Abstract
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract, and its pathogenesis is believed to be associated with an imbalance between commensal organisms and the intestinal immune system. This imbalance is significantly influenced by the intestinal microbiota and metabolites and plays a critical role in maintaining intestinal mucosal homeostasis. However, disturbances in the intestinal microbiota cause dysregulated immune responses and consequently induce intestinal inflammation. Recent studies have illustrated the roles of the intestinal microbiota in the pathogenesis of IBD and underscored the potential of precision diagnosis and therapy. This work summarises recent progress in this field and particularly focuses on the application of the intestinal microbiota and metabolites in the precision diagnosis, prognosis assessment, treatment effectiveness evaluation, and therapeutic management of IBD.
Keywords: inflammatory bowel disease, fecal microbiota, metabolite, diagnosis, treatment, pathogenesis
Introduction
Inflammatory bowel disease (IBD) is a chronic nonspecific inflammatory disease of the gastrointestinal tract that mainly includes Crohn's disease (CD) and ulcerative colitis (UC). Despite extensive study, the precise etiologies and pathologies of IBD, which are considered to be involved in a complex interplay of genetic predispositions, microbial alternations, dysregulated immune responses, and environmental factors, remain elusive [1]. Essential in the pathogenesis of IBD is the disruption of intestinal immune homeostasis, influenced by predisposing factors including the gut microbiota, diet, medications, and psychological stress [2, 3].
The gut microbiota, a key player in this ecosystem, performs critical functions such as nutrient metabolism, maintenance of mucosal barrier integrity, immune modulation, and pathogen defense. The delicate balance between the gut microbiota and metabolites and the intestinal immune system is crucial for intestinal health [4]. Disturbances in this balance have been reported to be linked to various gastrointestinal disorders, including IBD [5], indicating that the gut microbiota diversity and abundances are vital for maintaining intestinal homeostasis [6].
Emerging evidence underscores the pivotal role of the gut microbiota in the onset of IBD and its progression. This review discusses the impacts of the intestinal microbiota on the pathogenesis of IBD and explores their potential in refining the precision diagnosis and treatment of this complex disease.
Regulation of intestinal microbiota in intestinal mucosal homeostasis
Physiological functions of the intestinal microbiota
The intestinal microbiota, comprising bacteria, viruses, fungi, parasites, and archaea [7], encompasses >50 bacterial groups with ∼1100 species. The collective mass of the intestinal microbiota is ∼0.2 kg, making up 60% of stool's dry mass [8]. Predominantly, Firmicutes, Bacteroidetes, and Actinobacteria constitute 90% of the gut microbiota, with Proteobacteria and Fusobacteria being less common [6]. Notably, Firmicutes, known for their production of butyric acid, a type of short-chain fatty acid (SCFA), play a crucial role in sustaining the health of the intestinal epithelia [9].
The intestinal microbiota performs vital physiological functions that contribute to the host's overall well-being, including assisting in food digestion and energy production, regulating immune responses, protecting the intestinal lining, regulating fat storage, stimulating intestinal angiogenesis [9], producing a variety of growth factors, shielding against pathogen invasion, and ensuring the intestinal microecological balance [7]. For example, Bifidobacterium longum has the capacity to balance the intestinal flora, mitigate intestinal inflammation by reducing proinflammatory cytokines and reactive oxygen species, and fortify the intestinal epithelial barrier integrity [10], which is suggestive of its therapeutic potential in IBD management.
The intestinal microbiota also provides important metabolites such as SCFAs, indoles, and bile acids [11, 12], and produces SCFAs, mainly including acetate, propionate, and butyrate. Bacteroidetes mainly produce acetate and propionate, while Firmicutes mainly produce butyrate. SCFAs enhance the barrier function of intestinal epithelial cells and also have immunomodulatory effects, such as reducing pro-inflammatory cytokines and stimulating the generation of regulatory T cells (Tregs). The intestinal microbiota converts tryptophan into a range of indole metabolites, and indoles play a regulatory role in intestinal mucosal immunity [13]. Additionally, primary bile acids are processed by the intestinal microbiota into secondary bile acids, which play an important role in preventing bacterial overgrowth, modulating mucosal immunity, and protecting intestinal epithelial integrity [7].
Intestinal microbiota antigens maintain intestinal mucosal immune homeostasis
Intestinal microbiota-derived antigens play dichotomous roles in maintaining intestinal mucosal homeostasis by either boosting mucosal immune responses or fostering immune tolerance. Increasing lines of evidence have demonstrated that certain microbial antigens can escalate mucosal immune responses, thus inducing CD4+ T cell activation and proliferation in the gut mucosa and facilitating them to secrete proinflammatory cytokines [14]. This event results in intestinal mucosal inflammation and tissue damage. A notable example involves the segmented filamentous bacteria (SFB), known for their capacity to enhance the generation of T helper 17 (Th17) cells in the small intestinal lamina propria, a key area for mucosal defense and autoimmune disease development [15]. Studies using fecal microbiota transplantation (FMT) from SFB-infected mice into germ-free or normal mice have shown a significant Th17 cell accumulation, highlighting the potential role of SFB in the induction of inflammatory processes in gut mucosa [15, 16].
Conversely, other evidence has also shown that several microbiota components modulate immune responses towards immune tolerance, thus aiding in inflammation control. Bacteroides fragilis, for example, leverages its polysaccharide A antigen to mitigate experimental colitis in mice by reducing proinflammatory interleukin (IL)-17A production, a process dependent on IL-10 production from CD4+ T cells [17]. In addition, Enterococcus faecium could activate the nucleotide-binding oligomerization domain-containing protein 2 (NOD2) gene in myeloid cells, an important gene involved in the regulation of the inflammatory response to bacterial antigens [18], thereby upregulating IL-1β, which subsequently enhances the secretion of IL-22 by lymphoid cells and exerts anti-inflammatory effects [19]. Moreover, the intestinal Tregs, crucial for immunoprotection, are modulated by the intestinal microbiota. Previous studies have also proven that specific strains of bifidobacteria, lactobacilli, and B. fragilis as well as particular Clostridium clusters, could enhance Treg accumulation in gut mucosa, thus providing a promising approach in the prevention of colitis and other inflammatory conditions [20,21].
Intestinal microbial metabolites participate in intestinal mucosal homeostasis
The gastrointestinal tract serves as a complex ecosystem where a diverse community of microorganisms produces a variety of metabolites with significant impacts on host physiology [13]. These metabolites, particularly SCFAs like acetate, propionate, and butyrate, are pivotal in maintaining intestinal mucosal homeostasis. Accumulating lines of evidence have highlighted that SCFA levels are notably reduced in IBD patients and murine colitis models, whereas SCFA supplementation mitigates colitis symptoms [22]. SCFAs are instrumental in preserving intestinal health, providing energy to the colonic cells, and modulating immune responses to reduce intestinal mucosal inflammation [23], and play a key role in energy metabolism regulation. Acetate influences satiety and fat storage, and propionate affects intestinal motility and energy metabolism via glucagon-like peptide 1 (GLP1) secretion. Moreover, butyrate, as the primary energy source for colonic epithelial cells, enhances GLP1 production [13]. Beyond energy metabolism, SCFAs have profound effects on the intestinal immune system, fostering the development of B cells and Tregs, contributing to the intestinal mucosal integrity, and possessing anti-inflammatory properties through the production of IL-18 and inhibition of histone deacetylases [13]. Additionally, SCFAs regulate intestinal IgA production, essential for maintaining gut homeostasis and combating intestinal inflammation. This regulation is driven by acetate that acts through G protein-coupled receptor 43 (GPR43) to prompt dendritic cells to convert vitamin A into retinoic acid, thereby stimulating IgA production by B cells [24]. Furthermore, SCFAs influence cellular immunity by Treg cell differentiation, which further facilitates IL-10 and IL-22 production, essential for maintaining intestinal balance and controlling gut inflammation [25]. Specifically, butyrate has been shown to promote the differentiation of CD4+ T cells into Tregs, thus contributing to potential therapeutic benefits to IBD patients [26]. In addition, SCFAs also constrain the production of pro-inflammatory cytokines by neutrophils, thereby alleviating mucosal inflammatory damage [27].
