017. Two independent modes of chromatin organization revealed by cohesin removal. Nature. 551(7678):51–56. doi: 10.1038/nature24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick SG, Taylor IA, Adam AC, Spanos A, Howell S, Morgan BA, Treiber MK, Kanuga N, Banks GR, Foord R, et al. 1998. Structural and functional architecture of the yeast cell-cycle transcription factor swi6. J Mol Biol. 281(5):763–775. doi: 10.1006/jmbi.1998.1996. [DOI] [PubMed] [Google Scholar]
- Seitan VC, Banks P, Laval S, Majid NA, Dorsett D, Rana A, Smith J, Bateman A, Krpic S, Hostert A, et al. 2006. Metazoan Scc4 homologs link sister chromatid cohesion to cell and axon migration guidance. PLoS Biol. 4(8):e242. doi: 10.1371/journal.pbio.0040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitan VC, Hao B, Tachibana-Konwalski K, Lavagnolli T, Mira-Bontenbal H, Brown KE, Teng G, Carroll T, Terry A, Horan K, et al. 2011. A role for cohesin in T-cell-receptor rearrangement and thymocyte differentiation. Nature. 476(7361):467–471. doi: 10.1038/nature10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sept CE, Tak YE, Cerda-Smith CG, Hutchinson HM, Goel V, Blanchette M, Bhakta MS, Hansen AS, Joung JK, Johnstone S, et al. 2023. High-resolution CTCF footprinting reveals impact of chromatin state on cohesin extrusion dynamics. bioRxiv 563340. 10.1101/2023.10.20.563340, preprint: not peer reviewed. [DOI]
- Sjögren C, Nasmyth K. 2001. Sister chromatid cohesion is required for postreplicative double-strand break repair in Saccharomyces cerevisiae. Curr Biol. 11(12):991–995. doi: 10.1016/S0960-9822(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Skibbens RV, Corson LB, Koshland D, Hieter P. 1999. Ctf7p is essential for sister chromatid cohesion and links mitotic chromosome structure to the DNA replication machinery. Genes Dev. 13(3):307–319. doi: 10.1101/gad.13.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutani T, Kawaguchi T, Kanno R, Itoh T, Shirahige K. 2009. Budding yeast Wpl1(Rad61)-Pds5 complex counteracts sister chromatid cohesion-establishing reaction. Curr Biol. 19(6):492–497. doi: 10.1016/j.cub.2009.01.062. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Yonekawa T, Kawasaki Y, Kai M, Furuya K, Iwasaki M, Murakami H, Yanagida M, Okayama H. 2000. Fission yeast Eso1p is required for establishing sister chromatid cohesion during S phase. Mol Cell Biol. 20(10):3459–3469. doi: 10.1128/MCB.20.10.3459-3469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong K, Skibbens RV. 2014. Cohesin without cohesion: a novel role for Pds5 in Saccharomyces cerevisiae. PLoS One. 9(6):e100470. doi: 10.1371/journal.pone.0100470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong K, Skibbens RV. 2015. Pds5 regulators segregate cohesion and condensation pathways in Saccharomyces cerevisiae. Proc Natl Acad Sci. 112(22):7021–7026. doi: 10.1073/pnas.1501369112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin ET, Wang T-J, Lisgo S, Bamshad MJ, Strachan T. 2004. NIPBL, encoding a homolog of fungal Scc2-type sister chromatid cohesion proteins and fly nipped-B, is mutated in cornelia de lange syndrome. Nat Genet. 36(6):636–641. doi: 10.1038/ng1363. [DOI] [PubMed] [Google Scholar]
- Toth A, Ciosk R, Uhlmann F, Galova M, Schleiffer A, Nasmyth K. 1999. Yeast cohesin complex requires a conserved protein, Eco1p(Ctf7), to establish cohesion between sister chromatids during DNA replication. Genes Dev. 13(3):320–333. doi: 10.1101/gad.13.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travesa A, Kalashnikova TI, De Bruin RAM, Cass SR, Chahwan C, Lee DE, Lowndes NF, Wittenberg C. 2013. Repression of G 1/S transcription is mediated via interaction of the GTB motifs of Nrm1 and Whi5 with Swi6. Mol Cell Biol. 33(8):1476–1486. doi: 10.1128/MCB.01333-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann F, Lottspeich F, Nasmyth K. 1999. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature. 400(6739):37–42. doi: 10.1038/21831. [DOI] [PubMed] [Google Scholar]
- Uhlmann F, Nasmyth K. 1998. Cohesion between sister chromatids must be established during DNA replication. Curr Biol. 8(20):1095–1102. doi: 10.1016/S0960-9822(98)70463-4. [DOI] [PubMed] [Google Scholar]
- Vaddavalli PL, Schumacher B. 2022. The p53 network: cellular and systemic DNA damage responses in cancer and aging. Trends Genet TIG. 38(6):598–612. doi: 10.1016/j.tig.2022.02.010. [DOI] [PubMed] [Google Scholar]
