Abstract
Study Design.
A prospective single-arm clinical study.
Objective.
To explore the clinical utility of an intervertebral motion metric by determining the proportion of patients for whom it changed their surgical treatment plan from decompression only to decompression with fusion or vice versa.
Summary of Background Data.
Lumbar spinal stenosis from degenerative spondylolisthesis is commonly treated with decompression only or decompression with additional instrumented fusion. An objective diagnostic tool capable of establishing abnormal motion between lumbar vertebrae to guide decision-making between surgical procedures is needed. To this end, a metric based on the vertebral sagittal plane translation-per-degree-of-rotation calculated from flexion-extension radiographs was developed.
Materials and Methods.
First, spine surgeons documented their intended surgical plan. Subsequently, the participants’ flexion-extension radiographs were taken. From these, the translation-per-degree-of-rotation was calculated and reported as a sagittal plane shear index (SPSI). The SPSI metric of the spinal level intended to be treated was used to decide if the intended surgical plan needed to be changed or not.
Results.
SPSI was determined for 75 participants. Of these, 51 (68%) had an intended surgical plan of decompression only and 24 (32%) had decompression with fusion. In 63% of participants, the SPSI was in support of their intended surgical plan. For 29% of participants, the surgeon changed the surgical plan after the SPSI metric became available to them. A suggested change in the surgical plan was overruled by 8% of participants. The final surgical plan was decompression only for 59 (79%) participants and decompression with fusion for 16 (21%) participants.
Conclusion.
The 29% change in intended surgical plans suggested that SPSI was considered by spine surgeons as an adjunct metric in deciding whether to perform decompression only or to add instrumented fusion. This change exceeded the a priori defined 15% considered necessary to show the potential clinical utility of SPSI.
Key words: spinal stenosis, spinal fusion, orthopaedics, diagnostic imaging, decision support systems, clinical, neurosurgery, radiographic image interpretation, computer-assisted, surgical decompression, spondylolisthesis
Decompression alone or decompression with instrumented fusion are both common surgical procedures to treat patients with lumbar spinal stenosis (LSS) from degenerative spondylolisthesis. Analyses have shown that additional fusion in the management of LSS may be less cost-effective1 and yield no clinical improvements over decompression alone.2,3 However, research has also shown that fusion in addition to decompression may be of some benefit to a proportion of patients with LSS in terms of back pain or physical health-related quality of life.4,5 It is always challenging for surgeons to select which of the two procedures is best for the individual patient because, to date, there are no valid diagnostic tools and treatment algorithms that can reliably identify patients that will benefit from the addition of fusion.
Spinal instability is one of the signs most surgeons consider when deciding whether or not to combine decompression with fusion.6,7 Since a diagnostic test to objectively diagnose spinal instability is lacking,8–11 surgeons currently rely on a combination of clinical symptoms, tests, and subjective radiographic criteria to judge instability.12 There is some evidence that lateral flexion-extension radiographs can be a useful adjunct to neutral radiographs and magnetic resonance imaging,13 and that sagittal plane intervertebral translation between flexion and extension may be an objective metric for dynamic instability.14,15 Since the amount of translation can increase with the amount of intervertebral rotation,16–19 the magnitude of rotation must also be considered when interpreting translation. Determining the intervertebral translation-per-degree-of-rotation (TPDR)15 has previously been proposed as a potentially useful metric to quantify spinal instability. The TPDR is associated with the facet fluid sign14,15,20,21 which is currently considered one of the better indirect indicators of instability.22
Sagittal Plane Shear Index (SPSI; Medical Metrics Inc., Houston, TX) is a new and validated metric based on the TPDR to quantify abnormal spinal motion between two vertebrae. Mathematically, SPSI is the same metric as the previously developed Quantitative Stability Index.14 However, its name was changed because SPSI better indicates that shear motion in the sagittal plane is being measured, but also to avoid confusion about SPSI being an instability index (that increases with increasing instability) instead of a stability index, as the name Quantitative Stability Index was suggesting. SPSI is produced by a computer-assisted method measuring the ratio of TPDR between two lumbar vertebrae on lateral dynamic flexion-extension radiographs.23 SPSI is a ratio that is expressed as the number of SDs from the average TPDR that was found in people with radiographically normal spines.24 SPSI is intended to be used preoperatively to objectively assess the sagittal plane TPDR of the stenotic level. A SPSI >2 means that the degree of TPDR is more than two SDs (i.e. outside of the 95% CI) from the average asymptomatic person’s TPDR.14,24 Although the SPSI metric has not yet been used in in vivo studies, its use may have clinical value as an objective diagnostic indicator for the presence of spinal instability. As such, and as an adjunct to their existing diagnostic strategies, it may assist spine surgeons in deciding whether to perform decompression only or with instrumented fusion in patients with LSS from degenerative spondylolisthesis.
