Abstract
Background:
In China, acupuncture has been employed as an adjunctive therapy for coronavirus disease 2019 (COVID-19). Press needle acupuncture is a special type of acupuncture that provides prolonged stimulation to acupuncture points and simultaneously reduces the pain associated with traditional acupuncture. This study assessed the effectiveness of integrating press needles alongside pharmacologic treatment in patients with mild-to-moderate COVID-19.
Methods:
Patients hospitalized with mild-to-moderate COVID-19 symptoms between December 2022 and January 2023 were included in the study. The enrolled patients were randomly assigned to receive pharmacologic treatment alone (control group) or both pharmacologic treatment and press needle acupuncture (intervention group). Patients were evaluated for clinical outcomes, including symptom scores, deterioration rates, fever durations, and nucleic acid test results. The patients’ complete blood count and C-reactive protein levels were also analyzed using venous blood samples both before and after treatment.
Results:
Both groups exhibited a reduction in clinical symptom scores, but symptoms regressed faster in the intervention group. Nucleic acid test negativity was achieved faster in the intervention group than in the control group. The intervention group also had a lower deterioration rate. Furthermore, the increase in the lymphocyte count and decrease in C-reactive protein levels following treatment were more pronounced in the intervention group than in the control group.
Conclusion:
This study suggests that utilizing press needle acupuncture as an adjunct to pharmacologic treatment can be effective in patients with mild-to-moderate COVID-19 symptoms.
Keywords: acupuncture, COVID-19, press needles
1. Introduction
In late 2019, coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), rapidly spread globally, leading to more than 242 million confirmed cases and nearly 5 million fatalities.[1] Because of the lack of readily available cost-effective and proven treatment for COVID-19, extensive basic and clinical research has been conducted to identify viable treatments against this new virus.[2] A noteworthy approach has combined traditional Chinese medical practices with contemporary Western medicine. A large number of patients with COVID-19 have received such treatments in China throughout the pandemic.[3]
Acupuncture, a cornerstone of traditional Chinese medicine (TCM) and a prominent form of complementary and alternative medicine, has long been utilized to manage inflammatory conditions.[4] Acupuncture has been used as an adjunctive therapy for COVID-19 during the pandemic in China. However, there is little published clinical research exploring the efficacy of acupuncture in patients with COVID-19.[5] Specific protocols for how acupuncture and moxibustion should be utilized in COVID-19 prevention and treatment have been created and distributed by the China Association of Acupuncture–Moxibustion.[6]
Among the various acupuncture modalities, press needle implantation at acupoints has garnered attention for its ability to provide sustained stimulation without the discomfort associated with conventional acupuncture. In contrast to traditional acupuncture, which can cause temporary mild pain at the site of needle insertion, press needles are associated with less discomfort, and they can remain attached to acupoints for up to a day to provide prolonged stimulation.[7] This makes press needle acupuncture a safe and affordable alternative to traditional acupuncture. However, studies examining the effectiveness of press needles in clinical trials involving patients with COVID-19 are scarce.
In this study, patients hospitalized with mild-to-moderate COVID-19 symptoms were recruited to evaluate the effectiveness of adding press needle acupuncture as an adjunct to pharmacologic treatment. Press needles were administered once daily for 6 sessions. Clinical and laboratory outcomes were evaluated before and after treatment.
2. Materials and methods
2.1. Study design
This single-blind, randomized controlled clinical trial enrolled adults displaying mild-to-moderate COVID-19 symptoms who were hospitalized at Nanfang Hospital Baiyun Branch, Southern Medical University, between December 2022 and January 2023. All individuals provided written consent before enrollment. The study protocol was approved by the Ethics Committee of Nanfang Hospital Baiyun Branch, Southern Medical University (approval number: NFBY-Y-2023001-01).
The inclusion criteria were as follows:
(1) Admitted to the hospital with a COVID-19 diagnosis as confirmed by a RT-PCR (nucleic acid) test using swabs.
(2) Presence of mild-to-moderate symptoms as defined by the national guideline for diagnosis and treatment of COVID-19.[5,6]
18 to 90 years old.
The exclusion criteria of the study were as follows:
(1) Known or suspected allergic history or serious adverse reactions to metal and tape in intradermal needle, or allergic constitution.