Bile acids are derivatives of cholesterol, and the intestinal microbiota markedly influences the synthesis and metabolism of bile acids [28]. The primary bile acids are converted into secondary bile acids by the intestinal microbiota, thus greatly expanding the molecular diversity in the intestinal environment. Previous study has demonstrated that B. longum CECT 7894, which contains bile salt hydrolase genes, improves the efficacy of anti-TNF monoclonal antibody [i.e. infliximab (IFX)] in a murine colitis model through the regulation of the metabolism of bile acids [29]. Several therapeutic approaches, such as diet, probiotics, FMT, and ursodeoxycholic acid, could alleviate intestinal mucosal inflammation by regulating bile acids and the intestinal microbiota [30]. Evidence has shown that bile acids play an important role in the maintenance of intestinal microbiota homeostasis and the delicate balance of the mucosal immune system, and generally possess an anti-inflammatory effect. They inhibit the assembly of inflammatory vesicles, decrease the expression of proinflammatory cytokines in macrophages and dendritic cells, and prevent the proinflammatory capacity of monocytes. Moreover, bile acids constrain the differentiation and function of Th17 cells [31], and upregulate the differentiation of Tregs, downregulate proinflammatory cytokines such as IL-17A/F and TNF-α, and upregulate anti-inflammatory cytokines such as IL-10 and transforming growth factor-β (TGF-β). Additionally, bile acids also protect intestinal barrier integrity by enhancing tight junction molecule expression [32].
Tryptophan is an essential aromatic amino acid that humans must obtain from their diet. Poultry, fish, oats, and dairy products are common sources of dietary tryptophan. Plasma tryptophan is decreased in IBD patients due to dysregulated gut microbiota metabolism [13] and is negatively correlated with IBD disease severity [33]. Tryptophan can be converted into a series of indole metabolites by the intestinal microbiota. Indoles promote the release of GLP1, and indole derivatives act as agonists of the transcription factor aryl hydrocarbon receptor, thereby affecting mucosal immunity and CD4+ T cell activation and proliferation [13]. Consistently, supplementation with Lactobacillus-producing tryptophan catabolite could reduce susceptibility to inflammatory disease [34].
The intestinal microbiota also produces other metabolites, such as histamine, which plays a role in maintaining immune responses in gut mucosa. Histamine is a biogenic amine found in a wide variety of cells. The main source of histamine is food, but it can also be synthesized and regulated by the intestinal microbiota. Histamine has proinflammatory effects, and is found to be increased in the inflamed mucosa of IBD patients [35]. Moreover, mast cells also produce histamine upon antigen stimulation in the inflamed mucosa of IBD patients, indicating that mast cells are also involved in the pathogenesis [36].
Dysbiosis of the intestinal microbiota is involved in the development of IBD
Alterations in the intestinal microbiota in IBD patients
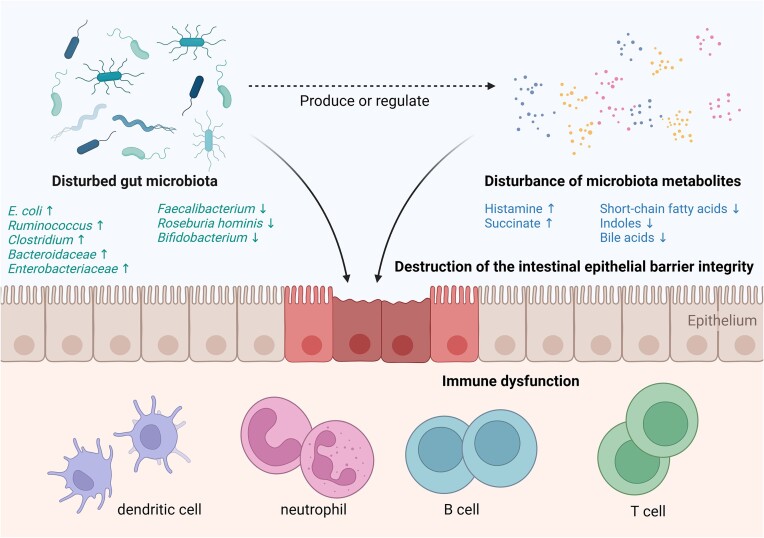
IBD patients have disrupted intestinal microbiota and metabolites, characterized by reduced bacterial diversity, increased invasive microbiota (such as Proteus and Escherichia coli) and decreased protective microbiota (such as Bifidobacterium). Moreover, the content of SCFAs in the stool of IBD patients is significantly lower than that of healthy donors, which is consistent with the low frequencies of SCFA-producing microbiota (such as Faecalibacterium and Methanogenic archaea) in IBD patients [37]. Such alterations are involved in the pathogenesis of IBD [38], causing damage to the intestinal barrier and dysregulated immune response in the gut mucosa [39, 40] (Fig. 1).
Figure 1.
Gut microbiota and metabolites are involved in the pathogenesis of IBD. Gut microbiota and metabolite disturbance in IBD includes a decrease in commensal bacteria and anti-inflammatory metabolites such as SCFAs, and an increase in pathogenic bacteria and proinflammatory metabolites such as histamine and succinate, which then induce compromised intestinal barrier integrity and dysregulated immune responses in gut mucosa, contributing to the pathogenesis of IBD.
In addition to the alterations in intestinal microbiota observed in IBD patients compared to healthy individuals, distinct microbial profiles are associated with each IBD behavior. IBD encompasses two primary subtypes, namely UC and CD, which can be further stratified based on disease location and comorbidities. A recent Mendelian randomization analysis exploring gut microbiota across 10 IBD subtypes revealed that the family Defluviitaleaceae is associated with a reduced risk of colonic CD, while the genus Lachnospiraceae ND3007 group and the genus Hungatella are linked to a decreased risk of left-sided UC. Generally, Hungatella, Acidaminococcaceae, and 13 other microbial taxa are identified as protective factors for various IBD subtypes, whereas Terrisporobacter, Anaerostipes, and 21 other microbial taxa are associated with an increased risk for different IBD subtypes [41]. These findings underscore the potential for modifying gut microbiota composition as a strategy for preventing and improving the prognosis of IBD.
Many other aspects of the altered intestinal microbiota in patients with IBD are also apparant. Increasing lines of evidence have shown that bacterial levels are disturbed in the inflamed mucosa [42], such as an increase in facultative anaerobes such as E. coli but a decrease in beneficial obligate anaerobes like Faecalibacterium prausnitzii and Roseburia hominis. Moreover, higher numbers of Ruminococcus torques and Ruminococcus gnavus are present in IBD patients, along with increased abundance and activity of certain Clostridium species [43]. In addition, a reduction in fumarate and its derivatives in murine colitis models, along with a decrease in fumarate-producing bacteria, suggests a metabolic shift associated with disease status [44]. At the ecological level, some bacterial species in IBD patients have specific co-abundance relationships compared to healthy controls. For example, E. coli shows a positive co-abundance relationship with pro-inflammatory bacteria, but a negative co-abundance relationship with anti-inflammatory bacteria [45], suggesting a disturbance of intestinal microbial interactions that plays an important role in the pathogenesis of IBD.