As a first step in exploring the clinical utility25 of the SPSI metric in surgical decision-making, we established the SPSI metric in a target group of patients and explored whether their SPSI results would be considered by spine surgeons26 as an adjunct to their existing diagnostic strategies. In particular, we explored whether the patients’ SPSI value of the level intended to treat would change the surgical treatment plan that was recorded by the surgeons before the SPSI metric was available to them. This outcome was deemed critical for the design and reporting of future research,27 because, for SPSI to have a future chance of being adopted into routine clinical practice, it would first have to show its clinical utility.
MATERIALS AND METHODS
Design and Participants
This was a prospective, single-arm clinical study. Patients from four tertiary nonacademic hospitals in the Netherlands were recruited by employing a nonprobability purposive sampling method. Only patients referred to the spine surgeons involved in the study were checked against the eligibility criteria and approached for participation. Patients were eligible for participation if they had (i) symptoms consistent with single-level LSS, (ii) central and/or foraminal stenosis between two adjacent vertebrae confirmed by magnetic resonance imaging, (iii) grades 1 (10%–25%) or 2 (26%–50%) anterior or retrospondylolisthesis according to the Meyerding classification,28 (iv) no history of prior lumbar spinal surgery and (v) were suitable for posterior lumbar interbody fusion surgery using posterior pedicle screws, rods, and/or a spinal cage in case instrumented fusion was considered. Patients were excluded if they had (i) stenosis at the level of a transitional vertebra or severe stenosis that required a wide decompression that was judged to destabilize the spine, (ii) lateral spondylolisthesis, (iii) scoliosis involving a lumbar curve >10°, (iv) an American Society of Anesthesiologists class IV or higher disease, or (v) were pregnant. Written informed consent was obtained from all participants.
Sample Size
The sample size calculation was based on the assumption that, for the SPSI metric to be accepted as clinically useful and as a threshold for deciding whether to proceed to a large clinical trial after this study, at least 15% of treatment plans would need to be changed by the inclusion of SPSI metrics. With alpha set at 5% and beta at 90%, a simple test of proportion indicated that a sample size of 59 participants would be required to determine if this change was at least 25%, under the assumption that 10% (n=6) of treatment plans would change anyway due to variability in how surgeons establish treatment plans. It was also estimated that in a maximum of about 25% (n=15) of the cases, the SPSI metric would not be followed. Another 20% drop-out was expected due to participants having insufficient (<5°) intervertebral rotation.29 This ≥5° threshold is required since, without sufficient intervertebral motion, it is not possible to determine whether the intervertebral motion restraints are competent or whether abnormal motion can occur during flexion to extension. Motion of <5° is considered to be within the neutral zone of intervertebral motion,30–32 where the spine is not sufficiently stressed to allow for detection of incompetent intervertebral motion restraints. To offset all of these possible reasons for participant drop-out we aimed to recruit a minimum of 100 participants.
Study Procedures
A detailed description of the study procedures, including two representative examples describing how the SPSI metric was utilized during this study and two stabilized flexion-extension radiographs, can be found in Supplemental File 1, http://links.lww.com/BRS/C388 and Supplemental Digital Content 1, http://links.lww.com/BRS/C387, respectively.
