ABSTRACT
The classic peripheral chemoreflex response is a critical homeostatic mechanism. In healthy individuals, appropriate chemoreflex responses are triggered by acute activation of the carotid body - the principal chemosensory organ in mammals. However, the aberrant chronic activation of the carotid body can drive the elevated sympathetic activity underlying cardio-respiratory diseases such as hypertension, diabetes and heart failure. Carotid body resection induces intolerable side effects and so understanding how to modulate carotid body output without removing it, and whilst maintaining the physiological chemoreflex response, represents the next logical next step in the development of effective clinical interventions. By definition, excessive carotid body output must result from altered intra-carotid body inter-cellular communication. Alongside the canonical synaptic transmission from glomus cells to petrosal afferents, many other modes of information exchange in the carotid body have been identified, for example bidirectional signalling between type I and type II cells via ATP-induced ATP release, as well as electrical communication via gap junctions. Thus, herein we review the carotid body as an integrated circuit, discussing a variety of different inter-cellular signalling mechanisms and highlighting those that are potentially relevant to its pathological hyperactivity in disease with the aim of identifying novel therapeutic targets.
KEYWORDS: Carotid body, inter-cellular signalling, visceral sensory neurons, type I cells, glomus cells, type II cells, sustentacular cells, chemosensation, chemoreflex
Introduction
Maintenance of homeostasis is crucial for mammalian health. Multiple parameters including pH, the partial pressures of dissolved blood gases, temperature and others must be maintained within a narrow range of values if the organs of the body are to function optimally. This is achieved using a network of sensors that provide feedback to motor effectors via neuronal circuits that compute the adjustments in motor output required to achieve a pre-determined set point.
The carotid bodies are examples of such sensors. Although best known as regulators of blood gases, the carotid bodies are in fact multi-modal sensors (Conde et al. 2014; Holmes et al. 2019; Prabhakar and Peng 2017; Shin et al. 2019). Located bilaterally at the bifurcation of the two common carotid arteries, they are innervated by the glossopharyngeal and vagal nerves, as well as by a sympathetic nerve that originates in the nearby superior cervical ganglion (Schulz et al. 2016). Petrosal ganglion neuron projections carried by the carotid sinus and then glossopharyngeal nerves act as the only afferents and route carotid body output to the brainstem (Schulz et al. 2016). Activation of the carotid body by various stimuli, including acidosis, hypoxia, hypercapnia, hypoglycaemia and others, drives an increase in minute ventilation, as well as an increase in sympathetic activity (via a reflex loop that includes the nucleus of the solitary tract and ventral lateral medulla areas of the brainstem) that results in elevated blood pressure (Marshall 1994). Interestingly, carotid body stimulation also activates the nucleus ambiguus, which induces bradycardia (Marshall 1994) and bronchoconstriction (Moraes et al. 2021) via the vagus nerve. Collectively, these changes constitute the classical primary peripheral chemoreflex response.
Despite the attention afforded to components of the efferent system such as the sympathetic nervous system, recent work suggests that the aetiologies of diseases involving peripheral dysautonomia may involve afferent rather than efferent pathways (Ford et al. 2015; Koeners et al. 2016). In these cases, the observed changes in efferent output result from alterations in afferent drive. It is now commonly accepted that over-activation of the sympathetic nervous system contributes to hypertension (Grassi and Ram 2016; Esler 2000; Abboud 1982) and so research has largely been focused on the efferent system. Interestingly however, inactivation of the carotid body by hyperoxia has been shown to dramatically reduce both sympathetic nerve activity (renal sympathetic nerve) and blood pressure specifically in the spontaneously hypertensive rat (SHR) (but not in Wistar rats), suggesting that the carotid body is tonically active in this model of hypertension (McBryde et al. 2013). Further, carotid body de-afferentation lowers blood pressure and renal sympathetic nerve activity chronically in SHRs (McBryde et al. 2013). In addition to these data from the SHR, it has also been reported that hyperoxia reduces the elevated mean sympathetic nerve activity of hypertensive humans (Siński et al. 2012) and that both carotid body tumours and obstructive sleep apnoea induce primary hypertension in human patients (Wang et al. 2000; Prabhakar et al. 2015). These results support the hypothesis that the increased sympathetic activity associated with hypertension results from carotid body hyperactivity - a combination of hyperreflexia (exacerbated responses to acute stimuli) and aberrant hypertonicity (chronic hyperactivation in the absence of an acute stimuli) (Paton et al. 2013). Elevated sympathetic activity driven by carotid body hyperactivity is also thought to exacerbate chronic heart failure (Schultz et al. 2015) and diabetes (Ribeiro et al. 2013), suggesting carotid body dysfunction may contribute to multiple cardiovascular and metabolic diseases.
Although these data are promising, resection of the carotid bodies can prolong oxygen desaturations in heart failure patients with sleep apnoea (Lugliani et al. 1971; Chang et al. 1978). This has raised awareness of the side effects caused by surgical removal of the carotid body and research efforts are now focused on identifying an alternative, pharmacological approach. Modulating carotid body activity to selectively alleviate the excessive sympathetic activity underlying cardiovascular diseases may be possible. This is especially important given that some current anti-hypertensive treatments activate the sympathetic nervous system, which likely accounts for why treated and controlled hypertensives remain at cardiovascular risk (Guido 2004). Thus, we now require a thorough understanding of how carotid body hyperactivity occurs in disease. The aspiration is to discover if the carotid body pathway to the sympathetic nervous system can be selectively targeted.
By inspection, inter-cellular communication within the carotid body must be altered relative to the normotensive state if its output is increased in disease. The mechanisms underlying these inter-cellular communication deficits are not fully understood: aberrant transmission mechanisms may be involved or this altered communication may simply be a result of increased upstream signalling. Regardless, an understanding of physiological inter-cellular communication in the carotid body, as well as miscommunication in disease, could prove informative when designing therapeutic interventions targeting the carotid body.
Structure of the carotid body
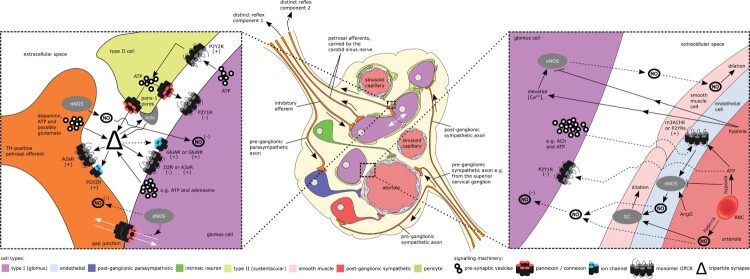
The carotid body is comprised of several cell types (Figure 1). Neuron-like Chemoreceptive type I (glomus) cells, sheathed by glial-like type II (sustentacular) cells, are innervated by petrosal afferents that transmit information to the brainstem (Duchen et al. 1988; Gonzalez et al. 1994). A subset of glomus cells, the so-called type Ib cells, receive minimal innervation (Mcdonald and Mitchell 1975). Clusters of afferent-innervated type I cells and type II cells are arranged around the many sinusoidal capillaries and arterioles found throughout the carotid body (Gonzalez et al. 1994). Arterioles comprise a monolayer of endothelial cells, which is in turn is encased in a monolayer of smooth muscle cells. Sinusoidal capillaries, however, lack this outer layer. Autonomic neurons, and axons from autonomic neurons localised outside the carotid body in other ganglia, are also present in the carotid body and are thought to innervate both the carotid body vasculature and type I cells; however, the autonomic innervation of the carotid body has been recently reviewed by us (Brognara et al. 2020) and will not be considered in any detail here. The relatively large number of cell types present in such a small organ, combined with their interspersion and close apposition, as well as the presence of one of the densest capillary networks in the body, suggests that intra-carotid body inter-cellular communication is potentially complex and may represent an opportunity for the development of fine-tuned interventions.
Figure 1.
information exchange in the carotid body. Known and hypothesised routes of information flow (non-exhaustive) are represented by solid and dashed arrow shafts respectively. White arrows indicate exclusively intracellular pathways i.e. mediated by gap junctions, whilst black arrows denote all other pathways. See main text for more detailed descriptions of individual signalling machinery components. The presence of pericytes in the carotid body requires confirmation. Note that type II cells provide feedback to glomus cells via retrograde transmission following glomus cell stimulation of type II cells. Petrosal afferents may similarly use anti-dromic signalling to provide feedback (not shown) to glomus cells following the stimulation of petrosal afferents by glomus cells. GC, guanalyl cyclase, ecto, ectonucleotidases, eNOS, endothelial nitric oxide synthase, nNOS, neuronal nitric oxide synthases, NO, nitric oxide, ACh, acetyl choline, TH, tyrosine hydroxylase, AngII, angiotensin II, (+), excitatory, (-), inhibitory. Adapted, using the CC BY license provided with the publication, from (Taha 2015).
Extracellular signalling in the carotid body: molecular mechanisms
This section is not exhaustive and if extra detail is desired, the neurotransmitter / receptor complement of the carotid body has been reviewed more extensively elsewhere (Nurse 2010; Iturriaga and Alcayaga 2004).
As with all cellular events, inter-cellular communication relies on, and is a product of, underlying molecular processes. Similar to nuclei in the central nervous system, the molecular machinery that facilities communication in the carotid body is diverse and so facilitates several different modes of information exchange (Figure 1). Extracellular signalling in the carotid body is achieved by two main structures: synapses and large pores embedded in the cell membrane. Both of these structure types have a critical role in mediating inter-cellular communication in the carotid body.
Classical synapses are used in the carotid body to link type I cells to petrosal afferents, as well as to connect pre- and post- ganglionic autonomic efferents to their various targets. The role of membrane pores in carotid body inter-cellular signalling is less well understood but it is nevertheless clear that they contribute significantly, for example by permitting the release of signalling molecules from type II cells (a mechanism analogous to glio-transmission in the central nervous system) (Murali et al. 2017; Zhang et al. 2012; Murali and Nurse 2016). Current evidence suggests that the majority of the functional pores in the carotid body are pannexons (Murali et al. 2017), although the involvement of the analogous connexons remain a possibility. Pannexons (and connexons) are integral cell membrane hexamers that facilitate the exchange of ions, small molecules or small peptides through large diameter, hydrated channels which may have a preferential direction of current flux (Thompson 2015; Penuela et al. 2013).
Excitatory ionotropic channels
A veritable cornucopia of signalling molecules with functional roles, along with their cognate receptors, have been detected in the carotid body. The principle excitatory neurotransmitters in the carotid body are ATP (Buttigieg and Nurse 2004; Zhang et al. 2000; Pijacka et al. 2016), acetyl choline (ACh) (Zhang et al. 2000; Zhong and Nurse 1997; Alcayaga et al. 2007) and dopamine (Verna et al. 1995; Iturriaga et al. 1994). ATP activates petrosal afferents by agonising the ionotropic purinergic receptors P2X2 and P2X3, which are expressed in the petrosal afferent post-synaptic membrane (Buttigieg and Nurse 2004; Zhang et al. 2000; Pijacka et al. 2016). Alongside ATP, ACh also activates ionotropic receptors in order to induce an excitatory post-synaptic response, in this case by binding to, and opening, the α7 nicotinic ACh receptor (nAChR) (and possibly other isoforms) expressed by petrosal sensory afferents (Zhang et al. 2000; Zhong and Nurse 1997; Alcayaga et al. 2007; Kåhlin et al. 2014).
Recent work has also established that glutamate, the major excitatory neurotransmitter in the brain, has a functional role in the carotid body. N-methyl-D-aspartic acid (NMDA) receptor activation has been found to augment hypoxic chemotransmission (Liu et al. 2009) (where neurotransmitter binding to cognate receptors then induces an action potential in the petrosal afferents innervating the glomus cells in question), whilst it has been proposed that α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) activates extracellular signal-related kinases 1/2 in rat glomus cells (Liu et al. 2018). Consistent with this, the expression of VGluT2 has been observed in petrosal afferents (Yokoyama et al. 2014), whilst the GluA1 and GluN1, 2A and 2B glutamate receptor subunits are now known to be expressed in glomus cells (Liu et al. 2009, 2018). mRNA transcripts encoding other excitatory ionotropic glutamate receptors (including AMPA, NMDA and kainate receptor subunits) were also detected in the same studies (Liu et al. 2009, 2018).
Inhibitory ionotropic channels
Not all of the ionotropic channels in the carotid body are excitatory. There are Cl- permeable GABA(A) receptors on petrosal afferent terminals that carry hyperpolarising outward currents when opened by γ-aminobutyric acid (GABA) (Igarashi et al. 2009; Zhang et al. 2009).
Excitatory metabotropic receptors
Metabotropic receptors are also critical for physiological carotid body function. ATP activates not only the ionotropic P2X2/3 channels but also the metabotropic P2Y2 receptor, which is expressed by type II cells (Zhang et al. 2012). P2Y2 receptors are Gq-coupled, which means ATP binding to P2Y2Rs leads to an increase in intracellular calcium that then drives the opening of Pannexin-1 pores, releasing gliotransmitters including more ATP into the synapse (Zhang et al. 2012). Thus, ATP from glomus cells can act to amplify P2X2/3 receptor signalling.
