Abstract
Intestinal mucosa is a portal for many infectious pathogens. Systemic immunization, in general, does not induce a cytotoxic T-lymphocyte (CTL) response at the mucosal surface. Because papillomavirus (PV) naturally infects mucosa and skin, we determined whether PV pseudovirus, i.e., PV-like particles in which unrelated DNA plasmids are packaged, could generate specific mucosal immunity. We found that the pseudovirus that encoded the lymphocytic choriomeningitis virus gp33 epitope induced a stronger CTL response than a DNA vaccine (plasmid) encoding the same epitope given systemically. The virus-like particles that were used to make the pseudoviruses provided an adjuvant effect for induction of CTLs by the DNA vaccine. The PV pseudovirus pseudoinfected mucosal and systemic lymphoid tissues when administered orally. Oral immunization with the pseudovirus encoding human PV type 16 mutant E7 induced mucosal and systemic CTL responses. In comparison, a DNA vaccine encoding E7, when given orally, did not induce a CTL response in intestinal mucosal lymphoid tissue. Further, oral immunization with the human PV pseudovirus encoding E7 protected mice against mucosal challenge with an E7-expressing bovine PV pseudovirus. Thus, PV pseudovirus can be used as a novel vaccine to induce mucosal and systemic CTL responses.
The mucosal surfaces of the body are readily infected with many pathogenic viruses and bacteria. In particular, the intestinal mucosa is an important portal for infectious agents. Most pathogens initiate their infectious processes by interaction with epithelial cells at mucosal surfaces and then spread systemically. To prevent initial infections by those pathogens, antibodies and cytotoxic T lymphocytes (CTLs) specific for the pathogens induced at the mucosal surface are of great importance. Because some pathogens continue to replicate in the mucosa, it is advantageous to induce mucosa-specific CTLs to clear the pathogens at initial infection and during the early stage of disease.
Intestinal mucosal lymphoid cells are located in organized lymphoid tissue, such as Peyer's patches, or in diffuse lymphoid tissue, such as lamina propria. Peyer's patches are considered the site where a mucosal immune response is induced after a pathogen invades the mucosa (24). In general, systemic immunization, such as subcutaneous vaccination, does not effectively induce mucosal immune responses; instead, mucosal immunization is required to generate an intestinal mucosal immune response.
DNA (plasmid)-based immunization induces host humoral and cellular immune responses (1, 3, 5, 12, 13, 31, 39, 43). Because antigens encoded by plasmid DNA vaccines are produced in the host, the antigens retain their natural form, unlike those of attenuated whole-organism vaccines, which are denatured and modified. Because the antigens are expressed in the immunized host, there is prolonged exposure to the host immune system and sustained immune responses. However, DNA vaccines do not reach gut-associated lymphoid tissues via oral immunization because they do not survive degradation in the gastric and intestinal environment. Furthermore, DNA vaccines induced relatively low amounts of CTLs and generated CTLs in some but not all immunized individuals when given intramuscularly to mice and humans (4, 29, 34, 38, 51).
Papillomaviruses (PVs) are a group of small DNA viruses that naturally infect skin and mucosal surfaces (52). More than 95 types have been characterized so far (45). PV major protein L1 can be assembled spontaneously into virus-like particles (VLPs) when expressed in insect cells, yeasts, and even bacteria (10, 15, 27, 35, 37, 46). It has been shown that PV VLPs can induce strong humoral and cellular immune responses when used for systemic immunization (6, 10, 11, 18, 20, 33, 37, 40, 44, 49, 50). Further, VLPs can be used to package unrelated plasmids to form PV pseudoviruses (14, 42). Because many PVs are mucosatropic and can induce cellular immune responses, we hypothesized that PV pseudoviruses would reach the mucosal immune system and induce mucosal immune responses. Because PV VLPs were shown to induce strong T-helper responses, we hypothesized that the concurrent T-helper responses to the VLPs might enhance the CTL response against the antigen encoded by the plasmid in the pseudoviruses. In this study, we found that when administered orally, PV pseudoviruses reached Peyer's patches, lamina propria, and spleen. By systemic immunization, PV pseudoviruses induced a stronger CTL response than plasmid DNA vaccines alone, and by oral immunization, they generated specific mucosal and systemic CTL responses and protected mice against mucosal challenge.
MATERIALS AND METHODS
Cells.
RMA, RMA-neo, and RMA-E7 cells were maintained in RPMI 1640 medium (GIBCO-BRL, Gaithersburg, Md.) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml.
Plasmids.
Plasmid pCI-neo was purchased from Promega (Madison, Wis.). The expression cassette for the green lantern protein (GLP) was constructed by inserting the full-length GLP cDNA into the NotI site of plasmid pCI-neo. The expression cassette for a fusion protein, GLP fused with lymphocytic choriomeningitis virus (LCMV) gp33 major histocompatibility complex (MHC) class I H-2Db-restricted epitope (amino acids [aa] 33 to 41; KAVYNFATC), was constructed by using PCR with pCI-GLP as the template, oligonucleotide 5′ primer GCCACCATGAGCAAGGGCGAGGAACTGT, and 3′ primer TCAACAGGTGGCAAAAT TG TAGACAGCC T TAGATCCGCCGCCACCGCCACCCT TGTACAGCTCGTCCAT, containing the linker sequences (Gly6Ser1; underlined) between GLP and LCMV gp33 epitope. The amplification mixtures (50 μl) contained dGTP, dATP, dTTP, dCTP (200 μM each), oligonucleotide primers (1 μM), template DNA (25 ng), and Taq DNA polymerase (Promega) (5 μM). The reaction mixture was subjected to 30 cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min and a final 10 min at 72°C. The amplified DNAs were gel purified and ligated into T-easy vector (Promega). Then the DNAs were digested with EcoRI and ligated into pCI-neo, which had been digested with the corresponding enzyme. The human PV type 16 (HPV-16) E7 open reading frame was fused to the GLP sequence by PCR using the same linker (Gly6Ser1). The fragment was then inserted into pCI-neo to form pCI-GLP-E7. The E7 open reading frame was inserted into pCMV.
Generation of recombinant baculoviruses.
Briefly, Spodoptera frugiperda (Sf9) cells were grown in monolayer cultures at 27°C in TNMFH medium (Sigma, St. Louis, Mo.) supplemented with 10% FCS and 2 mM glutamine. Ten micrograms of transfer plasmid (pVL1933 BPV-1 L1Δ or pVL1932 HPV-16 L1Δ) was used to transfect Sf9 cells together with 0.2 μg of linearized Baculo-Gold DNA (Pharmingen, San Diego, Calif.). Recombinant viruses were purified using methods modified as described previously (26, 28).
Purification of PV VLPs.