Advancements in 16S rRNA and shotgun metagenomic sequencing have revolutionized our understanding of the intestinal microbiota, offering a high-resolution view that distinguishes IBD patients from healthy individuals. These technologies accurately analyze intestinal microbial composition from stool or mucosal biopsy samples, generate extensive bioinformatics data through gene sequencing [46], and facilitate the exploration of underlying mechanisms by which intestinal microbes contribute to the onset and progression of IBD. Recently, several studies have identified new loci associated with IBD [47–49], paving the way for better understanding of host–microbe abnormalities in IBD [50]. Some antimicrobial genes are observed to be overexpressed in IBD [51], e.g. expression of the antimicrobial gene regenerating family member 3 (REG3) is abnormally increased in IBD patients [19], while GPR65, which has antibacterial effects, is significantly reduced in the inflamed intestinal epithelium [52]. GPR84, which can be stimulated by lipopolysaccharide, is increased in inflamed colon tissue [53]. These studies help to reveal the mechanism of the involvement of intestinal microbiota in the occurrence and development of IBD and provide an avenue for potential therapeutic intervention.
Dysregulated intestinal microbiota facilitates intestinal inflammation in IBD
Significant alterations in the composition and functionality of the gut microbiota are present in IBD patients. An upsurge in Bacteroidaceae [54] and Enterobacteriaceae families has been observed in an experimental colitis model in mice, linked with an increase in intestinal formic acid [55]. Such dysbiosis aggravates the inflammatory processes, highlighting a vicious cycle between the intestinal microbiota imbalance and intestinal inflammation.
Further studies have underscored the intricate relationship between microbiota dysfunction and intestinal inflammation. For example, mice deficient in caspase recruitment domain family member 9 (CARD9) exhibit altered tryptophan metabolism by the gut microbiota, leading to a decrease in IL-22 production and a heightened vulnerability to colitis [56], suggesting that the intestinal microbial metabolite activities are crucial for modulating the immune response and maintaining mucosal integrity. Additionally, experiments involving Lactobacillus strains capable of metabolizing tryptophan have shown promising results in mitigating intestinal inflammation, thus underscoring the potential therapeutic benefits of modulating the intestinal microbial metabolism [57]. Moreover, the role of specific microbial metabolites, such as butyrate produced by Clostridium strains, has been highlighted in controlling intestinal mucosal inflammation. In line with this, limiting the beneficial microbes exacerbates intestinal inflammation, whereas supplementation of the gut with butyrate-producing probiotics has shown efficacy in alleviating colitis symptoms, indicating that strategic manipulation of the gut microbiota to enhance the presence of beneficial metabolite-producing microbes could offer a novel approach to managing IBD [58].
Regulating the intestinal microbiota of IBD patients appears to directly impact the degree of intestinal inflammation. When a large number of bacteria invade the gut mucosa, neutrophils migrate into the intestinal epithelial area to clear bacterial infection. However, dysfunctional regulation of neutrophils in mucosal homeostasis not only promotes susceptibility to intestinal epithelial damage (e.g. cryptitis and crypt abscess), but also affects intestinal inflammation [59]. Enterobacteriaceae is found to be increased in the intestines of early CD patients, and elimination of these bacteria from the intestines has been shown to reduce the degree of mucosal inflammation [60]. A decrease in the abundance of intestinal F. prausnitzii, an anti-inflammatory bacterium, in patients with CD has been linked to a higher risk of recurrent inflammation [61]. The supernatants of F. prausnitzii have been observed to downregulate nuclear factor (NF)-κB activity [62], and stimulation of peripheral blood mononuclear cells by F. prausnitzii in vitro significantly reduces the production of IL-12 and interferon-γ, but facilitates IL-10 production [61]. Notably, oral administration of F. prausnitzii or its supernatants to mice significantly reduces the severity of colitis and tends to correct colitis-related dysbiosis. Moreover, a previous study has shown that the supernatant of F. prausnitzii culture also produces a microbial anti-inflammatory molecule (MAM), which is a seven peptide anti-inflammatory that significantly reduces the activation of the NF-κB pathway. Consistently, the delivery of the MAM plasmid by Lactobacillus has been demonstrated to markedly reduce experimental intestinal inflammation in mice [62]. These data indicate F. prausnitzii as a potential treatment option in IBD.
Dysbiosis of the intestinal microbiota contributes to disease progression by promoting creeping fat formation in CD patients
A previous study highlighted an intriguing aspect of CD pathology: the formation of creeping fat in the mesenteric adipose tissue (MAT) surrounding the intestine [63]. This phenomenon is closely linked to the altered interactions between the host and its intestinal microbiota, along with defects in the intestinal epithelial barrier functions, which facilitates the translocation of the gut microbes into MAT [64]. The mesentery, which connects the intestine to the abdominal wall and supports its blood and lymphatic supply, becomes encased in the creeping fat. Initially, this fat hypertrophy plays a protective role in alleviating intestinal damage and preventing the systemic dissemination of bacterial antigens [65]. However, the dual nature of the creeping fat in CD patients presents a complex scenario. It could mitigate the spread of inflammation and bacteria initially, whereas prolonged accumulation of the creeping fat detrimentally affects the intestinal wall and nearby normal tissues [65]. Accordingly, the adipose tissue in CD patients is not just a passive fat deposit but is actively involved in the immune response, and is enriched with activated immune cells like T cells, B cells, neutrophils, and macrophages. Microbial translocation (e.g. Clostridium innocuum) prompts significant pathological changes in the mesentery, leading to tissue remodeling and fibrosis via macrophage activation [65, 66].
This aspect of CD underlines the intricate relationships between the gut microbiota dynamics, immune responses, and the pathological changes in intestinal and adjacent tissues. Understanding the mechanisms underlying creeping fat formation and its effects on disease progression will open new avenues for potential target therapies in CD, focusing on restoring the balance of the intestinal microbiota and reinforcing intestinal barrier functions.
Oral microbiota translocation and transintestinal migration affect intestinal homeostasis
The intricate connection between the oral microbiota and IBD highlights the complex interplay between different microbial communities within the human body. The oral microbiota, ranking second in abundance only to the gut microbiota, exerts a profound influence on overall health and has been linked to the development of IBD [67]. Alterations in oral microbial composition, often resulting from conditions like periodontitis, have been associated with the pathogenesis of IBD. Studies have shown that periodontitis participates in the pathogenesis of colitis through the swallowing of salivary microbiota [68]. Previous studies have demonstrated that periodontitis exacerbates experimental colitis by introducing oral pathogens, such as Klebsiella and Enterobacteriaceae, into the gastrointestinal tract, where they incite gut inflammation [69, 70]. Moreover, the migration of Th17 cells, activated in response to oral pathogens, to the gut further underscores the systemic impact of oral microbial imbalances on intestinal health [70]. Porphyromonas gingivalis, a notable periodontal pathogen, has been implicated in altering the gut's microbial landscape and immune balance, specifically the Th17/Treg cell ratio, thereby aggravating intestinal inflammation [71].
