Abstract
Human immunodeficiency virus type 1 (HIV-1) primary infection is characterized by the use of CCR5 as a coreceptor for viral entry, which is associated with the non-syncytium-inducing (NSI) phenotype in lymphoid cells. Syncytium-inducing (SI) variants of HIV-1 appear in advanced stages of HIV-1 infection and are characterized by the use of CXCR4 as a coreceptor. The emergence of SI variants is accompanied by a rapid decrease in the number of T cells. However, it is unclear why SI variants emerge and what factors trigger the evolution of HIV from R5 to X4 variants. Interleukin-7 (IL-7), a cytokine produced by stromal cells of the thymus and bone marrow and by keratin, is known to play a key role in T-cell development. We evaluated IL-7 levels in plasma of healthy donors and HIV-positive patients and found significantly higher levels in HIV-positive patients. There was a negative correlation between circulating IL-7 levels and CD4+ T-cell count in HIV-positive patients (r = −0.621; P < 0.001), suggesting that IL-7 may be involved in HIV-induced T-cell depletion and disease progression. IL-7 levels were higher in individuals who harbored SI variants and who had progressed to having CD4 cell counts of lower than 200 cells/μl than in individuals with NSI variants at a similar stage of disease. IL-7 induced T-cell proliferation and up-regulated CXCR4 expression in peripheral blood mononuclear cells in vitro. Taken together, our results suggest a role for IL-7 in the maintenance of T-cell regeneration and depletion by HIV in infected individuals and a possible relationship between IL-7 levels and the emergence of SI variants.
Human immunodeficiency virus type 1 (HIV-1) primary infection is characterized by the presence of non-syncytium-inducing (NSI) variants with low replication kinetics, capable of infecting macrophages and CD4+ memory T cells, that use the receptor CCR5 as a coreceptor for viral entry (21). Later, as the disease progresses, the syncytium-inducing (SI) variants emerge (2). SI variants are characterized by high replication kinetics in vitro and the capacity to infect naive CD4+ T cells by using CXCR4 as a coreceptor (4, 26). The emergence of SI variants is accompanied by an accelerated decrease of CD4+ cell count, rapid disease progression, and the establishment of AIDS (10, 13). However, it remains unclear why SI variants emerge and how this relates to CXCR4 expression in vivo. It is probable that multiple host factors affect HIV-1 coreceptor levels or function; interleukin-4 (IL-4) has been shown to decrease the expression of CCR5 and increase CXCR4 expression, favoring the propagation of X4 strains (42). Other factors that may induce overexpression of CXCR4 or block the replication of R5-NSI variants may favor the selection of X4-SI HIV variants and could determine when SI variants will arise.
During clinical latency, HIV-1 replicates, inducing the destruction of CD4+ T cells and immature cells in the bone marrow, thymus, and lymph nodes, where T cells are produced (25). The immune system responds by inducing the proliferation of T cells, and hence the CD4+ T-cell number is maintained relatively constant during this stage of the infection (11, 20, 23, 43). The development of AIDS was thought to be caused by exhaustion of the immune system. That is, at a certain point, the immune system cannot maintain the high rate of T-cell production necessary to compensate for HIV-induced T-cell depletion (27). Nevertheless, HIV-1 infection destroys T-cell supplies in the periphery by direct infection and killing of cells and through hyperactivation of the immune system (15), suggesting that it may be not exhaustion but rather homeostatic inability, along with gradual wasting of T-cell supplies, that leads to T-lymphocyte depletion in HIV-1 infection (16, 17).
It is known that cytokines play an important role in HIV-1 infection. However, determination of their function in viral dynamics, replication, and disease progression is very complex, because different cytokines have opposite effects on viral replication (19, 24, 32, 34, 42). IL-7 is a cytokine produced by stromal cells of the thymus and the bone marrow and by keratinocytes (18, 38, 39, 45). IL-7 has recognized functions in B-cell lymphopoiesis (30) and has been shown to take part in the differentiation of thymocytes into mature T cells that will leave the thymus and move to the periphery (7, 29). Similarly, IL-7 contributes to the development, proliferation, and homeostatic maintenance of T cells (12, 14, 33, 35, 40). IL-7 is known to enhance viral replication (6, 41) and may induce CXCR4 expression on resting CD4+ memory T cells in vitro (22). IL-7 production by human stromal cells is induced by IL-1 and by tumor necrosis factor alpha (44), which have been found to also enhance HIV production in vitro (32).
The role of IL-7 as a marker of disease progression has not been well established. Napolitano et al. (31) have shown that increased circulating levels of IL-7 are strongly associated with CD4+ T lymphopenia in HIV-1 disease. Nevertheless, the importance of these results to the understanding of HIV pathogenesis requires further confirmation. Furthermore, we need to assess the effect of IL-7 on the evolution of HIV in vivo.
We have evaluated IL-7 levels in plasma of healthy individuals and HIV-1-infected patients and correlated their expression in HIV-positive individuals to CD4+ T-cell depletion, disease progression, and emergence of the SI phenotype.
MATERIALS AND METHODS
Patient and donor samples.