Data Collection and Analysis
Data collection was managed by an independent Clinical Research Organization (Avania, https://www.avaniaclinical.com). Data was analyzed using IBM SPSS Statistics for Windows (Version 25.0; IBM Corp., Armonk, NY). Participant characteristics were summarized descriptively. The proportion of changed treatment plans was calculated using frequencies (percentages).
RESULTS
Participant Flow and Characteristics
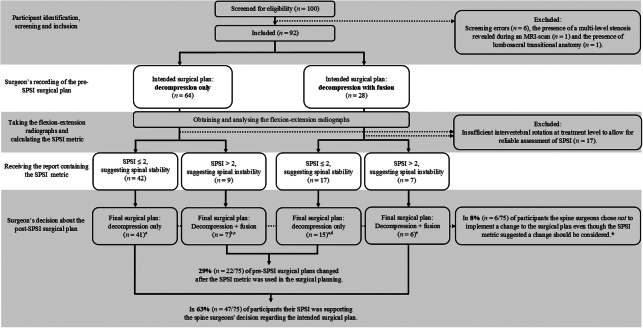
Between March 2019 and November 2022, a total of 100 patients were identified and screened for study eligibility. Eight participants had to be excluded shortly after initial inclusion, resulting in 92 participants included in the study. The flow of the participants and their SPSI metric and surgical planning results are shown in Figure 1.
Figure 1.
The flow of the participants and their Sagittal Plane Shear Index (SPSI) metric and surgical planning results. * These cases explain why the sample sizes in the “Final surgical plan” boxes decreased. The spine surgeons’ reasons reported for not implementing a change to the surgical plan in cases where the SPSI metric suggested a change should be considered were: a the decision to perform decompression with fusion despite a SPSI of exactly 2 which was suggesting spinal stability (n=2); b concerns about the invasiveness of fusion surgery in a participant at risk of complications (n=1); c doubts about the presence of instability based on the radiographic images despite a SPSI of 4.1 (n=1); d the decision to perform a decompression with fusion at two levels (n=1); and e the refusal of a participant to undergo fusion surgery (n=1). MRI indicates magnetic resonance imaging.
Another 17 participants had to be excluded shortly after having documented their intended (pre-SPSI) surgical plans and having analyzed their flexion-extension radiographs because they had <5° of rotation at the level intended to treat. The characteristics of the 75 participants, for whom all the required data was available to answer the main research question, are presented in Table 1.
TABLE 1.
Baseline Characteristics of the 75 Analyzable Participants
| Characteristic | Value |
|---|---|
| Age, yr | 66.7 (9.3) |
| Sex, n (%) | |
| Male | 28 (37) |
| Female | 47 (63) |
| BMI, kg/m2 | 28.2 (4.8) |
| Lumbar spinal level treated, n (%) | |
| L3-L4 | 12 (16) |
| L4-L5 | 61 (81) |
| L5-S1 | 2 (3) |
| Leg pain intensity NRS score* | 6.6 (2) |
| Use of pain medication, yes, n (%) | 51 (68) |
| ODI score† | 38.5 (16) |
| Centers, participants, n (%) | |
| Amsterdam | 20 (26.7) |
| Arnhem | 48 (64) |
| Den Haag | 5 (6.7) |
| Zwolle | 2 (2.7) |
Values are reported as mean (SD) unless stated otherwise.
Scores range from 0 to 10 where a higher score indicates a higher leg pain intensity experienced in the past week.
Original scores range from 0 to 50, and these are subsequently recalculated into a percentage by using the equation [total ODI score /(5×number of completed items)]/100, where a higher percentage indicates more disability related to low back pain.
BMI indicates body mass index; NRS, numeric rating scale; ODI, Oswestry disability index.
Outcomes
For 51 (68%) of the analyzable 75 participants, the eight senior spine surgeons involved in the study documented an intended (pre-SPSI) surgical plan of decompression only. In 24 (32%) participants, the intended surgical plan was decompression with fusion.