Several studies have revealed the host–microbe interactions across multiple tissues in CD patients based on spatial omics analysis. Hazardous conditions such as intestinal inflammation, transmural inflammation, and impaired intestinal vascular barrier integrity may promote bacterial dissemination to intestinal and external tissues, accompanied by immune cell activation, and further contribute to the onset and persistence of intestinal inflammation [72, 73]. These data suggest that host microbes not only affect the intestinal mucosa, but also participate in the extraintestinal progression of inflammation by affecting MAT, mesentery, and mesenteric lymph nodes.
The evidence mentioned above underscores the importance of both local and distant microbial communities in the etiology and pathology of IBD, suggesting a broader therapeutic perspective that manages oral health to maintain intestinal homeostasis and prevent disease exacerbation.
Intestinal microbiota in the precision diagnosis and treatment of IBD
Intestinal microbiota and metabolites are used for precision diagnosis of IBD
Advances in understanding the intestinal microbiota and metabolites offer new avenues for the precision diagnosis and treatment of IBD. Notably, the genetic signatures of fecal microbes from CD patients are promising as non-invasive biomarkers [74]. One pivotal study analyzed fecal samples from 1418 CD patients and healthy individuals across multiple cohorts and demonstrated the utility of microbial genes in the precision diagnosis, achieving a high accuracy in distinguishing CD from other conditions. Specifically, the phosphotransferase system, particularly the celB and manY genes, has emerged as a significant contributor to diagnostic performance, thus underscoring the potential of these microbial genes as reliable, non-invasive diagnostic tools for CD [75].
Moreover, analysis of bacterial profiles in fecal samples has shown significant potential for differential diagnosis. A multinational study involving 2045 stool samples identified distinct microbial patterns in CD patients, characterized by reduced diversity and stability. This study identified that eight microorganisms can be used to discriminate CD patients from other diseases. Another study showed that a machine-learning predictive model, developed from fecal microbiota analysis, can effectively diagnose and classify UC and CD [76], highlighting the diagnostic potential of fecal bacterial analysis in clinical practice [77].
Additionally, the discovery of anti-paratuberculosis-nocardia polypeptide antibody as a biomarker opens new doors for assessing CD severity and activity. This antibody, produced in response to Mycobacterium avium subsp. paratuberculosis infection, has shown excellent predictive value for active CD, further illustrating the burgeoning role of microbial and immune markers in diagnosing and understanding IBD [78].
Bile acids, regulated by the intestinal microbiota, have an excellent diagnostic efficacy in stratifying IBD activity. The ratio of serum primary to secondary bile acids was found to be an ideal index for stratifying IBD activity [79]. These data suggest that microbial metabolites (e.g. bile acids) can be used for the differential diagnosis of IBD.
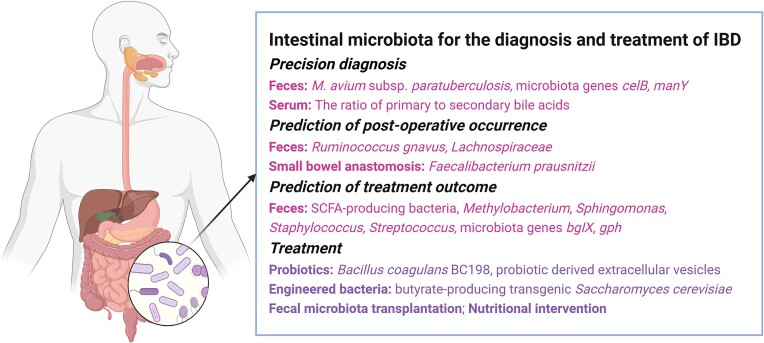
The heterogeneity of the host genes and gut microbiota in the population leads to the heterogeneity of the host–microbiota interaction. Current progress on host genomics, epigenomics, microbiome, metabolomics, and other omics of IBD, is expected to deepen the understanding of IBD disease characteristics in different cohorts [80] and achieve precision and personalized medicine in IBD. These insights into the microbial and metabolic underpinnings of IBD not only enhance diagnostic accuracy but also pave the way for personalized treatment approaches, leveraging the unique microbial signatures of individuals to tailor interventions (Fig. 2).
Figure 2.
Intestinal microbiota and metabolites have multiple applications in IBD, including precision diagnosis, prediction of post-surgical occurrence and treatment outcome, and effective therapeutic strategies. Feces is a commonly used test sample, and bacteria and bacterial genes in feces are potential biomarkers for IBD.
Intestinal microbiota predicts post-operative occurrence of IBD
The predictive power of the intestinal microbiota extends to forecasting surgical outcomes in IBD treatment. For UC patients undergoing colectomy with ileal pouch–anal anastomosis, preoperative fecal bacterial profiles have been linked to postoperative complications like pouchitis. Specific bacterial shifts, an increase in pathogens like R. gnavus, and a decrease in beneficial microbes such as those from the Lachnospiraceae family, have emerged as potential indicators of post-surgical prognosis, offering a new method to assess the risk of postoperative complications [81].
Moreover, the recurrence of CD after ileocolonic resection can be anticipated by analyzing microbial patterns in the small intestine and at the anastomosis site. Previous studies have identified distinct microbial compositions associated with either recurrence or sustained remission, pointing to the role of specific microbes like F. prausnitzii in potentially protecting against mucosal inflammation recurrence [82].
These insights into the diagnostic and prognostic capabilities of the intestinal microbiota underscore their potential as a tool in managing IBD more effectively. By predicting surgical outcomes, clinicians can tailor treatment plans more accurately, enhancing patient care and optimizing treatment efficacy.
Intestinal microbiota predicts the therapeutic outcome in IBD
The intestinal microbiota and metabolites hold promise in predicting responses to biotherapy for IBD. With fecal microbiota serving as a mirror to the gut's microbial ecosystem, an analysis can inform on the likely success of biological treatments [83].
The characteristics of the fecal microbiota in IBD patients at baseline have the potential to predict multi-level treatment effects [84]. A more diverse intestinal microbiota at baseline may indicate a more abundant anti-inflammatory microbiome, and that microbial diversity at baseline may predict treatment outcomes [85]. A good anti-inflammatory effect is more likely to be achieved when SCFA-producing bacteria are abundant. In addition, microorganisms that cause succinate accumulation are often associated with alleviating intestinal inflammation [86]. Some fecal bacteria and metabolites may predict the outcome of IFX therapy in pediatric CD patients. A multi-omics analysis of stool samples from 49 children with CD and healthy controls showed that the disease severity of CD and the outcome of IFX treatment are correlated with the abundance of certain intestinal bacteria and the levels of metabolites. Methylobacterium, Sphingomonas, Staphylococcus, and Streptococcus were abundant in the intestines of pediatric CD patients who achieved sustained remission after IFX treatment. At the same time, pediatric CD patients who achieved sustained remission after IFX treatment had higher concentrations of amino acids and butyrate in fecal samples [87]. These alterations in feces may be potential prognostic biomarkers in pediatric CD patients.
Fecal microbiota may also predict the efficacy of anti-TNF antibody [i.e. adalimumab (ADA)] in CD. Increased protective microbiota (such as Barnesiella, Anaerostipes, Tyzzerella, Lachnoclostridium) and decreased pathogenic bacteria (E. coli-Shigella) were observed in ADA-responsive fecal samples. Moreover, the gene bgIX, encoding b-glucosidase, and the gene gph, encoding phosphoglycolate phosphatase, were also enriched in fecal samples from the ADA-responsive group. However, the abundance of genes encoding ATP-binding cassette was significantly increased in fecal samples from the ADA non-responsive group [88].