Sf9 cells were grown to a density of 1 × 106 to 2 × 106 cells/ml in TNMFH medium supplemented with 10% FCS and 2 mM glutamine in a spinner flask. Approximately 2 × 108 cells were pelleted at 1,500 × g for 5 min, resuspended in 10 ml of medium, and then added to 10 ml of recombinant baculoviruses at a multiplicity of infection of 2 to 5 for 1 h at room temperature. After addition of 125 ml of medium, the cells were plated on five round dishes (150 mm in diameter) and incubated for 3 to 4 days at 27°C. Cells were harvested, pelleted, and suspended in 10 ml of extraction buffer (5 mM MgCl2, 5 mM CaCl2, 150 mM NaCl, 20 mM HEPES, 0.01% Triton X-100). The cells were sonicated for 1 min at speed 3; then the extract was pelleted at 10,000 rpm in a Sorvall RC5B centrifuge at 4°C for 30 min. The pellet was suspended in 8 ml of extraction buffer, sonicated again for 30 s at speed 4.5, and centrifuged again. Combined supernatants were layered on a two-step gradient with 14 ml of 40% sucrose on top of 8 ml of CsCl solution (4.6 g of CsCl per 8 ml of extraction buffer) and centrifuged in a Sorvall AH629 swinging-bucket rotor for 2 h at 27,000 rpm at 10°C. The interphase between CsCl and sucrose and the complete layer of CsCl were collected and placed in 13.4-ml Quickseal tubes filled with extraction buffer. Samples were centrifuged overnight at 50,000 rpm at 20°C. Gradients were fractionated by puncturing tubes on top and bottom with a 21-gauge needle, and 5 μl of each fraction was analyzed by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.
Western blot analysis.
The extracts from infected insert cells were separated by SDS–10% PAGE and transferred to nitrocellulose by using a semidry blotting system (Semi Dry blotting unit; Fisher Biotech, Hanover Park, Ill.). The membranes were blocked overnight with 5% nonfat dry milk and incubated with mouse anti-HPV-16 L1 monoclonal antibody (Pharmingen) or rabbit anti–bovine PV type 1 (BPV-1) L1 antibody. Then the membranes were incubated with horseradish peroxidase-conjugated anti-mouse immunoglobulin G (IgG) or anti-rabbit IgG. Finally, the membranes were processed with the ECL system (Amersham, Arlington Heights, Ill.). Positive fractions were tested for the presence of VLPs by electron microscopy.
Production of PV pseudoviruses.
Disassembly and reassembly of the recombinant HPV-16 VLPs and BPV-1 VLPs were done according to a modification of the procedure of Touze and Coursaget (42). Briefly, 5 μg of purified HPV-16 VLPs or BPV-1 VLPs (theoretically 1.5 × 1011 particles) was incubated in 50 mM Tris-HCl buffer (pH 7.5) containing 150 mM NaCl, 10 mM EGTA, and 20 mM dithiothreitol (DTT) in a final volume of 100 μl at room temperature for 30 min. At this step, 1 μg of expression plasmid in 50 mM Tris-HCl buffer and 150 mM NaCl were added to the disrupted VLPs. The preparation was then diluted with CaCl2 (25 mM) and 20% dimethyl sulfoxide in equal volume at room temperature for 1 h. The preparations were treated with 10 U of Benzonase (Bz) with or without proteinase K (pK; 1 mg/ml) for 1 h at room temperature, and the presence of plasmid DNA was determined by agarose gel electrophoresis to verify whether the DNA plasmid was packaged into the VLPs. Additionally, 0.5 μg of plasmid DNA (about 7 × 1010 copies of plasmid) was incorporated into 200 μl of pseudoviruses.
Electron microscopy.
Twenty microliters of each fraction from CsCl gradients was dialyzed against 10 mM HEPES for 45 min on floating filter pads (0.02-μm pore size; Millipore, Bedford, Mass.). Carbon-coated copper grids (200 mesh size; EM Sciences, Gibbstown, N.J.) were treated with 20 μl of poly-l-lysine (1 mg/ml; Sigma) for 2 min. The sample was placed onto the grid for 2 min. Spotted grids were then stained with 30 μl of uranyl acetate solution for 2 min. Excess stain was removed, and grids were air dried. Specimens were examined with a Zeiss EM 900 electron microscope.
Mice.
Six- to eight-week-old female C57BL/6 mice (purchased from the Jackson Laboratory, Bar Harbor, Maine, or Harlan, Indianapolis, Ind.) were used. All mice were kept under pathogen-free conditions. The protocol was approved by the Institutional Animal Care and Use Committees.
Immunization.
For systemic immunization, mice were immunized subcutaneously with 100 μl of HPV pseudoviruses (about 3.5 × 1010 pseudoviruses or 0.25 μg of plasmid), 100 μl of HPV VLPs, 20 μg of plasmid in 100 μl of phosphate-buffered saline (PBS), or 100 μg of peptide (LCMV glycoprotein [gp] aa 33 to 41) in 100 μl of incomplete Freund's adjuvant (IFA). On day 14 after immunization, each group of five mice was given a booster of 100 μl of BPV pseudoviruses (about 3.5 × 1010 pseudoviruses or 0.25 μg of plasmid), 100 μl of BPV VLPs, 20 μg of plasmid, or 100 μg of peptide (LCMV gp aa 33 to 41) in IFA. For mucosal immunization, mice were immunized orally by gavage with 100 μl of PV pseudovirus, 100 μl of VLPs, or 20 μg of plasmids in 100 μl of PBS as a negative control and boosted in the same way on day 14.
Detection of systemic CTLs.
Two weeks after the booster immunization, mice (five per group) were sacrificed, and spleen cells were isolated from each mouse. After incubation in nylon wool columns for 1 h at 37°C and 5% CO2, enriched T cells were washed through the column with complete cell culture medium (RPMI 1640 medium, including 10% heat-inactivated FCS, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml). Cells were cultured at 37°C and 5% CO2 for 7 days in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 10 U of interleukin 2 (IL-2) per ml, and 5 μg of E7 peptide aa 49 to 57 (RAHYNIVTF, H-2Db-restricted epitope) per ml, or the LCMV gp peptide. Specific cytolytic activity was determined by a 51Cr release assay (see below).
Isolation of Peyer's patches and MLN cells.
Briefly, after mice were sacrificed, mesenteric lymph nodes (MLN) were removed from the mesenteric tissue, and Peyer's patches were identified and removed from small intestine. Single-cell suspensions were prepared in complete cell culture medium. Because freshly isolated mucosal T cells undergo apoptosis in vitro, their specific cytolytic activity was determined immediately by 51Cr release assay.
In vitro cytotoxicity assay.
Target cells (106 RMA-E7, RMA-neo, or RMA cells) were labeled with 51Cr (100 μCi) for 1 h at 37°C and washed three times. The RMA cells were further loaded with peptides by directly adding peptides to the cells at 5 μg/ml. Target cells (2,000 cells per well) were then incubated with effector cells at different effector/target ratios in V-bottomed 96-well microtiter plates for 6 h at 37°C. Supernatant was collected, and 51Cr release was quantified by γ counter (ICN Biomedical Inc., Huntsville, Ala.). Specific lysis was calculated according to the formula [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. Spontaneous release was determined in control microcultures containing 51Cr-labeled target cells in culture medium with no effector cells. Maximum release was determined by lysing 51Cr-labeled target cells with 0.5% (vol/vol) NP-40.
ELISPOT assay for IFN-γ-secreting cells.