In 63% (n=47) of all participants, the resulting SPSI was in support of the surgeons’ decision regarding their intended (pre-SPSI) surgical plan. Twenty-nine percent (n=22) of pre-SPSI surgical plans changed after the SPSI metric was used in the surgical planning. In 8% (n=6) of all participants, the surgeons chose not to implement a change to the surgical plan, even though this was supported by the SPSI metric. Reasons reported for not changing it (all explained in the legend of Fig. 1) included doubts about how to interpret SPSI values at the cutoff value for spinal (in)stability. Including the data of the six participants for whom the surgeons chose not to implement a change to the surgical plan, where the SPSI metric suggested a change should be considered, for 79% (n=59) of participants, the final surgical plan was decompression only, and for 21% (n=16), it was decompression with fusion. There were no changes in any of the participants’ surgical plans because of intraoperative findings.
DISCUSSION
SPSI is a new and validated metric based on the TPDR to quantify abnormal spinal motion between two vertebrae. It was developed as an objective diagnostic indicator for the presence of spinal instability, and it may offer spine surgeons a valuable adjunct metric in deciding whether to perform decompression only or to add instrumented fusion in patients with LSS from degenerative spondylolisthesis. The results of this clinical study showed that the use of SPSI by spine surgeons led to a change of 29% in their originally intended (pre-SPSI) surgical plans. This change was almost twice the a priori defined 15% considered acceptable for the SPSI metric to have potential clinical utility and considered a threshold for proceeding to a future large clinical trial. Knowledge about the SPSI metric led spine surgeons to perform fewer instrumented fusions than they had originally intended because SPSI suggested spinal stability in a proportion of the participants judged by the spine surgeons to have spinal instability. Overall, the results of this study suggest that SPSI has potential clinical utility in surgical decision-making for patients with LSS. However, before the use of SPSI in daily clinical practice and surgical decision-making is considered, some important issues, gaps in knowledge, and study limitations need to be discussed.
The Potential Clinical Utility of SPSI in Surgical Decision-making
The results of this study show that the SPSI metric was considered in the clinical decision-making process for the study participants and that spine surgeons were willing to change their surgical treatment plan from decompression only to decompression with fusion or vice versa if this was supported by SPSI. Concerns about the invasiveness of additional fusion surgery for a patient or doubts about the presence of (in)stability based on regular radiology led surgeons to not implement a change to the surgical plan in 8% (n=6) of the participants, even when a change was supported by the SPSI metric. For example, SPSI values around the cutoff value 2 of two participants were not just used as a dichotomy between stability or instability, but interpreted in the context of other relevant clinical factors and as an adjunct to the surgeons’ existing diagnostic strategies. These six cases arguably exemplify the surgeons’ learning process in using SPSI.
During this study, 68% of the analyzable 75 participants were initially planned for decompression only and 32% for decompression with fusion. The latter rate is clearly lower than the 82% to 97% fusion rates sometimes reported in the literature,33,34 and it also contrasts with the results of a recent international survey that showed that surgeons utilized fusion for the treatment of degenerative spondylolisthesis more commonly than performing primary decompression.7 However, our 32% fusion rate does lie within the 22% to 44% range reported in more recently published studies.1,35,36 These latter lower rates may reflect a decrease in the utilization of fusion surgery ever since evidence has accumulated that decompression with fusion is not superior to conventional decompression alone,2,3,37,38 that it remains unclear as to which patients may still benefit from fusion surgery39 and that fusion procedures are associated with increased costs but not clinical benefits.40,41 As long as there is no robust evidence that adding fusion to decompression is beneficial for patients, it has been suggested that it should be restricted to patients with radiographically proven mechanical instability.42 With this study, we explored the clinical utility of a diagnostic indicator for the presence of such mechanical instability. By using the SPSI metric for surgical decision-making, the number of participants who ultimately underwent decompression with fusion surgery was 11% lower than what the surgeons had intended before SPSI was known to them.