Extra-cellular adenosine is either generated via ectonucleotidase degradation of ATP or pumped in to the extra-cellular space through equilibrative nucleoside transporters expressed in the glomus cell plasma membrane (Conde and Monteiro 2004; Conde et al. 2012). Adenosine acts on excitatory A2a receptors localised both to the petrosal afferent post-synaptic membrane and the glomus cell plasma membrane (where A2b receptors are also expressed). Activation of petrosal afferent A2a receptors (which is essential for physiological afferent firing Murali and Nurse 2016) induces depolarisation and thus augments firing (Conde et al. 2006), whilst the binding of adenosine to glomus cell A2a/b receptors raises glomus cell intracellular calcium via the inhibition of background TWIK-related acid sensitive K+ (TASK) channels. This increases membrane excitability (Zhang et al. 2018) and, consequently, also increases the release of neurotransmitters from type I cells (Nurse 2005) that then modulate the effects of adenosine on innervating petrosal afferents. Thus, as with ATP-induced ATP release for amplification, adenosine (which can be ATP-derived) can also provide positive complementary excitation.
The list of other excitatory metabotropic receptors with functional roles in the carotid body includes the serotonin-activated 5-HT2a receptor, expressed by glomus cells. Serotonin binding to this receptor leads to protein kinase C-mediated inhibition of resting K+ conductances, as well as calcium dependent K+ conductances (Zhang et al. 2003), thus enhancing hypoxia-evoked depolarisation. 5-HT3 receptors are known to have an excitatory role in rat aortic bodies (Brophy et al. 1999), although whether they have a similar function in the carotid body remains to be seen. M1 and M2 muscarinic acetyl choline receptors (mAChRs) are known to be expressed by type I cells (Shirahata et al. 2004; Bairam et al. 2006), but, similar to 5-HT2a receptor activation, muscarinic stimulation appears to elevate type I cell [Ca2+]i (Dasso et al. 1997) via the inhibition of background K+ leak TASK channels (Ortiz and Varas 2010), suggesting that Gq-coupled mAChRs are functionally dominant in type I cells. α1 adrenoreceptors are expressed by type I cells, as well as cells of the carotid body vasculature and act to amplify the carotid sinus nerve output signal in response to hypoxia (Felippe et al. 2022). Transcripts encoding the excitatory D1 receptor have also been detected in the carotid body by quantitative-reverse transcriptase PCR (Bairam et al. 1998).
Inhibitory metabotropic receptors and nitric oxide
Given the multiple excitatory signalling mechanisms above it is perhaps not surprising that there are inhibitory systems in the carotid body that prevent excess chemosensory afferent discharge. The best characterised inhibitory transmitter found in the carotid body is dopamine. In the cat, dopamine acts via metabotropic D2 receptors to inhibit what would otherwise be tonic activation of the petrosal afferents innervating type I cells (D2 receptors are Gi coupled and therefore reduce cAMP production) (Verna et al. 1995; Iturriaga et al. 1994). Of note, D2 receptors appear to be expressed both on the terminals of innervating petrosal afferent axons, as well as other cells localised to the carotid body (Dinger et al. 1981). It has been proposed that glomus cell D2R activation prevents neurotransmitter secretion via the inhibition of L-type Ca2+ channels (Benot and López-Barneo 1990; Carroll et al. 2005). The presence of excitatory D1 receptors alongside inhibitory D2 receptors may explain why dosing petrosal ganglion neurons with high concentrations of dopamine prior to ACh application was found to be inhibitory, whilst lower doses of dopamine potentiated the response to subsequent cholinergic stimulation (Alcayaga et al. 1999). The exception to this is the rabbit carotid body, in which dopamine appears to act via D2 receptors to increase chemotransmission frequency (Alcayaga et al. 2006).
Of note, ATP has inhibitory effects in addition to its excitatory actions described above. ATP acts via glomus cell metabotropic P2Y1 and P2Y1/2 receptors (which may act as autoreceptors) to inhibit the rise in intracellular calcium that would otherwise be triggered by a stimulus (Xu et al. 2005; Carroll et al. 2012), reducing the likelihood of chemotransmission. Other inhibitory metabotropic receptors include GABA(B) receptors, which are expressed by glomus cells. Activation of these receptors drives a background TASK-like K+ conductance that inhibits glomus cell depolarisation (Fearon et al. 2003). Another inhibitory transmitter of note is nitric oxide (NO) (Alcayaga et al. 1999; Fung et al. 2001), which is synthesised in the carotid body by both endothelial nitric oxide synthase (eNOS) and neuronal nitric oxide synthase (nNOS) (discussed further below) (Valdés et al. 2003). The classical signalling cascade triggered by NO involves the activation of cytoplasmic NO-sensitive guanylyl cyclase, however it has been reported that glomus cells are inhibited by NO via a cGMP-independent mechanism that involves the blockade of L-type Ca2+ channels (Summers et al. 1999).
Similar to ATP, glutamate also has inhibitory as well as excitatory effects on the carotid body. It has recently been reported that the metabotropic glutamate receptor mGluR1 is expressed in type I cells (Li et al. 2021). The activation of this receptor by a selective agonist was shown to inhibit hypoxia-driven chemotransmission (Li et al. 2021).
Histamine is also released by the carotid body during acute hypoxia (Koerner et al. 2004; Del Rio et al. 2008) and the presence of H1, 2 and 3 receptors has been confirmed via immunohistochemistry (Lazarov et al. 2006). However, the functional role of histamine remains uncertain with studies reporting both inhibitory and excitatory responses to H3R activation (Del Rio et al. 2008; Lazarov et al. 2006). Of note, dopamine (Fishman et al. 1985; Almaraz et al. 1993), ACh (Zhang et al. 2000), ATP (Zhang et al. 2000), adenosine (Conde and Monteiro 2004), serotonin (Ramirez et al. 2012), GABA (Igarashi et al. 2009; Zhang et al. 2009), histamine (Koerner et al. 2004; Del Rio et al. 2008) and NO (Fung et al. 2001) are all also released in response to acute hypoxia, raising the intriguing possibility that the exact degree of hypoxia may determine the relative amounts of the different neurotransmitters in the secreted neurotransmitter pool.
Regulation of neurotransmitter systems: ectonucleotidases
All of the above listed neurotransmitters and receptors are regulated by mechanisms controlling their synthesis, transport and degradation - the dynamic modulation of which can drastically alter output signals. Of particular recent interest is the role of ectonucleotidases in the carotid body, such as ectonucleoside triphosphate diphosphohydrolase 1, 2 and 3 and ecto-5′-nucleotidase, which collectively convert ATP into adenosine (Salman et al. 2017). Interestingly, ATP can inhibit some glomus cells (Xu et al. 2005; Carroll et al. 2012) but its metabolite adenosine excites glomus cells (Conde et al. 2006). As a consequence, we should expect that if the equilibrium of this pathway is disturbed then changes in carotid body activity will follow.
Additional receptors
In addition to the receptors discussed here, it has also been proposed that there are specialised receptors (e.g. adrenoreceptors) expressed by cells of non-vasculature lineage (e.g. glomus cells) for receiving inputs directly from autonomic neurons (Brognara et al. 2020). Further, neuropeptides such as substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide, endothelin-1 and neuropeptide Y have also been detected in the carotid body (Iturriaga and Alcayaga 2004; Takahashi et al. 2011).
Although the specific mechanisms underlying the function of each neurotransmitter system are of interest (further such details can be found elsewhere Nurse 2010; Iturriaga and Alcayaga 2004), it is perhaps more interesting to appreciate the potential for a vast combinatorial code (and the possible fine tuning this implies) that having an array of different signalling systems provides.
Direct communication in the carotid body: molecular mechanisms
Gap junctions are formed by the binding of a pair of connexons on apposed cell membranes and enable the direct exchange of information, in the form of signalling molecules, between two coupled cells. The diameter of the gap junction pore is estimated to be around 1.5nm (Weber et al. 2004), which is large enough to permit the passage of molecules up to 1KDa in size (Weber et al. 2004). The presence of functional gap junctions in the carotid body has been demonstrated using dye and current transfer experiments (Jiang and Eyzaguirre 2003, 2006) (Figure 1). Further, both connexin 43 (Cx43) (Abudara et al. 1999), the canonical glial connexin, and connexin 36 (Cx36) (Frinchi et al. 2013), the classical neuronal connexin, are expressed in the carotid body. The spatial distribution of reported immunohistochemistry staining patterns suggests glomus cells express both (Abudara et al. 1999; Frinchi et al. 2013), although further work is needed to confirm this. Petrosal ganglion neuron projections are another possibility. Importantly, homomer connexons formed of Cx36 are incompatible with homomer connexons formed of Cx43, meaning Cx36 / 43 heteromer gap junctions never assemble and so cannot couple cells. Homotypic Cx43 and Cx36 gap junctions are both possible.
Type I cells (Duchen et al. 1988; Ureña et al. 1989), petrosal afferents, smooth muscle cells and autonomic neurons are all electrically excitable, whereas type II cells and endothelial cells are not. Direct electrical and chemical communication is possible between the electrically excitable cells, whereas, with minor exceptions, only direct chemical communication is possible between the remaining non-excitable cells. By facilitating the sharing of electrical and chemical signalling states between cells, the direct coupling of cells by gap junctions enables synchronicity, signal amplification and the propagation of excitability in the carotid body and thus adds further complexity to its computational code.
Bidirectional type I - petrosal afferent signalling
Canonical orthodromic signalling
The canonical model of carotid body function involves the detection of a change in the chemical composition of circulating blood and the transduction of this information into petrosal afferent firing. A drop in blood pO2 (or other stimuli) is detected by type I cells, which causes the inhibition of K+ channels (López-Barneo et al. 1988; Buckler 1997). The resulting depolarisation triggers an action potential (Duchen et al. 1988; Ureña et al. 1989), which leads to Ca2+ influx into the cytoplasm via voltage-sensitive calcium channels (Buckler and Vaughan-Jones 1994) and the secretion of excitatory neurotransmitters, for example adenosine and / or ATP (Iturriaga and Alcayaga 2004; Nurse 2005). Chemotransmission follows (Figure 1) and this in turn drives reflex arcs that activate the motor outputs that define the classic chemoreflex response, which includes hyperventilation.
Antidromic signalling
Less widely recognised is the antidromic signalling of petrosal afferents in the carotid body (Figure 1). Stimulation of the carotid sinus nerve has been found to depress carotid body sensory discharges (Fidone and Sato 1970; Eyzaguirre and Koyano 1965) and this phenomena persists when autonomic efferents are eliminated via surgery, but is abolished by dopamine antagonists (McDonald and Mitchell 1981). Together, these data suggest that dopamine could mediate antidromic synaptic communication between petrosal afferent terminals and type I cells. Consistent with this, tyrosine-hydroxylase-containing type axons that innervate type I cells were found to be maintained following the removal of autonomic efferents but were lost when the carotid sinus nerve was severed (McDonald and Mitchell 1981). It has since been demonstrated that a sub-population of petrosal afferents that innervate type I cells are definitively tyrosine hydroxylase positive (Ichikawa 2002; Katz and Black 1986). It should be noted, however, that due to the surgical procedures employed in this study (McDonald and Mitchell 1981), it is possible that autonomic efferents remaining in the carotid sinus nerve were involved in the depression of chemotransmission following post-‘efferent removal’ sinus nerve stimulation.
As has been pointed out (Brognara et al. 2020), if dopamine does mediate the depression of chemotransmission that is triggered by sinus nerve stimulation then it could be acting either as a retrograde or autocrine signal (Gerdeman 2008; Tao and Poo 2001). In the retrograde case, dopamine may be released by the afferent terminal as result of depolarisation induced by orthodromic transmission (Brognara et al. 2020): if so, it is likely that this mechanism exists to limit the rate of afferent firing locally. Interestingly, in this situation, antidromic synaptic transmission could be induced by (but would not require) a preceding antidromic action potential.
Evidence suggests that ATP is also released by petrosal afferents at the level of the nucleus tractus solitarii (Paton et al. 2002; Braga et al. 2007). This, together with the fact that petrosal afferents are purine-sensitive (Buttigieg and Nurse 2004; Zhang et al. 2000; Pijacka et al. 2016) pseudo-unipolar neurons, raises the intriguing possibility that antidromic action potentials may be triggered via the autocrine action of transmitters including ATP in the nucleus tractus solitarii. Consequently, this central activation may trigger retrograde dopamine signalling in the carotid body, which could potentially effect local control of afferent firing.
NO may also be used as an inhibitory neurotransmitter during antidromic synaptic transmission: a sub-population of the petrosal ganglia neurons that innervate type I cells are nitric oxide synthase (NOS) – positive (Wang et al. 1993, 1994), the presence of the P2 receptor blocker suramin reduces hypoxia-driven release of NO by around 50% (Fung et al. 2007) and glomus cells are inhibited by NO (Summers et al. 1999). Of note, NOS activity is positively correlated with O2 saturation levels (Wang et al. 1994; Prabhakar et al. 1993), which is consistent with the idea that NO signalling is used as a regulator that helps to ensure pO2 and chemosensory discharge are negatively correlated.