Blood samples from healthy donors and from HIV-positive individuals were collected from our hospital blood blank and from the HIV unit, respectively. Samples were collected with informed consent and processed immediately after collection. Briefly, 10 to 20 ml of whole blood was collected in EDTA-Vacutainer tubes (Becton Dickinson [BD], Madrid, Spain). Plasma was isolated from each sample after centrifugation of blood samples at 400 × g for 10 min and was immediately cryopreserved and stored at −80°C until use. Peripheral blood mononuclear cells (PBMC) were obtained by separation on Ficoll-Hypaque density gradient and either used immediately in fractional studies or cryopreserved in liquid nitrogen for further determinations. Some patients were enrolled in clinical trials with monotherapy (zidovudine [AZT] or dideoxyinosine [ddI]) or dual therapy (AZT plus dideoxycytosine, AZT plus ddI, or AZT plus lamivudine [3TC]) and were later included in triple antiretroviral therapy. These patients usually initiated treatment at a late stage of disease and the treatment options were not efficacious, increasing the possibility selecting SI variants.
T-lymphocyte proliferation.
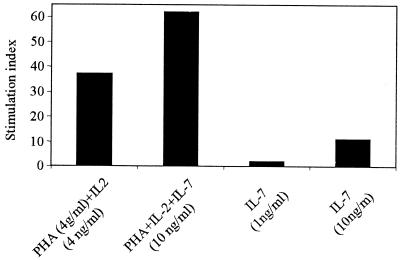
Fresh PBMC (106) from two healthy donors were cultured with different antigens as follows: medium control, phytohemagglutinin (PHA) (4 ng/ml) plus IL-2 (4 ng/ml) as a positive control, PHA plus IL-2 plus IL-7 (10 ng/ml), and IL-7 alone (1 and 10 ng/ml). The cultures were maintained at 37°C in a 5% CO2 incubator for 5 days. [3H]thymidine was then added to each well and incubated overnight. The cells were harvested, and the amount of incorporated [3H]thymidine was measured in a liquid scintillation counter (1450 Microbeta; Wallac, Turku, Finland). The stimulation index was calculated by dividing the counts per minute of PBMC after specific stimulation by the counts per minute of PBMC incubated with medium control. A stimulation index of >5 was considered to be a positive response in this assay.
CXCR4 expression.
Fresh PBMC from healthy donors were cultured with IL-7 at different concentrations and with stromal cell-derived factor 1 (SDF-1) (500 ng/ml) or medium alone as controls. After 5 days of incubation, PBMC were collected and CXCR4 and CD4 expression was analyzed by flow cytometry as described below.
Detection of IL-7 and RANTES levels in plasma.
Plasma IL-7 levels were determined by an ultrasensitive commercial enzyme-linked immunosorbent assay (ELISA) (Quantikine HS Human IL-7 Immunoassay; R&D Systems, Minneapolis, Minn.) according to the manufacturer instructions. RANTES levels were measured by a commercial ELISA (Endogen, Barcelona, Spain).
Viral isolation and phenotype in MT-2 cells.
PBMC (10 × 106) from HIV-infected individuals were cocultured with PBMC (5 × 106) from healthy donors stimulated with 3 μg of PHA per ml and 25 IU of IL-2 per ml. Viral replication was quantified by evaluation of antigen p24 production in coculture supernatants, using a commercial ELISA (Innogenetics, Madrid, Spain). Coculture supernatants that were positive for p24 were collected after centrifugation at 400 × g for 5 min, and the SI or NSI phenotype was determined in MT-2 cells as was previously described (9). For simplicity, individuals from whom SI or NSI variants were isolated are referred to hereafter as SI or NSI individuals, respectively.
Flow cytometry.
CD4+ and CXCR4+ T-cell subpopulations were determined by flow cytometry analysis. Aliquots of 50 μl of whole-blood samples were stained with monoclonal antibodies CD4-PerCP and CXCR4-PE (BD) for 15 min, and then the samples were washed twice in phosphate-buffered saline, resuspended in phosphate-buffered saline containing 1% formaldehyde, and analyzed in a FACScalibur flow cytometer (BD).
Measurement of viral load.
Plasma HIV RNA levels were determined using a commercial assay (Amplicor VIH-1 Monitor Assay; Roche Molecular Systems, Somerville, N.J.) according to the manufacturer's instructions. Undetectable levels of RNA in plasma were considered equivalent to 200 copies/ml.
Statistical analysis.
Statistical analysis was performed using parametric and nonparametric tests (Spearman r and Mann-Whitney U tests). P values of <0.05 were considered to have statistical significance. Data were analyzed using the SPSS version 9.0 software package.
RESULTS
T-lymphocyte proliferation.
IL-7 has been described as an indispensable factor for T-cell development (33). As can be observed in Fig. 1, IL-7 induced the proliferation of T cells at 10 ng/ml (stimulation index = 11) and boosted PHA- and IL-2-induced cell proliferation activity in vitro, confirming a known property of this cytokine. Our data support previous studies in which IL-7 has been described as an important agent in T-cell proliferation (14).
FIG. 1.