Alteration in the intestinal microbiota is observed in UC patients in response to treatment with steroids and biological agents, indicating the potential for gut microbiota profiling to predict UC treatment outcomes. Responders to steroid therapy exhibit changes in β-diversity and enrichment of beneficial bacteria, including Blautia, Anaerostipes, and Bifidobacterium, compared to non-responders [89]. In UC patients receiving anti-α4β7 monoclonal antibody (mAb) (i.e. vedolizumab), responders at 14 weeks show increased abundances of an unannotated genus from the Barnesiellaceae family, whereas non-responders at 14 weeks are more associated with Collinsella. Moreover, the genus cc_115 from the Erysipelotrichaceae family was more prevalent in non-responders at 52 weeks [90].
Taken together, these approaches have revealed that microbial composition and metabolic activity are significantly correlated with therapeutic outcomes, paving the way for personalized treatment strategies based on an individual's microbiota profile.
Adjustment of the intestinal microbiota is used in the clinical treatment of IBD
Modulation of the gut microbiota presents a promising therapeutic avenue in treating IBD, as reflected in the beneficial effects of probiotics. Commonly used strains in the probiotic industry, including Lactobacillus, Bifidobacterium, Lactococcus, Streptococcus thermophilus, E. coli Nissle 1917, and Saccharomyces boulardii, have shown potential in maintaining gut health. Specifically, Bacillus coagulans BC198 has demonstrated its ability to fortify intestinal barrier functions, mitigate inflammatory cell infiltration in the colon, and rebalance the gut microbiota, marking it as a viable probiotic candidate for the management of IBD [91]. The efficacy of probiotics is attributed to their multifaceted roles in immune modulation, stress resistance, pathogen inhibition, microbiota adjustment, and bolstering the intestinal epithelial barrier, making them effective in both inducing and maintaining remission in IBD. Probiotics have been used in many intestinal disorders, such as irritable bowel syndrome [92], and have also shown promise in the treatment of IBD patients [93]. The utilization of probiotics, prebiotics, and synbiotics has been demonstrated to be effective in inducing and maintaining remission in IBD and decreasing the UC disease activity index [94]. Intestinal microbiota-derived extracellular vesicles are thought to play a key role in bacteria–host communication [95], and extracellular vesicles derived from probiotic Clostridium butyricum have been proven to protect the intestinal barrier function in UC [96]. In addition, probiotic agents, such as paraprobiotics (inactivated bacteria or their components) and postprobiotics (bacterial metabolites or equivalent synthetic products), are easier to store and manufacture, and cause fewer side effects, making them a potential adjunctive treatment modality [97].
Innovations in microbial engineering have expanded the therapeutic potential of microorganisms [98]. For instance, genetically modifying Saccharomyces cerevisiae to produce butyrate has resulted in increased intestinal levels of this beneficial metabolite, offering promise in both clinical and preclinical settings [99]. Furthermore, artificial-enzyme-modified B. longum has been developed to enhance antioxidant activity within the gut, showcasing the ability to modulate the immune system and reduce inflammation in IBD models [100]. In another study, an oral probiotic E. coli Nissle 1917 was genetically engineered to overexpress catalase and superoxide dismutase for the treatment of intestinal inflammation [101].
FMT represents another frontier in IBD treatment. By transferring fecal microbiota from healthy donors to patients, FMT aims at restoring a healthy gut microbial balance, thus offering a novel therapeutic strategy that has already proven successful in treating recurrent Clostridium difficile infections with high cure rates [7]. Several lines of evidence have demonstrated the efficacy of FMT in the treatment of IBD, especially UC, inducing significant remissions and improving the intestinal microbial diversity of patients. A recent study has found that its preparation method may be related to treatment outcomes, e.g. frozen FMT samples or washed microbiota transplantation may have better efficacy or safety [102], which requires further investigation. As a new treatment modality, more randomized, double-blind clinical trials should be conducted in the future to establish clear indications and treatment options for FMT in IBD. The potential of FMT in IBD treatment lies in its capacity to modify the gut ecosystem favorably, demonstrating the critical role of host–microbiota interactions in managing the disease and achieving remission.
Nutritional intervention can regulate the intestinal microbiota and is a common adjuvant treatment strategy for IBD [103]. Several nutritional interventions, including enteral nutrition and dietary control, significantly affect the intestinal microbiota, thus promoting clinical remission and mucosal healing [104]. Notably, other dietary interventions, including a specific carbohydrate diet, a mediterranean diet, and a low-fat diet, also affect the intestinal microbiota, disease activity, and gastrointestinal symptoms in IBD [105]. These emerging therapeutic approaches underscore the significant impact of gut microbiota modulation on treatment strategies for IBD, offering a new horizon for patient care through the restoration of microbial balance and function.
Conclusions
The intestinal microbiota plays an important role in the physiological regulation of intestinal mucosal homeostasis, and alterations in the intestinal microbiota are involved in the pathogenesis of IBD. Study of the intestinal microbiota and metabolites helps us elucidate the pathogenesis of IBD and has the potential to be applied in the precision diagnosis, prognosis, and drug efficacy evaluation of IBD. By adjusting the intestinal microbiota, selecting specific microorganisms, or FMT, the intestinal flora can be used in the clinical treatment of IBD.
Despite remarkable progress in the field of IBD microbiology, many issues remain to be resolved before precision microbial diagnosis and treatment can be achieved. Evidence for microbial therapies such as probiotics and FMT is insufficient, so more prospective studies are warranted in the future, along with further discovery of microbial predictors to identify appropriate treatment populations. It is also necessary to combine multi-omics analysis to develop precision and personalized microbial medicine for IBD. In addition, if microbial therapy is to be attempted in clinical application in the future, it is essential to optimize the composition, dosage form, and administration regimen of microbial agents. While the intestinal microbiota is a promising diagnostic and therapeutic target for IBD, its clinical application remains a long-term goal.
Abbreviations
IBD: inflammatory bowel disease; CD: Crohn's disease; UC: ulcerative colitis; SCFA: short-chain fatty acids; Tregs: regulatory T cells; SFB: segmented filamentous bacteria; Th: helper T cells; FMT: fecal microbiota transplantation; GPR: G protein-coupled receptor; IFX, infliximab; MAM: microbial anti-inflammatory molecule; MAT: mesenteric adipose tissue; ADA: adalimumab.
Acknowledgement
The study was supported by grants from the National Natural Science Foundation of China (Grant Nos. 82370532, 82341219) and Shanghai Hospital Development Center Foundation (Grant No. SHDC12022118). We thank all the physicians, nurses, and students of the Shanghai Tenth People's Hospital Affiliated to Tongji University for their assistance.
Contributor Information
Long Ju, Center for Inflammatory Bowel Disease Research and Department of Gastroenterology, Shanghai Tenth People's Hospital of Tongji University, Shanghai 200072, China.
Zhimin Suo, Department of Gastroenterology, Huaihe Hospital of Henan University, Kaifeng 475000, China.
Jian Lin, Center for Inflammatory Bowel Disease Research and Department of Gastroenterology, Shanghai Tenth People's Hospital of Tongji University, Shanghai 200072, China; Department of Gastroenterology, Affiliated Hospital of Putian University, Putian 351100, China.
Zhanju Liu, Center for Inflammatory Bowel Disease Research and Department of Gastroenterology, Shanghai Tenth People's Hospital of Tongji University, Shanghai 200072, China.
Author contributions
L.J., Z.S., and J.L. contributed to conceptualization and writing of the manuscript. Z.L. contributed to conceptualization and review and editing of the manuscript.