The role of gap junctions
The injection of hyperpolarising current into type I cells during paired recordings has been shown to hyperpolarise the Em of neighbouring petrosal ganglion neuron projections as well as the Em of the type I cell, demonstrating gap junction coupling between type I cells and petrosal afferents (Jiang and Eyzaguirre 2003, 2006). The transfer of intracellular dye between type I cells and these afferents has also been described, although the low quality of the reported data makes this evidence less convincing (Jiang and Eyzaguirre 2003, 2006). Of note, the transfer of voltage between type I cells and afferents was found to be asymmetrical: voltage was transferred much more efficiently from type I cells to afferents than vice versa. At first glance, this may suggest that the observed transmission might be mostly synaptic, but, importantly, the voltage change transferred (in both directions) was hyperpolarising and so was very unlikely to have been transmitted via any other route than via a gap junction. Additional studies have shown that a combination of suramin and hexamethonium only partially inhibits chemosensory excitation (Reyes et al. 2007), which is consistent with the existence of direct electrical synapses that link type I cells and petrosal afferents (Figure 1).
When examined together, the multiple possible modes of information exchange between glomus cells and petrosal afferents strongly suggest that communication between these two cells types is complex and likely highly regulated. Although this makes understanding the carotid body more challenging, it also provides us with opportunities to create highly targeted interventions.
Plasticity
Evidence that intra-carotid body signalling is dynamic is mounting. Carotid bodies from rats exposed to mild chronic intermittent hypoxia (such as in sleep apnoea), but not those exposed to multiple episodes of a more severe sustained hypoxia (such as experienced at high altitude), exhibit a sustained but temporary increase in activity in the period following mild acute intermittent hypoxia (Peng et al. 2003). This raises the intriguing possibility that the carotid body is capable of plastic responses. Additional experiments presented in the same publication revealed that complex I (of the mitochondrial electron transport chain) activity in these carotid bodies was depressed and that the long-term facilitation of the chemosensory response following mild chronic intermittent hypoxia could be ameliorated by pre-treatment with a potent scavenger of oxygen free radicals (Peng et al. 2003). If the strength of the chemosensory response is indeed positively correlated with the concentration of free radicals (which is inversely correlated with pO2 and so in effect reports on the efficacy of the chemoreflex response) this relationship could be viewed as a form of plasticity.
Chronic sustained hypoxia and chronic intermittent hypoxia are known to have different effects. For example, the above described potentiation of the response to acute intermittent hypoxia following chronic intermittent hypoxia was found to be absent from the carotid bodies of rats subjected to chronic sustained hypoxia (Peng et al. 2003). Nevertheless, it is well established that carotid body facilitation occurs during, and is crucial for, acclimatisation to chronic sustained hypoxia (Bisgard 1994; Bishop et al. 2013). Specifically, long-term (but reversible), hypoxia-inducible factor (HIF)-dependent carotid body facilitation mediates the sustained hypoxia-driven progressive tachypnoea that ameliorates what would otherwise be low blood pO2 in these conditions (Smith et al. 1986; Olson et al. 1988; Hodson et al. 2016; Kline et al. 2002) Many have reported differences in transmitters, their receptors and other channels in the carotid body following chronic sustained hypoxia. NO, ACh, ATP, dopamine, and endothelin-1 signalling, ectonucleotidase expression (discussed further below) and K+ and Na+ channels are all now known to be altered (Bisgard 2000; Pulgar-Sepúlveda et al. 2018) following chronic sustained hypoxia. However, the studies involved in identifying these changes largely failed to address whether they were reversible. This is an important point given the reversible nature of the ventilatory hypoxic response (Janssen et al. 1998), which implies that the cellular and molecular processes responsible for carotid body facilitation during chronic sustained hypoxia are most likely also reversible. To the best of our knowledge, carotid body hyperplasia, which is driven by HIF-2a dependent type I cell proliferation (Hodson et al. 2016; Fielding et al. 2018) as well as by vasculature changes (Fielding et al. 2018) in the rat, is the only carotid body adaptation induced by chronic sustained hypoxia that is known to be reversible (Heath et al. 1985). In addition, it is interesting to note that the HIF proteins, which are oxygen-sensing transcription factors, regulate the expression of key components of several of the signalling systems observed to be altered by chronic sustained hypoxia (Semenza 2000), including P2X and P2Y purinergic receptors (Tak et al. 2016; Hirayama et al. 2015). Could chronic sustained hypoxia – driven carotid body plasticity be mediated by activation of the HIF system (Pulgar-Sepúlveda et al. 2018)?
Perhaps consistent with this (as HIF-1 is universally expressed), chemosensitive petrosal afferent neurons in the SHR exhibit a pathological P2X3 receptor-dependent tonicity and ATP hyper-sensitivity (Pijacka et al. 2016; Moraes et al. 2018). This finding is likely explained by the 5-fold up-regulation of P2×3 mRNA levels that was detected in these neurons (Pijacka et al. 2016; Moraes et al. 2018). It is possible that petrosal afferent neurites become enlarged in the SHR to enable synaptic contacts with all glomus cells in the hyperplasic carotid body. Thus, it remains to be seen whether this up-regulation of P2×3 mRNA levels reflects, either in part or in whole, the synthesis of additional receptors necessary to maintain expression levels per unit of cell membrane area in petrosal afferent neurites. It is also unclear if the apparent increased expression of P2X3-containing receptors can be reversed if normotension can be re-established. Either way, it is becoming apparent that significant modulation of synaptic efficacy in the carotid body is possible and that the mechanisms underlying this modulation can become aberrant in disease (Pijacka et al. 2016). Further, blockade of the P2X3 receptor entirely inhibits the pathological tonic carotid body activity observed in the SHR and eliminates differences in ATP-sensitivity between the petrosal afferent neurons of Wistar rats and SHRs (Pijacka et al. 2016; Moraes et al. 2018), leading to reduced arterial pressure in the SHR (whilst leaving the physiological chemoreflex intact) and demonstrating that targeting the mechanisms that control the efficacy of intra carotid body inter-cellular communication could well represent an effective clinical intervention.
One additional potential form of sustained chronic hypoxia-driven carotid body plasticity is gap junction modulation: sustained hypobaric hypoxia has been found to increase type I – petrosal neuron afferent coupling efficiency, whilst normoxia reportedly induces intra-type I gap junction coupling that is then lost during hypoxia (Jiang and Eyzaguirre 2006). These changes have the potential to reconfigure the carotid body circuit and so could have important implications for carotid body output (see below for further discussion).
Understanding the root cause of the differences in the plastic responses of the carotid body to intermittent or sustained hypoxia is critical as although sustained hypoxia induces beneficial adaptations (Bisgard 1994), the carotid body response to chronic intermittent hypoxia is maladaptive and associated with hypertension (Del Rio et al. 2014). In the future, additional insight into how low pO2 can drive a plastic response in the carotid body may come from studies of the human cortico-spinal pathway, where mild acute intermittent hypoxia has been shown to accentuate spike timing dependent plasticity (Christiansen et al. 2018).
The paradox of encoding hypoxia detection using ATP
The observation that scavenging free radicals can apparently attenuate long-term facilitation of the carotid body chemosensory response suggests that free radicals generated by hypoxic inhibition of the electron transport chain act as one of the detectors of pO2 in the carotid body (Peng et al. 2003). By definition, hypoxic inhibition of the electron transport chain reduces ATP availability and yet, surprisingly, ATP is one of the primary neurotransmitters used to encode hypoxia. Exactly how hypoxia induces both an increase in ATP-signalling and a (presumed) decrease in ATP synthesis, remains an open question - as we discussed recently (Bardsley et al. 2021). One possibility is regulated catabolism: the rate at which ectonucleotidase enzymes catabolise ATP into adenosine appears to be inversely correlated with pO279. As explained above, whilst adenosine and ATP act in synergistic fashion to excite petrosal afferents (Buttigieg and Nurse 2004; Zhang et al. 2000; Pijacka et al. 2016; Conde et al. 2006), they have juxta-posed effects on glomus cells, with adenosine being excitatory (Conde et al. 2006) and ATP being inhibitory (Xu et al. 2005; Carroll et al. 2012). Thus, augmenting the conversion of ATP to adenosine under hypoxic conditions should boost purinergic signalling without demanding the synthesis of additional ATP. Of note, it has been proposed that excess purinergic signalling leads to carotid body hyperactivity in disease (Moraes et al. 2018), consistent with the aberrant tonic activation of mechanisms that might ordinarily boost purine signalling during acute hypoxia. Another potential mechanism is the inhibition of ATP-dependent TASK channel K+ conductances (Varas et al. 2007) as a result of the reduced intracellular ATP availability that generally defines hypoxia. Similar to the effects of adenosine on glomus cells (Conde et al. 2006), this would also lead to cell membrane depolarisation and increased excitability.
Systems must also exist to inhibit carotid body output during hyperoxia (or even normoxia), or at other times when mitochondrial efficiency improves. ATP does inhibit complex IV (Arnold and Kadenbach 1997) and the above described ectonucleotidase-driven shift from adenosine to ATP (which inhibits glomus cells) during normoxia may also contribute, but one other additional mechanism might be a concomitant rise in the levels of inhibitory NO during normoxia / hyperoxia: by inspection NO production should fall during hypoxia, which presumably leads to disinhibition. Additional experiments are required if we are to resolve this paradox.
Type II cell interactions
Type II cells have historically been considered supporting cells, however, many now accept that they act to modulate carotid body chemotransmission (Ortega-Sáenz and López-Barneo 2020; Nurse and Piskuric 2013; Leonard et al. 2018). This revelation is largely the result of the establishment of the ATP-induced ATP release hypothesis (Nurse and Piskuric 2013). Here (and as stated above), type II P2Y2 receptor activation by ATP released from type I cells leads to a [Ca2+]i-dependent ATP release through type II cell pannexons (Zhang et al. 2012; Murali and Nurse 2016; Xu et al. 2003) and this in turn induces afferent firing, potentially by amplifying synaptic ATP and / or adenosine signalling (Zhang et al. 2012) (Figure 1). Because glomus cells express P2Y1 receptors, secreted ATP may also concurrently inhibit the hypoxia-induced rise in [Ca2+]i in glomus cells (Xu et al. 2005), providing negative feedback (Figure 1). Consistent with the ATP-induced ATP release theory, electron microscopy has revealed dense core vesicles clustered around regions of the glomus cell plasma membrane apposed to type II cells (Platero-Luengo et al. 2014). Other signalling molecules such as serotonin and angiotensin induce pannexon opening by raising type II cell [Ca2+]i (Murali et al. 2017; Murali et al. 2014), although it is not yet known if these pathways affect purine release. It is also currently unclear if pannexon channels are specifically localised to synapses or if the population is wholly or partially extra-synaptic, with released ATP diffusing into nearby synapses formed between type I cell and petrosal afferents. Panx1 and P2X2 receptors do co-immunoprecipitate together, implying at least a fraction of expressed pannexons are localised to the synapse (Li et al. 2018), but further research to determine which model is the case is warranted as the balance of synaptic (localised) and extra-synaptic (global) type II cell ATP signalling has important consequences for information processing in the carotid body.
Quantitative PCR and immunofluorescence data indicates that ectonucleotidases are expressed by type II cells (Salman et al. 2017). Given that ectonucleotidases convert glomus cell-inhibiting ATP to glomus cell-activating adenosine (see above), this raises the intriguing prospect that type II cells could modulate carotid body output via regulated secretion of these enzymes (Figure 1). In support of this idea, the activation of type I cells following type II cell activation is often delayed and can be inhibited using Panx-1, A2a receptor and 5′-ectonucleotidase blockers (Murali and Nurse 2016).
The observation that type II cells can trigger afferent firing via ATP release leads directly to the idea that type II cell signalling may trigger petrosal afferent firing even in the absence of direct afferent excitation by glomus cells. If this is the case, dysfunction of type II signalling alone could be sufficient to cause carotid body sensitisation (Leonard et al. 2018).
The glial cells of the CNS share several characteristics with type II cells, including glial fibrillary acidic protein expression and the modulation of synaptic transmission (Murali and Nurse 2016). Of note, Western blotting of carotid body tissue has shown that Cx43 expression is upregulated during chronic hypoxia (Abudara et al. 1999; Chen et al. 2002) and, at least in cultured rat astrocytes, mild hypoxia also causes an upregulation in Cx43, as well as a concomitant rise in ATP efflux following extracellular Ca2+ depletion (Ca2+ depletion opens hemi-channels) (Lin et al. 2008). This implies that mild hypoxia triggers a rise in the number of functional gap junction hemi-channels (functionally equivalent to pannexons) expressed by glia. A similar mechanism affecting the glial-like type II cells of the carotid body would be consistent with the enhanced petrosal afferent firing observed during hypoxia, given the importance of purines released by type II cells for chemotransmission.
Interestingly, it has recently been reported that astrocytes in the CNS act as ‘intracranial baroreceptors’ (Marina et al. 2020); a finding which raises the possibility that type II cells may also transduce pressure information, given the similarities between these cells and CNS glia. If so, this could effectively provide a feedback loop, enabling homeostatic control of carotid body output affecting blood pressure.