Strengths, Limitations, and Future Research
By conducting this study, we have taken some of the recommended25,26 first steps to explore the clinical utility of a new intervertebral motion metric. The participants included in this study resembled those planned for decompression only or with fusion used in previous studies in terms of leg pain intensity and disability related to low back pain. For example, for both of these patient groups, the baseline mean leg pain numeric rating scale scores typically range between 6 and 8.43–45 Our participants had numeric rating scale scores of around 6.6, which was within the exact same range. Similarly, Oswestry Disability Index scores typically ranged between 36 and 484,41,43,44,46 while our participants’ Oswestry Disability Index scores of around 38 also fell within this range.
The most important limitation of this study was that it was neither designed nor powered to test the hypothesis that SPSI can help to improve clinical outcomes. Data regarding the clinical outcomes of this study’s participants will be presented separately after the two-year follow-up has been completed (results expected in 2025). Those results will arguably assist in gaining a better understanding of the likely role of SPSI in terms of a replacement or add-on diagnostic27 and in the design of further research into the accuracy of SPSI. In order to reliably calculate the SPSI metric, patients need to have ≥5° of lumbar rotation. This requires that a patient flexes and extends the spine with maximal effort while the flexion-extension radiographs are taken. During this study, the participants received their instructions on how to achieve this through a short video. Although these instructions were standardized and staff were also trained on this protocol, it was not possible to physically check whether each participant sufficiently stressed their spine during radiography. Some of the 17 excluded participants may actually have had <5° of lumbar rotation,29 but some may also not have exerted maximal effort, for example, due to back pain. The exclusion of these participants negatively impacted the sample size of this study. Also, other factors can influence a patient’s SPSI values, such as minor (<10°) rotational deformities. Even though SPSI is tolerant of a fair amount of malalignment of the central X-ray beam with respect to vertebral bodies,47 future research into this and potential other confounding factors is warranted. The presence of a certain degree of measurement error around TPDR values could thus not be ruled out. Especially around the SPSI cutoff value of 2, such errors could result in the misclassification of spinal (in)stability.
We recommend conducting a study where SPSI is used during the clinical decision-making process in symptomatic patients with LSS and to investigate its effect on clinical outcomes such as pain, disability, reoperation, and revision rates, postoperative complications, and their associated health care costs. We further recommend establishing TPDR values in a large sample of patients with symptomatic LSS and conducting research into the methodological qualities (i.e. psychometric properties) of SPSI.
CONCLUSION
The 29% change in intended surgical plans suggested that SPSI, a metric based on the TPDR to quantify abnormal spinal motion between two vertebrae, was considered by spine surgeons as an adjunct metric in deciding whether to perform decompression only or with instrumented fusion for patients with LSS from degenerative spondylolisthesis. This change exceeded the a priori defined 15% considered necessary to show the potential clinical utility of SPSI and to proceed with further research. By using the SPSI metric for surgical decision-making, the number of participants who ultimately underwent decompression with fusion surgery was 11% lower than what the surgeons had intended before SPSI was known to them. Future research should focus on establishing the methodological qualities of SPSI and whether utilizing it during clinical decision-making will yield better patient outcomes.
Key Points
Sagittal Plane Shear Index (SPSI) is a new and validated metric based on the translation-per-degree-of-rotation calculated from flexion-extension radiographs that aims to quantify abnormal spinal motion between two vertebrae and to objectively diagnose spinal instability.
This study explored whether the patients’ SPSI value of the level intended to treat would change the surgical treatment plan that was recorded by the surgeons before the SPSI metric was available to them.
A 29% change in intended surgical plans suggests that SPSI was considered by spine surgeons as an adjunct metric in deciding whether to perform decompression only or with instrumented fusion. This change exceeded the a priori defined 15% considered necessary to show the potential clinical utility of SPSI.
Supplementary Material
Footnotes
J.F.H.R. and L.D.d.J. contributed equally to this work.
Ethics approval was granted by METC Oost-Nederland (NL67222.091.18) and all the participating hospitals’ Institutional Research Boards. The study was registered on November 14, 2018 (NCT03754972; https://clinicaltrials.gov/ct2/show/NCT03754972).