The enzyme-linked immunospot (ELISPOT) assay described by Taguchi et al. (41) was modified to detect specific CD8 T lymphocytes. First, 96-well filtration plates (Millipore) were coated with rat anti-mouse gamma interferon (IFN-γ) antibody (Pharmingen). Threefold dilutions of spleen cells in RPMI 1640 medium supplemented with 10% FCS, l-glutamine, 2-mercaptoethanol, and antibiotics were added to the wells along with 105 γ-irradiated (50 Gy) feeder spleen cells and 10 U of recombinant human IL-2 (Pharmingen) per well. Cells were incubated for 48 h with peptide stimulation. After culture, the plates were washed followed by incubation with biotinylated anti-mouse IFN-γ antibody (Pharmingen). Spots were developed using freshly prepared substrate buffer (0.33 mg of 3-amino-9-ethyl-carbazole per ml and 0.015% H2O2 in 0.1 M sodium acetate, pH 5).
Confocal microscopy.
The tissue slides (∼5 to 7 μm) were fixed in cold PBS containing 2% formaldehyde for 10 min and examined with a Zeiss EM 900 confocal microscope. Images were captured and recorded with software provided by Zeiss.
Statistical analysis.
The differences among groups were compared by analysis of variance. Between-group comparisons were made with the Duncan test. A two-sided alpha level of 0.05 was considered statistically significant.
RESULTS
Production of PV pseudoviruses.
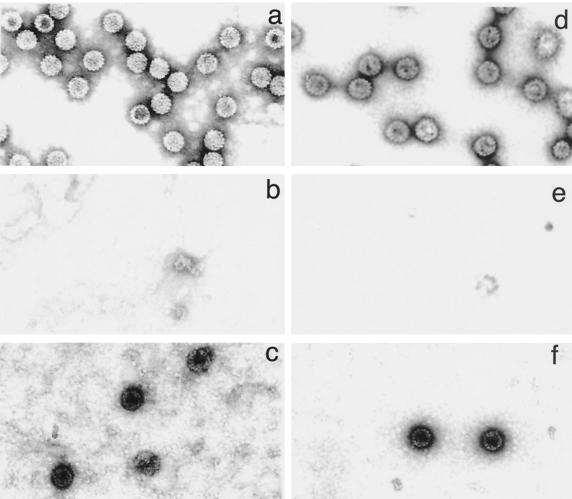
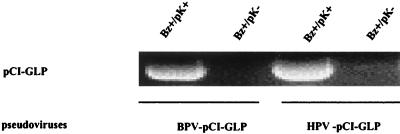
HPV-16 L1 and BPV-1 L1 VLPs were produced in Sf9 cells using recombinant baculoviruses (26, 28). Briefly, the cells were infected by recombinant baculoviruses encoding either HPV-16 L1 or BPV-1 L1 with a C-terminus deletion. The C-terminus deletion has been shown to enhance production of PV VLPs (28). Three days after infection, the cells were lysed and VLPs were purified on CsCl and sucrose gradients. Gradients were fractionated (1 ml per fraction); then 5 μl of each fraction was analyzed by SDS–10% PAGE and Western blotting. The fractions positive for the L1 protein were examined for the presence of VLPs by electron microscopy. The fractions containing VLPs were dialyzed for 1 h against 10 mM HEPES (pH 7.5). BPV-1 and HPV-16 VLPs (Fig. 1a and d) were added to an equal volume of buffer containing EGTA and DTT and then incubated at room temperature for 30 min. Under these conditions, VLPs were completely disrupted (Fig. 1b and e). Plasmid DNA (pCI-GLP) was then added, and the preparation was incubated with CaCl2 and dimethyl sulfoxide in order to refold VLPs (Fig. 1c and f). Most of the L1 proteins seemed to reassemble into VLPs under those conditions. To determine whether the plasmid DNA was packaged in the VLPs or on their surfaces, Bz was used after the refolding to digest DNA on the surfaces of the VLPs. Then the pseudovirions were treated with pK so that the VLPs were disrupted, and the presence of plasmid DNA inside the VLPs was determined by agarose gel electrophoresis. We found DNA plasmid in Bz- and pK-treated pseudovirus, indicating that the plasmid DNA was packaged in the VLPs (Fig. 2).
FIG. 1.
Electron micrographs of VLPs derived from BPV-1 L1 or HPV-16 L1, EGTA- and DTT-disrupted VLPs, and pseudoviruses. The VLPs were disrupted with EGTA and DTT; then the plasmid pCI-GLP was added. The VLPs were refolded by adding increasing concentrations of CaCl2 to form PV pseudoviruses. (a) BPV VLPs; (b) disrupted BPV VLPs; (c) BPV pseudoviruses; (d) HPV VLPs; (e) disrupted HPV VLPs; (f) HPV pseudoviruses. Magnification, ×84,000.
FIG. 2.
Encapsidation of plasmid pCI-GLP DNA by PV VLPs. The pseudoviruses were treated with Bz to digest the DNA on the surfaces of VLPs and with pK to verify whether the plasmid DNA was packaged inside the VLPs and then subjected to electrophoresis. Initially, 1 μg of the plasmid DNA was used to make the pseudovirus, and after digestion of 200 μl of pseudovirus with Bz and pK, 0.5 μg of the plasmid remained.
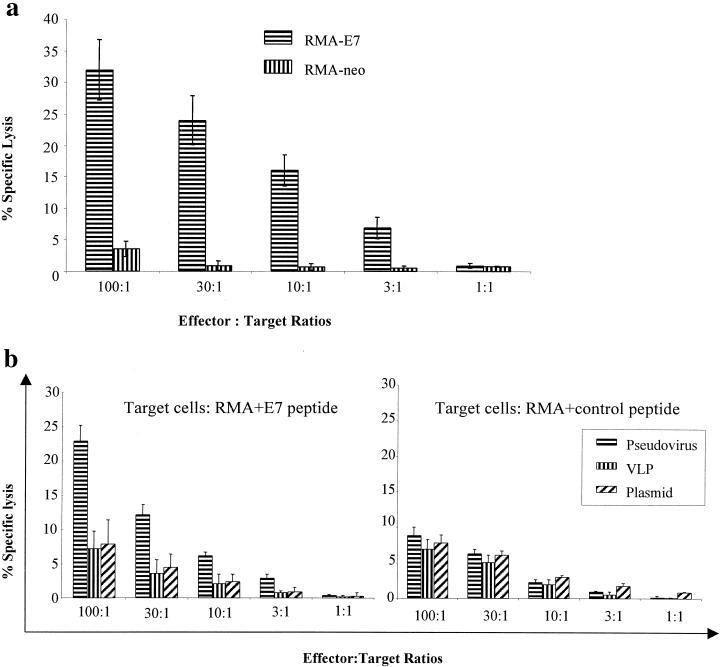
PV pseudoviruses induced a stronger CTL response than a DNA vaccine.