- Valton A-L, Venev SV, Mair B, Khokhar ES, Tong AHY, Usaj M, Chan K, Pai AA, Moffat J, Dekker J. 2022. A cohesin traffic pattern genetically linked to gene regulation. Nat Struct Mol Biol. 29(12):1239–1251. doi: 10.1038/s41594-022-00890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ruiten MS, Van Gent D, Sedeño Cacciatore Á, Fauster A, Willems L, Hekkelman ML, Hoekman L, Altelaar M, Haarhuis JHI, Brummelkamp TR, et al. 2022. The cohesin acetylation cycle controls chromatin loop length through a PDS5A brake mechanism. Nat Struct Mol Biol. 29(6):586–591. doi: 10.1038/s41594-022-00773-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega H, Waisfisz Q, Gordillo M, Sakai N, Yanagihara I, Yamada M, Van Gosliga D, Kayserili H, Xu C, Ozono K, et al. 2005. Roberts syndrome is caused by mutations in ESCO2, a human homolog of yeast ECO1 that is essential for the establishment of sister chromatid cohesion. Nat Genet. 37(5):468–470. doi: 10.1038/ng1548. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Nogami S, Ohya Y, Kanno Y, Zhou Y, Akao T, Shimoi H. 2011. Ethanol fermentation driven by elevated expression of the G1 cyclin gene CLN3 in sake yeast. J Biosci Bioeng. 112(6):577–582. doi: 10.1016/j.jbiosc.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Wendt KS, Yoshida K, Itoh T, Bando M, Koch B, Schirghuber E, Tsutsumi S, Nagae G, Ishihara K, Mishiro T, et al. 2008. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature. 451(7180):796–801. doi: 10.1038/nature06634. [DOI] [PubMed] [Google Scholar]
- Whelan G, Kreidl E, Wutz G, Egner A, Peters J-M, Eichele G. 2012. Cohesin acetyltransferase Esco2 is a cell viability factor and is required for cohesion in pericentric heterochromatin: Esco2 acetylates PCH cohesin. EMBO J. 31(1):71–82. doi: 10.1038/emboj.2011.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BC, Garrett-Engele CM, Li Z, Williams EV, Rosenman ED, Goldberg ML. 2003. Two putative acetyltransferases, San and Deco, are required for establishing sister chromatid cohesion in Drosophila. Curr Biol. 13(23):2025–2036. doi: 10.1016/j.cub.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Wutz G, Ladurner R, St Hilaire BG, Stocsits RR, Nagasaka K, Pignard B, Sanborn A, Tang W, Várnai C, Ivanov MP, et al. 2020. ESCO1 and CTCF enable formation of long chromatin loops by protecting cohesinSTAG1 from WAPL. eLife. 9:e52091. doi: 10.7554/eLife.52091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz G, Várnai C, Nagasaka K, Cisneros DA, Stocsits RR, Tang W, Schoenfelder S, Jessberger G, Muhar M, Hossain MJ, et al. 2017. Topologically associating domains and chromatin loops depend on cohesin and are regulated by CTCF, WAPL, and PDS5 proteins. EMBO J. 36(24):3573–3599. doi: 10.15252/embj.201798004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang S, Koshland D. 2021. Cohesin architecture and clustering in vivo. eLife. 10:e62243. doi: 10.7554/eLife.62243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Jiang Y, Mao Q, Demeler B, Tao YJ, Pati D. 2013. Characterization of the interaction between the cohesin subunits Rad21 and SA1/2. PLoS One. 8(7):e69458. doi: 10.1371/journal.pone.0069458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Kuznetsov SG, Sharan SK, Li K, Rao PH, Pati D. 2008. A handcuff model for the cohesin complex. J Cell Biol. 183(6):1019–1031. doi: 10.1083/jcb.200801157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shi D, Li X, Ding L, Tang J, Liu C, Shirahige K, Cao Q, Lou H. 2017. Rtt101-Mms1-Mms22 coordinates replication-coupled sister chromatid cohesion and nucleosome assembly. EMBO Rep. 18(8):1294–1305. doi: 10.15252/embr.201643807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yeung CHL, Wu L, Yuen KWY. 2017. E3 ubiquitin ligase Bre1 couples sister chromatid cohesion establishment to DNA replication in Saccharomyces cerevisiae. eLife. 6:e28231. doi: 10.7554/eLife.28231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang Y, Ba Z, Kyritsis N, Casellas R, Alt FW. 2019. Fundamental roles of chromatin loop extrusion in antibody class switching. Nature. 575(7782):385–389. doi: 10.1038/s41586-019-1723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Shi B. 2022. New roles of DNA-binding and forkhead-associated domains of Fkh1 and Fkh2 in cellular functions. Cell Biochem Funct. 40(8):888–902. doi: 10.1002/cbf.3750. [DOI] [PubMed] [Google Scholar]
- Zuilkoski CM, Skibbens RV. 2020. PCNA antagonizes cohesin-dependent roles in genomic stability. PLoS One. 15(10):e0235103. doi: 10.1371/journal.pone.0235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables with the following exceptions. Supplementary Table 1 contains yeast strains and genotypes used in this study. Supplementary Table 2 contains DNA oligo sequences used in this study. Supplementary Figs. 1–4 contain supplemental figures and figure legends. Illumina sequencing data was deposited into the short read archive (SRA): BioSample SAMN42761258 within the BioProject PRJNA836598.
Supplemental material available at GENETICS online.