Highlights
-
•
The PUL-Br is valid for assessing upper limb function in Brazilian patients with DMD.
-
•
The PUL-Br presents excellent inter-rater reliability.
-
•
The PUL-Br will standardise the clinical assessment of Brazilian patients with DMD.
-
•
The PUL-Br may guide rehabilitation professionals’ therapeutic decisions.
Keywords: Function, Neuromuscular diseases, Physical therapy, Rehabilitation, Scale, Upper limbs
Abstract
Background
Duchenne muscular dystrophy (DMD) is one of the most common and disabling childhood genetic diseases. The course of DMD involves progressive muscular degeneration and weakness, leading to functional decline. The Performance of the Upper Limb scale (PUL) is a specific instrument designed to assess the upper limb function of patients with DMD.
Objective
To adapt the PUL cross-culturally to Brazilian Portuguese (PUL-Br) and assess the convergent validity, structural validity, inter-rater reliability, and internal consistency for Brazilian patients with DMD
Methods
The cross-cultural adaptation involved six steps: translation to Brazilian Portuguese, Brazilian Portuguese translation synthesis, back-translation to English, back-translation synthesis, an expert committee review, and a pre-final version test (n = 12). The convergent validity of the PUL-Br was evaluated by examining its correlation to the Motor Function Measure scale (MFM) using 30 patients with DMD. Confirmatory factor analysis was conducted to assess structural validity. Intraclass correlation coefficient (ICC) verified the PUL-Br interrater reliability. Cronbach's alpha was calculated to verify internal consistency.
Results
The PUL was cross-culturally adapted to Brazilian Portuguese. A strong and positive correlation was found between the PUL-Br total score and the total score on the MFM (r = 0.83; 95% CI: 0.67, 0.91). The PUL-Br showed a satisfactory fit of the data to the three-factor model, excellent inter-rater reliability (ICC: 0.94), and good internal consistency (Cronbach's: 0.91).
Conclusion
The PUL-Br is valid and reliable for assessing the upper limb function of Brazilian patients with DMD.
Introduction
Duchenne muscular dystrophy (DMD) is one of the most common and disabling childhood myopathies, and affects 1 in every 5000 boys born alive.1 DMD has a clinical presentation with progressive muscular weakness and proximal to distal involvement.2 The lower limbs are less affected in the initial phase of the disease, but gait deterioration begins to emerge around 9–12 years of age.2, 3, 4 In the non-ambulatory stage, the upper limbs become extremely necessary for transfers and mobility.5 The decline of the upper limbs’ function compromises patients’ performance of daily life activities, affecting their independence, socialisation, and quality of life.6,7
Specific instruments, such as the Performance of Upper Limb scale (PUL)8,9 and the Brooke Upper Extremity scale,10,11 which assess upper limb function, are needed to follow disease progression and to select therapeutic strategies to improve function. A recent systematic review identified the PUL as the most suitable instrument for assessing upper limbs function of children and adolescents with DMD.12 Studies indicated an association between upper limbs function using the PUL and other clinical findings.13, 14, 15 Moreover, this scale detected the efficacy of physical therapeutic approaches.16,17
The PUL has not been cross-culturally adapted and validated in Brazilian Portuguese, but it has already been used to assess Brazilian patients with DMD.18,19 Cross-cultural adaptation is crucial to guarantee equivalence between the original version (English language) and the translated version of the instrument.20 The process of cross-cultural adaptation should follow a standard method because mistakes may change the original construct of the instrument, invalidating the results.
The measurement properties of the original PUL presented excellent methodological quality indices according to the Risk of Bias checklist of the Consensus-based Standards for the selection of health Measurement Instruments.12 Nevertheless, it is fundamental to assess measurement properties after cross-cultural adaptation.21, 22, 23 Validity guarantees that the new version of the instrument measures the same construct as the original. Reliability ensures that the new version is free from measurement errors.22,23
The cross-cultural adaptation of the PUL to Brazilian Portuguese will provide a valid and reliable instrument to assess the upper limb function of Brazilian patients with DMD. Therefore, this study aimed to adapt the PUL cross-culturally to Brazilian Portuguese (PUL-Br) and assess the convergent validity, structural validity, inter-rater reliability, and internal consistency. We hypothesize that there will be a strong positive correlation (0.70 ≥ r ≤ 0.89) between the PUL-Br total score and the Motor Function Measure scale (MFM); there will also be good internal consistency (Cronbach's alpha 0.70 - 0.90) and excellent reproducibility (intraclass correlation coefficient [ICC] > 0.90).