Lymphocyte proliferative response to PHA (4 μg/ml) plus IL-2 (4 ng/ml), PHA plus IL-2 plus IL-7 (10 ng/ml), and IL-7 alone (1 or 10 ng/ml). The stimulation index was calculated by dividing the counts per minute of PBMC in stimulated wells by the counts per minute of PBMC in medium alone. The results are from a representative experiment of two performed.
IL-7 up-regulates CXCR4 expression in vitro
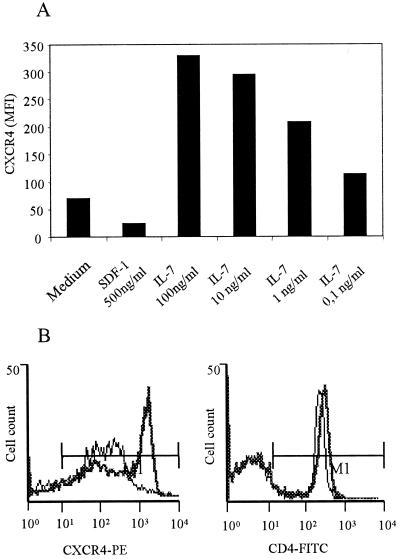
To determine if IL-7 modulates CXCR4 expression in T cells, we incubated PBMC of two uninfected donors for 5 days with different concentrations of this cytokine. IL-7 up-regulated the expression of the CXCR4 receptor in a dose-dependent manner, whereas SDF-1, the natural ligand of CXCR4, down-regulated CXCR4 expression (Fig. 2A). Although IL-7 caused the greatest up-regulation of CXCR4 expression at 100 ng/ml (Fig. 2B), 0.1 ng/ml was sufficient to up-regulate CXCR4 expression in vitro. However, CD4 expression was not modified by IL-7 (Fig. 2B). This finding suggests a possible role of IL-7 in selection of SI variants in HIV-positive patients with high IL-7 levels in plasma through up-regulation of CXCR4.
FIG. 2.
(A) Effect of IL-7 and SDF-1 on expression of CXCR4 in PBMC. IL-7 was evaluated at concentrations of 100, 10, 1, and 0.1 ng/ml. SDF-1 was evaluated at 500 ng/ml. CXCR4 expression is represented as mean fluorescence intensity (MFI). (B) Comparison of CXCR4 and CD4 expression in PBMC from a healthy donor, either stimulated with 100 ng of IL-7 per ml (thick lines) or without stimulation (thin lines). The results are from a representative experiment of two performed.
IL-7 levels in HIV-1-infected patients and healthy donors.
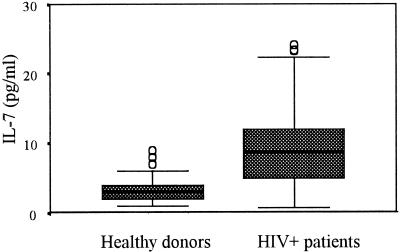
IL-7 levels in 49 plasma samples from healthy volunteers and in 131 plasma samples from HIV-positive patients were analyzed in a cross-sectional study. The HIV-positive group had significantly (P < 0.001) higher levels of IL-7 than the healthy donor group (Fig. 3). The IL-7 levels measured in plasma were 3.6 ± 3.05 and 9.4 ± 5.7 pg/ml (means and standard deviations [SD]) for the healthy donor and HIV-positive groups, respectively. Other immunological and virological characteristics were evaluated for HIV-positive patients, with the findings that the means and SD of CD4 and CD8 T-cell counts and the log10 viral load were 243 ± 221 cells/μl, 796 ± 586 cells/μl, and 5.26 ± 5.5 copies/ml, respectively. RANTES levels were evaluated for healthy donors (12.7 ± 16.1 ng/ml) and HIV-positive patients (27.7 ± 21.2 ng/ml); the differences between the groups were significant (P < 0.001), as previously described (1).
FIG. 3.
Expression of IL-7 levels in plasma of healthy donors and HIV-positive patients. Results are depicted in box plot diagrams, where the box represents the 25th and 75th quartiles and the line represents the median value. Bars indicate 5th and 95th percentiles, and circles indicate atypical values. The means ± SD of IL-7 levels in plasma for the healthy donor group and the HIV-positive patient group were 3.6 ± 3.05 and 9.4 ± 5.7 pg/ml, respectively.
IL-7 as a marker of disease progression.