Conflict of interest
None declared. In addition, as an Editorial Board Member of Precision Clinical Medicine, the corresponding author Z.L. was blinded from reviewing and making decisions on this manuscript.
References
- 1. Ahlawat S, Kumar P, Mohan Het al. . Inflammatory bowel disease: tri-directional relationship between microbiota, immune system and intestinal epithelium. Crit Rev Microbiol. 2021;47:254–73. 10.1080/1040841x.2021.1876631 [DOI] [PubMed] [Google Scholar]
- 2. Khalili H, Chan SSM, Lochhead Pet al. . The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2018;15:525–35. 10.1038/s41575-018-0022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu W, Gao X, Liu Z. Psychological stress as a detrimental factor in colitis. Med (New York, NY). 2023;4:401–3. 10.1016/j.medj.2023.06.008 [DOI] [PubMed] [Google Scholar]
- 4. Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17. 10.1038/nature10209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Quaglio AEV, Grillo TG, De Oliveira ECSet al. . Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol. 2022;28:4053–60. 10.3748/wjg.v28.i30.4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen H, Li H, Liu Z. Interplay of intestinal microbiota and mucosal immunity in inflammatory bowel disease: a relationship of frenemies. Therapeutic Advances in Gastroenterology. 2020;13:1756284820935188. 10.1177/1756284820935188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ooijevaar RE, Terveer EM, Verspaget HWet al. . Clinical application and potential of fecal microbiota transplantation. Annu Rev Med. 2019;70:335–51. 10.1146/annurev-med-111717-122956 [DOI] [PubMed] [Google Scholar]
- 8. Sender R, Fuchs S, Milo R. Revised estimates for the number of Human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. 10.1371/journal.pbio.1002533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eckburg PB, Bik EM, Bernstein CNet al. . Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. 10.1126/science.1110591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yao S, Zhao Z, Wang Wet al. . Bifidobacterium longum: protection against inflammatory bowel disease. J Immunol Res. 2021;2021:1. 10.1155/2021/8030297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vich Vila A, Hu S, Andreu-Sánchez Set al. . Faecal metabolome and its determinants in inflammatory bowel disease. Gut. 2023;72:1472–85. 10.1136/gutjnl-2022-328048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Dong Q, Hu Set al. . Decoding microbial genomes to understand their functional roles in human complex diseases. iMeta. 2022;1: e14. 10.1002/imt2.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:223–37. 10.1038/s41575-019-0258-z [DOI] [PubMed] [Google Scholar]
- 14. Prame Kumar K, Ooi JD, Goldberg R. The interplay between the microbiota, diet and T regulatory cells in the preservation of the gut barrier in inflammatory bowel disease. Front Microbiol. 2023;14:1291724. 10.3389/fmicb.2023.1291724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang Y, Torchinsky MB, Gobert Met al. . Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152–6. 10.1038/nature13279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ivanov II, Atarashi K, Manel Net al. . Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. 10.1016/j.cell.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. 10.1038/nature07008 [DOI] [PubMed] [Google Scholar]
- 18. Lu H, Suo Z, Lin Jet al. . Monocyte-macrophages modulate intestinal homeostasis in inflammatory bowel disease. Biomark Res. 2024;12:76. 10.1186/s40364-024-00612-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He X, Zhou H. Decoding the IBD paradox: A triadic interplay between REG3, enterococci, and NOD2. Cell Host Microbe. 2023;31:1425–7. 10.1016/j.chom.2023.08.008 [DOI] [PubMed] [Google Scholar]
- 20. Atarashi K, Tanoue T, Shima Tet al. . Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–41. 10.1126/science.1198469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sefik E, Geva-Zatorsky N, Oh Set al. . MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of rorγ⁺ regulatory T cells. Science. 2015;349:993–7. 10.1126/science.aaa9420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sugihara K, Kamada N. Metabolic network of the gut microbiota in inflammatory bowel disease. Inflamm Regener. 2024;44:11. 10.1186/s41232-024-00321-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu X, Chen H, Gao Xet al. . Natural herbal remedy wumei decoction ameliorates intestinal mucosal inflammation by inhibiting Th1/Th17 cell differentiation and maintaining microbial homeostasis. Inflamm Bowel Dis. 2022;28:1061–71. 10.1093/ibd/izab348 [DOI] [PubMed] [Google Scholar]
- 24. Wu W, Sun M, Chen Fet al. . Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunology. 2017;10:946–56. 10.1038/mi.2016.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang W, Yu T, Huang Xet al. . Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020;11:4457. 10.1038/s41467-020-18262-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun M, Wu W, Chen Let al. . Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:3555. 10.1038/s41467-018-05901-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li G, Lin J, Zhang Cet al. . Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes. 2021;13:1968257. 10.1080/19490976.2021.1968257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guo X, Okpara ES, Hu Wet al. . Interactive relationships between intestinal flora and bile acids. Int J Mol Sci. 2022;23:8343. 10.3390/ijms23158343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiao F, Dong F, Li Xet al. . Bifidobacterium longum CECT 7894 improves the efficacy of Infliximab for DSS-induced colitis via regulating the gut microbiota and bile acid metabolism. Front Pharmacol. 2022;13:902337. 10.3389/fphar.2022.902337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang M, Gu Y, Li Let al. . Bile acid-gut microbiota axis in inflammatory bowel disease: from bench to bedside. Nutrients. 2021;13:3143. 10.3390/nu13093143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paik D, Yao L, Zhang Yet al. . Human gut bacteria produce T(H)17-modulating bile acid metabolites. Nature. 2022;603:907–12. 10.1038/s41586-022-04480-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun R, Xu C, Feng Bet al. . Critical roles of bile acids in regulating intestinal mucosal immune responses. Therap Adv Gastroenterol. 2021;14:175628482110180. 10.1177/17562848211018098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu Y, Chen Z, Xu Cet al. . Disturbances of the gut microbiota and microbiota-derived metabolites in inflammatory bowel disease. Nutrients. 2022;14:5140. 10.3390/nu14235140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang R, Wu F, Zhou Qet al. . Lactobacillus and intestinal diseases: mechanisms of action and clinical applications. Microbiol Res. 2022;260:127019. 10.1016/j.micres.2022.127019 [DOI] [PubMed] [Google Scholar]
- 35. Raithel M, Matek M, Baenkler HWet al. . Mucosal histamine content and histamine secretion in Crohn's disease, ulcerative colitis and allergic enteropathy. Int Arch Allergy Immunol. 1995;108:127–33. 10.1159/000237129 [DOI] [PubMed] [Google Scholar]
- 36. Smolinska S, Winiarska E, Globinska Aet al. . Histamine: A mediator of intestinal disorders-A review. Metabolites. 2022;12:895. 10.3390/metabo12100895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sartor RB, Wu GD. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology. 2017;152:327–39. 10.1053/j.gastro.2016.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu M, Song Y, Xu Yet al. . Manipulating microbiota in inflammatory bowel disease treatment: clinical and natural product interventions explored. Int J Mol Sci. 2023;24:11004. 10.3390/ijms241311004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gao H, Peng K, Shi Yet al. . Development and validation of a novel criterion of histologic healing in ulcerative colitis defined by inflammatory cell enumeration in lamina propria mucosa: A multicenter retrospective cohort in China. Chin Med J (Engl). 2024;137:1316–23. 10.1097/cm9.0000000000003154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lu H, Zhang C, Wu Wet al. . MCPIP1 restrains mucosal inflammation by orchestrating the intestinal monocyte to macrophage maturation via an ATF3-AP1S2 axis. Gut. 2023;72:882–95. 10.1136/gutjnl-2022-327183 [DOI] [PubMed] [Google Scholar]