Methods
Study design
The present study was completed in two parts: first, the PUL was cross-culturally adapted from its original English version24 to Brazilian Portuguese. Second, the measurement properties of the PUL-Br were tested for children and adolescents with DMD.21, 22, 23 The Ethical Committee of the Clinical Hospital of Ribeirão Preto Medical School, Universidade de Sao Paulo, Ribeirão Preto, SP, Brazil (CAEE: 28600919.1.0000.5440) approved the methods for this study. All legal guardians and patients signed the consent form.
PUL description
The PUL is a performance measure developed to assess upper limb function of patients with DMD greater than five years old.8 The current version of the PUL, version 2.0, consists of 23 items.24 The first item is used to classify the higher functional capacity of the patient. Therefore, a more impaired patient with a lower score on this item will be excluded from testing at the higher and more complex level—namely, the proximal/shoulder level. The other 22 items are divided into the following three domains: proximal (six items), medium (nine items), and distal (seven items). The item scores vary from 0 to 1 or 0 to 2.24 The maximal scores for the domains are 12 (proximal domain), 17 (medium domain), and 13 (distal domain), and the highest total score on the PUL is 42. During the scale application, the patient remains seated in a chair or wheelchair without arm support, and the physical therapist instructs the patient on how to perform each item task. The tester must enter the score for each of the items on the scale. The average time to complete the PUL is 15 min.24
Eligibility criteria
Patients were included if they had a confirmed diagnosis of DMD, were aged 6–18 years and were able to understand the evaluator's instructions and signed, with their guardians, an informed consent form. Patients were excluded if they had a cognitive deficit, as well as if they had sustained a fracture or undergone surgery on the upper limbs in the previous year.
Part 1. cross-cultural adaptation of PUL (2.0)
Participants
The pre-test of the pre-final version of the PUL-Br was completed for 12 patients with DMD. This sample size was based on the study of Okama et al.,25 who performed a cross-cultural adaptation of the North Star scale for the same population.
Procedures
Permission to translate and use the PUL was requested by email from Anna Mayhew (A.M.), the first author of the original scale.8 After permission, the original PUL version 2.0 was translated to Brazilian Portuguese, following international guidelines.20 Two independent translators (G.D. for translation 1 [T1] and B.B. for translation 2 [T2]), who were fluent in both languages (English as the original language of the scale and Brazilian Portuguese as the target language of the scale), but native Brazilian Portuguese speakers, forward-translated the scale. The first translator (G.D.) was also a physical therapist specialising in neurological paediatrics; the second translator (B.B.) was not experienced in healthcare or health research. In sequence, both translations (T1 and T2) were synthesised into a single translation (T1–2). The third step consisted of the back-translation of T1–2 from Brazilian Portuguese to English by two native English speakers (A.C. and P.S.) fluent in Brazilian Portuguese, blind to the original PUL scale and without background in healthcare or health research. Finally, the back-translation of PUL was synthesised in a single back-translation and sent by email to A.M. to ensure the consistency of the terms used. After approval of this version, an expert committee composed of rehabilitation professionals met to check the Brazilian Portuguese terms from the pre-final version of the PUL-Br.
A well-trained researcher (G.C.) trialled the pre-final version of the PUL-Br among Brazilian patients with DMD (n = 12). First, the rater read the item for the patient, and if the patient had comprehension problems, the rater demonstrated how to perform the task. At the end of each assessment, the rater asked the patients about a question or misunderstanding to identify comprehension problems in the pre-final version of the PUL-Br. A 20% threshold of doubts in the same item was used to determine which suggestions should be incorporated into the final version of the scale.26
Part 2. measurement properties
Participants
Thirty patients with DMD were enrolled to determine the convergent validity, structural validity, and internal consistency of the PUL-Br (Supplementary material online). In the pre-test, comprehension problems did not reach 20% for any of the items; as described above, meaning that all items could be included in the final version. Thus, the sample (30 patients) included the 12 patients from the pre-test. To verify the reliability of the PUL-Br, 10 patients with DMD were enrolled. Because DMD is a rare condition, this study's sample size was based on previous research.25,27
Assessments
The assessment was conducted at the Rehabilitation Centre of the Clinical Hospital of Ribeirão Preto Medical School (CER-HC-FMRP-USP). First, measurements were taken of the patient's weight and height, as well as the arm span of some patients (wheelchair users only). A trained researcher applied the PUL-Br and MFM to the patients.