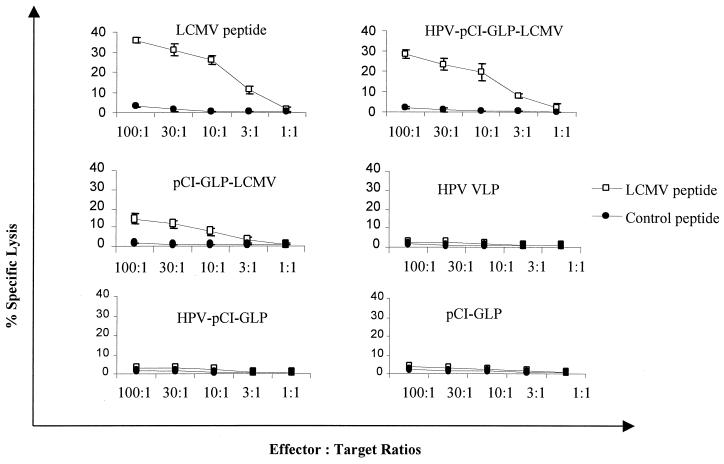
To test whether PV pseudoviruses induce a stronger CTL response than a plasmid DNA vaccine, we immunized mice subcutaneously with an HPV-16 pseudovirus containing a plasmid encoding the H-2Db-restricted epitope (9) of LCMV gp (aa 33 to 41) fused to GLP sequences (HPV-pCI-GLP-LCMV) or the plasmid alone (pCI-GLP-LCMV). pCI-GLP, HPV-16 VLPs, and the pseudovirus encoding GLP (HPV-pCI-GLP) were used as negative controls, and LCMV gp peptide (aa 33 to 41) in IFA was the positive control. Fourteen days after immunization, mice were given subcutaneous boosters of BPV-1 pseudovirus encoding GLP-LCMV or GLP, the plasmid pCI-GLP-LCMV or pCI-GLP, the BPV-1 VLPs, or the gp33 peptide. Fourteen days after the booster, spleen cells were isolated and then incubated with LCMV gp33 peptide (aa 33 to 41) in T-Stim culture supplement (Collaborative Biomedical Products, Bedford, Mass.) (without concanavalin A) in 5% CO2 at 37°C for 1 week. Standard 51Cr release assay was used to detect LCMV-specific CTLs using murine RMA cells loaded with LCMV peptide or control peptide (HPV-16 E7 aa 49 to 57) as target cells. We found that PV pseudoviruses encoding the LCMV epitope induced a stronger CTL response than the plasmid encoding the LCMV epitope (Fig. 3). Furthermore, by using the ELISPOT assay, we found that PV pseudoviruses generated three times more IFN-γ-producing CD8+ cells specific for the LCMV peptide than the plasmid alone (Table 1).
FIG. 3.
PV pseudovirus induced a stronger LCMV-specific CTL response than a DNA vaccine. Mice were subcutaneously immunized with PV pseudovirus with a plasmid encoding the GLP-LCMV gp33 epitope (aa 33 to 41) fusion protein or GLP, with VLPs alone, with the plasmid alone (pCI-GLP-LCMV or pCI-GLP), or with the LCMV gp peptide (aa 33 to 41) in IFA. A 51Cr release assay was used to measure the LCMV gp33 epitope-specific CTLs in spleen lymphocytes. The target cells (RMA) were pulsed with LCMV peptide (aa 33 to 41) or with a control peptide (HPV-16 E7 aa 49 to 57). The data are means ± standard deviations (five mice per group).
TABLE 1.
PV pseudovirus induced more specific IFN-γ-producing CD8+ T cells than a DNA vaccinea
| Immunization | No. of spots per 2 × 104 spleen lymphocytes |
|---|---|
| LCMV gp peptide (aa 33–41) + IFA | 45.6 ± 2.09 |
| PV-pCI-GLP-LCMV (pseudovirus) | 23.0 ± 1.13 |
| pCI-GLP-LCMV | 7.9 ± 2.7 |
| VLPs | 0 |
| PV-pCI-GLP (pseudovirus) | 0 |
| pCI-GLP | 0 |
Mice were immunized with HPV pseudovirus containing a plasmid encoding GLP fused to LCMV gp33 epitope (aa 33 to 41) or GLP, with VLPs alone, with the plasmid pCI-GLP-LCMV, pCI-GLP alone, or LCMV peptide plus IFA (aa 33 to 41) and then given boosters of BPV pseudoviruses, VLPs alone, or plasmids alone. Two weeks after the boosters, spleen lymphocytes pooled for five mice were incubated in plates that were coated with anti-IFN-γ antibody. Two days later, biotin anti-IFN-γ antibody was added and incubated overnight. Then streptavidin-horseradish peroxidase was added. The spots were counted after being developed by 3-amino-9-ethylcarbazole substrate. Data are means ± standard deviations for triplicates.
PV VLPs serve as an adjuvant for a DNA vaccine to induce CTL response.
To test whether VLPs had an adjuvant effect on CTL induction by the DNA vaccine, we immunized mice (five per group) with the plasmids alone (20 μg), with the plasmids (20 μg) plus BPV VLPs (2.5 μg), or with VLPs alone (2.5 μg) as a control and then measured the generation of LCMV gp33-specific CTLs by IFN-γ ELISPOT. In the group immunized with the plasmids alone, 10.7 ± 7.25 (mean ± standard deviation) LCMV gp33-specific CTLs per 2 × 104 spleen cells were generated. In contrast, 23.35 ± 1.26 gp33-specific CTLs per 2 × 104 spleen cells were induced in mice immunized with the plasmids plus the VLPs. VLPs alone did not induce specific T cells. Thus, coimmunization with the plasmids and VLPs induced significantly more CTLs than immunization with the plasmids alone (P < 0.05), indicating that the VLPs are an adjuvant for generating CTLs by the DNA vaccine.
PV pseudoviruses pseudoinfect mucosal and systemic lymphoid tissues.
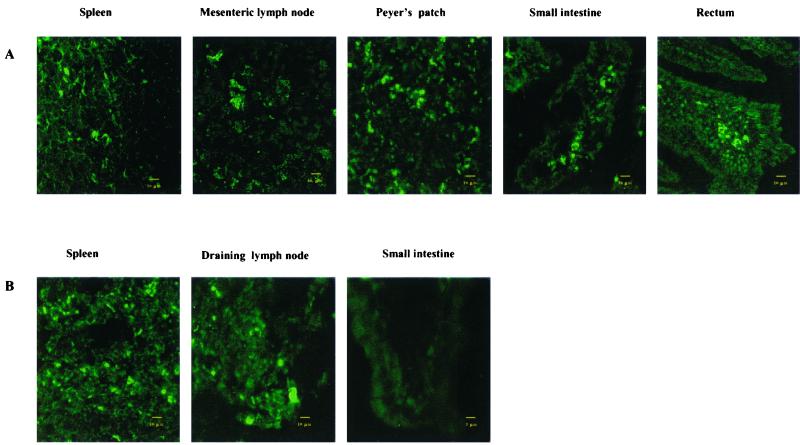
To test whether PV pseudoviruses pseudoinfect mucosal and systemic lymphoid tissues, we determined whether oral administration with the pseudovirus containing a plasmid encoding GLP (PV-pCI-GLP) resulted in expression of GLP in the mucosal and systemic lymphoid tissues. If the pseudoviruses pseudoinfected mucosal and systemic lymphoid tissues, we would be able to detect the expression of GLP by confocal microscopy. Thus, we fed mice (five per group) with HPV or BPV pseudoviruses (HPV-pCI-GLP or BPV-pCI-GLP), the VLPs alone (HPV VLPs or BPV VLPs), or the plasmid alone (pCI-GLP). At 1 and 7 days after feeding, mice were sacrificed; small intestines, rectums, spleens, MLN, and muscles were removed immediately and frozen. Tissue sections were made, and GLP expression was determined by confocal microscopy. We found expression of GLP in Peyer's patches, lamina propria, rectum, spleen, and MLN at day 1 (Fig. 4A) and at day 7 (data not shown). When the PV pseudoviruses were given subcutaneously, GLP expression was found in draining lymph nodes and spleen but not in the mucosal tissues (Fig. 4B). To determine which cells were infected by the pseudoviruses, we stained the tissues with phycoerythrin-labeled antibodies directed against CD11b, CD11c, CD3, and CD19 and determined whether they colocalized with GLP. Some of the CD11b+ and CD11c+ cells colocalized with the GLP (Fig. 5), suggesting that macrophages and dendritic cells were pseudoinfected with PV pseudoviruses. No CD3+ or CD19+ cells colocalized with the GLP.