The interface between type II cells and the vasculature
As well as sensing pressure, astrocytes couple blood flow to metabolism in the brain. When increased neuronal activity creates additional metabolic demands, nearby astrocytes induce vasodilation in the vessels they ensheath and thus elevate blood flow to the area (Harder et al. 2002; Alkayed et al. 1996; Bhardwaj et al. 2000) overspill glutamate released by transmitting neurons is detected by astrocytes, which respond by secreting arachidonic acid. This acid is metabolised in the extracellular space to produce a set of epoxyeicosatrienoic acids (Alkayed et al. 1996), which drive the hyperpolarisation and consequent relaxation and dilation of vascular muscle by potentiating smooth muscle cell K+ conductances (Gebremedhin et al. 1992). In the carotid body, type II cells, and possibly also pericytes, encircle vessels in the carotid body in a similar fashion to the way astrocytes surround vessels in the brain (Heath et al. 1983; McDonald and Larue 1983) (Figure 1). This related anatomy suggests that the carotid type II cells may also control blood flow, possibly in response to the metabolic state of the surrounding tissue or possibly as a consequence of some other factor. If so, this role could be relevant to carotid body pathology, as the inhibition of any vasodilation-inducing mechanism would be expected to contribute to the carotid body sensitisation associated with hypertension by inducing a hypoperfusion-driven local hypoxia. Of note, it has recently been proposed that the dysfunction of autonomic inputs to the carotid body may lead to exactly this situation (Brognara et al. 2020).
Research examining the role of type II cells as part of an integrated carotid body circuit is at an early stage, but it is clear that these cells should be afforded the same attention as glial cells in the central nervous system, if we are to fully understand peripheral chemosensation.
Nitric oxide: parenchyma-vasculature cross talk and nitric oxide synthase microdomains
Type I cells and petrosal afferents collectively release several neurotransmitters NO (Wang et al. 1993; Del Rio et al. 2011) and ATP (Buttigieg and Nurse 2004; Grygorczyk and Orlov 2017; Buvinic et al. 2002) as well as ACh (Zhang et al. 2000) and noradrenaline (Gomez-Niño et al. 1990; Schamel and Verna 1992) known to stimulate or inhibit vascular smooth muscle and so it may be that parenchymal cells engage in paracrine communication with the local vasculature and that this modulates carotid body perfusion with consequences for chemoreceptor sensitivity and ultimately petrosal afferent firing (Figure 1).
If parenchymal signalling to the vasculature is to be considered then so too should the possibility of the reverse, with NO being worthy of particular attention here. Although it is now widely accepted that NO has a critical inhibitory role in the carotid body (Alcayaga et al. 1999; Fung et al. 2001), attention has mostly focused on NO released from inhibitory efferents, petrosal afferents and intrinsic neurons (Brognara et al. 2020; Wang et al. 1993, 1994; Campanucci et al. 2006), with little attention paid to the possibility that NO released from other sources such as endothelial cells (Boulanger et al. 1995; Chistiakov et al. 2015) may also be relevant to intra-carotid body inter-cellular communication (Figure 1). This is despite the carotid body being one of the most densely vascularised human organs (meaning vascular and non-vascular tissues are closely apposed throughout the carotid body) (Barnett et al. 1988) and classical paracrine endothelial cell signalling being mediated by NO. Illustrating the importance of non-neuronal NO in the nervous system, previous work has demonstrated that eNOS-derived NO in the nucleus tractus solitarius is critical for the modulation of the baroreflex by circulating angiotensin II, for example (Paton et al. 2001; Wong et al. 2002; Waki et al. 2003).
The relative contributions of eNOS and nNOS to NO signalling
Interestingly, it has been shown via the use of non-selective and specific inhibitors of nNOS and eNOS, that eNOS activity is in fact the main source of functional NO in the intact carotid body following carotid body stimulation (Valdés et al. 2003). At first glance, this result seems to imply that the endothelial cells of the vasculature might release the majority of the functionally relevant NO in the carotid body, raising the intriguing possibility that endothelial cell-derived NO may significantly influence the activity of carotid body parenchymal cells (Figure 1). An important caveat to this logic however, is that this difference in nNOS- and eNOS-driven NO activity may be the result of the eNOS isoform being expressed by glomus cells (Del Rio et al. 2011; Yamamoto et al. 2006). Despite this, the prospect of vasculature endothelial cells communicating chemosensory information to the parenchyma is appealing because of the direct contact between the vasculature and circulating blood. One speculative hypothesis is that endothelial cells may contribute to the putative NO - mediated inhibition described above, where the level of inhibition is proportional to [NO], which in turn depends on pO2 as this determines the availability of O2 for NO synthesis by NOS. It should be noted that this mechanism might be temporarily counter-acted by the fact that hypoxia drives both the release of NO from erythrocytes (Stamler et al. 1997; McMahon et al. 2002; Thomas et al. 2001; Pawloski et al. 2001; Ulker et al. 2013), as well as an acute [Ca2+]i-dependent increase in the quantity of NO synthesised by glomus cells (Yamamoto et al. 2006). Further, hypoxia also drives the release of ATP from erythrocytes, which then acts via endothelial P2YRs to activate eNOS and thus amplify acute endothelial NO production (Grygorczyk and Orlov 2017; Buvinic et al. 2002). It is also possible that this ATP may ‘spill over’ and activate the P2Rs expressed by type I cells (inhibitory P2Y1Rs (Xu et al. 2005; Carroll et al. 2012)), type II cells (excitatory P2Y2Rs (Zhang et al. 2012)), petrosal afferents (excitatory P2XRs (Pijacka et al. 2016)) and other cells of the parenchyma (Figure 1).
Regardless of whether parenchyma-vasculature cross-talk is functionally relevant in the carotid body, the balance of nNOS and eNOS activity has significant implications for carotid body NO signalling. It is already well established that nNOS and eNOS have distinct functions (Valdés et al. 2003; Martin et al. 2006) and in particular it is intriguing to note that Nos3-/- (encodes eNOS), but not Nos1-/- (encodes nNOS) mice develop hypertension (Barouch et al. 2002), a finding consistent with constitutive eNOS-mediated inhibition being required to prevent the development of carotid body hyperactivity.
NOS isoform microdomains
Understanding the difference in the functions of the two NOS isoforms requires an understanding of enzyme microdomains. In this case, a NOS microdomain is the area within which the NO synthesised by a particular NOS moiety is likely to have an effect and is primarily defined by the localisation of the enzyme population and the diffusion distance of NO.
NO diffusion distance is a product of the half-life of NO and of the NO diffusion rate. Although many factors contribute to the regulation of the NO diffusion rate in vivo, NO has a very short lifetime under physiological conditions with estimates of half-life ranging from of under 1s in blood-free isolated perfused heart (maximum diffusion distance estimated to be 130um) down to only a few ms in the presence of oxygenated blood (Beckman 1996). This short half-life dominates the diffusion distance calculation and ensures that NOS isoform microdomains are relatively small in volume, which in turn permits tight spatial and temporal regulation of the NO signal produced by NOS.
As described, eNOS is found in endothelial cells in the carotid body (as would be expected) and is also highly expressed in carotid body glomus cells (Del Rio et al. 2011; Yamamoto et al. 2006), whilst nNOS has been detected in glossopharyngeal nerve inhibitory efferent neurons and is presumed to be present in intrinsic neurons and NOS-positive petrosal afferents (Wang et al. 1993, 1994; Campanucci et al. 2006). The sub-cellular expression patterns of the NOS isoforms are also known to be segregated: nNOS is soluble and is usually localised to the sarcoplasmic / endoplasmic reticulum via its PDZ domain (Elfering et al. 2002), but it has also been detected at pre- and post- synaptic structures (Atkinson et al. 2003), whilst eNOS is palmitoylated and so associates with calveolar rafts in the plasma membrane (Shaul et al. 1996) as well as potentially with carotid body mitochondrial membranes, but only in response to hypoxia (Yamamoto et al. 2006).
By inspection, this differential cellular and sub-cellular localisation of the two NOS isoforms combined with the restricted volume of NOS microdomains should result in eNOS and nNOS having segregated microdomains and thus divergent functions in the carotid body, despite being apparently similar enzymes. This is indeed consistent with experimental results, as described above (Valdés et al. 2003). Further, the localisation of the NOS isoform populations should be expected to be the dominant factor in defining their separate functions.
Future work will be required if we are to elucidate the exact roles of eNOS and nNOS in their respective cellular compartments in the carotid body. However, based on the fact that glomus cells and petrosal afferents express eNOS (Del Rio et al. 2011; Yamamoto et al. 2006) and nNOS (Campanucci et al. 2006) respectively (meaning that these cells are at least partially included in the eNOS and nNOS microdomains by definition) and are inhibited by NO (Alcayaga et al. 1999; Summers et al. 1999), it is fairly certain that both eNOS and nNOS act as ‘auto-inhibitors’, with eNOS inhibiting glomus cells and nNOS inhibiting petrosal afferents. It is also likely that the microdomains of both NOS isoforms include a volume of extracellular space – eNOS by virtue of its association with caveolin (Shaul et al. 1996), which is an integral part of the plasma membrane and nNOS because it interacts with the synaptic machinery (Atkinson et al. 2003). As a result, both eNOS and nNOS microdomains may well also include neighbouring cells and thus may facilitate inter-cellular communication. For the eNOS microdomain, inter-cellular communication would likely involve the inhibition of petrosal afferents (Alcayaga et al. 1999) via eNOS localised to glomus cells (Del Rio et al. 2011; Yamamoto et al. 2006) and the induction of vasodilation via smooth muscle cell relaxation via eNOS (Wang et al. 1994) expressed by endothelial cells (see Figure 1 for circuit diagram). nNOS microdomain inter-cellular signalling probably also causes vasodilation in this way (Wang et al. 1994), but via nNOS found in the inhibitory efferents (Wang et al. 1993, 1994) and intrinsic neurons (Wang et al. 1993, 1994) that target the carotid body microvasculature instead of via eNOS. In addition, nNOS microdomain inter-cellular communication may also include the inhibition of glomus cells (Summers et al. 1999) via nNOS expressed by inhibitory efferents (Campanucci et al. 2006) and petrosal afferents (Wang et al. 1993, 1994), with the latter case being discussed earlier as a form of antidromic signalling at the glomus cell-petrosal afferent synapse. Although this means the collective functional microdomains of both NOS isoforms may possibly incorporate an identical set of cell types, it should be noted that intercellular communication within a NOS microdomain requiring diffusion across an extracellular space is probably significantly less robust than NO-mediated intracellular communication within that same microdomain.
In addition, as both eNOS and Cx43 associate with cavaeolin (Shaul et al. 1996; Langlois et al. 2008), these three proteins may form a tripartite complex and thus provide access to a high-speed (Figueroa et al. 2013) and direct pathway to apposed, coupled cells for eNOS-synthesised NO, greatly increasing the likelihood of the eNOS microdomain facilitating inter-cellular communication between specific cell types. Based on the gap junction coupling patterns of cells known to express eNOS (Del Rio et al. 2011; Yamamoto et al. 2006) and Cx43 (Abudara et al. 1999) (i.e. glomus cells), this inter-cellular intra-eNOS microdomain signalling via gap junctions would probably involve intra-glomus cell and glomus cell-petrosal afferent communication (Figure 1). Of note, the eNOS microdomain may not be static: it has been reported that eNOS may be translocated to the mitochondrial membrane during hypoxia (Yamamoto et al. 2006) i.e. away from the plasma membrane and neighbouring cells. If true, this may help explain how disinhibition of petrosal afferents (which are inhibited by NO (Alcayaga et al. 1999)) occurs during carotid body stimulation. Importantly, given that eNOS is the apparent source of the majority of the NO capable of inhibiting stimulus-driven chemotransmission (Valdés et al. 2003), most functionally relevant NOS signalling in the carotid body is likely contained within eNOS microdomains (the area surrounding an eNOS moiety within which the NO synthesised by that moiety could be expected to have a functional effect).
Distributed networks
It is widely acknowledged that syncytial systems formed of multiple cells of the same type exist in the CNS. Links between astrocytes, for example, can be formed via gap junctions (Simard and Nedergaard 2004; Wallraff et al. 2006; Dallérac et al. 2018). When many astrocytes are connected in this way, the resulting syncytial system effectively acts as a single cell with an expansive receptive field, permitting homeostasis of global conditions and thus global activity (Simard and Nedergaard 2004; Wallraff et al. 2006; Dallérac et al. 2018). Until this point, however, most studies have conceptualised the carotid body as either a complete organ or a collection of individual cells that communicate using the linear logic of the canonical model of carotid body function. As a result, few have considered the possibility that an arrangement analogous to the astrocytic syncytial system might exist in the carotid body.