Convergent validity
The MFM was used to assess overall function28 and to determine the convergent validity of the PUL-Br. The MFM consists of 32 items divided into three dimensions. Dimension one relates to aspects of standing position and transfers, with 13 items and a maximal score of 39. Dimension two relates to axial and proximal motor function aspects, with 12 items and a maximal score of 36. Dimension three assesses distal motor function with seven items and a maximal score of 21.28
Structural validity
Using the “lavaan” package in the computer program R, a diagonally weighted least squares confirmatory factor analysis (DWLS-CFA) was conducted to assess the fit between the PUL-Br data and the original three-factor structure (proximal, medium, and distal domains).29 The adequacy of the model was assessed by the following fit indices: the chi-square test, the root mean square error of approximation (RMSEA) with 90% confidence intervals (CI), the adjusted goodness of fit (AGFI), the comparative fit index (CFI), and the Tucker-Lewis index (TLI).30 CFI and TLI values greater than 0.95 and RMSEA values lower than 0.06 are indicators of good model fit.31
Test-retest
The reliability of the PUL-Br was assessed by testing inter-rater reliability. Two trained researchers (G.C. and V.A.) applied the PUL-Br in the sample of patients with DMD. Minimum and maximum intervals of 30 and 60 min between testing were used.
Internal consistency
The internal consistency was assessed among the 23 items of the PUL-Br.
Statistical analysis
A descriptive statistical analysis (median, standard deviation) and an analytical analysis using the R Studio® software version 4.1.3 were performed to determine the results. Pearson correlation coefficient (r) determined the association among the PUL-Br, the MFM total score, and the score on dimension three of the MFM. Dimension three of the MFM is the most specific dimension to assess function of the upper limbs. For this analysis, r = 1.00 indicated a perfect, r = 0.80 strong, 0.50 moderate, 0.20 weak, and 0.00 inexistent correlation.32 A 95% CI that does not include 0 suggests evidence of difference. Intraclass correlation coefficient [ICC (3,1)] was calculated using the irr package in R33 and indicated the inter-rater reliability of the PUL-Br. ICC values < 0.40 represented low, between 0.40 and 0.75 moderate, between 0.75 and 0.90 substantial, and > 0.90 excellent reliability.34 Cronbach's alpha was calculated using the alpha function of the psych package in R.35 Values between 0.7 and 0.9 represented good internal consistency.21,23
Results
Cross-cultural adaptation of PUL (2.0)
The expert committee's assessment of the T1–2 instrument suggested that there should be a modification in a few Brazilian Portuguese terms in favour of specific synonyms. These modifications were intended to facilitate patients’ and future professionals’ understanding of the items. The modifications included changes to the words elevar, estender, levantar and pinça de dedos, which were changed to levantar, esticar, levar, and pinça polpa-polpa, respectively. The average time to complete the scale in the pre-final version was 15 min; this varied among patients according to the stage of the disease. The doubts presented by the patients did not exceed 20%. Therefore, there was no need for modifications between the pre-final version to the final version of the PUL-Br.
Convergent validity of the PUL-Br
Table 1 shows the patients’ characteristics. Table 2 presents the mean values obtained from the functional assessments.
Table 1.
Sample characteristics.
| DMD | Mean (SD) |
|---|---|
| Age (years) | 12.7 (3.0) |
| Body mass (kg) | 48.5 (20.9) |
| Height (cm)⁎ | 139.2 (14.9) |
| Arm span (cm)⁎⁎ | 144.0 (16.5) |
| Male sex (%) | 100% |
| Ambulatory patients (n) | 11 |
| Wheelchair user patients (n) | 19 |
| Physical Therapy (n) | 21 |
n = 13.
n = 18.
Table 2.
Functional assessments of patient performance.
| Scales | Mean (SD) |
|---|---|
| PUL-Br total score | 32.0 (9.0) |
| MFM total score | 56.4 (15.9) |
| Score on dimension 3 of the MFM | 17.3 (3.1) |
MFM, Motor Function Measure scale; PUL, Performance of Upper Limbs scale.