FIG. 4.
PV pseudovirus pseudoinfected intestinal mucosa and systemic lymphoid tissue when given orally. (A) HPV and BPV pseudoviruses encoding GLP were administered orally to mice. GLP expression was determined in the indicated tissues by confocal microscopy. GLP was found in the lamina propria of small and large intestines, Peyer's patches, MLN, rectum, and spleen but not in the muscles (data not shown). The data from mice given HPV pseudoviruses are shown. (B) BPV and HPV pseudoviruses encoding GLP were administered to mice by subcutaneous injection. GLP expression was found in draining lymph nodes and spleen but not in mucosa. The data from mice given BPV pseudoviruses are shown.
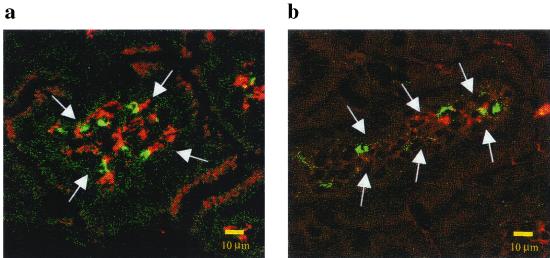
FIG. 5.
Intestinal CD11b- and CD11c-positive cells colocalized with GLP expression in mucosal tissues of mice orally fed PV pseudoviruses encoding GLP. One day after oral administration with the pseudovirus encoding GLP, the mucosal tissues of the mice were removed and stained with anti-mouse CD11b or CD11c antibody. Phycoerythrin-labeled second antibodies were used after washing. GLP, CD11b, and CD11c expression was determined in the mucosal tissues by confocal microscopy. The expression of GLP (green), CD11c (red in panel a), and CD11b (red in panel b) in lamina propria (lamina propria is indicated by arrows) is shown.
Induction of specific CTLs in mucosal and systemic lymphoid tissues after oral immunization with PV pseudoviruses.
To determine whether orally administered PV pseudoviruses induced mucosal and systemic CTL responses, we used a plasmid encoding an HPV-16 E7 mutant that has been shown to be highly effective in inducing CTL responses systemically (38). Mice (five per group) were fed by gavage with HPV-16 pseudovirus encoding the E7 mutant (HPV-pCMV-E7), a plasmid encoding the E7 mutant (pCMV-E7), or HPV-16 VLPs only. Fourteen days after feeding, they were given boosters of BPV-1 pseudovirus encoding the E7 mutant (BPV-pCMV-E7), the plasmid pCMV-E7 only, or BPV-1 VLPs only. Fourteen days after the booster, lymphocytes were isolated from MLN and Peyer's patches or spleen. The lymphocytes from MLN and Peyer's patches were used immediately to detect E7-specific CTLs. Spleen lymphocytes were stimulated with an E7 peptide (aa 49 to 57; RAHYNIVTF) for 1 week. A standard 51Cr release assay was performed. We found that T cells from the mice immunized orally with PV-pCMV-E7 had mucosal and systemic CTL responses against E7-expressing target cells (Fig. 6). Oral immunization with the plasmid alone or PV VLPs did not induce an E7-specific CTL response. Pseudoviruses did not induce a mucosal immune response when mice were immunized by subcutaneous injection (data not shown).
FIG. 6.
Oral immunization with PV pseudovirus encoding E7 induced mucosal and systemic E7-specific CTLs. Mice were orally immunized with HPV-16 pseudovirus with a plasmid encoding a mutant E7, VLPs alone, or the plasmid alone and then given a booster of BPV pseudovirus, VLPs alone, or the plasmid alone. (a) Peyer's patches and MLN cells were isolated and immediately used to test E7-specific CTLs without in vitro restimulation. A 51Cr release assay was used to measure E7-specific CTLs. The target cells were RMA-E7, which express the E7 antigen, and RMA-neo cells (negative controls). RMA-E7 and RMA-neo expressed comparable MHC class I levels (data not shown). The lymphoid cells from mice fed the plasmid or VLPs did not lyse the RMA-E7 cells (data not shown). (b) Spleen lymphocytes were isolated and restimulated with E7 peptides (aa 49 to 57) in vitro. The 51Cr release assay was used to measure E7-specific CTLs. The target cells (RMA) were pulsed with an E7 peptide (aa 49 to 57) or with a control peptide. The data are means ± standard deviations for five mice per group.
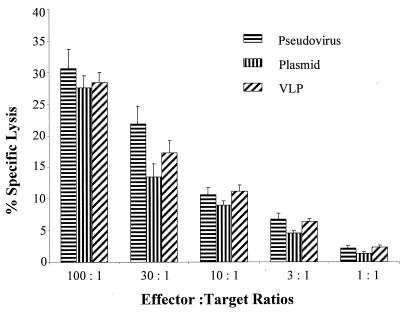
Oral immunization with PV pseudovirus did not induce systemic tolerance.
Because oral administration with soluble proteins might induce systemic tolerance (16, 22, 47, 48), we tested whether PV pseudoviruses induced systemic tolerance after oral immunization. We fed mice (five per group) with HPV-pCMV-E7, pCMV-E7, or HPV VLPs alone. Fourteen days after oral immunization, we immunized mice subcutaneously with BPV-pCMV-E7. Spleen T cells were isolated and then incubated with the E7 peptide, T-stim medium (without concanavalin A) in 5% CO2 at 37°C for 1 week. A standard 51Cr release assay was used to detect specific CTLs by using RMA-E7 and RMA-neo as target cells. All three groups of mice had CTLs against target RMA-E7 cells but not against RMA-neo cells (Fig. 7).
FIG. 7.
Oral immunization with PV pseudovirus did not induce systemic tolerance. Mice were orally fed HPV-16 pseudovirus with a plasmid encoding HPV-16 E7, VLPs alone, or the plasmid alone; then mice were systemically immunized with BPV pseudovirus (PV) encoding the E7 protein. Spleen lymphocytes were isolated and restimulated with E7 peptides (aa 49 to 57) in vitro. A 51Cr release assay was used to detect E7-specific CTLs. The target cells (RMA) were pulsed with an E7 peptide (aa 49 to 57) or with a control peptide (data not shown). The data are means ± standard deviations for five mice per group.
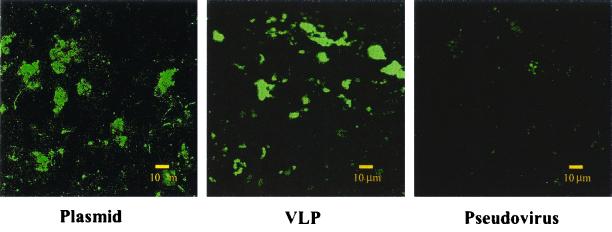
Oral immunization with PV pseudovirus provided immunity against mucosal challenge.