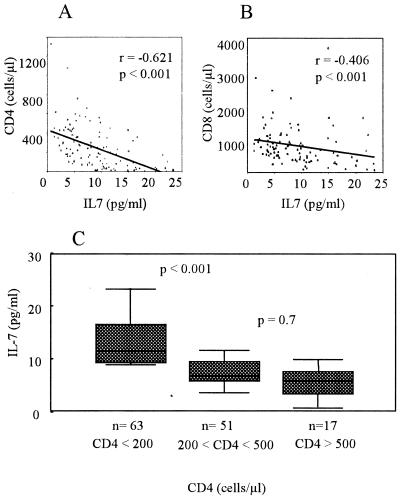
It has been recently shown that increased production of IL-7 accompanies HIV-1-mediated T-cell depletion (31) Similarly, we have found a clear negative correlation between IL-7 levels in plasma and absolute CD4+ T-cell counts in HIV-positive individuals (r = −0.621; P < 0.001) (Fig. 4A). A similar but weaker correlation was found between IL-7 levels and CD8+ T-cell levels (r = −0.406; P < 0.001) (Fig. 4B). When we grouped HIV-positive individuals according to their immunological status (that is, stratifying HIV-positive patients according to their CD4+ T-cell count) (Fig. 4C), the subset with <200 CD4 cells/μl had significantly (P < 0.001) higher levels of IL-7 in plasma (12.26 ± 5.86 pg/ml) than the subsets with CD4 cell counts of between 200 and 500 and above 500 cells/μl (6.67 ± 4.10 and 5.69 ± 2.75 pg/ml, respectively). These data suggest that CD4+ T-cell depletion, caused by HIV-1 replication, may alter IL-7 levels in plasma as a means to regenerate T-cell numbers.
FIG. 4.
(A and B) Correlation between IL-7 levels and CD4 T-cell count (r = −0.621) (A) and between IL-7 levels and CD8 T-cell count (r = −0.406) (B) in HIV-positive patients. (C) Levels of IL-7 in plasma of HIV-positive patients stratified according to CD4 T-cell count in three subsets, i.e., <200, 200 to 500, and >500 CD4 cells/μl. The means ± SD of IL-7 levels in plasma for the three subsets were 12.26 ± 5.9, 6.67 ± 4.1, and 5.69 ± 2.7 pg/ml, respectively. Subsets are depicted in box plots as described for Fig. 3.
IL-7 as a marker for the emergence of the SI phenotype.
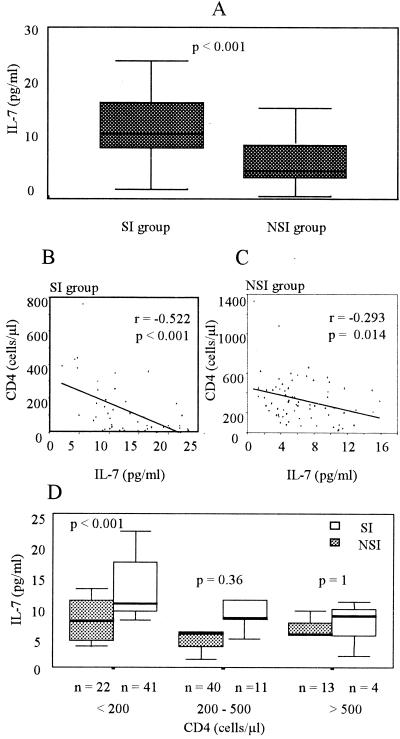
PBMC from HIV-positive individuals were cocultured with PBMC from healthy donors to isolate virus. HIV-1 p24 antigen-containing supernatant from each coculture was then used for evaluation of the SI phenotype in MT-2 cells. Fifty-six samples tested as SI, and 75 samples tested as NSI. When we analyzed IL-7 levels in the HIV-positive group separated into groups that harbored SI and NSI viruses, we found significantly (P < 0.001) higher levels of IL-7 in the SI group (13 ± 6 pg/ml) than in the NSI group (7 ± 4 pg/ml) (Table 1; Fig. 5A). Significant differences were also found between the SI group and the NSI group in CD4+ T-cell count, CD8+ T-cell count, and viral load, suggesting a more advanced stage of disease in the SI group than in the NSI group. There were no significant differences in RANTES levels between the groups.
TABLE 1.
Immunological and virological variables for the patients in the NSI and SI groupsa
| Group | n | IL-7 (pg/ml) | CD4 (cells/μl) | CD8 (cells/μl) | RANTES (ng/ml) | Viral load (log10) |
|---|---|---|---|---|---|---|
| SI | 56 | 13.04 ± 5.9 | 125 ± 155 | 619 ± 420 | 29.3 ± 19.3 | 5.49 ± 5.6 |
| NSI | 75 | 6.7 ± 3.6 | 329 ± 223 | 909 ± 649 | 26.5 ± 22.7 | 4.9 ± 5.2 |
| Pb | <0.001 | <0.001 | <0.05 | >0.1 | <0.001 |
Results are expressed as means and SD.
Statistical significance (Mann-Whitney U test) between the SI and NSI groups.
FIG. 5.
(A) IL-7 levels in plasma in HIV-positive patients separated according to the viral phenotype (SI or NSI). IL-7 levels are depicted in a box plot as described for Fig. 3. (B and C) Linear regression between IL-7 levels and CD4 T-cell counts in patients of the SI group (r = −0.522) (B) and the NSI group (r = −0.293) (C). (D) Levels of IL-7 in plasma of HIV-positive individuals of the SI and NSI groups stratified according to CD4 T-cell count in three subsets as described for Fig. 4C.
We correlated CD4+ T-cell counts with IL-7 levels separately in the SI and NSI groups, finding a higher correlation between the two parameters in the SI group (r = −0.522; P < 0.001) than in the NSI group (r = −0.293; P = 0.014) (Fig. 5B and C).