- 41. Li F, Yu C, Zhao Qet al. . Exploring the intestinal ecosystem: from gut microbiota to associations with subtypes of inflammatory bowel disease. Front Cell Infect Microbiol. 2023;13:1304858. 10.3389/fcimb.2023.1304858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rhodes JM The role of Escherichia coli in inflammatory bowel disease. Gut. 2007;56:610–2. 10.1136/gut.2006.111872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Conrad MA, Bittinger K, Ren Yet al. . The intestinal microbiome of inflammatory bowel disease across the pediatric age range. Gut microbes. 2024;16:2317932. 10.1080/19490976.2024.2317932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen H, Wu X, Sun Ret al. . Dysregulation of CD177(+) neutrophils on intraepithelial lymphocytes exacerbates gut inflammation via decreasing microbiota-derived DMF. Gut Microbes. 2023;15:2172668. 10.1080/19490976.2023.2172668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen L, Collij V, Jaeger Met al. . Gut microbial co-abundance networks show specificity in inflammatory bowel disease and obesity. Nat Commun. 2020;11:4018. 10.1038/s41467-020-17840-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wiredu Ocansey DK, Hang S, Yuan Xet al. . The diagnostic and prognostic potential of gut bacteria in inflammatory bowel disease. Gut microbes. 2023;15:2176118. 10.1080/19490976.2023.2176118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu Z, Liu R, Gao Het al. . Genetic architecture of the inflammatory bowel diseases across East Asian and European ancestries. Nat Genet. 2023;55:796–806. 10.1038/s41588-023-01384-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gao H, Liu Z. The latest breakthrough on genetic characteristics of inflammatory bowel disease in Chinese and other East Asian ancestries. Precision Clinical Medicine. 2023;6:pbad017. 10.1093/pcmedi/pbad017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gao H, Liu R, Huang Het al. . Susceptibility gene profiling elucidates the pathogenesis of inflammatory bowel disease and provides precision medicine. Clinical & Translational Med. 2023;13:e1404. 10.1002/ctm2.1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Qiu P, Ishimoto T, Fu Let al. . The gut microbiota in inflammatory bowel disease. Front Cell Infect Microbiol. 2022;12:733992. 10.3389/fcimb.2022.733992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lloyd-Price J, Arze C, Ananthakrishnan ANet al. . Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–62. 10.1038/s41586-019-1237-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li G, Lin J, Gao Xet al. . Intestinal epithelial pH-sensing receptor GPR65 maintains mucosal homeostasis via regulating antimicrobial defense and restrains gut inflammation in inflammatory bowel disease. Gut microbes. 2023;15:2257269. 10.1080/19490976.2023.2257269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang Q, Chen LH, Yang Het al. . GPR84 signaling promotes intestinal mucosal inflammation via enhancing NLRP3 inflammasome activation in macrophages. Acta Pharmacol Sin. 2022;43:2042–54. 10.1038/s41401-021-00825-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Berry D, Schwab C, Milinovich Get al. . Phylotype-level 16S rRNA analysis reveals new bacterial indicators of health state in acute murine colitis. ISME J. 2012;6:2091–106. 10.1038/ismej.2012.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hughes ER, Winter MG, Duerkop BAet al. . Microbial respiration and formate oxidation as metabolic signatures of inflammation-associated dysbiosis. Cell Host Microbe. 2017;21:208–19. 10.1016/j.chom.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sokol H, Conway KL, Zhang Met al. . Card9 mediates intestinal epithelial cell restitution, T-helper 17 responses, and control of bacterial infection in mice. Gastroenterology. 2013;145:591–601.e3. 10.1053/j.gastro.2013.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lamas B, Richard ML, Leducq Vet al. . CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22:598–605. 10.1038/nm.4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tye H, Yu CH, Simms LAet al. . NLRP1 restricts butyrate producing commensals to exacerbate inflammatory bowel disease. Nat Commun. 2018;9:3728. 10.1038/s41467-018-06125-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen H, Wu X, Xu Cet al. . Dichotomous roles of neutrophils in modulating pathogenic and repair processes of inflammatory bowel diseases. Precision Clinical Medicine. 2021;4:246–57. 10.1093/pcmedi/pbab025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhu W, Winter MG, Byndloss MXet al. . Precision editing of the gut microbiota ameliorates colitis. Nature. 2018;553:208–11. 10.1038/nature25172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sokol H, Pigneur B, Watterlot Let al. . Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–6. 10.1073/pnas.0804812105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Quévrain E, Maubert MA, Michon Cet al. . Identification of an anti-inflammatory protein from faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn's disease. Gut. 2016;65:415–25. 10.1136/gutjnl-2014-307649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Crohn BB, Ginzburg L, Oppenheimer GD. Landmark article Oct 15, 1932. Regional ileitis. A pathological and clinical entity. By Burril B. Crohn, Leon Ginzburg, and Gordon D. Oppenheimer. JAMA. 1984;251:73–9. 10.1001/jama.251.1.73 [DOI] [PubMed] [Google Scholar]
- 64. Giambra V, Pagliari D, Rio Pet al. . Gut microbiota, inflammatory bowel disease, and cancer: the role of guardians of innate immunity. Cells. 2023;12:2654. 10.3390/cells12222654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ha CWY, Martin A, Sepich-Poore GDet al. . Translocation of viable gut microbiota to mesenteric adipose drives formation of creeping fat in humans. Cell. 2020;183:666–83. 10.1016/j.cell.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Spencer SP, Sonnenburg JL. When gut microbiota creep into fat, the fat creeps back. Cell. 2020;183:589–91. 10.1016/j.cell.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tanwar H, Gnanasekaran JM, Allison Det al. . Unraveling the oral-gut axis: interconnection between periodontitis and IBD, current challenges, and future perspective. Journal of Crohn's & Colitis. 2024; 18: 1319–41. 10.1093/ecco-jcc/jjae028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Qian J, Lu J, Huang Yet al. . Periodontitis salivary microbiota worsens colitis. J Dent Res. 2022;101:559–68. 10.1177/00220345211049781 [DOI] [PubMed] [Google Scholar]