(2) Complicated with serious heart and lung diseases, kidney disease, diabetes, advanced tumors, hematological and hematopoietic system diseases, or serious or progressive diseases of other systems.
(3) Patients with comorbid neurological or mental disorders who were unable to cooperate or unwilling to cooperate.
(4) Pregnant and lactating women.
(5) Other conditions that the investigator considered inappropriate to participate in the trial.
(6) Participants enrolled in other COVID-19 intervention clinical trials.
2.2. Randomization and blinding
Patients were recruited through direct interviews conducted by researchers and randomly assigned to the intervention or control group after recruitment. Randomization was conducted by giving each patient a serial number that matched a computer-generated random number list. Patients were not stratified prior to randomization. The intervention group consisted of 58 patients who received pharmacologic treatment together with press needle acupuncture, whereas the control group included the same number of patients who only received pharmacologic treatment.
The research adopted a single-blind approach, specifically with the blinding of evaluators responsible for measuring outcomes. Laboratory personnel tasked with examining test outcomes, physicians in the COVID-19 units assessing clinical results, and the nursing staff were all uninformed of the participants’ group assignments.
2.3. Treatment
The treatment lasted 6 days. Patients in the intervention group received daily pharmacologic treatment and completed 6 sessions of press needle acupuncture. For each session, press needles were inserted into acupoints and kept in place for approximately 24 hours. Only 1 side of the body (left or right) was treated in each session. The following day, press needles were removed from 1 side of the body, and new press needles were placed on acupoints on the other side of the body. Press needle acupuncture was performed by a qualified physician who had completed comprehensive acupuncture training. By contrast, patients in the control group solely received daily pharmacologic treatment, such as Lianhua Qingwen granules, with ibuprofen added as needed for fever management. Study participants were instructed to notify researchers of the appearance of clinical symptoms, and they were prohibited from participating in other studies during the trial period.
Press needles (Huatuo, Suzhou, China) were applied to specific acupoints, including LI4 (Hegu), LI11 (Quchi), LU7 (Lieque), LU9 (Taiyuan), GV14 (Dazhui), CV4 (Guanyuan), CV6 (Qihai), and ST36 (Zusanli).
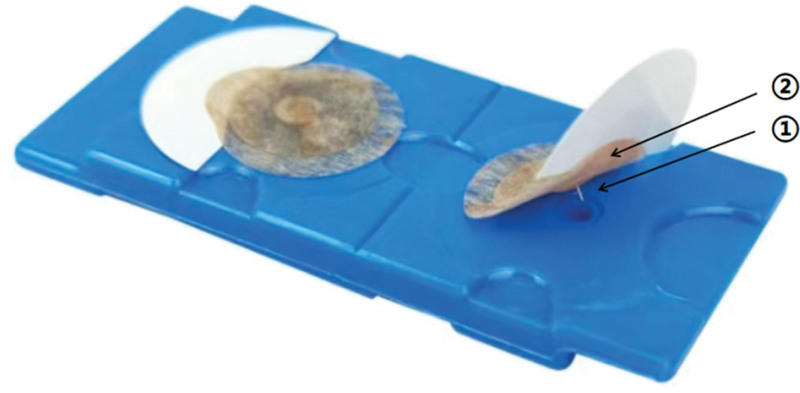
Figure 1 presents the structure of the press needles used in this study. Each needle measured 0.2 mm in diameter and 0.6 mm in length. The needle was placed on the acupoint with the sheath attached, gently pressed down, and inserted. The sheath was aligned at a right angle to the skin surface.
Figure 1.
The components of the press needles included: ① a small stainless steel needle and ② medical adhesive tape.
2.4. Outcomes
Clinical evaluations, including assessments of clinical symptom scores and laboratory tests (complete blood count and C-reactive protein [CRP]), were conducted before the initiation of treatment. Body temperature measurements and COVID-19 PCR tests were performed daily. Clinical symptom scores, encompassing symptoms such as sore throat, productive cough, fever, headache, muscle soreness, nasal congestion, runny nose, weakness, constipation or diarrhea, and diminished sense of smell and/or taste, were evaluated again on day 3 after the initiation of treatment. Laboratory tests (complete blood count and CRP) were performed again on day 6 after the initiation of treatment.