Previous experiments have failed to detect dye transfer between cultured type II cells (Chou et al. 1998), suggesting that the ‘glial’ cells of the carotid body are not connected. This is in contrast to brain astrocytes (although gap junctions between multiple type II cells remains a possibility: discussed here (Reyes et al. 2014). Type I cells, however, do appear to be linked by gap junctions, as evidenced by the transfer of Lucifer yellow dye between apposed cells that were all later confirmed, via post-hoc immunostaining, to express tyrosine hyrdoxylase (Jiang and Eyzaguirre 2006). In addition, it has been demonstrated via paired sharp microelectrode recordings that neighbouring type I cells exhibit bidirectional electrical coupling (Chou et al. 1998; Monti-Bloch et al. 1993). This charge transfer phenomenon was also observed when multiple petrosal afferent terminals were recorded from simultaneously, suggesting that gap junctions also link the individual petrosal neuron projections that innervate type I cells (Jiang and Eyzaguirre 2006). As described above, initial evidence suggests glomus cells express both Cx36 and Cx43, whilst petrosal afferents may express Cx36 (Abudara et al. 1999; Frinchi et al. 2013).
In addition to achieving a syncytial system via information transfer through gap junctions, a similar system using coded chemical transmitters with both paracrine and autocrine features is also possible. Given that type I cells are known to be sensitive to the transmitters that they release when depolarised, and that autocrine stimulation by several of these ligands induces a positive feedback loop, such a system regulating type I cells likely operates in the carotid body.
For example: type I cells release ACh when stimulated (Zhang et al. 2000) and the activation of type I cell α4 and α7 nAChRs depolarises type I cells (Wyatt and Peers 1993; Meza et al. 2012). Further, the activation of either nAChRs or mAChRs expressed by type I cells elevates type I cell [Ca2+]i (Dasso et al. 1997). In the case of mAChRs, the [Ca2+]i response was found to be biphasic, with a rapid initial phase that was dependent on internal Ca2+ stores and a slower secondary phase that was dependent on external Ca2+ (Dasso et al. 1997). [Ca2+]i is increased above baseline in both phases (Dasso et al. 1997). These observations are consistent with the mAChRs in question being Gq coupled (i.e. either M1, M3 or M5 mAChRs) as the first and second phases are well explained by inositol trisphosphate receptor-mediated Ca2+ release from the ER and the inhibition of background K+ leak TASK channels via diacylglycerol respectively (Ortiz and Varas 2010). Indeed, it does appears that M1 mAChRs have a functional, excitatory role in the carotid body (Bairam et al. 1985, 2006; Wang and Fitzgerald 2002) and are expressed by glomus cells (Shirahata et al. 2004) (M2 mAChRs are seemingly also expressed by glomus cells (Shirahata et al. 2004 but are presumably functionally dominated either by M1 mAChRs alone or M1 mAChRs working in concert with M3 and / or M5 mAChRs). The combination of stimulation-induced release of ACh from type I cells together with ACh providing excitatory input to those same cells strongly suggests an ACh-specific positive feedback loop via ACh autoreceptors. This mechanism would be expected to spread an excited state amongst localised type I cells, thus effectively creating a syncytial system.
Interestingly, ACh activation of type I cells also induces a catecholamine release (Meza et al. 2012) that may mostly consist of NA (Vicario et al. 2000), which itself may represent another example of a transmitter that facilitates a syncytial state. Type I cells release NA when activated (Gomez-Niño et al. 1990) and recent data suggests α1 adrenoreceptors are expressed by type I cells, as well as cells of the carotid body vasculature (Felippe et al. 2022). The pre-activation of these receptors amplifies carotid sinus nerve discharges in response to hypoxia (Felippe et al. 2022) and so, as the hypoxic state is communicated directly to petrosal afferents by type I cells, it is possible that type I cell α1 adrenoreceptors can also act to propagate excitatory NA signalling within a local group of type I cells via a positive feedback mechanism. However, it remains possible that carotid body α1 adrenoreceptor activation mediates a vasculature-only effect. If the type I cell cholinergic system does act both to initiate and maintain a syncytial state directly, as well as trigger a localised, noradrenergic positive feedback loop then this would serve to amplify syncytial function fidelity.
A final example of a neurotransmitter that may induce a syncytial state amongst localised type I cells via a positive feedback loop is adenosine: type I cells release adenosine when stimulated (Conde and Monteiro 2004; Conde et al. 2012), but also express A2 receptors, which, when bound by ligand, induce the downstream inhibition of TASK channels and so increase excitability (Zhang et al. 2018).
These positive autocrine signals may directly offset the negative D2 auto-receptor feedback that likely results from type I cells both releasing dopamine upon being stimulated (Fishman et al. 1985) and being inhibited by it (Carroll et al. 2005). They may also offset the inhibitory retrograde dopamine transmission that is thought to occur following petrosal afferent activation (see above).
Functional consequences of a syncytial network in the carotid body
The cellular and molecular bases for gap junction coupling and neurotransmission in the carotid body suggest the presence of syncytial structures; the implications of this for information processing are significant. Individual clusters of linked glomus cells would in effect act as a single cell with a large receptive field. This could, for example, amplify the sensitivity of the glomus cell detection system and / or allow coincidence detection, which would improve the accuracy of the chemoreflex response by rejecting errant activation of an individual glomus cell. Further, the collective activation of a syncytial network of glomus cells potentially innervated by multiple different petrosal afferents (Figure 1) may help synchronise petrosal afferent output in the brainstem, which could in turn improve transmission fidelity, provide signal patterning that contributes to plasticity mechanisms such as LTP and LDP or synchronise downstream motor effector outputs. Similar information processing changes as a result of linked petrosal afferents can also be imagined.
Interestingly, hypoxia reduces intra-type I gap junction coupling (Jiang and Eyzaguirre 2006) whilst simultaneously increasing intra-afferent and type I – afferent gap junction coupling (Jiang and Eyzaguirre 2006). This latter change effectively exchanges lower fidelity synaptic signalling for much higher fidelity gap junction-mediated communication and so may represent one of the mechanisms underlying the increase in carotid body discharge in hypoxic conditions. The molecular processes underlying these changes in gap junction coupling are currently unclear, but interestingly, chronic hypoxia has been shown to upregulate Cx43 expression (Abudara et al. 1999; Chen et al. 2002). If the different connexins represent a computational code for specifying cell coupling in the carotid body, as they do in other organs, then a change in the differential translation of distinct connexin isoforms could well be what facilitates the switch from intra-type I and / or intra-afferent gap junction coupling to type I – afferent gap junction coupling during sustained hypoxia. Another proposal is that glomus cells may de-couple due to the inactivation of gap junction channels by hypoxia, an acidic pH or hypercapnia (Reyes et al. 2014), however this does not explain the surge in coupling efficiency between other cell types induced by low pO2. This apparent discrepancy may be explained by the hypothesis that whilst certain connexin isoforms are inhibited by specific environmental conditions, others (which are localised elsewhere) are activated by those same stimuli. In support of this idea, the activation of medullary connexin 26 by elevated PaCO2 is required for 30% of the total ventilatory response to hypercapnia (Van de Wiel et al. 2020).
These findings together suggest that, in future, models of the carotid body may need to incorporate the idea that individual clusters of cells in the carotid body function as isolated distributed networks (see below for evidence supporting the notion of multiple confined networks rather than a single syncytial system). Further, the active modulation of cell coupling and de-coupling appears to regulate afferent firing and so an examination of whether the disruption of these mechanisms is involved in pathological carotid body tonicity and hyperreflexia is warranted.
Specialised sub-circuits facilitate heterogeneous carotid body output pathways
The responses of individual components of the peripheral chemoreflex response differ depending on the stimuli involved, consistent with specific carotid body sub-circuits responding to certain stimuli and these sub-populations of cells then communicating with specific motor output pathways (Zera et al. 2019). For example, hypoxia leads to tachypnoea whereas, surprisingly, ATP causes apnoea (Spiller et al. 2021). Further, some highly branched petrosal afferents form multiple groups of calcyform synapses that each envelope 20–60 glomus cells, an arrangement that presumably produces functional cluster units (likely separated from other clusters by septal tissue, with the glomus cells in such clusters possibly being linked via gap junctions to form a syncytial detector as described above) (Torrealba and Alcayaga 1986). Other thinner afferents, with far simpler arborisation patterns, project to only a few closely apposed glomus cells (Torrealba and Alcayaga 1986; Torrealba 1996; Nishi and Stensaas 1974). It has been suggested that the larger fibres are likely myelinated A-type fibres that are characterised by a lower rheobase and a phasic firing pattern, whilst the smaller fibres are proposed to be C-type fibres, which have a higher rheobase and a tonic firing pattern (Zera et al. 2019; Belmonte and Gallego 1983). If correct, this could enable the system to encode heterogeneous responses to quantitative differences in stimulation intensity in addition to differentiating between distinct modes of stimulation. Finally, as mentioned above, inhibition of the P2X3 purinergic receptor reduces sympathetic tone and blood pressure in the SHR but does not affect reflex bradycardia or hyperventilation, which supports the separate reflex transmission circuits proposal (Pijacka et al. 2016; Zera et al. 2019). Taken together, these data suggest that differential responses to carotid body activation may be defined by a functional computational code that combines the variety of neurotransmitters described earlier with anatomical segregation of the carotid body and downstream structures into chemosensory units. This idea has been termed the ‘SCART lead’ or ‘ribbon cable’ hypothesis and is discussed further here (Zera et al. 2019; Paton et al. 2013) (also see Figure 1). If correct, this logic could provide a basis for the selective modulation of different chemoreflex components and thus enable highly targeted treatments with reduced off-target effects.
Conclusions and future perspectives
Further research is needed to reveal how the interplay between the different cells of the carotid body culminates in the output that drives the chemoreflex response and how this inter-cellular communication is altered in disease. Addressing the carotid body as a circuit by, for example, exploring how its receptor fields are organised or if vasculature signalling within the carotid body is influential, will be critical in achieving this goal. By clarifying exactly how manipulation of carotid body activity can influence sympathetic activity, we will be able to establish a basis for more specific interventions to control the elevated sympathetic activity underlying a variety of cardio-respiratory and metabolic diseases.