- 69. Atarashi K, Suda W, Luo Cet al. . Ectopic colonization of oral bacteria in the intestine drives T(H)1 cell induction and inflammation. Science. 2017;358:359–65. 10.1126/science.aan4526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kitamoto S, Nagao-Kitamoto H, Jiao Yet al. . The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell. 2020;182:447–62. 10.1016/j.cell.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jia L, Jiang Y, Wu Let al. . Porphyromonas gingivalis aggravates colitis via a gut microbiota-linoleic acid metabolism-Th17/treg cell balance axis. Nat Commun. 2024;15:1617. 10.1038/s41467-024-45473-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gao X, Sun R, Jiao Net al. . Integrative multi-omics deciphers the spatial characteristics of host-gut microbiota interactions in Crohn's disease. Cell Reports Medicine. 2023;4:101050. 10.1016/j.xcrm.2023.101050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vujkovic-Cvijin I, Welles HC, Ha CWYet al. . The systemic anti-microbiota IgG repertoire can identify gut bacteria that translocate across gut barrier surfaces. Sci Transl Med. 2022;14:eabl3927. 10.1126/scitranslmed.abl3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ning L, Zhou YL, Sun Het al. . Microbiome and metabolome features in inflammatory bowel disease via multi-omics integration analyses across cohorts. Nat Commun. 2023;14:7135. 10.1038/s41467-023-42788-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gao S, Gao X, Zhu Ret al. . Microbial genes outperform species and SNVs as diagnostic markers for Crohn's disease on multicohort fecal metagenomes empowered by artificial intelligence. Gut microbes. 2023;15:2221428. 10.1080/19490976.2023.2221428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kang DY, Park JL, Yeo MKet al. . Diagnosis of Crohn's disease and ulcerative colitis using the microbiome. BMC Microbiol. 2023;23:336. 10.1186/s12866-023-03084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pascal V, Pozuelo M, Borruel Net al. . A microbial signature for Crohn's disease. Gut. 2017;66:813–22. 10.1136/gutjnl-2016-313235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gao H, He Q, Xu Cet al. . The development and validation of anti-paratuberculosis-nocardia polypeptide antibody [Anti-pTNP] for the diagnosis of Crohn's Disease. Journal of Crohn's & Colitis. 2022;16:1110–23. 10.1093/ecco-jcc/jjac008 [DOI] [PubMed] [Google Scholar]
- 79. Chen W, Wang D, Deng Xet al. . Bile acid profiling as an effective biomarker for staging in pediatric inflammatory bowel disease. Gut microbes. 2024;16:2323231. 10.1080/19490976.2024.2323231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mu C, Zhao Q, Zhao Qet al. . Multi-omics in Crohn's disease: new insights from inside. Comput Struct Biotechnol J. 2023;21:3054–72. 10.1016/j.csbj.2023.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Machiels K, Sabino J, Vandermosten Let al. . Specific members of the predominant gut microbiota predict pouchitis following colectomy and IPAA in UC. Gut. 2017;66:79–88. 10.1136/gutjnl-2015-309398 [DOI] [PubMed] [Google Scholar]
- 82. Mondot S, Lepage P, Seksik Pet al. . Structural robustness of the gut mucosal microbiota is associated with Crohn's disease remission after surgery. Gut. 2016;65:954–62. 10.1136/gutjnl-2015-309184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang C, Gu Y, Chu Qet al. . Gut microbiota and metabolites as predictors of biologics response in inflammatory bowel disease: A comprehensive systematic review. Microbiol Res. 2024;282:127660. 10.1016/j.micres.2024.127660 [DOI] [PubMed] [Google Scholar]
- 84. Franzin M, Stefančič K, Lucafò Met al. . Microbiota and drug response in inflammatory bowel disease. Pathogens (Basel, Switzerland). 2021;10:211. 10.3390/pathogens10020211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mah C, Jayawardana T, Leong Get al. . Assessing the relationship between the gut microbiota and inflammatory bowel disease therapeutics: A systematic review. Pathogens (Basel, Switzerland). 2023;12:262. 10.3390/pathogens12020262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lee JWJ, Plichta D, Hogstrom Let al. . Multi-omics reveal microbial determinants impacting responses to biologic therapies in inflammatory bowel disease. Cell Host Microbe. 2021;29:1294–304.e4. 10.1016/j.chom.2021.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang Y, Gao X, Zhang Xet al. . Microbial and metabolic features associated with outcome of infliximab therapy in pediatric Crohn's disease. Gut Microbes. 2021;13:1–18. 10.1080/19490976.2020.1865708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chen L, Lu Z, Kang Det al. . Distinct alterations of fecal microbiota refer to the efficacy of adalimumab in Crohn's disease. Front Pharmacol. 2022;13:913720. 10.3389/fphar.2022.913720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Blesl A, Wurm P, Waschina Set al. . Prediction of response to systemic corticosteroids in active UC by microbial composition-A prospective multicenter study. Inflamm Bowel Dis. 2024;30:9–19. 10.1093/ibd/izad126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Palmieri O, Bossa F, Castellana Set al. . Deciphering microbial composition in patients with inflammatory bowel disease: implications for therapeutic response to biologic agents. Microorganisms. 2024;12:1260. 10.3390/microorganisms12071260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Koh YC, Chang YC, Lin WSet al. . Efficacy and mechanism of the action of live and heat-killed Bacillus coagulans BC198 as potential probiotic in ameliorating dextran sulfate sodium-induced colitis in mice. ACS Omega. 2024;9:10253–66. 10.1021/acsomega.3c07529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Suez J, Zmora N, Segal Eet al. . The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25:716–29. 10.1038/s41591-019-0439-x [DOI] [PubMed] [Google Scholar]
- 93. Glassner KL, Abraham BP, Quigley EMM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. 2020;145:16–27. 10.1016/j.jaci.2019.11.003 [DOI] [PubMed] [Google Scholar]
- 94. Zhang XF, Guan XX, Tang YJet al. . Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: a systematic review and meta-analysis. Eur J Nutr. 2021;60:2855–75. 10.1007/s00394-021-02503-5 [DOI] [PubMed] [Google Scholar]
- 95. Liang X, Dai N, Sheng Ket al. . Gut bacterial extracellular vesicles: important players in regulating intestinal microenvironment. Gut Microbes. 2022;14:2134689. 10.1080/19490976.2022.2134689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ma L, Shen Q, Lyu Wet al. . Clostridium butyricum and its derived extracellular vesicles modulate gut homeostasis and ameliorate acute experimental colitis. Microbiol Spectr. 2022;10:e0136822. 10.1128/spectrum.01368-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Martyniak A, Medyńska-Przęczek A, Wędrychowicz Aet al. . Prebiotics, probiotics, synbiotics, paraprobiotics and postbiotic compounds in IBD. Biomolecules. 2021;11:1903. 10.3390/biom11121903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Feng Z, Hua J, Guo Fet al. . A retrospective analysis of vitamin B6 deficiency and associated changes of gut microbes in Crohn's disease. Eur J Clin Nutr. 2023;77:1034–43. 10.1038/s41430-023-01324-5 [DOI] [PubMed] [Google Scholar]
- 99. Wu J, Huang H, Wang Let al. . A tailored series of engineered yeasts for the cell-dependent treatment of inflammatory bowel disease by rational butyrate supplementation. Gut Microbes. 2024;16:2316575. 10.1080/19490976.2024.2316575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cao F, Jin L, Gao Yet al. . Artificial-enzymes-armed bifidobacterium longum probiotics for alleviating intestinal inflammation and microbiota dysbiosis. Nat Nanotechnol. 2023;18:617–27. 10.1038/s41565-023-01346-x [DOI] [PubMed] [Google Scholar]
- 101. Zhou J, Li M, Chen Qet al. . Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat Commun. 2022;13:3432. 10.1038/s41467-022-31171-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Seida I, Al Shawaf M, Mahroum N. Fecal microbiota transplantation in autoimmune diseases—an extensive paper on a pathogenetic therapy. Autoimmun Rev. 2024;23:103541. 10.1016/j.autrev.2024.103541 [DOI] [PubMed] [Google Scholar]
- 103. Haneishi Y, Furuya Y, Hasegawa Met al. . Inflammatory bowel diseases and gut microbiota. Int J Mol Sci. 2023;24:3817. 10.3390/ijms24043817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li G, Wu X, Gao Xet al. . Long-term exclusive enteral nutrition remodels the gut microbiota and alleviates TNBS-induced colitis in mice. Food & Function. 2022;13:1725–40. 10.1039/d1fo03579g [DOI] [PubMed] [Google Scholar]
- 105. Sugihara K, Kamada N. Diet-microbiota interactions in inflammatory bowel disease. Nutrients. 2021;13:1533. 10.3390/nu13051533 [DOI] [PMC free article] [PubMed] [Google Scholar]