To test whether oral immunization with the PV pseudoviruses induced mucosal protection, we immunized mice with HPV-16 pseudovirus encoding E7 and challenged them with BPV-1 pseudovirus encoding GLP-E7. We hypothesized that if HPV-16 pseudovirus induced a protective immune response, the mucosal immune system would clear BPV-1 pseudovirus-infected cells. Such a response would be indicated by an absence or decrease of GLP expression in the mucosal tissue of HPV-16 pseudovirus-immunized mice compared to the control-immunized group. To this end, we fed mice (five per group) with HPV-pCMV-E7, pCMV-E7, or HPV-16 VLPs. Fourteen days after oral immunization, the mice were given boosters of HPV-pCMV-E7, pCMV-E7 only, or HPV-16 VLPs. Fourteen days after the booster, all three groups of mice were challenged with BPV-1 pseudovirus encoding the GLP-E7 fusion protein. One day later, mice were sacrificed, and GLP expression in Peyer's patches was determined. The GLP expression was markedly lower in Peyer's patches of mice immunized with PV pseudovirus (4 ± 1 [mean ± standard deviation] green spots in each microscopic field) than in mice immunized with VLP (20 ± 3 green spots) or plasmid (21 ± 4 green spots) (P < 0.05) (Fig. 8).
FIG. 8.
Oral immunization with pseudovirus protected mice against mucosal challenge. Mice were orally immunized with an HPV-16 pseudovirus with a plasmid encoding E7, VLP alone, or the plasmid alone and then given boosters of the same agent 14 days later. On day 28, all three groups of mice were orally challenged with BPV pseudovirus encoding the GLP-E7 fusion protein. One day later, mice were sacrificed to detect GLP expression in Peyer's patches. The GLP expression was markedly lower in the Peyer's patches from the pseudovirus-immunized mice than in those from the VLP- and the plasmid-immunized mice.
DISCUSSION
One of the most important features of the PV pseudoviruses is their ability to reach mucosal and systemic lymphoid tissues. We showed that the plasmids themselves could not reach mucosal and systemic lymphoid tissues when administered orally, possibly because they did not survive degradation in the gastric and intestinal environment. Our data suggest that PV VLPs are resistant to the low-pH environment in the stomach and to proteolysis in the intestine.
Although we do not know which cells take up the pseudoviruses, it is likely that M cells in the follicle-associated epithelium have an important role in sampling the pseudoviruses and delivering them into the Peyer's patches. It is also possible that epithelial cells take up the pseudoviruses, because we observed GLP expression in lamina propria of the small intestine and rectum of mice fed PV pseudoviruses encoding GLP. PV VLPs have been shown to bind cells from different tissues and species (25, 32). We also found that PV pseudoviruses pseudoinfect dendritic cells and macrophages in lamina propria and Peyer's patches, which suggests that the pseudoviruses might cross the epithelial layers. It remains to be investigated how pseudoviruses pass through the epithelium and get into lamina propria dendritic cells and macrophages. We also found that the pseudoviruses reached MLN and spleen. It is possible that the dendritic cells and macrophages in lamina propria that had taken up the pseudoviruses moved to MLN and spleen directly. However, we cannot exclude the possibility that the pseudoviruses themselves directly reached MLN and spleen.
We also administered PV pseudoviruses encoding GLP to nostrils of mice and vaginal mucosae of rabbits and found that GLP was expressed in the mucosae of the respiratory tract and female reproductive tract (data not shown). It is thus likely that immunization with the pseudoviruses in these mucosal tissues might also generate CTL responses in respiratory tract and cervicovaginal mucosa. In fact, it has been shown that intranasal immunization with PV VLPs resulted in mucosal antibody responses (2, 19), suggesting that mucosal cellular immune responses can be induced at those sites.
Another important feature of the PV pseudoviruses is that they can induce a stronger CTL response than a DNA vaccine when administered systemically in mice. We have also shown that coinjection of PV VLPs with a plasmid DNA vaccine induced a stronger CTL response than immunization with the DNA vaccine alone. This demonstrates that PV VLPs actually serve as an adjuvant for the DNA plasmids to induce CTL responses. Because the VLPs that are used to package the plasmid DNA can induce VLP-specific T-helper responses, the T-helper cells might enhance the generation of CTLs specific for the antigen encoded by the plasmid through bystander action. Indeed, the VLPs can induce a strong Th1 response (7, 8, 17, 21); thus, it is likely that IL-2 produced by the Th1 cells amplifies the proliferation of CTLs. After the uptake of the pseudoviruses, antigen-presenting cells might be activated by the VLPs of the pseudoviruses, thereby expressing more costimulatory molecules, such as CD80 or CD86. Indeed, PV VLPs are shown to infect and activate human and murine dendritic cells (17, 36). The PV VLP-activated murine dendritic cells expressed an enhanced level of costimulatory molecules such as CD80 and produced proinflammatory cytokines, such as IL-6 and tumor necrosis factor alpha (17). These activated antigen-presenting cells could have an enhanced capacity to activate naïve CTLs specific for the antigen encoded by the plasmid compared with dendritic cells transduced by the plasmid (DNA vaccine) alone.
Oral immunization with PV pseudoviruses induced mucosal and systemic CTL responses. The intestinal mucosal CTLs were detectable among freshly isolated lymphocytes from Peyer's patches, suggesting that a significantly large number of specific CTLs were generated in Peyer's patches. Furthermore, oral immunization with the pseudoviruses protected mice against a mucosal pseudoviral challenge, strongly suggesting that the oral immunization with the pseudoviruses was protective. The protection is probably not a result of anti-HPV L1 VLP IgA, which cross-reacts with BPV pseudovirus and prevents its uptake or promotes its clearance, because there was no protection in mice immunized with HPV L1 VLPs alone. Further, the protection cannot be from E7-specific antibody responses, because E7 is a cytoplasmic protein (30) and the mutant E7 we used here was an unstable protein and was degraded intracellularly (38). Thus, protection is apparently mediated by CTLs specific for the E7 protein. The loss in GLP expression in the Peyer's patches was detected only 1 day after mucosal challenge in mice, which reflects the immediate antigen-specific CTL effector activity in mucosal tissues. Our data confirm those of a study that found that mucosal CTL responses are sustained once induced (23).
Because PV pseudoviruses pseudoinfect mucosal and systemic lymphoid tissues, they can be used as a gene delivery vector. After mice were fed with PV pseudoviruses encoding GLP, expression of GLP was found from the following day until week 3. Those data suggest that the expression of the gene delivered to the mucosal and systemic lymphoid tissues is transient. Thus, PV pseudoviruses can be used to deliver genes that are needed in the immune system for a short period. For example, they might be used to deliver immunomodulatory cytokine genes, such as the IL-10 gene to intestinal mucosa for Crohn's disease to suppress the Th1 type mucosal immune responses or the IL-12 gene to the upper respiratory tract to switch off the Th2 immune responses for asthma.
PV pseudoviruses are nonreplicating vectors. Their advantage over other live vectors is that they are composed of PV VLPs and plasmids, so there is no danger that they will revert to a virulent form. Although there are concerns about the integration of the DNA plasmids into the host genome, so far it has not been shown that the DNA plasmids cause any neoplasm. PV VLPs are made of L1 protein, which has not been shown to have detrimental effects on the host. The cells infected by the pseudoviruses, in general, will be deleted by specific CTLs because they induce CTL responses to the pseudoviruses. Although the pseudoviruses do not replicate in the host, the immunogen is expressed in the host antigen-presenting cells, leading to longer exposure of antigens to T cells.