This study was designed and sponsored by Medical Metrics Inc. (Houston, Texas). The sponsor analyzed the flexion-extension images to produce the SPSI metric. The remaining authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.spinejournal.com.
Contributor Information
Joey F.H. Reijmer, Email: JReijmer@rijnstate.nl.
Lex D. de Jong, Email: lexddejong@gmail.com.
Diederik H.R. Kempen, Email: D.H.R.Kempen@olvg.nl.
Mark P. Arts, Email: m.arts@haaglandenmc.nl.
Job L.C. van Susante, Email: JvanSusante@Rijnstate.nl.
References
- 1. Won YI, Kim CH, Park HP, et al. A cost-utility analysis between decompression only and fusion surgery for elderly patients with lumbar spinal stenosis and sagittal imbalance. Sci Rep. 2022;12:20408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang W, Yuwen P, Zhu Y, et al. Effectiveness of decompression alone versus decompression plus fusion for lumbar spinal stenosis: a systematic review and meta-analysis. Arch Orthop Trauma Surg. 2017;137:637–650. [DOI] [PubMed] [Google Scholar]
- 3. Kaiser R, Kantorova L, Langaufova A, et al. Decompression alone versus decompression with instrumented fusion in the treatment of lumbar degenerative spondylolisthesis: a systematic review and meta-analysis of randomised trials. J Neurol Neurosurg Psychiatry. 2023;94:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ghogawala Z, Dziura J, Butler WE, et al. Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med. 2016;374:1424–1434. [DOI] [PubMed] [Google Scholar]
- 5. Dijkerman ML, Overdevest GM, Moojen WA, et al. Decompression with or without concomitant fusion in lumbar stenosis due to degenerative spondylolisthesis: a systematic review. Eur Spine J. 2018;27:1629–1643. [DOI] [PubMed] [Google Scholar]
- 6. Strube P, Putzier M, Siewe J, et al. To fuse or not to fuse: a survey among members of the German Spine Society (DWG) regarding lumbar degenerative spondylolisthesis and spinal stenosis. Arch Orthop Trauma Surg. 2019;139:613–621. [DOI] [PubMed] [Google Scholar]
- 7. Morse KW, Steinhaus M, Bovonratwet P, et al. Current treatment and decision-making factors leading to fusion vs decompression for one-level degenerative spondylolisthesis: survey results from members of the Lumbar Spine Research Society and Society of Minimally Invasive Spine Surgery. Spine J. 2022;22:1778–1787. [DOI] [PubMed] [Google Scholar]
- 8. Leone A, Cassar-Pullicino VN, Guglielmi G, et al. Degenerative lumbar intervertebral instability: what is it and how does imaging contribute? Skeletal Radiol. 2009;38:529–533. [DOI] [PubMed] [Google Scholar]
- 9. Simmonds AM, Rampersaud YR, Dvorak MF, et al. Defining the inherent stability of degenerative spondylolisthesis: a systematic review. J Neurosurg Spine. 2015;23:178–189. [DOI] [PubMed] [Google Scholar]
- 10. Siebert E, Pruss H, Klingebiel R, et al. Lumbar spinal stenosis: syndrome, diagnostics and treatment. Nat Rev Neurol. 2009;5:392–403. [DOI] [PubMed] [Google Scholar]
- 11. Cabraja M, Mohamed E, Koeppen D, et al. The analysis of segmental mobility with different lumbar radiographs in symptomatic patients with a spondylolisthesis. Eur Spine J. 2012;21:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alqarni AM, Schneiders AG, Hendrick PA. Clinical tests to diagnose lumbar segmental instability: a systematic review. J Orthop Sports Phys Ther. 2011;41:130–140. [DOI] [PubMed] [Google Scholar]