Since HIV disease may progress in the absence of the SI phenotype, it was important to compare IL-7 levels between individuals in the NSI or SI group in a similar stage of disease. Thus, we stratified the individuals of both groups according to their CD4 T-cell count (Fig. 5D). The NSI group showed no significant differences in IL-7 levels in the three subsets of CD4 levels (7.3 ± 3.2, 6.3 ± 4, and 5.7 ± 2.9 pg/ml). In contrast, in the SI group, we found significantly (P < 0.001) higher levels of IL-7 (14.6 ± 5.4 pg/ml) in the subset with the lower CD4 cell count (<200 cells/μl) than in the two other subsets (7.8 ± 4.5 and 5.6 ± pg/ml, respectively). It is interesting that the SI subset with a CD4 cell level of <200 cells/μl showed significantly (P < 0.001) higher levels of IL-7 than the NSI subset with a similar CD4 cell count, whereas there were no significant differences in IL-7 levels between the NSI and the SI groups when the CD4 cell count was above 200 cells/μl.
High IL-7 levels are associated with SI variants.
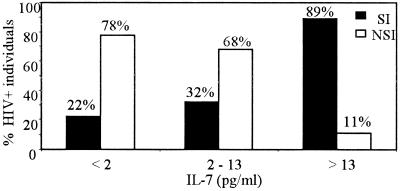
To characterize the IL-7 level in plasma as a marker of the emergence of the SI phenotype, we stratified patients according to the IL-7 level in plasma (Fig. 6) The results showed that 89% of individuals with more than 13 pg of IL-7 per ml belonged to the SI group and that this decreased to 32 and 22% when IL-7 levels were below 13 and 2 pg/ml, respectively. The 78% of the individuals with IL-7 levels below 2 pg/ml belonged to the NSI group. As the IL-7 level increased (2 to 13 pg/ml and >13 pg/ml), the proportion of NSI individuals was lower (68 and 11%, respectively). Thus, the IL-7 level in plasma may be a marker for the emergence of the SI phenotype.
FIG. 6.
Percentages of individuals belonging to the SI and NSI groups, stratified according to the levels of IL-7 in plasma in three subsets (<2, 2 to 13, and >13 pg/ml.
Longitudinal study.
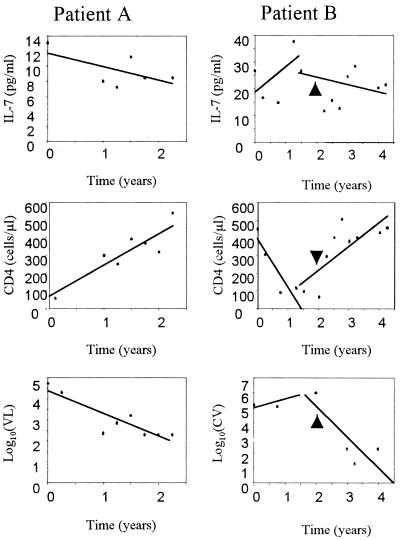
A longitudinal study was designed to evaluate changes in IL-7 levels with respect to standard virological and immunological markers of HIV-1 disease (viral load and CD4 cell count) and to better characterize IL-7 as a marker of disease progression. Frozen plasma samples that were collected from five HIV-positive individuals for a period of 2 to 7 years were used to evaluate IL-7 levels in plasma. Patients were selected on the basis of availability of plasma samples taken at least every 3 months for the period of the study, in which IL-7 levels and viral load could be evaluated. All patients had received antiretroviral therapy during the course of the disease; therefore, the CD4+ T-cell count, viral load, and IL-7 levels in plasma were expected to be influenced by drug treatment. In Fig. 7, we show IL-7, CD4+ T-cell count, and viral load trends for two patients as a representative sample of the longitudinal study. In patient A, a decrease in IL-7 level corresponded to an increase in CD4 T-cell count and to a decrease in viral load, indicating an effective response to the treatment with indinavir. The data for patient B revealed biphasic trends in the measured parameters; that is, this patient did not respond satisfactorily to the first treatment with double therapy (AZT plus 3TC), which caused a initial increase in IL-7 level and in viral load and a decrease in CD4 T-cell count. After 2 years the patient began receiving triple antiretroviral therapy, which caused a decrease in viral load and in IL-7 levels and an increase in CD4 T-cell count. Taken together, these data suggest that the plasma IL-7 level may be an effective marker of disease progression in HIV-positive patients.
FIG. 7.
IL-7 levels, CD4 T-cell counts, and log10 viral load in two patients along the course of the longitudinal study. In patient A, time zero corresponds to the initiation of treatment (AZT plus ddI plus 3TC plus indinavir). In patient B, time zero corresponds to the initiation of the first treatment (AZT plus 3TC plus d4T), which was continued for 2 years; the second treatment (AZT plus 3TC plus d4T plus indinavir) (time of initiation is shown by the arrowhead) covers the following 2 years. The lines represent the trends calculated by linear regression.