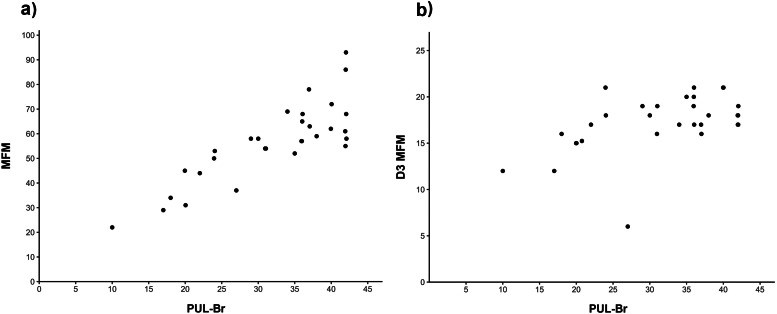
A strong positive correlation was obtained between the PUL-Br total score and the MFM total score (r = 0.83; 95% CI: 0.67, 0.91; Fig. 1a). A moderate positive correlation was obtained between the PUL-Br total score and the score on dimension three of the MFM (r = 0.50; 95% CI: 0.17, 0.73; Fig. 1b).
Fig. 1.
Correlation between the PUL-Br total score and (a) MFM total score; PUL-Br total score and (b) score on dimension three of the MFM (D3 MFM).
Structural validity of the PUL-Br
Because all participants answered that they were able to hold six coins in one hand for item 20 of the PUL-Br, this item was not included in the CFA because it had no variability. Considering the other items and the three-factor structure, the CFA value of CFI was 0.99, TLI was 0.99, AGFI was 0.96, and RMSEA was 0.07 (90% CI: 0.00 to 0.11, p-value that RMSEA ≤ 0.05 = 0.23). The chi-squared test results were X2= 214.14 with 186 degrees of freedom (p = 0.07). The chi-squared test results (p > 0.05) would indicate a good fit; however, this may be due to the small sample size rather than a true satisfactory fit. On the other hand, considering the good performance described by the CFI, TLI, AGFI, and RMSEA indices, we assume that the three-factor model adequately describes our data.
Test-retest of the PUL-Br
For the inter-rater reliability of the PUL-Br, an ICC of 0.94 (95% CI: 0.70, 0.98; p < 0.01) was observed, indicating excellent reliability.
Internal consistency of the PUL-Br
Cronbach's alpha value of the total PUL-Br score was 0.91 (95% CI: 0.81, 0.94), which showed good internal consistency.
Discussion
The findings of the present study confirmed our initial hypotheses, showing a strong and positive correlation between the PUL-Br and the MFM. The PUL-Br exhibited good convergent validity, a satisfactory fit of the data to the three-factor model, excellent inter-rater reliability, and good internal consistency.
Beaton et al.20 indicated that an expert committee should make decisions based on the areas of semantic equivalence between the original and the cross-culturally adapted version. In the present study, after discussing with the expert committee, the translated term pinça de dedos was changed to pinça polpa-polpa to adjust the idiomatic equivalence. The words elevar, estender, and levantar were also replaced by levantar, esticar, and levar, respectively, for adequate semantic equivalence. These last modifications ensured the patients’ understanding. No problems were observed with experiential equivalence or conceptual equivalence.
With the advance of therapeutic approaches for DMD treatment, measuring upper limbs function is increasingly relevant. In the present study, most patients were non-ambulatory (63%) and the mean PUL-Br total score was higher than the mean reported by Mayhew et al.23 (32.0 points vs 28.9). Mayhew et al.24 analysed 177 ambulatory (49%) and non-ambulatory (51%) youths with DMD. Despite this difference in the mean values of the PUL total score between the studies, we may consider that in both studies, the PUL score demonstrated a decline in patient's upper limb function. Moreover, in agreement with this statement, the mean of the MFM total score (56.4) indicates their low functional level.
Concerning the measurement properties, the convergent validity of the present study showed a strong positive correlation of the PUL-Br total score with the MFM total score (r = 0.83; 95% CI: 0.67, 0.91) and a moderate positive correlation with dimension three of the MFM (r = 0.50; 95% CI: 0.17, 0.73). Consistent with our findings, Chiu et al.36 assessed the validity of the PUL version 1.2 in 33 Chinese patients with DMD. They found a strong and positive correlation (r = 0.84) between the PUL and the Hammersmith motor scale.36 About the moderate positive correlation between the total PUL-Br score and dimension three of the MFM, it is important to consider item 4, which is specific to assessing the distal motor function of the lower limbs.