Funding Statement
This work was supported by Health Research Council of New Zealand: [Grant Number 19/687]; Sidney Taylor Trust: [Grant Number].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abboud FM. 1982. The sympathetic system in hypertension. state-of-the-art review. Hypertens. Dallas Tex 1979. 4:208–225. [PubMed] [Google Scholar]
- Abudara V, Garcés G, Sáez JC.. 1999. Cells of the carotid body express connexin43 which is up-regulated by cAMP. Brain Research. 849:25–33. [DOI] [PubMed] [Google Scholar]
- Alcayaga C, Varas R, Valdés V, Cerpa V, Arroyo J, Iturriaga R, Alcayaga J.. 2007. ATP- and ACh-induced responses in isolated cat petrosal ganglion neurons. Brain Research. 1131:60–67. [DOI] [PubMed] [Google Scholar]
- Alcayaga J, Soto CR, Vargas RV, Ortiz FC, Arroyo J, Iturriaga R.. 1999a. Modulatory effect of nitric oxide on acetylcholine-induced activation of cat petrosal ganglion neurons in vitro. Brain Research. 825:194–198. [DOI] [PubMed] [Google Scholar]
- Alcayaga J, Soto CR, Vargas RV, Ortiz FC, Arroyo J, Iturriaga R.. 2006. Carotid body transmitters actions on rabbit petrosal ganglion in vitro. In: Hayashida Y, Gonzalez C, Kondo H, editor. THE ARTERIAL CHEMORECEPTORS. Boston, MA: Springer; p. 331–337. [DOI] [PubMed] [Google Scholar]
- Alcayaga J, Varas R, Arroyo J, Iturriaga R, Zapata P.. 1999b. Dopamine modulates carotid nerve responses induced by acetylcholine on the cat petrosal ganglion in vitro. Brain Research. 831:97–103. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Birks EK, Hudetz AG, Roman RJ, Henderson L, Harder DR.. 1996. Inhibition of brain P-450 arachidonic acid epoxygenase decreases baseline cerebral blood flow. American Journal of Physiology-Heart and Circulatory Physiology. 271:H1541–H1546. [DOI] [PubMed] [Google Scholar]
- Almaraz L, Wang Z-Z, Stensaas LJ, Fidone SJ.. 1993. Release of dopamine from carotid sinus nerve fibers innervating type I cells in the Cat carotid body. Neurosignals. 2:16–26. [DOI] [PubMed] [Google Scholar]
- Arnold S, Kadenbach B.. 1997. Cell Respiration is controlled by ATP, an allosteric inhibitor of cytochrome-c oxidase. European Journal of Biochemistry. 249:350–354. [DOI] [PubMed] [Google Scholar]
- Atkinson L, Batten TFC, Corbett EKA, Sinfield JK, Deuchars J.. 2003. Subcellular localization of neuronal nitric oxide synthase in the rat nucleus of the solitary tract in relation to vagal afferent inputs. Neuroscience. 118:115–122. [DOI] [PubMed] [Google Scholar]
- Bairam A, Frenette J, Dauphin C, Carroll JL, Khandjian EW.. 1998. Expression of dopamine D1-receptor mRNA in the carotid body of adult rabbits, cats and rats. Neuroscience Research. 31:147–154. [DOI] [PubMed] [Google Scholar]
- Bairam A, Joseph V, Lajeunesse Y, Kinkead R.. 2006. Developmental pattern of M1 and M2 muscarinic gene expression and receptor levels in cat carotid body, petrosal and superior cervical ganglion. Neuroscience. 139:711–721. [DOI] [PubMed] [Google Scholar]
- Bairam, A, Néji, H. & Marchal, F.. Cholinergic dopamine release from the in vitro rabbit carotid body. Journal of Applied Physiology 1985. 88, 1737–1742 (2000). [DOI] [PubMed] [Google Scholar]
- Bardsley EN, Pen DK, McBryde FD, Ford AP, Paton JFR.. 2021. The inevitability of ATP as a transmitter in the carotid body. Autonomic Neuroscience. 234:102815. [DOI] [PubMed] [Google Scholar]
- Barnett S, Mulligan E, Wagerle LC, Lahiri S.. 1988. Measurement of carotid body blood flow in cats by use of radioactive microspheres. Journal of Applied Physiology Bethesda Md 1985. 65:2484–2489. [DOI] [PubMed] [Google Scholar]
- Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER.. 2002. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 416:337–339. [DOI] [PubMed] [Google Scholar]
- Beckman JS. 1996. 1 - The physiological and pathological chemistry of nitric oxide. In: Lancaster J, editor. Nitric oxide. San Diego, CA: Academic Press; p. 1–82. [Google Scholar]
- Belmonte C, Gallego R.. 1983. Membrane properties of cat sensory neurones with chemoreceptor and baroreceptor endings. The Journal of Physiology. 342:603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benot AR, López-Barneo J.. 1990. Feedback Inhibition of Ca2+ currents by dopamine in glomus cells of the carotid body. European Journal of Neuroscience. 2:809–812. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Northington FJ, Carhuapoma JR, Falck JR, Harder DR, Traystman RJ, Koehler RC.. 2000. P-450 epoxygenase and NO synthase inhibitors reduce cerebral blood flow response toN-methyl-d-aspartate. American Journal of Physiology-Heart and Circulatory Physiology. 279:H1616–H1624. [DOI] [PubMed] [Google Scholar]
- Bisgard GE. 1994. The role of Arterial Chemoreceptors in ventilatory acclimatization to hypoxia. In: O’Regan RG, Nolan P, McQueen DS, Paterson DJ, editors. Arterial Chemoreceptors: cell to system. Boston, MA: Springer; p. 109–122. [DOI] [PubMed] [Google Scholar]
- Bisgard GE. 2000. Carotid body mechanisms in acclimatization to hypoxia. Respiration Physiology. 121:237–246. [DOI] [PubMed] [Google Scholar]
- Bishop T, Talbot NP, Turner PJ, Nicholls LG, Pascual A, Hodson EJ, Douglas G, Fielding JW, Smith TG, Demetriades M, Schofield CJ.. 2013. Carotid body hyperplasia and enhanced ventilatory responses to hypoxia in mice with heterozygous deficiency of PHD2. The Journal of Physiology. 591:3565–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CM, Caputo L, Lévy BI.. 1995. Endothelial AT1–mediated release of nitric oxide decreases angiotensin II contractions in Rat carotid artery. Hypertension. 26:752–757. [DOI] [PubMed] [Google Scholar]
- Braga VA, Soriano RN, Braccialli AL, De Paula PM, Bonagamba LG, Paton JF, Machado BH.. 2007. Involvement of l-glutamate and ATP in the neurotransmission of the sympathoexcitatory component of the chemoreflex in the commissural nucleus tractus solitarii of awake rats and in the working heart–brainstem preparation. The Journal of Physiology. 581:1129–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brognara F, Felippe ISA, Salgado HC, Paton JFR.. 2020. Autonomic innervation of the carotid body as a determinant of its sensitivity: implications for cardiovascular physiology and pathology. Cardiovascular Research. 117:1015–1032. [DOI] [PubMed] [Google Scholar]
- Brophy S, Ford TW, Carey M, Jones JFX.. 1999. Activity of aortic chemoreceptors in the anaesthetized rat. The Journal of Physiology. 514:821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ. 1997. A novel oxygen-sensitive potassium current in rat carotid body type I cells. The Journal of Physiology. 498:649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD.. 1994. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. The Journal of Physiology. 476:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttigieg J, Nurse CA.. 2004. Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochemical and Biophysical Research Communications. 322:82–87. [DOI] [PubMed] [Google Scholar]
- Buvinic S, Briones R, Huidobro-Toro JP.. 2002. P2y1 and P2Y2 receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. British Journal of Pharmacology. 136:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanucci VA, Zhang M, Vollmer C, Nurse CA.. 2006. Expression of multiple P2X receptors by glossopharyngeal neurons projecting to Rat carotid body O2-chemoreceptors: role in nitric oxide-mediated efferent inhibition. Journal of Neuroscience. 26:9482–9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JL, Agarwal A, Donnelly DF, Kim I.. 2012. Purinergic modulation of carotid body glomus cell hypoxia response during postnatal maturation in rats. In: Nurse CA, Gonzalez C, Peers C, Prabhakar N, editor. Arterial chemoreception. Netherlands: Springer; p. 249–253. [DOI] [PubMed] [Google Scholar]
- Carroll JL, Boyle KM, Wasicko MJ, Sterni LM.. 2005. Dopamine D2 receptor modulation of carotid body type 1 cell intracellular calcium in developing rats. American Journal of Physiology-Lung Cellular and Molecular Physiology. 288:L910–L916. [DOI] [PubMed] [Google Scholar]
- Chang KC, Morrill CG, Chai H.. 1978. Impaired response to hypoxia after bilateral carotid body resection for treatment of bronchial asthma. Chest. 73:667–669. [DOI] [PubMed] [Google Scholar]
- Chen J, He L, Dinger B, Stensaas L, Fidone S.. 2002. Chronic hypoxia upregulates connexin43 expression in rat carotid body and petrosal ganglion. Journal of Applied Physiology. 92:1480–1486. [DOI] [PubMed] [Google Scholar]
- Chistiakov DA, Ashwell KW, Orekhov AN, Bobryshev YV.. 2015. Innervation of the arterial wall and its modification in atherosclerosis. Autonomic Neuroscience. 193:7–11. [DOI] [PubMed] [Google Scholar]
- Chou C-L, Sham JSK, Schofield B, Shirahata M.. 1998. Electrophysiological and immunocytological demonstration of cell-type specific responses to hypoxia in the adult cat carotid body. Brain Research. 789:229–238. [DOI] [PubMed] [Google Scholar]
- Christiansen L, Urbin M, Mitchell GS, Perez MA.. 2018. Acute intermittent hypoxia enhances corticospinal synaptic plasticity in humans. eLife. 7:e34304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde SV, Monteiro EC.. 2004. Hypoxia induces adenosine release from the rat carotid body. Journal of Neurochemistry. 89:1148–1156. [DOI] [PubMed] [Google Scholar]
- Conde SV, Monteiro EC, Rigual R, Obeso A, Gonzalez C.. 2012. Hypoxic intensity: a determinant for the contribution of ATP and adenosine to the genesis of carotid body chemosensory activity. Journal of Applied Physiology. 112:2002–2010. [DOI] [PubMed] [Google Scholar]
- Conde SV, Obeso A, Vicario I, Rigual R, Rocher A, Gonzalez C.. 2006. Caffeine inhibition of rat carotid body chemoreceptors is mediated by A2A and A2B adenosine receptors. Journal of Neurochemistry. 98:616–628. [DOI] [PubMed] [Google Scholar]
- Conde SV, Sacramento JF, Guarino MP, Gonzalez C, Obeso A, Diogo LN, Monteiro EC, Ribeiro MJ.. 2014. Carotid body, insulin, and metabolic diseases: unraveling the links. Frontiers in Physiology. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallérac G, Zapata J, Rouach N.. 2018. Versatile control of synaptic circuits by astrocytes: where, when and how? Nature Reviews Neuroscience. 19:729–743. [DOI] [PubMed] [Google Scholar]
- Dasso LL, Buckler KJ, Vaughan-Jones RD.. 1997. Muscarinic and nicotinic receptors raise intracellular Ca2+ levels in rat carotid body type I cells. The Journal of Physiology. 498(Pt 2):327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio R, Moya EA, Iturriaga R.. 2011. Differential expression of pro-inflammatory cytokines, endothelin-1 and nitric oxide synthases in the rat carotid body exposed to intermittent hypoxia. Brain Research. 1395:74–85. [DOI] [PubMed] [Google Scholar]
- Del Rio R, Moya EA, Iturriaga R.. 2014. Carotid body potentiation during chronic intermittent hypoxia: implication for hypertension. Front. Physiol. 5(434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio R, Moya EA, Koenig CS, Fujiwara K, Alcayaga J, Iturriaga R.. 2008. Modulatory effects of histamine on cat carotid body chemoreception. Respiratory Physiology & Neurobiology. 164:401–410. [DOI] [PubMed] [Google Scholar]
- Dinger B, Gonzalez C, Yoshizaki K, Fidone S.. 1981. [3h]spiroperidol binding in normal and denervated carotid bodies. Neuroscience Letters. 21:51–55. [DOI] [PubMed] [Google Scholar]
- Duchen MR, Caddy KWT, Kirby GC, Patterson DL, Ponte J, Biscoe TJ.. 1988. Biophysical studies of the cellular elements of the rabbit carotid body. Neuroscience. 26:291–311. [DOI] [PubMed] [Google Scholar]
- Elfering SL, Sarkela TM, Giulivi C.. 2002. Biochemistry of mitochondrial nitric-oxide synthase *. Journal of Biological Chemistry. 277:38079–38086. [DOI] [PubMed] [Google Scholar]
- Esler M. 2000. The sympathetic system and hypertension. American Journal of Hypertension. 13:99S–105S. [DOI] [PubMed] [Google Scholar]
- Eyzaguirre C, Koyano H.. 1965. Effects of electrical stimulation on the frequency of chemoreceptor discharges. The Journal of Physiology. 178:438–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon IM, Zhang M, Vollmer C, Nurse CA.. 2003. GABA mediates autoreceptor feedback inhibition in the Rat carotid Body Via Presynaptic GABAB receptors and TASK-1. The Journal of Physiology. 553:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felippe IS, Zera T, da Silva MP, Moraes DJ, McBryde F, Paton JF.. 2022. The sympathetic nervous system exacerbates carotid body sensitivity in hypertension. Cardiovascular Research. doi: 10.1093/cvr/cvac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidone SJ, Sato A.. 1970. Efferent inhibition and antidromic depression of chemoreceptor A-fibers from the cat carotid body. Brain Research. 22:181–193. [DOI] [PubMed] [Google Scholar]
- Fielding JW, Hodson EJ, Cheng X, Ferguson DJ, Eckardt L, Adam J, Lip P, Maton-Howarth M, Ratnayaka I, Pugh CW, Buckler KJ.. 2018. PHD2 inactivation in type I cells drives HIF-2α-dependent multilineage hyperplasia and the formation of paraganglioma-like carotid bodies. The Journal of Physiology. 596:4393–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa XF, Lillo MA, Gaete PS, Riquelme MA, Sáez JC.. 2013. Diffusion of nitric oxide across cell membranes of the vascular wall requires specific connexin-based channels. Neuropharmacology. 75:471–478. [DOI] [PubMed] [Google Scholar]
- Fishman MC, Greene WL, Platika D.. 1985. Oxygen chemoreception by carotid body cells in culture. Proceedings of the National Academy of Sciences. 82:1448–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AP, Undem BJ, Birder LA, Grundy D, Pijacka W, Paton JF.. 2015. P2x3 receptors and sensitization of autonomic reflexes. Autonomic Neuroscience. 191:16–24. [DOI] [PubMed] [Google Scholar]
- Frinchi M, Di Liberto V, Turimella S, D’Antoni F, Theis M, Belluardo N, Mudò G.. 2013. Connexin36 (Cx36) expression and protein detection in the mouse carotid body and myenteric plexus. Acta Histochemica. 115:252–256. [DOI] [PubMed] [Google Scholar]