- 13. Alvarez AP, Anderson A, Farhan SD, et al. The utility of flexion-extension radiographs in degenerative cervical spondylolisthesis. Clin Spine Surg. 2022;35:319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hipp JA, Guyer RD, Zigler JE, et al. Development of a novel radiographic measure of lumbar instability and validation using the facet fluid sign. Int J Spine Surg. 2015;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weiler PJ, King GJ, Gertzbein SD. Analysis of sagittal plane instability of the lumbar spine in vivo. Spine (Phila Pa 1976). 1990;15:1300–1306. [DOI] [PubMed] [Google Scholar]
- 16. Byrne RM, Aiyangar AK, Zhang X. A dynamic radiographic imaging study of lumbar intervertebral disc morphometry and deformation in vivo. Sci Rep. 2019;9:15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dombrowski ME, Rynearson B, LeVasseur C, et al. ISSLS PRIZE IN BIOENGINEERING SCIENCE 2018: dynamic imaging of degenerative spondylolisthesis reveals mid-range dynamic lumbar instability not evident on static clinical radiographs. Eur Spine J. 2018;27:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Byrne RM, Zhou Y, Zheng L, et al. Segmental variations in facet joint translations during in vivo lumbar extension. J Biomech. 2018;70:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahmadi A, Maroufi N, Behtash H, et al. Kinematic analysis of dynamic lumbar motion in patients with lumbar segmental instability using digital videofluoroscopy. Eur Spine J. 2009;18:1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aggarwal A, Garg K. Lumbar facet fluid-does it correlate with dynamic instability in degenerative spondylolisthesis? A systematic review and meta-analysis. World Neurosurg. 2021;149:53–63. [DOI] [PubMed] [Google Scholar]
- 21. Chaput C, Padon D, Rush J, et al. The significance of increased fluid signal on magnetic resonance imaging in lumbar facets in relationship to degenerative spondylolisthesis. Spine (Phila Pa 1976). 2007;32:1883–1887. [DOI] [PubMed] [Google Scholar]
- 22. Hasegawa K, Shimoda H, Kitahara K, et al. What are the reliable radiological indicators of lumbar segmental instability? J Bone Joint Surg Br. 2011;93:650–657. [DOI] [PubMed] [Google Scholar]
- 23. Zhao K, Yang C, Zhao C, et al. Assessment of non-invasive intervertebral motion measurements in the lumbar spine. J Biomech. 2005;38:1943–1946. [DOI] [PubMed] [Google Scholar]
- 24. Staub BN, Holman PJ, Reitman CA, et al. Sagittal plane lumbar intervertebral motion during seated flexion-extension radiographs of 658 asymptomatic nondegenerated levels. J Neurosurg Spine. 2015;23:731–738. [DOI] [PubMed] [Google Scholar]
- 25. Smart A. A multi-dimensional model of clinical utility. Int J Qual Health Care. 2006;18:377–382. [DOI] [PubMed] [Google Scholar]
- 26. Ferrante di Ruffano L, Hyde CJ, McCaffery KJ, et al. Assessing the value of diagnostic tests: a framework for designing and evaluating trials. BMJ. 2012;344:e686. [DOI] [PubMed] [Google Scholar]
- 27. Bossuyt PM, Irwig L, Craig J, et al. Comparative accuracy: assessing new tests against existing diagnostic pathways. BMJ. 2006;332:1089–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meyerding HW. Spondylolisthesis as an Etiologic Factor in Backache. J Am Med Assoc. 1938;111:1971–1976. [Google Scholar]
- 29. Sawa AGU, Lehrman JN, Crawford NR, et al. Variations among human lumbar spine segments and their relationships to in vitro biomechanics: a retrospective analysis of 281 motion segments from 85 cadaveric spines. Int J Spine Surg. 2020;14:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crawford NR, Peles JD, Dickman CA. The spinal lax zone and neutral zone: measurement techniques and parameter comparisons. J Spinal Disord. 1998;11:416–429. [PubMed] [Google Scholar]