Additionally, our study showed a satisfactory fit of the data to the three-factor model. The three domains of PUL were originally selected to reflect the progression of weakness and the natural history of functional decline in DMD.8 Our findings are consistent with the division proposed by Mayhew et al.8 It should be noted that item 20 of the PUL (pick up coins) had the same score for all patients and was therefore not included in the CFA. The clinical impairment of DMD progresses from proximal to distal,2 which may explain the good performance of all patients on item 20. Excluding this item from the factor analysis would not affect the findings because the distal domain comprises seven items that measure the same construct.
Our study showed good internal consistency (Cronbach's alpha value of 0.91). This finding is consistent with the literature.24,36 Chiu et al.,36 showed a Cronbach's alpha value of 0.97 in the PUL version 1.2. Mayhew et al.24 also showed good reliability of the PUL version 2.0, with Pearson's separation index of 0.95. To our knowledge, this is the first research that assesses the reliability of PUL version 2.0. The inter-rater reliability analysis of the PUL-Br showed excellent reliability (ICC: 0.94). This is consistent with results by Chiu et al.36 (ICC: 0.95), Pane et al.37 (ICC: 0.95), and Gandolla et al.38 (ICC: 0.99) who used the PUL version 1.2. It is worth noting that both versions of the PUL (1.2 and 2.0) presented the same construct and maintained reliability.24
Concerning some limitations, we analysed a limited sample size because of the rarity of the disease. However, our study followed previous studies' sample size and data characteristics that validated and quantified the measurement properties of other instruments for this population.25,27 It was not possible to analyse the intra-rater reliability because the patients had already spent a long time in the outpatient assessment, which had to include other clinical parameters. Thus, the intra-rater reliability of the PUL-Br should be reported in future studies. The data from the present study will provide an important instrument that is cross-culturally adapted and reliable for assessing upper limb function in Brazilian patients with DMD. The PUL has been widely used in clinical practice,9,13,14,39,40 and it can now assist in standardized upper limb function assessments by health professionals in Brazil.
Conclusion
The PUL (2.0) was cross-culturally adapted to Brazilian Portuguese in this study. The PUL-Br showed good convergent validity, a satisfactory fit of the data to the original three-factor model, good internal consistency, and excellent inter-examiner reliability in assessing upper limb function of Brazilian patients with DMD. Based on this, we recommend the PUL-Br for use in clinical practice and future research.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
Silva received support through a scholarship from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, financial code 20/13380-0). Mattiello-Sverzut was fellows of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (process numbers 309058/2018-0). Financial support was provided by Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FAEPA). These funders have no authority over this study. The authors alone decided to submit the data and analysis for publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.bjpt.2024.101118.
Appendix. Supplementary materials
References
- 1.Romitti P.A., Zhu Y., Puzhankara S., et al. Prevalence of Duchenne and Becker muscular dystrophies in the United States. Pediatrics. 2015;135(3):513–521. doi: 10.1542/peds.2014-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verhaart I.E.C., Aartsma-Rus A. Therapeutic developments for Duchenne muscular dystrophy. Nat Rev Neurol. 2019;15(7):373–386. doi: 10.1038/s41582-019-0203-3. [DOI] [PubMed] [Google Scholar]
- 3.Janssen M.M.H.P., Geurts A.C.H., de Groot I.J.M. Towards a short questionnaire for stepwise assessment of upper limb function, pain and stiffness in Duchenne muscular dystrophy. Disabil Rehabil. 2018;40(7):842–847. doi: 10.1080/09638288.2016.1274336. [DOI] [PubMed] [Google Scholar]