DISCUSSION
In our cross-sectional study, we have found a significant difference in plasma IL-7 levels between HIV-negative donors and HIV-positive patients. Confirming recently published results (12, 31), we have found a negative correlation between IL-7 levels in plasma and CD4+ T-cell counts in HIV-positive patients (r = −0.621), suggesting that HIV infection may mask the proliferative effect of IL-7 (Fig. 1) (31).
In addition, a longitudinal study with HIV-positive patients showed that variations in CD4+ T-cell counts caused by the response to treatment were accompanied by similar variations in plasma IL-7 levels. These data support the idea of IL-7 as an indicator of CD4+ T-cell depletion and consequently as a marker of disease progression.
There is controversy about the origin of the T-cell renewal that compensates for T-cell depletion in HIV infection. Some evidence points to a persistent immune activation induced by viral replication that causes proliferation of existing naive CD4+ T cells in the periphery (16). Other evidence points to thymic output of new naive T cells (8, 29) caused by a homeostatic response to T-cell depletion. Previous observations have associated abundant thymic tissue in HIV-positive individuals with increased numbers of naive T cells (8, 36). Since IL-7 is produced by stromal cells of the thymus and is implicated in thymocyte maturation, our data may indicate a homeostatic response that is mediated by IL-7. Alternatively, IL-7 produced by extrathymic tissue or induced by other factors (e.g., tumor necrosis factor alpha) could explain the observations made here. The fact that some individuals with <200 CD4 cells/μl have low IL-7 levels in plasma may support the latter hypothesis.
Our results suggest a relationship between IL-7 levels in plasma and HIV phenotype, since HIV-positive patients with high IL-7 levels had a high probability (0.89) of having the SI phenotype. It is unclear why individuals with low CD4 cell counts of the NSI phenotype have significantly lower IL-7 levels than those individuals with SI variants. If IL-7 increases in response to CD4 cell depletion, NSI individuals with CD4 cell counts of <200/μl should have plasma IL-7 levels similar to those of SI individuals in the same immunological status. One possible explanation is that NSI and SI variants may have different tropisms for cell subpopulations that produce IL-7 (3, 5, 37). In addition, HIV-1 may induce a collapse of the regulatory signals that control CD4 cell number, allowing for IL-7 production without a concomitant effect on CD4 proliferation. Our data indicate that, unlike CD4 cell counts, IL-7 levels could discriminate between those individuals with the NSI phenotype and those with the SI phenotype in that subset of individuals with advanced disease progression.
IL-7 may be considered a causal factor for the emergence of the SI variants, together with other factors such as SDF-1. We have shown that individuals with high levels of SDF-1 were at a lower risk of developing HIV variants of the SI phenotype (28).
The immune system may respond to CD4 T-cell depletion caused by HIV replication by inducing the proliferation of circulating naive CD4 T cells and by producing homeostatic signals (such as IL-7) that induce production of new naive CD4 T cells. However, at a certain time during infection, the immune system may not be able to respond to the signals induced by decreased CD4 T-cell number, which in turn act on existing CD4 cells. Consequently, higher IL-7 levels may induce the overexpression of CXCR4 (Fig. 2A), allowing SI variants to grow. High SDF-1 levels could maintain lower expression of CXCR4 and keep SI variants at bay, and at the same time, high SDF-1 levels could block the effect of IL-7 and/or other factors that affect CXCR4 expression. This hypothesis could help to explain the correlation between the emergence of SI variants and the rapid CD4 T-cell decline and why SI variants appear late after infection. The effect of IL-7 on CXCR4 expression could be masked by the same principle governing IL-7 and CD4 cell count: it is possible that during HIV-1 infection, increased IL-7 could lead to the selective destruction of CXCR4-expressing cells. The regulation of CXCR4 expression appears to be governed by multiple factors that may be active at any given time and that require further study.
In conclusion, our observations on the increased production of IL-7 in HIV-positive individuals and its correlation to T-cell depletion suggest that IL-7 may have an important role in the maintenance of T-cell homeostasis in HIV infection. Intrapatient IL-7 production may be an effective marker of the disease progression and a causal factor for the emergence of SI HIV variants.
ACKNOWLEDGMENTS
This work was supported in part by Fundación para la Investigación y la Prevención del SIDA en España (FIPSE) project 3111/00, Ministerio de Ciencia y Tecnología project BFM2000-1382, and the Fundació irsiCaixa. J. Blanco is an FIS researcher from the Fundació para la Recerca Biomédica Hospital Germans Trias i Pujol. A. Llano and J. Barretina hold predoctoral scholarships from FIS.