- 31. Sengupta DK, Fan H. The basis of mechanical instability in degenerative disc disease: a cadaveric study of abnormal motion versus load distribution. Spine (Phila Pa 1976). 2014;39:1032–1043. [DOI] [PubMed] [Google Scholar]
- 32. Panjabi MM. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5:390–396. [DOI] [PubMed] [Google Scholar]
- 33. Bae HW, Rajaee SS, Kanim LE. Nationwide trends in the surgical management of lumbar spinal stenosis. Spine (Phila Pa 1976). 2013;38:916–926. [DOI] [PubMed] [Google Scholar]
- 34. Kepler CK, Vaccaro AR, Hilibrand AS, et al. National trends in the use of fusion techniques to treat degenerative spondylolisthesis. Spine (Phila Pa 1976). 2014;39:1584–1589. [DOI] [PubMed] [Google Scholar]
- 35. Ulrich NH, Burgstaller JM, Valeri F, et al. Incidence of revision surgery after decompression with vs without fusion among patients with degenerative lumbar spinal stenosis. JAMA Netw Open. 2022;5:e2223803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Melcher C, Paulus AC, Rossbach BP, et al. Lumbar spinal stenosis—surgical outcome and the odds of revision-surgery: is it all due to the surgeon? Technol Health Care. 2022;30:1423–1434. [DOI] [PubMed] [Google Scholar]
- 37. Machado GC, Ferreira PH, Yoo RI, et al. Surgical options for lumbar spinal stenosis. Cochrane Database Syst Rev. 2016;11:CD012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharif S, Shaikh Y, Bajamal AH, et al. Fusion surgery for lumbar spinal stenosis: WFNS Spine Committee Recommendations. World Neurosurg X. 2020;7:100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Austevoll IM, Ebbs E. Fusion is not a safeguard to prevent revision surgery in lumbar spinal stenosis. JAMA Netw Open. 2022;5:e2223812. [DOI] [PubMed] [Google Scholar]
- 40. Austevoll IM, Hermansen E, Fagerland MW, et al. Decompression with or without fusion in degenerative lumbar spondylolisthesis. N Engl J Med. 2021;385:526–538. [DOI] [PubMed] [Google Scholar]
- 41. Forsth P, Olafsson G, Carlsson T, et al. A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med. 2016;374:1413–1423. [DOI] [PubMed] [Google Scholar]
- 42. Pisano AJ, Butler JS, Sebastian A, et al. Does surgically managed grade i degenerative lumbar spondylolisthesis require fusion? Clin Spine Surg. 2019;32:133–136. [DOI] [PubMed] [Google Scholar]
- 43. Thomas K, Faris P, McIntosh G, et al. Decompression alone vs. decompression plus fusion for claudication secondary to lumbar spinal stenosis. Spine J. 2019;19:1633–1639. [DOI] [PubMed] [Google Scholar]
- 44. Austevoll IM, Gjestad R, Brox JI, et al. The effectiveness of decompression alone compared with additional fusion for lumbar spinal stenosis with degenerative spondylolisthesis: a pragmatic comparative non-inferiority observational study from the Norwegian Registry for Spine Surgery. Eur Spine J. 2017;26:404–413. [DOI] [PubMed] [Google Scholar]
- 45. Ulrich NH, Burgstaller JM, Pichierri G, et al. Decompression surgery alone versus decompression plus fusion in symptomatic lumbar spinal stenosis: a Swiss prospective multicenter cohort study with 3 years of follow-up. Spine (Phila Pa 1976). 2017;42:E1077–E1086. [DOI] [PubMed] [Google Scholar]
- 46. Sigmundsson FG, Jonsson B, Stromqvist B. Outcome of decompression with and without fusion in spinal stenosis with degenerative spondylolisthesis in relation to preoperative pain pattern: a register study of 1,624 patients. Spine J. 2015;15:638–646. [DOI] [PubMed] [Google Scholar]
- 47. Zhao KD, Ben-Abraham EI, Magnuson DJ, et al. Effect of off-axis fluoroscopy imaging on two-dimensional kinematics in the lumbar spine: a dynamic in vitro validation study. J Biomech Eng. 2016;138:054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.