Infection with SARS-CoV-2 was confirmed by RT-PCR using swabs before admission to the hospital.
The primary outcomes included improvements in laboratory test results (such as increased lymphocyte percentage [LYM%] and decreased CRP levels), and clinical improvements (such as shorter duration of clinical symptoms, time to a negative nucleic acid tests, and lower deterioration rate).
2.5. Sample size calculation
According to the results of our pilot study, the incidence of deterioration was 22.4% in the control group, versus 5% in the intervention group. The sample size estimation was performed using Power Analysis and Sample Size 15.0 software. Using a two-sided test, the test efficacy value was set to 80%, and the test level was set to 0.05. The sample size for each group was calculated to be 57 patients. Considering a 5% rate of loss to follow-up, 60 patients were included in each group.
2.6. Statistical analysis
Baseline data were summarized as the mean ± SD (normally distributed data) or median (interquartile range [IQR], non-normally distributed data) for continuous variables and percentages for categorical variables. The chi-squared test and independent-samples t test were used to analyze categorical and continuous variables, respectively, and normality was checked using the Shapiro–Wilk test. Repeated-measures ANOVA was used for normally distributed data, and the Kruskal–Wallis test for applied for non-normally distributed data. The effect of press needle acupuncture on clinical indicators such as blood counts and CRP levels, along with symptom duration and deterioration rates, was determined using appropriate tests, including the Kruskal–Wallis test and chi-squared test for non-normally distributed data and rate comparisons, respectively. Clinical symptom scores were analyzed using two-factor ANOVA.
All statistical analyses were performed using SPSS version 21. P < .05 indicated statistical significance.
3. Results
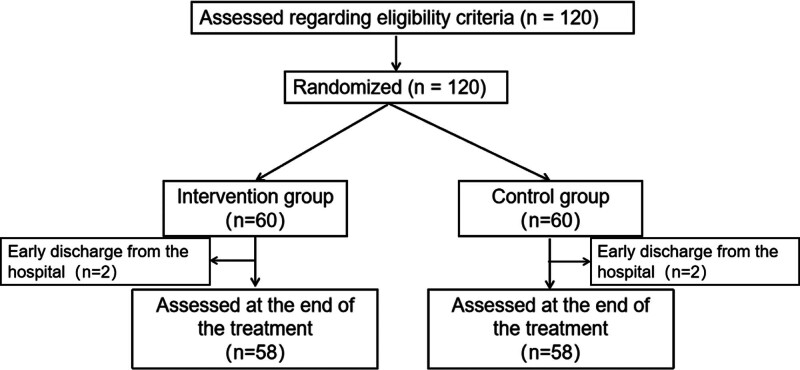
In total, 120 patients hospitalized with mild-to-moderate COVID-19 symptoms were recruited from Nanfang Hospital Baiyun Branch, Southern Medical University. All 120 patients were randomly assigned to 1 of 2 groups. However, 4 patients did not undergo outcome measurement post-intervention nor complete laboratory tests.
The mean age of the patients was 63.85 years, and the sex distribution was nearly equal (female, 53.45%; male, 46.55%). The baseline characteristics of the intervention and control groups were similar, with no statistically significant differences detected (Table 1, Fig. 2).
Table 1.
Baseline characteristics.
| Characteristics | Intervention group (n = 58) |
Control group (n = 58) |
P | |
|---|---|---|---|---|
| Age (years) | 62.81 ± 17.58 | 64.89 ± 18.66 | .537 | |
| Sex | Male | 30/58 | 32/58 | .71 |
| Female | 28/58 | 26/58 | ||
| Symptom Severity | Mild | 13/58 | 16/58 | .396 |
| Moderate | 45/58 | 42/58 | ||
Age was compared using the independent-samples t test. Sex and symptom severity were compared using the chi-squared test.
Figure 2.
Study flow chart.
3.1. Clinical outcomes
3.1.1. Decreased deterioration rate
Following press needle acupuncture, the intervention group exhibited fewer cases of deterioration and no reported deaths. The deterioration rate was significantly lower in the intervention group than in the control group (χ2 = 4.172, P = .041), as presented in Table 2. Deterioration was defined as a change in COVID-19 severity from mild-to-moderate or from moderate to severe or death.
Table 2.