- Fung M-L, Li M, Lahiri S.. 2007. Increased endogenous nitric oxide release by iron chelation and purinergic activation in the Rat carotid body. Open Biochem. J. 1:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung M-L, Ye J-S, Fung P.. 2001. Acute hypoxia elevates nitric oxide generation in rat carotid body in vitro. Pflgers Archiv European Journal of Physiology. 442:903–909. [DOI] [PubMed] [Google Scholar]
- Gebremedhin DEBEBE, Ma YH, Falck JR, Roman RJ, VanRollins MIKE, Harder DR.. 1992. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. American Journal of Physiology-Heart and Circulatory Physiology. 263:H519–H525. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL. 2008. Endocannabinoids at the synapse: retrograde Signaling and Presynaptic plasticity in the brain. In: Köfalvi A, editor. Cannabinoids and the brain. Boston, MA: Springer; p. 203–236. [Google Scholar]
- Gomez-Niño A, Dinger B, Gonzalez C, Fidone SJ.. 1990. Differential stimulus coupling to dopamine and norepinephrine stores in rabbit carotid body type I cells. Brain Research. 525:160–164. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R.. 1994. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiological Reviews. 74:829–898. [DOI] [PubMed] [Google Scholar]
- Grassi G, Ram VS.. 2016. Evidence for a critical role of the sympathetic nervous system in hypertension. Journal of the American Society of Hypertension. 10:457–466. [DOI] [PubMed] [Google Scholar]
- Grygorczyk R, Orlov SN.. 2017. Effects of hypoxia on erythrocyte membrane properties—implications for intravascular hemolysis and purinergic control of blood flow. Frontiers in Physiology. eCollection 2017. doi: 10.3389/fphys.2017.01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido G. 2004. Sympathetic and baroreflex function in hypertension: implications for current and New drugs. Current Pharmaceutical Design. 10:3579–3589. [DOI] [PubMed] [Google Scholar]
- Harder DR, Zhang C, Gebremedhin D.. 2002. Astrocytes function in matching blood flow to metabolic activity. Physiology. 17:27–31. [DOI] [PubMed] [Google Scholar]
- Heath D, Jago R, Smith P.. 1983. The vasculature of the carotid body. Cardiovascular Research. 17:33–42. [DOI] [PubMed] [Google Scholar]
- Heath D, Smith P, Fitch R, Harris P.. 1985. Comparative pathology of the enlarged carotid body. Journal of Comparative Pathology. 95:259–271. [DOI] [PubMed] [Google Scholar]
- Hirayama Y, Ikeda-Matsuo Y, Notomi S, Enaida H, Kinouchi H, Koizumi S.. 2015. Astrocyte-Mediated ischemic tolerance. Journal of Neuroscience. 35:3794–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson EJ, Nicholls LG, Turner PJ, Llyr R, Fielding JW, Douglas G, Ratnayaka I, Robbins PA, Pugh CW, Buckler KJ, Ratcliffe PJ.. 2016. Regulation of ventilatory sensitivity and carotid body proliferation in hypoxia by the PHD2/HIF-2 pathway. The Journal of Physiology. 594:1179–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Ray CJ, Thompson EL, Alshehri Z, Coney AM, Kumar P.. 2019. Adrenaline activation of the carotid body: Key to CO2 and pH homeostasis in hypoglycaemia and potential pathological implications in cardiovascular disease. Respiratory Physiology & Neurobiology. 265:92–99. [DOI] [PubMed] [Google Scholar]
- Ichikawa H. 2002. Innervation of the carotid body: immunohistochemical, denervation, and retrograde tracing studies. Microscopy Research and Technique. 59:188–195. [DOI] [PubMed] [Google Scholar]
- Igarashi A, Zadzilka N, Shirahata M.. 2009. Benzodiazepines and GABA-GABAA receptor system in the Cat carotid body. In: Gonzalez C, Nurse CA, Peers C, editors. Arterial Chemoreceptors: arterial chemoreceptors. Dordrecht, Netherlands: Springer; p. 169–175. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Alcayaga J.. 2004. Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Research Reviews. 47:46–53. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Larraín C, Zapata P.. 1994. Effects of dopaminergic blockade upon carotid chemosensory activity and its hypoxia-induced excitation. Brain Research. 663:145–154. [DOI] [PubMed] [Google Scholar]
- Janssen PL, Dwinell MR, Pizarro J, Bisgard GE.. 1998. Intracarotid dopamine infusion does not prevent acclimatization to hypoxia. Respiration Physiology. 111:33–43. [DOI] [PubMed] [Google Scholar]
- Jiang RG, Eyzaguirre C.. 2003. Dye and electric coupling between carotid nerve terminals and glomus cells. In: Pequignot J-M, Gonzalez C, Nurse CA, Prabhakar NR, Dalmaz Y, editors. Chemoreception. Boston, MA: Springer; p. 247–253. [DOI] [PubMed] [Google Scholar]
- Jiang RG, Eyzaguirre C.. 2006. Effects of prolonged hypobaric hypoxia on carotid nerve endings and glomus cells. changes in intercellular coupling. Brain Research. 1076:198–208. [DOI] [PubMed] [Google Scholar]
- Katz DM, Black IB.. 1986. Expression and regulation of catecholaminergic traits in primary sensory neurons: relationship to target innervation in vivo. The Journal of Neuroscience. 6:983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kåhlin J, Mkrtchian S, Ebberyd A, Hammarstedt-Nordenvall L, Nordlander B, Yoshitake T, Kehr J, Prabhakar N, Poellinger L, Fagerlund MJ, Eriksson LI.. 2014. The human carotid body releases acetylcholine, ATP and cytokines during hypoxia. Experimental Physiology. 99:1089–1098. [DOI] [PubMed] [Google Scholar]
- Kline DD, Peng Y-J, Manalo DJ, Semenza GL, Prabhakar NR.. 2002. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. Proceedings of the National Academy of Sciences. 99:821–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeners MP, Lewis KE, Ford AP, Paton JF.. 2016. Hypertension: a problem of organ blood flow supply–demand mismatch. Future Cardiology. 12:339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner P, Hesslinger C, Schaefermeyer A, Prinz C, Gratzl M.. 2004. Evidence for histamine as a transmitter in rat carotid body sensor cells. Journal of Neurochemistry. 91:493–500. [DOI] [PubMed] [Google Scholar]
- Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW.. 2008. Caveolin-1 and -2 interact with Connexin43 and regulate Gap junctional intercellular communication in keratinocytes. Molecular Biology of the Cell. 19:912–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov N, Rozloznik M, Reindl S, Rey-Ares V, Dutschmann M, Gratzl M.. 2006. Expression of histamine receptors and effect of histamine in the rat carotid body chemoafferent pathway. European Journal of Neuroscience. 24:3431–3444. [DOI] [PubMed] [Google Scholar]
- Leonard EM, Salman S, Nurse CA.. 2018. Sensory processing and integration at the carotid body tripartite synapse: neurotransmitter functions and effects of chronic hypoxia. Frontiers in Physiology. eCollection. doi: 10.3389/fphys.2018.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhao B, Zhao C, Huang L, Liu Y.. 2021. Metabotropic glutamate receptors 1 regulates Rat carotid body response to acute hypoxia via Presynaptic mechanism. Frontiers in Neuroscience. 15:1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Bjelobaba I, Stojilkovic SS.. 2018. Interactions of Pannexin1 channels with purinergic and NMDA receptor channels. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1860:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JHC, Lou N, Kang N, Takano T, Hu F, Han X, Xu Q, Lovatt D, Torres A, Willecke K, Yang J.. 2008. A central role of connexin 43 in hypoxic preconditioning. Journal of Neuroscience. 28:681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ji ES, Xiang S, Tamisier R, Tong J, Huang J, Weiss JW.. 2009. Exposure to cyclic intermittent hypoxia increases expression of functional NMDA receptors in the rat carotid body. Journal of Applied Physiology. 106:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li C, Jia X, Huang L, Weiss JW.. 2018. AMPA Receptor-Dependent glutamatergic Signaling is present in the carotid chemoreceptor. Neuroscience. 382:59–68. doi: 10.1016/j.neuroscience.2018.04.032 [DOI] [PubMed] [Google Scholar]
- López-Barneo J, López-López JR, Ureña J, González C.. 1988. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 241:580–582. [DOI] [PubMed] [Google Scholar]
- Lugliani R, Whipp BJ, Seard C, Wasserman K.. 1971. Effect of bilateral carotid-body resection on ventilatory control at rest and during exercise in man. New England Journal of Medicine. 285:1105–1111. [DOI] [PubMed] [Google Scholar]
- Marina N, Christie IN, Korsak A, Doronin M, Brazhe A, Hosford PS, Wells JA, Sheikhbahaei S, Humoud I, Paton JF, Lythgoe MF.. 2020. Astrocytes monitor cerebral perfusion and control systemic circulation to maintain brain blood flow. Nature Communications. 11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM. 1994. Peripheral chemoreceptors and cardiovascular regulation. Physiological Reviews. 74:543–594. [DOI] [PubMed] [Google Scholar]
- Martin SR, Emanuel K, Sears CE, Zhang Y-H, Casadei B.. 2006. Are myocardial eNOS and nNOS involved in the β-adrenergic and muscarinic regulation of inotropy? A systematic investigation. Cardiovascular Research. 70:97–106. [DOI] [PubMed] [Google Scholar]
- McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJ, Sobotka PA, Paton JF.. 2013. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nature Communications. 4:2395. [DOI] [PubMed] [Google Scholar]
- Mcdonald D, Mitchell R.. 1975. The innervation of glomus cells, ganglion cells and blood vessels in the rat carotid body: A quantitative ultrastructural analysis. Journal of Neurocytology. 4(2):177–230. [Google Scholar]
- McDonald DM, Larue DT.. 1983. The ultrastructure and connections of blood vessels supplying the rat carotid body and carotid sinus. Journal of Neurocytology. 12:117–153. [DOI] [PubMed] [Google Scholar]
- McDonald DM, Mitchell RA.. 1981. The neural pathway involved in ‘efferent inhibition’ of chemoreceptors in the cat carotid body. The Journal of Comparative Neurology. 201:457–476. [DOI] [PubMed] [Google Scholar]
- McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, Piantadosi CA.. 2002. Nitric oxide in the human respiratory cycle. Nature Medicine. 8:711–717. [DOI] [PubMed] [Google Scholar]
- Meza RC, Ortiz FC, Bravo E, Iturriaga-Vásquez P, Eugenín JL, Varas R.. 2012. Functional expression of the α7 and α4-containing nicotinic acetylcholine receptors on the neonatal rat carotid body. Neurochemistry International. 60:115–124. [DOI] [PubMed] [Google Scholar]
- Monti-Bloch L, Abudara V, Eyzaguirre C.. 1993. Electrical communication between glomus cells of the rat carotid body. Brain Research. 622:119–131. [DOI] [PubMed] [Google Scholar]
- Moraes DJA, da Silva MP, de Souza DP, Felintro V, Paton JFR.. 2021. Heightened respiratory-parasympathetic coupling to airways in the spontaneously hypertensive rat. The Journal of Physiology. 599:3237–3252. [DOI] [PubMed] [Google Scholar]
- Moraes DJA, da Silva MP, Spiller PF, Machado BH, Paton JFR.. 2018. Purinergic plasticity within petrosal neurons in hypertension. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 315:R963–R971. [DOI] [PubMed] [Google Scholar]
- Murali S, Nurse CA.. 2016. Purinergic signalling mediates bidirectional crosstalk between chemoreceptor type I and glial-like type II cells of the rat carotid body. The Journal of Physiology. 594:391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali S, Zhang M, Nurse CA.. 2014. Angiotensin II mobilizes intracellular calcium and activates pannexin-1 channels in rat carotid body type II cells via AT1 receptors. The Journal of Physiology. 592:4747–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali S, Zhang M, Nurse CA.. 2017. Evidence that 5-HT stimulates intracellular Ca2+ signalling and activates pannexin-1 currents in type II cells of the rat carotid body. The Journal of Physiology. 595:4261–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi K, Stensaas LJ.. 1974. The ultrastructure and source of nerve endings in the carotid body. Cell and Tissue Research. 154:303–319. [DOI] [PubMed] [Google Scholar]
- Nurse CA. 2005. Neurotransmission and neuromodulation in the chemosensory carotid body. Autonomic Neuroscience. 120:1–9. [DOI] [PubMed] [Google Scholar]
- Nurse CA. 2010. Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Experimental Physiology. 95:657–667. [DOI] [PubMed] [Google Scholar]
- Nurse CA, Piskuric NA.. 2013. Signal processing at mammalian carotid body chemoreceptors. Seminars in Cell & Developmental Biology. 24:22–30. [DOI] [PubMed] [Google Scholar]
- Olson EB, Vidruk EH, Dempsey JA.. 1988. Carotid body excision significantly changes ventilatory control in awake rats. Journal of Applied Physiology Bethesda Md 1985. 64:666–671. [DOI] [PubMed] [Google Scholar]
- Ortega-Sáenz P, López-Barneo J.. 2020. Physiology of the carotid body: from molecules to disease. Annual Review of Physiology. 82:127–149. [DOI] [PubMed] [Google Scholar]
- Ortiz FC, Varas R.. 2010. Muscarinic modulation of TASK-like background potassium channel in rat carotid body chemoreceptor cells. Brain Research. 1323:74–83. [DOI] [PubMed] [Google Scholar]
- Paton JF, Deuchars J, Ahmad Z, Wong LF, Murphy D, Kasparov S.. 2001. Adenoviral vector demonstrates that angiotensin II-induced depression of the cardiac baroreflex is mediated by endothelial nitric oxide synthase in the nucleus tractus solitarii of the rat. The Journal of Physiology. 531:445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF, Ratcliffe L, Hering D, Wolf J, Sobotka PA, Narkiewicz K.. 2013a. Revelations about carotid body function through its pathological role in resistant hypertension. Current Hypertension Reports. 15:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N.. 2013b. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension. 61:5–13. [DOI] [PubMed] [Google Scholar]
- Paton JFR, De Paula PM, Spyer KM, Machado BH, Boscan P.. 2002. Sensory afferent selective role of P2 receptors in the nucleus tractus solitarii for mediating the cardiac component of the peripheral chemoreceptor reflex in rats. The Journal of Physiology. 543:995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawloski JR, Hess DT, Stamler JS.. 2001. Export by red blood cells of nitric oxide bioactivity. Nature. 409:622–626. [DOI] [PubMed] [Google Scholar]