In conclusion, PV pseudoviruses generated a stronger CTL response than a DNA vaccine and induced protective mucosal and systemic CTL responses; thus, PV pseudoviruses represent a novel vaccine for preventing and treating infections by pathogens at the mucosal surface.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants CA81254 and AI43214 from the National Institutes of Health.
REFERENCES
- 1.Bagarazzi M L, Boyer J D, Ayyavoo V, Weiner D B. Nucleic acid-based vaccines as an approach to immunization against human immunodeficiency virus type-1. Curr Top Microbiol Immunol. 1998;226:107–143. doi: 10.1007/978-3-642-80475-5_8. [DOI] [PubMed] [Google Scholar]
- 2.Balmelli C, Roden R, Potts A, Schiller J, De Grandi P, Nardelli-Haefliger D. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J Virol. 1998;72:8220–8229. doi: 10.1128/jvi.72.10.8220-8229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benton P A, Kennedy R C. DNA vaccine strategies for the treatment of cancer. Curr Top Microbiol Immunol. 1998;226:1–20. doi: 10.1007/978-3-642-80475-5_1. [DOI] [PubMed] [Google Scholar]
- 4.Calarota S, Bratt G, Nordlund S, Hinkula J, Leandersson A C, Sandstrom E, Wahren B. Cellular cytotoxic response induced by DNA vaccination in HIV-1 infected patients. Lancet. 1998;351:1320–1325. doi: 10.1016/S0140-6736(97)09440-3. [DOI] [PubMed] [Google Scholar]
- 5.Chen C H, Wang T L, Hung C F, Yang Y, Young R A, Pardoll D M, Wu T C. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
- 6.De Bruijn M L, Greenstone H L, Vermeulen H, Melief C J, Lowy D R, Schiller J T, Kast W M. L1-specific protection from tumor challenge elicited by HPV16 virus-like particles. Virology. 1998;250:371–376. doi: 10.1006/viro.1998.9372. [DOI] [PubMed] [Google Scholar]
- 7.Dupuy C, Frazer I H, Payne E, Qi Y M, Hengst K, McMillan N A. Cell mediated immunity induced in mice by HPV 16 L1 virus-like particles. Microb Pathog. 1997;22:219–225. doi: 10.1006/mpat.1996.0113. [DOI] [PubMed] [Google Scholar]
- 8.Dupuy C, Buzoni-Gatel D, Touze A, Bout D, Coursaget P. Nasal immunization of mice with human papillomavirus type 16 (HPV-16) virus-like particles or with the HPV-16 L1 gene elicits specific cytotoxic T lymphocytes in vaginal draining lymph nodes. J Virol. 1999;73:9063–9071. doi: 10.1128/jvi.73.11.9063-9071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallimore A, Hombach J, Dumrese T, Rammensee H G, Zinkernagel R M, Hengartner H. A protective cytotoxic T cell response to a subdominant epitope is influenced by the stability of the MHC class I/peptide complex and the overall spectrum of viral peptides generated within infected cells. Eur J Immunol. 1998;28:3301–3311. doi: 10.1002/(SICI)1521-4141(199810)28:10<3301::AID-IMMU3301>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 10.Galloway D A. Is vaccination against human papillomavirus a possibility? Lancet. 1998;351:22–24. doi: 10.1016/s0140-6736(98)90007-1. [DOI] [PubMed] [Google Scholar]
- 11.Greenstone H L, Nieland J D, de Visser K E, De Bruijn M L, Kirnbauer R, Roden R B, Lowy D R, Kast W M, Schiller J T. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc Natl Acad Sci USA. 1998;95:1800–1805. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassett D E, Whitton J L. DNA immunization. Trends Microbiol. 1996;4:307–312. doi: 10.1016/0966-842x(96)10048-2. [DOI] [PubMed] [Google Scholar]
- 13.Ji H, Chang E Y, Lin K Y, Kurman R J, Pardoll D M, Wu T C. Antigen-specific immunotherapy for murine lung metastatic tumors expressing human papillomavirus type 16 E7 oncoprotein. Int J Can. 1998;78:41–45. doi: 10.1002/(sici)1097-0215(19980925)78:1<41::aid-ijc8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Kawana K, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. In vitro construction of pseudovirions of human papillomavirus type 16: incorporation of plasmid DNA into reassembled L1/L2 capsids. J Virol. 1998;72:10298–10300. doi: 10.1128/jvi.72.12.10298-10300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirnbauer R, Booy F, Cheng N, Lowy D R, Schiller J T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyburz D, Aichele P, Speiser D E, Hengartner H, Zinkernagel R M, Pircher H. T cell immunity after a viral infection versus T cell tolerance induced by soluble viral peptides. Eur J Immunol. 1993;23:1956–1962. doi: 10.1002/eji.1830230834. [DOI] [PubMed] [Google Scholar]
- 17.Lenz P, Day P M, Pang Y S, Frye S A, Jensen P N, Lowy D R, Schiller J T. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166:5346–5355. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- 18.Liu W J, Liu X S, Zhao K N, Leggatt G R, Frazer I H. Papillomavirus virus-like particles for the delivery of multiple cytotoxic T cell epitopes. Virology. 2000;273:374–382. doi: 10.1006/viro.2000.0435. [DOI] [PubMed] [Google Scholar]
- 19.Liu X S, Abdul-Jabbar I, Qi Y M, Frazer I H, Zhou J. Mucosal immunisation with papillomavirus virus-like particles elicits systemic and mucosal immunity in mice. Virology. 1998;252:39–45. doi: 10.1006/viro.1998.9442. [DOI] [PubMed] [Google Scholar]
- 20.Lowe R S, Brown D R, Bryan J T, Cook J C, George H A, Hofmann K J, Hurni W M, Joyce J G, Lehman E D, Markus H Z, Neeper M P, Schultz L D, Shaw A R, Jansen K U. Human papillomavirus type 11 (HPV-11) neutralizing antibodies in the serum and genital mucosal secretions of African green monkeys immunized with HPV-11 virus-like particles expressed in yeast. J Infect Dis. 1997;176:1141–1145. doi: 10.1086/514105. [DOI] [PubMed] [Google Scholar]
- 21.Marais D, Passmore J A, Maclean J, Rose R, Williamson A L. A recombinant human papillomavirus (HPV) type 16 L1-vaccinia virus murine challenge model demonstrates cell-mediated immunity against HPV virus-like particles. J Gen Virol. 1999;80:2471–2475. doi: 10.1099/0022-1317-80-9-2471. [DOI] [PubMed] [Google Scholar]
- 22.Marth T, Strober W, Kelsall B L. High dose oral tolerance in ovalbumin TCR-transgenic mice: systemic neutralization of IL-12 augments TGF-beta secretion and T cell apoptosis. J Immunol. 1996;157:2348–2357. [PubMed] [Google Scholar]
- 23.Masopust D, Jiang J, Shen H, Lefrancois L. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J Immunol. 2001;166:2348–2356. doi: 10.4049/jimmunol.166.4.2348. [DOI] [PubMed] [Google Scholar]
- 24.McGhee J R, Mestecky J, Elson C O, Kiyono H. Regulation of IgA synthesis and immune response by T cells and interleukins. J Clin Immunol. 1989;9:175–199. doi: 10.1007/BF00916814. [DOI] [PubMed] [Google Scholar]