REFERENCES
- 1.Aukrust P, Muller F, Froland S S. Circulating levels of RANTES in human immunodeficiency virus type 1 infection: effect of potent antiretroviral therapy. J Infect Dis. 1998;177:1091–1096. doi: 10.1086/517402. [DOI] [PubMed] [Google Scholar]
- 2.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz R D, Alexander S, McCune J M. Causal relationships between HIV-1 coreceptor utilization, tropism, and pathogenesis in human thymus. AIDS Res Hum Retroviruses. 2000;16:1039–1045. doi: 10.1089/08892220050075291. [DOI] [PubMed] [Google Scholar]
- 4.Blaak H, van't Wout A B, Brouwer M, Hooibrink B, Hovenkamp E, Schuitemaker H. In vivo HIV-1 infection of CD45RA(+)CD4(+) T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4(+) T cell decline. Proc Natl Acad Sci USA. 2000;97:1269–1274. doi: 10.1073/pnas.97.3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonyhadi M L, Rabin L, Salimi S, Brown D A, Kosek J, McCune J M, Kaneshima H. HIV induces thymus depletion in vivo. Nature. 1993;363:728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 6.Chene L, Nugeyre M T, Barre-Sinoussi F, Israel N. High-level replication of human immunodeficiency virus in thymocytes requires NF-κB activation through interaction with thymic epithelial cells. J Virol. 1999;73:2064–2073. doi: 10.1128/jvi.73.3.2064-2073.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chene L, Nugeyre M T, Guillemard E, Moulian N, Barre-Sinoussi F, Israel N. Thymocyte-thymic epithelial cell interaction leads to high-level replication of human immunodeficiency virus exclusively in mature CD4+ CD8− CD3+ thymocytes: a critical role for tumor necrosis factor and interleukin-7. J Virol. 1999;73:7533–7542. doi: 10.1128/jvi.73.9.7533-7542.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douek D C, McFarland R D, Keiser P H, Gage E A, Massey J M, Haynes B F, Polis M A, Haase A T, Feinberg M B, Sullivan J L, Jamieson B D, Zack J A, Picker L J, Koup R A. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 9.Este J A, Cabrera C, Blanco J, Gutierrez A, Bridger G, Henson G, Clotet B, Schols D, De Clercq E. Shift of clinical human immunodeficiency virus type 1 isolates from X4 to R5 and prevention of emergence of the syncytium-inducing phenotype by blockade of CXCR4. J Virol. 1999;73:5577–5585. doi: 10.1128/jvi.73.7.5577-5585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fauci A S. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 11.Fauci A S. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 12.Fry T J, Connick E, Falloon J, Lederman M M, Liewehr D J, Spritzler J, Steinberg S M, Wood L V, Yarchoan R, Zuckerman J, Landay A, Mackall C L. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–2990. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 13.Glushakova S, Grivel J C, Fitzgerald W, Sylwester A, Zimmerberg J, Margolis L B. Evidence for the HIV-1 phenotype switch as a causal factor in acquired immunodeficiency. Nat Med. 1998;4:346–349. doi: 10.1038/nm0398-346. [DOI] [PubMed] [Google Scholar]
- 14.Grabstein K H, Namen A E, Shanebeck K, Voice R F, Reed S G, Widmer M B. Regulation of T cell proliferation by IL-7. J Immunol. 1990;144:3015–3020. [PubMed] [Google Scholar]
- 15.Hazenberg M D, Hamann D, Schuitemaker H, Miedema F. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat Immunol. 2000;1:285–289. doi: 10.1038/79724. [DOI] [PubMed] [Google Scholar]
- 16.Hazenberg M D, Otto S A, Stuart J W, Verschuren M C, Borleffs J C, Boucher C A, Coutinho R A, Lange J M, de Wit T F, Tsegaye A, van Dongen J J, Hamann D, de Boer R J, Miedema F. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat Med. 2000;6:1036–1042. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 17.Hazenberg M D, Stuart J W, Otto S A, Borleffs J C, Boucher C A, de Boer R J, Miedema F, Hamann D. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95:249–255. [PubMed] [Google Scholar]
- 18.Heufler C, Topar G, Grasseger A, Stanzl U, Koch F, Romani N, Namen A E, Schuler G. Interleukin 7 is produced by murine and human keratinocytes. J Exp Med. 1993;178:1109–1114. doi: 10.1084/jem.178.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho D D, Hartshorn K L, Rota T R, Andrews C A, Kaplan J C, Schooley R T, Hirsch M S. Recombinant human interferon alfa-A suppresses HTLV-III replication in vitro. Lancet. 1985;i:602–604. doi: 10.1016/s0140-6736(85)92144-0. [DOI] [PubMed] [Google Scholar]
- 20.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 21.Jansson M, Backstrom E, Bjorndal A, Holmberg V, Rossi P, Fenyo E M, Popovic M, Albert J, Wigzell H. Coreceptor usage and RANTES sensitivity of non-syncytium-inducing HIV-1 isolates obtained from patients with AIDS. J Hum Virol. 1999;2:325–338. [PubMed] [Google Scholar]
- 22.Jourdan P, Vendrell J P, Huguet M F, Segondy M, Bousquet J, Pene J, Yssel H. Cytokines and cell surface molecules independently induce CXCR4 expression on CD4+ CCR7+ human memory T cells. J Immunol. 2000;165:716–724. doi: 10.4049/jimmunol.165.2.716. [DOI] [PubMed] [Google Scholar]