- 4.Filiz M.B., Toraman N.F., Kutluk M.G., et al. Effects of lumbar lordosis increment on gait deterioration in ambulant boys with Duchenne Muscular Dystrophy: a cross-sectional study. Braz J Phys Ther. 2021;25(6):749–755. doi: 10.1016/j.bjpt.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzone E.S., Vasco G., Palermo C., et al. A critical review of functional assessment tools for upper limbs in Duchenne muscular dystrophy. Dev Med Child Neurol. 2012;54(10):879–885. doi: 10.1111/j.1469-8749.2012.04345.x. [DOI] [PubMed] [Google Scholar]
- 6.Savas D., Simsek T.T. Functional level and its relationship to upper limb function, pain, and muscle stiffness in children with Duchenne muscular dystrophy. Ir J Med Sci. 2022 doi: 10.1007/s11845-022-03162-z. [DOI] [PubMed] [Google Scholar]
- 7.Janssen M.M., Hendriks J.C., Geurts A.C., et al. Variables associated with upper extremity function in patients with Duchenne muscular dystrophy. J Neurol. 2016;263(9):1810–1818. doi: 10.1007/s00415-016-8193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayhew A., Mazzone E.S., Eagle M., et al. Development of the performance of the upper limb module for Duchenne muscular dystrophy. Dev Med Child Neurol. 2013;55(11):1038–1045. doi: 10.1111/dmcn.12213. [DOI] [PubMed] [Google Scholar]
- 9.Santos A.L.Y.D.S., Maciel F.K.L., Fávero F.M., et al. Trunk control and upper limb function of walking and non-walking Duchenne muscular dystrophy individuals. Dev Neurorehabil. 2021;24(7):435–441. doi: 10.1080/17518423.2020.1869337. [DOI] [PubMed] [Google Scholar]
- 10.Brooke M.H., Fenichel G.M., Griggs R.C., et al. Clinical investigation of Duchenne muscular dystrophy. Interesting results in a trial of prednisone. Arch Neurol. 1987;44(8):812–817. doi: 10.1001/archneur.1987.00520200016010. [DOI] [PubMed] [Google Scholar]
- 11.Bulut N., Aydin G., Alemdaroglu-Gurbuz I., et al. Pulmonary and limbs function in children with early stage Duchenne muscular dystrophy compared to their healthy peers. Braz J Phys Ther. 2021;25(3):251–255. doi: 10.1016/j.bjpt.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davoli G.B.Q., Cardoso J., Silva G.C., et al. Instruments to assess upper-limb function in children and adolescents with neuromuscular diseases: a systematic review. Dev Med Child Neurol. 2021;63(9):1030–1037. doi: 10.1111/dmcn.14887. [DOI] [PubMed] [Google Scholar]
- 13.Pane M., Mazzone E.S., Sivo S., et al. The 6 minute walk test and performance of upper limb in ambulant Duchenne muscular dystrophy boys. PLoS Curr. 2014;6 doi: 10.1371/currents.md.a93d9904d57dcb08936f2ea89bca6fe6. ecurrents.md.a93d9904d57dcb08936f2ea89bca6fe6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naarding K.J., Keene K.R., Sardjoe Mishre A.S.D., et al. Preserved thenar muscles in non-ambulant Duchenne muscular dystrophy patients. J Cachexia Sarcopenia Muscle. 2021;12(3):694–703. doi: 10.1002/jcsm.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naarding K.J., Janssen M.M.H.P., Boon R.D., et al. The black box of technological outcome measures: an example in Duchenne muscular dystrophy. J Neuromuscul Dis. 2022;9(4):555–569. doi: 10.3233/JND-210767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heutinck L., Jansen M., Elzen Y., et al. Virtual reality computer gaming with dynamic arm support in boys with Duchenne muscular dystrophy. J Neuromuscul Dis. 2018;5(3):359–372. doi: 10.3233/JND-180307. [DOI] [PubMed] [Google Scholar]
- 17.Janssen M.M.H.P., Horstik J., Klap P., et al. Feasibility and effectiveness of a novel dynamic arm support in persons with spinal muscular atrophy and Duchenne muscular dystrophy. J Neuroeng Rehabil. 2021;18(1):84. doi: 10.1186/s12984-021-00868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos A.L.Y.D.S., Maciel F.K.D.L., Fávero F.M., et al. Upper limb function in ambulatory and non-ambulatory patients with Duchenne muscular dystrophy. Fisioterapia e Pesquisa. 2020:27188–27193. doi: 10.1590/1809-2950/19017427022020. [DOI] [Google Scholar]
- 19.Maciel F.K.L., Santos A.L.Y.S., Sá C.S.C. Responsiveness of Upper Limb Scales and trunk control for the evolution of patients with Duchenne muscular dystrophy. Rev Paul Pediatr. 2021;39(1–7) doi: 10.1590/1984-0462/2021/39/2020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaton D.E., Bombardier C., Guillemin F., et al. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. 2000;25(24):3186–3191. doi: 10.1097/00007632-200012150-00014. [DOI] [PubMed] [Google Scholar]