- 25.Muller M, Gissmann L, Cristiano R J, Sun X Y, Frazer I H, Jenson A B, Alonso A, Zentgraf H, Zhou J. Papillomavirus capsid binding and uptake by cells from different tissues and species. J Virol. 1995;69:948–954. doi: 10.1128/jvi.69.2.948-954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller M, Zhou J, Reed T D, Rittmuller C, Burger A, Gabelsberger J, Braspenning J, Gissmann L. Chimeric papillomavirus-like particles. Virology. 1997;234:93–111. doi: 10.1006/viro.1997.8591. [DOI] [PubMed] [Google Scholar]
- 27.Nardelli-Haefliger D, Roden R B, Benyacoub J, Sahli R, Kraehenbuhl J P, Schiller J T, Lachat P, Potts A, De Grandi P. Human papillomavirus type 16 virus-like particles expressed in attenuated Salmonella typhimurium elicit mucosal and systemic neutralizing antibodies in mice. Infect Immun. 1997;65:3328–3336. doi: 10.1128/iai.65.8.3328-3336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paintsil J, Muller M, Picken M, Gissmann L, Zhou J. Carboxyl terminus of bovine papillomavirus type-1 L1 protein is not required for capsid formation. Virology. 1996;223:238–244. doi: 10.1006/viro.1996.0473. [DOI] [PubMed] [Google Scholar]
- 29.Pedroza Martins L, Lau L L, Asano M S, Ahmed R. DNA vaccination against persistent viral infection. J Virol. 1995;69:2574–2582. doi: 10.1128/jvi.69.4.2574-2582.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phelps W C, Munger K, Yee C L, Barnes J A, Howley P M. Structure-function analysis of the human papillomavirus type 16 E7 oncoprotein. J Virol. 1992;66:2418–2427. doi: 10.1128/jvi.66.4.2418-2427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson H L, Tores C A. DNA vaccines. Semin Immunol. 1997;9:271–283. doi: 10.1006/smim.1997.0083. [DOI] [PubMed] [Google Scholar]
- 32.Roden R B, Kirnbauer R, Jenson A B, Lowy D R, Schiller J T. Interaction of papillomaviruses with the cell surface. J Virol. 1994;68:7260–7266. doi: 10.1128/jvi.68.11.7260-7266.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roden R B, Greenstone H L, Kirnbauer R, Booy F P, Jessie J, Lowy D R, Schiller J T. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J Virol. 1996;70:5875–5883. doi: 10.1128/jvi.70.9.5875-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez F, Zhang J, Whitton J L. DNA immunization: ubiquitination of a viral protein enhances cytotoxic T-lymphocyte induction and antiviral protection but abrogates antibody induction. J Virol. 1997;71:8497–8503. doi: 10.1128/jvi.71.11.8497-8503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose R C, Bonnez W, Reichman R C, Garcea R L. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of virus-like particles. J Virol. 1993;67:1936–1944. doi: 10.1128/jvi.67.4.1936-1944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudolf M P, Fausch S C, Da Silva D M, Kast W M. Human dendritic cells are activated by chimeric human papillomavirus type-16 virus-like particles and induce epitope-specific human T cell responses in vitro. J Immunol. 2001;166:5917–5924. doi: 10.4049/jimmunol.166.10.5917. [DOI] [PubMed] [Google Scholar]
- 37.Schiller J, Lowy D. Papillomavirus-like particles and HPV vaccine development. Semin Cancer Biol. 1996;7:373–382. doi: 10.1006/scbi.1996.0046. [DOI] [PubMed] [Google Scholar]
- 38.Shi W, Bu P, Liu J, Polack A, Fisher S, Qiao L. Human papillomavirus type 16 E7 DNA vaccine: mutation in the open reading frame of E7 enhances specific cytotoxic T-lymphocyte induction and antitumor activity. J Virol. 1999;73:7877–7881. doi: 10.1128/jvi.73.9.7877-7881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sundaram P, Tigelaar R E, Brandsma J L. Inracutaneous vaccination of rabbits with the cottontail rabbit papillomavirus (CRPV) L1 gene protects against virus. Vaccine. 1998;15:664–671. doi: 10.1016/s0264-410x(96)00237-x. [DOI] [PubMed] [Google Scholar]
- 40.Suzich J A, Ghim S J, Palmer-Hill F J, White W I, Tamura J K, Bell J A, Newsome J A, Jenson A B, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taguchi T, McGhee J R, Coffman R L, Beagley K W, Eldridge J H, Takatsu K, Kiyono H. Detection of individual mouse splenic T cells producing IFN-γ and IL-5 using the enzyme-linked immunospot (ELISPOT) assay. J Immunol Methods. 1990;128:65–73. doi: 10.1016/0022-1759(90)90464-7. [DOI] [PubMed] [Google Scholar]
- 42.Touze A, Coursaget P. In vitro gene transfer using human papillomavirus-like particles. Nucleic Acids Res. 1998;26:1317–1323. doi: 10.1093/nar/26.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulmer J B, Sadoff J C, Liu M A. DNA vaccines. Curr Opin Immunol. 1996;8:531–536. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 44.Unckell F, Streeck R E, Sapp M. Generation and neutralization of pseudovirions of human papillomavirus type 33. J Virol. 1997;71:2934–2939. doi: 10.1128/jvi.71.4.2934-2939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Ranst M, Tachezy R, Rurk R D. Human papillomaviruses: a never-ending story? In: Lacey C, editor. Papillomavirus reviews: current research on papillomaviruses. Leeds, United Kingdom: Leeds University Press; 1996. pp. 1–19. [Google Scholar]
- 46.Volpers C, Schirmacher P, Streeck R E, Sapp M. Assembly of the major and the minor capsid protein of human papillomavirus type 33 into virus-like particles and tubular structures in insect cells. Virology. 1994;200:504–512. doi: 10.1006/viro.1994.1213. [DOI] [PubMed] [Google Scholar]
- 47.Weiner H L. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today. 1997;18:335–343. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 48.Whitacre C C, Gienapp I E, Orosz C G, Bitar D M. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J Immunol. 1991;147:2155–2163. [PubMed] [Google Scholar]
- 49.White W I, Wilson S D, Palmer-Hill F J, Woods R M, Ghim S J, Hewitt L A, Goldman D M, Burke S J, Jenson A B, Koenig S, Suzich J A. Characterization of a major neutralizing epitope on human papillomavirus type 16 L1. J Virol. 1999;73:4882–4889. doi: 10.1128/jvi.73.6.4882-4889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White W I, Wilson S D, Bonnez W, Rose R C, Koenig S, Suzich J A. In vitro infection and type-restricted antibody-mediated neutralization of authentic human papillomavirus type 16. J Virol. 1998;72:959–964. doi: 10.1128/jvi.72.2.959-964.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zarozinski C C, Fynan E F, Selin L K, Robinson H L, Welsh R M. Protective CTL-dependent immunity and enhanced immunopathology in mice immunized by particle bombardment with DNA encoding an internal virion protein. J Immunol. 1995;154:4010–4017. [PubMed] [Google Scholar]
- 52.zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991;184:9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]