- 23.Keet I P, Krijnen P, Koot M, Lange J M, Miedema F, Goudsmit J, Coutinho R A. Predictors of rapid progression to AIDS in HIV-1 seroconverters. AIDS. 1993;7:51–57. doi: 10.1097/00002030-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Kinter A L, Ostrowski M, Goletti D, Oliva A, Weissman D, Gantt K, Hardy E, Jackson R, Ehler L, Fauci A S. HIV replication in CD4+ T cells of HIV-infected individuals is regulated by a balance between the viral suppressive effects of endogenous beta-chemokines and the viral inductive effects of other endogenous cytokines. Proc Natl Acad Sci USA. 1996;93:14076–14081. doi: 10.1073/pnas.93.24.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitchen S G, Uittenbogaart C H, Zack J A. Mechanism of human immunodeficiency virus type 1 localization in CD4-negative thymocytes: differentiation from a CD4-positive precursor allows productive infection. J Virol. 1997;71:5713–5722. doi: 10.1128/jvi.71.8.5713-5722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koot M, Keet I P, Vos A H, de Goede R E, Roos M T, Coutinho R A, Miedema F, Schellekens P T, Tersmette M. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Leonard R, Zagury D, Desportes I, Bernard J, Zagury J F, Gallo R C. Cytopathic effect of human immunodeficiency virus in T4 cells is linked to the last stage of virus infection. Proc Natl Acad Sci USA. 1988;85:3570–3574. doi: 10.1073/pnas.85.10.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llano A, Barretina J, Gutierrez A, Blanco J, Clotet B, Esté J A. SDF-1 prevents the emergence of the syncytium inducing phenotype of HIV-1 in vivo. AIDS. 2001;15:1890–1892. doi: 10.1097/00002030-200109280-00023. [DOI] [PubMed] [Google Scholar]
- 29.McCune J M, Loftus R, Schmidt D K, Carroll P, Webster D, Swor-Yim L B, Francis I R, Gross B H, Grant R M. High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J Clin Invest. 1998;101:2301–2308. doi: 10.1172/JCI2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Namen A E, Lupton S, Hjerrild K, Wignall J, Mochizuki D Y, Schmierer A, Mosley B, March C J, Urdal D, Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333:571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 31.Napolitano L A, Grant R M, Deeks S G, Schmidt D, De Rosa S C, Herzenberg L A, Herndier B G, Andersson J, McCune J M. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–79. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 32.Osborn L, Kunkel S, Nabel G J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci USA. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plum J, De Smedt M, Leclercq G, Verhasselt B, Vandekerckhove B. Interleukin-7 is a critical growth factor in early human T-cell development. Blood. 1996;88:4239–4245. [PubMed] [Google Scholar]
- 34.Saha K, Bentsman G, Chess L, Volsky D J. Endogenous production of beta-chemokines by CD4+, but not CD8+, T-cell clones correlates with the clinical state of human immunodeficiency virus type 1 (HIV-1)-infected individuals and may be responsible for blocking infection with non-syncytium-inducing HIV-1 in vitro. J Virol. 1998;72:876–881. doi: 10.1128/jvi.72.1.876-881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schluns K S, Kieper W C, Jameson S C, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 36.Smith K Y, Valdez H, Landay A, Spritzler J, Kessler H A, Connick E, Kuritzkes D, Gross B, Francis I, McCune J M, Lederman M M. Thymic size and lymphocyte restoration in patients with human immunodeficiency virus infection after 48 weeks of zidovudine, lamivudine, and ritonavir therapy. J Infect Dis. 2000;181:141–147. doi: 10.1086/315169. [DOI] [PubMed] [Google Scholar]
- 37.Stanley S K, McCune J M, Kaneshima H, Justement J S, Sullivan M, Boone E, Baseler M, Adelsberger J, Bonyhadi M, Orenstein J, et al. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sudo T, Ito M, Ogawa Y, Iizuka M, Kodama H, Kunisada T, Hayashi S, Ogawa M, Sakai K, Nishikawa S. Interleukin 7 production and function in stromal cell-dependent B cell development. J Exp Med. 1989;170:333–338. doi: 10.1084/jem.170.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sudo T, Nishikawa S, Ohno N, Akiyama N, Tamakoshi M, Yoshida H. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci USA. 1993;90:9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan J T, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg K I, Surh C D. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;10:10. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uittenbogaart C H, Boscardin W J, Anisman-Posner D J, Koka P S, Bristol G, Zack J A. Effect of cytokines on HIV-induced depletion of thymocytes in vivo. AIDS. 2000;14:1317–1325. doi: 10.1097/00002030-200007070-00003. [DOI] [PubMed] [Google Scholar]
- 42.Valentin A, Lu W, Rosati M, Schneider R, Albert J, Karlsson A, Pavlakis G N. Dual effect of interleukin 4 on HIV-1 expression: implications for viral phenotypic switch and disease progression. Proc Natl Acad Sci USA. 1998;95:8886–8891. doi: 10.1073/pnas.95.15.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 44.Weitzmann M N, Cenci S, Rifas L, Brown C, Pacifici R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood. 2000;96:1873–1878. [PubMed] [Google Scholar]
- 45.Wolf S S, Cohen A. Expression of cytokines and their receptors by human thymocytes and thymic stromal cells. Immunology. 1992;77:362–368. [PMC free article] [PubMed] [Google Scholar]