- 21.Terwee C.B., Bot S.D.M., Boer M.R., et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Mokkink L.B., Terwee C.B., Patrick D.L., et al. The COSMIN study reached International consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63(7):737–745. doi: 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 23.De Vet H.C.W., Terwee C.B., Kokkink L.B., et al. Cambridge University Press; Cambridge, UK: 2011. Measurement in medicine: a Practical Guide. [Google Scholar]
- 24.Mayhew A.G., Coratti G., Mazzone E.S., et al. Performance of upper limb module for Duchenne muscular dystrophy. Dev Med Child Neurol. 2020;62(5):633–639. doi: 10.1111/dmcn.14361. [DOI] [PubMed] [Google Scholar]
- 25.Okama L.O., Zampieri L.M., Ramos C.L., et al. Reliability and validity analyses of the North Star Ambulatory Assessment in Brazilian Portuguese. Neuromuscul Disord. 2017;27(8):723–729. doi: 10.1016/j.nmd.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Nusbaum L., Natour J., Ferraz M.B., Goldenberg J. Translation, adaptation and validation of the Roland-Morris questionnaire. Braz J Med Biol Res. 2001;34(2):203–210. doi: 10.1590/S0100-879X2001000200007. [DOI] [PubMed] [Google Scholar]
- 27.Cruz K.L.T., Camargos A.C.R., Cardoso J., et al. Translation and cross-cultural adaptation of the Charcot-Marie-Tooth disease Pediatric Scale to Brazilian Portuguese and determination of its measurement properties. Braz J Phys Ther. 2021;25(3):303–310. doi: 10.1016/j.bjpt.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vuillerot C., Payan C., Girardot F., et al. Responsiveness of the motor function measure in neuromuscular diseases. Arch Phys Med Rehabil. 2012;93(12):2251–2256. doi: 10.1016/j.apmr.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 29.Rosseel Y. lavaan: an R package for structural equation modeling. J Stat Softw. 2012;48(2):1–36. doi: 10.18637/jss.v048.i02. [DOI] [Google Scholar]
- 30.Brown T.A. 2nd Edition. The Guilford Press; New York: 2015. Confirmatory Factor Analysis for Applied Research. [Google Scholar]
- 31.Hu L.T., Bentler P.M. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- 32.Zou K.H., Tuncali K., Silverman S.S. Correlation and simple linear regression. Radiology. 2003;227(3):617–622. doi: 10.1148/radiol.2273011499. [DOI] [PubMed] [Google Scholar]
- 33.Gamer M., Lemon J., Puspendra S. Irr: various coefficients of interrater reliability and agreement. R package version 0.84.1. Available at: https://cran.r-project.org/web/packages/irr/irr.pdf
- 34.Koo T.K., Li M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Revelle W. Psych: procedures for psychological, psychometric, and personality research. R package version 2.4.1. Available at: https://cran.r-project.org/web/packages/psych/index.html
- 36.Chiu Y.Y.A., Lo C.W., Hui C.K.C., et al. Performance of the upper limb module - a reliable and valid evaluation for chinese patients with duchenne muscular dystrophy. Research Square. 2020 doi: 10.21203/rs.2.22633/v1. [DOI] [Google Scholar]
- 37.Pane M., Mazzone E.S., Fanelli L., et al. Reliability of the performance of upper limb assessment in Duchenne muscular dystrophy. Neuromuscul Disord. 2014;24(3):201–206. doi: 10.1016/j.nmd.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Gandolla M., Antonietti A., Longatelli V., et al. Test-retest reliability of the Performance of Upper Limb (PUL) module for muscular dystrophy patients. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0239064. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brogna C., Cristiano L., Tartaglione T., et al. Functional levels and MRI patterns of muscle involvement in upper limbs in Duchenne muscular dystrophy. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0199222. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demir G., Bulut N., Yılmaz Ö., et al. Manual ability and upper limb performance in nonambulatory stage of Duchenne muscular dystrophy. Arch Pediatr. 2020;27(6):304–309. doi: 10.1016/j.arcped.2020.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.