- Peng Y-J, Overholt JL, Kline D, Kumar GK, Prabhakar NR.. 2003. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proceedings of the National Academy of Sciences. 100:10073–10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuela S, Gehi R, Laird DW.. 2013. The biochemistry and function of pannexin channels. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1828:15–22. [DOI] [PubMed] [Google Scholar]
- Pijacka W, Moraes DJ, Ratcliffe LE, Nightingale AK, Hart EC, da Silva MP, Machado BH, McBryde FD, Abdala AP, Ford AP, Paton JF.. 2016. Purinergic receptors in the carotid body as a new drug target for controlling hypertension. Nature Medicine. 22:1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platero-Luengo A, González-Granero S, Durán R, Díaz-Castro B, Piruat JI, García-Verdugo JM, Pardal R, López-Barneo J.. 2014. An O2-sensitive glomus cell-stem cell synapse induces carotid body growth in chronic hypoxia. Cell. 156:291–303. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Kumar GK, Chang CH, Agani FH, Haxhiu MA.. 1993. Nitric oxide in the sensory function of the carotid body. Brain Research. 625:16–22. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Peng Y-J.. 2017. Oxygen sensing by the carotid body: past and present. Adv. Exp. Med. Biol. 977:3–8. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Peng Y-J, Kumar GK, Nanduri J.. 2015. Peripheral Chemoreception and arterial pressure responses to intermittent hypoxia. In: Comprehensive physiology. American Cancer Society; 5:561–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulgar-Sepúlveda R, Varas R, Iturriaga R, Del Rio R, Ortiz FC.. 2018. Carotid body type-I cells under chronic sustained hypoxia: focus on metabolism and membrane excitability. Frontiers in Physiology. 9:1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez M, Gallego-Martin T, Olea E, Rocher A, Obeso A, Gonzalez C.. 2012. Serotonin dynamics and actions in the Rat carotid body: preliminary findings. In: Nurse CA, Gonzalez C, Peers C, Prabhakar N, editor. Arterial chemoreception. Netherlands: Springer; p. 255–263. [DOI] [PubMed] [Google Scholar]
- Reyes EP, Cerpa V, Corvalán L, Retamal MA.. 2014. Cxs and panx- hemichannels in peripheral and central chemosensing in mammals. Frontiers in Cellular Neuroscience. eCollection. doi: 10.3389/fncel.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes EP, Fernández R, Larraín C, Zapata P.. 2007. Effects of combined cholinergic-purinergic block upon cat carotid body chemoreceptors in vitro. Respiratory Physiology & Neurobiology. 156:17–22. [DOI] [PubMed] [Google Scholar]
- Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC, Conde SV.. 2013. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes. 62:2905–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman S, Vollmer C, McClelland GB, Nurse CA.. 2017. Characterization of ectonucleotidase expression in the rat carotid body: regulation by chronic hypoxia. American Journal of Physiology-Cell Physiology. 313:C274–C284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamel A, Verna A.. 1992. Norepinephrine-containing glomus cells in the rabbit carotid body. II. immunocytochemical evidence of dopamine-beta-hydroxylase and norepinephrine. Journal of Neurocytology. 21:353–362. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Marcus NJ, Rio RD.. 2015. Mechanisms of carotid body chemoreflex dysfunction during heart failure. Experimental Physiology. 100:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S-A, Wöhler A, Beutner D, Angelov DN.. 2016. Microsurgical anatomy of the human carotid body (glomus caroticum): features of its detailed topography, syntopy and morphology. Annals of Anatomy-Anatomischer Anzeiger. 204:106–113. [DOI] [PubMed] [Google Scholar]
- Semenza GL. 2000. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. Journal of Applied Physiology. 88:1474–1480. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RG, Michel T.. 1996. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae (∗). Journal of Biological Chemistry. 271:6518–6522. [DOI] [PubMed] [Google Scholar]
- Shin MK, Eraso CC, Mu YP, Gu C, Yeung BH, Kim LJ, Liu XR, Wu ZJ, Paudel O, Pichard LE, Shirahata M.. 2019. Leptin induces hypertension acting on transient receptor potential melastatin 7 channel in the carotid body. Circulation Research. 125:989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahata M, Hirasawa S, Okumura M, Mendoza JA, Okumura A, Balbir A, Fitzgerald RS.. 2004. Identification of M1 and M2 muscarinic acetylcholine receptors in the cat carotid body chemosensory system. Neuroscience. 128:635–644. [DOI] [PubMed] [Google Scholar]
- Simard M, Nedergaard M.. 2004. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 129:877–896. [DOI] [PubMed] [Google Scholar]
- Siński M, Lewandowski J, Przybylski J, Bidiuk J, Abramczyk P, Ciarka A, Gaciong Z.. 2012. Tonic activity of carotid body chemoreceptors contributes to the increased sympathetic drive in essential hypertension. Hypertension Research. 35:487–491. [DOI] [PubMed] [Google Scholar]
- Smith CA, Bisgard GE, Nielsen AM, Daristotle LEIGHANN, Kressin NA, Forster HV, Dempsey JA.. 1986. Carotid bodies are required for ventilatory acclimatization to chronic hypoxia. Journal of Applied Physiology Bethesda Md 1985. 60:1003–1010. [DOI] [PubMed] [Google Scholar]
- Spiller PF, da Silva MP, Moraes DJA.. 2021. Lactate does not activate the carotid body of Wistar rat. Respiratory Physiology & Neurobiology. 285:103593. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA.. 1997. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 276:2034–2037. [DOI] [PubMed] [Google Scholar]
- Summers BA, Overholt JL, Prabhakar NR.. 1999. Nitric oxide inhibits L-type Ca2+ current in glomus cells of the rabbit carotid Body Via a cGMP-independent mechanism. Journal of Neurophysiology. 81:1449–1457. [DOI] [PubMed] [Google Scholar]
- Taha AY. 2015. Carotid body tumours: A review. International Journal of Clinical Medicine. 6:119–131. [Google Scholar]
- Tak E, Jun DY, Kim SH, Park GC, Lee J, Hwang S, Song GW, Lee SG.. 2016. Upregulation of P2Y2 nucleotide receptor in human hepatocellular carcinoma cells. Journal of International Medical Research. 44:1234–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Matsuda H, Hayashida Y, Yamamoto Y, Tsukuda M, Kusakabe T.. 2011. Morphological characteristics and peptidergic innervation in the carotid body of spontaneously hypertensive rats. Histol. Histopathol. 26:369–375. [DOI] [PubMed] [Google Scholar]
- Tao HW, Poo M.. 2001. Retrograde signaling at central synapses. Proceedings of the National Academy of Sciences. 98:11009–11015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DD, Liu X, Kantrow SP, Lancaster JR.. 2001. The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proceedings of the National Academy of Sciences. 98:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ. 2015. Pannexin channels and ischaemia. The Journal of Physiology. 593:3463–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrealba F. 1996. Two morphological types of chemoreceptor afferents innervate the rabbit carotid body. Adv. Exp. Med. Biol. 410:51–54. [DOI] [PubMed] [Google Scholar]
- Torrealba F, Alcayaga J.. 1986. Nerve branching and terminal arborizations in the carotid body of the cat. A light microscopic study following anterograde injury filling of carotid nerve axons with horseradish peroxidase. Neuroscience. 19:581–595. [DOI] [PubMed] [Google Scholar]
- Ulker P, Gunduz F, Meiselman HJ, Baskurt OK.. 2013. Nitric oxide generated by red blood cells following exposure to shear stress dilates isolated small mesenteric arteries under hypoxic conditions. Clinical Hemorheology and Microcirculation. 54:357–369. [DOI] [PubMed] [Google Scholar]
- Ureña J, López-López J, González C, López-Barneo J.. 1989. Ionic currents in dispersed chemoreceptor cells of the mammalian carotid body. Journal of General Physiology. 93:979–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés V, Mosqueira M, Rey S, Del Rio R, Iturriaga R.. 2003. Inhibitory effects of NO on carotid body: contribution of neural and endothelial nitric oxide synthase isoforms. American Journal of Physiology-Lung Cellular and Molecular Physiology. 284:L57–L68. [DOI] [PubMed] [Google Scholar]
- Van de Wiel J, Meigh L, Bhandare A, Cook J, Nijjar S, Huckstepp R, Dale N.. 2020. Connexin26 mediates CO2-dependent regulation of breathing via glial cells of the medulla oblongata. Communications Biology. 3:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varas R, Wyatt CN, Buckler KJ.. 2007. Modulation of TASK-like background potassium channels in rat arterial chemoreceptor cells by intracellular ATP and other nucleotides. The Journal of Physiology. 583:521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verna A, Schamel A, Le Moine C, Bloch B.. 1995. Localization of dopamine D2 receptor mRNA in glomus cells of the rabbit carotid body by in situ hybridization. Journal of Neurocytology. 24:265–270. [DOI] [PubMed] [Google Scholar]
- Vicario I, Rigual R, Obeso A, Gonzalez C.. 2000. Characterization of the synthesis and release of catecholamine in the rat carotid body in vitro. American Journal of Physiology-Cell Physiology. 278:C490–C499. [DOI] [PubMed] [Google Scholar]
- Waki H, Kasparov S, Wong LF, Murphy D, Shimizu T, Paton JF.. 2003. Chronic inhibition of endothelial nitric oxide synthase activity in nucleus tractus solitarii enhances baroreceptor reflex in conscious rats. The Journal of Physiology. 546:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallraff A, Köhling R, Heinemann U, Theis M, Willecke K, Steinhäuser C.. 2006. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. Journal of Neuroscience. 26:5438–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-YJ, Fitzgerald RS.. 2002. Muscarinic modulation of hypoxia-induced release of catecholamines from the cat carotid body. Brain Research. 927:122–137. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Wang MB, Barauskas TM, Calcaterra TC.. 2000. Surgical management of carotid body tumors. Otolaryngology–Head and Neck Surgery. 123:202–206. [DOI] [PubMed] [Google Scholar]
- Wang Z-Z, Stensaas LJ, Bredt DS, Dinger B, Fidone SJ.. 1994. Localization and actions of nitric oxide in the cat carotid body. Neuroscience. 60:275–286. [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Bredt DS, Fidone SJ, Stensaas LJ.. 1993. Neurons synthesizing nitric oxide innervate the mammalian carotid body. The Journal of Comparative Neurology. 336:419–432. [DOI] [PubMed] [Google Scholar]
- Weber PA, Chang H-C, Spaeth KE, Nitsche JM, Nicholson BJ.. 2004. The permeability of Gap junction channels to probes of different size Is dependent on connexin composition and permeant-pore affinities. Biophysical Journal. 87:958–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L-F, Polson JW, Murphy D, Paton JFR, Kasparov S.. 2002. Genetic and pharmacological dissection of pathways involved in the angiotensin II-mediated depression of baroreflex function. The FASEB Journal. 16:1595–1601. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Peers C.. 1993. Nicotinic acetylcholine receptors in isolated type I cells of the neonatal rat carotid body. Neuroscience. 54:275–281. [DOI] [PubMed] [Google Scholar]
- Xu J, Tse FW, Tse A.. 2003. ATP triggers intracellular Ca2+ release in type II cells of the Rat carotid body. The Journal of Physiology. 549:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu F, Tse FW, Tse A.. 2005. ATP inhibits the hypoxia response in type I cells of rat carotid bodies. Journal of Neurochemistry. 92:1419–1430. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, König P, Henrich M, Dedio J, Kummer W.. 2006. Hypoxia induces production of nitric oxide and reactive oxygen species in glomus cells of rat carotid body. Cell and Tissue Research. 325:3–11. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Nakamuta N, Kusakabe T, Yamamoto Y.. 2014. Vesicular glutamate transporter 2-immunoreactive afferent nerve terminals in the carotid body of the rat. Cell and Tissue Research. 358:271–275. [DOI] [PubMed] [Google Scholar]
- Zera T, Moraes DJA, da Silva MP, Fisher JP, Paton JFR.. 2019. The logic of carotid body connectivity to the brain. Physiology. 34:264–282. [DOI] [PubMed] [Google Scholar]
- Zhang M, Clarke K, Zhong H, Vollmer C, Nurse CA.. 2009. Postsynaptic action of GABA in modulating sensory transmission in co-cultures of rat carotid body via GABAA receptors. The Journal of Physiology. 587:329–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Fearon IM, Zhong H, Nurse CA.. 2003. Presynaptic modulation of rat arterial chemoreceptor function by 5-HT: role of K+ channel inhibition via protein kinase C. The Journal of Physiology. 551:825–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Piskuric NA, Vollmer C, Nurse CA.. 2012. P2y2 receptor activation opens pannexin-1 channels in rat carotid body type II cells: potential role in amplifying the neurotransmitter ATP. The Journal of Physiology. 590:4335–4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Vollmer C, Nurse CA.. 2018. Adenosine and dopamine oppositely modulate a hyperpolarization-activated current Ih in chemosensory neurons of the rat carotid body in co-culture. The Journal of Physiology. 596:3101–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhong H, Vollmer C, Nurse CA.. 2000. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. The Journal of Physiology. 525:143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Nurse CA.. 1997. Nicotinic acetylcholine sensitivity of rat petrosal sensory neurons in dissociated cell culture. Brain Research. 766:153–161. [DOI] [PubMed] [Google Scholar]