Deteriorations.
| Intervention group (n = 58) |
Control group (n = 58) |
|
|---|---|---|
| Mild to moderate progression | 1 | 2 |
| Moderate to severe progression | 4 | 10 |
| Death | 0 | 1 |
| Deterioration | 5 | 13 |
| Rate of deterioration | 8.62% | 22.41% |
3.1.2. Decreased duration of sustained positive PCR test results
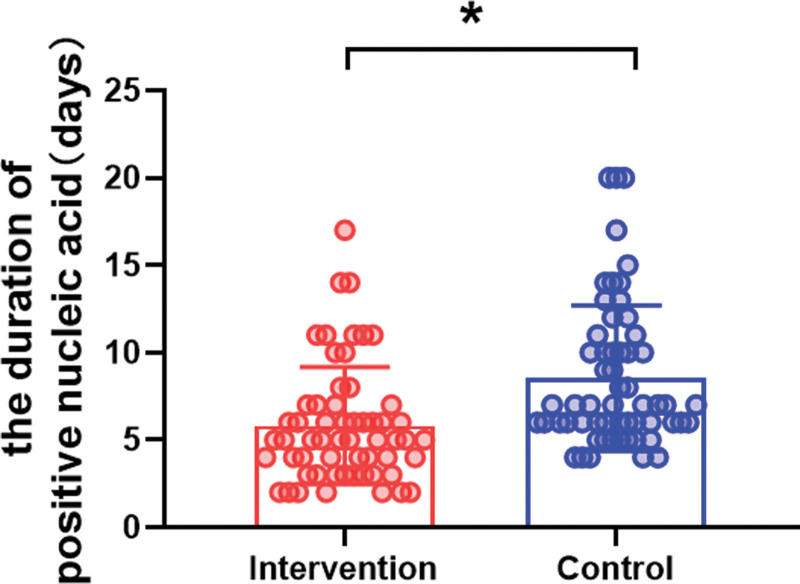
Upon enrollment, the presence of SARS-CoV-2 was confirmed by PCR, and subsequent tests were conducted daily thereafter. The aim was to scrutinize the difference in the duration for which PCR tests remained positive between the intervention and control groups. As presented in Figure 3, after a 6-day treatment period, the median duration of PCR positivity was notably reduced in the intervention group at 5 days (IQR = 3–7), compared to 7 days (IQR = 6–10.25) for the control group (P = .005).
Figure 3.
Comparison of the duration of PCR positivity between the groups. Data are presented as the median and IQR. *P < .05.
3.1.3. Decreased duration of fever
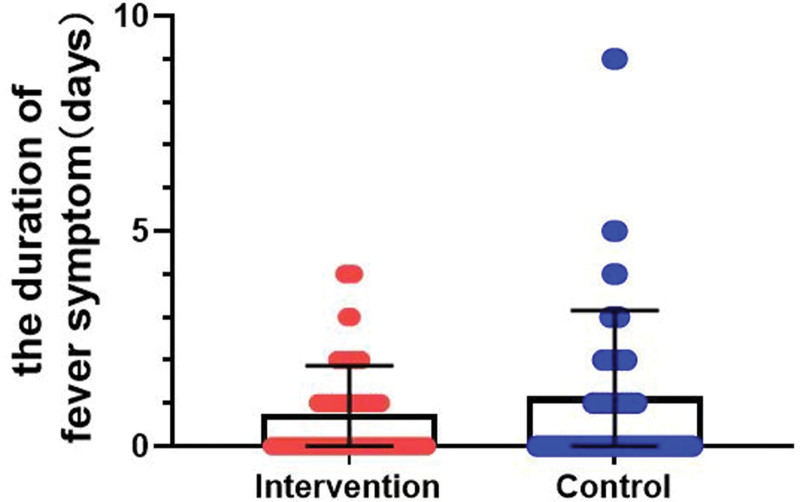
Throughout the trial, body temperature was monitored on a daily basis. As presented in Figure 4, following the 6-day treatment period, the median duration of fever symptoms was 0 days (IQR = 0–1) in the intervention group, versus 0 days (IQR = 0–2) in the control group (P = .851).
Figure 4.
Comparison of the duration of fever between the groups. Data are presented as the median and IQR.
3.1.4. Decreased clinical symptom score
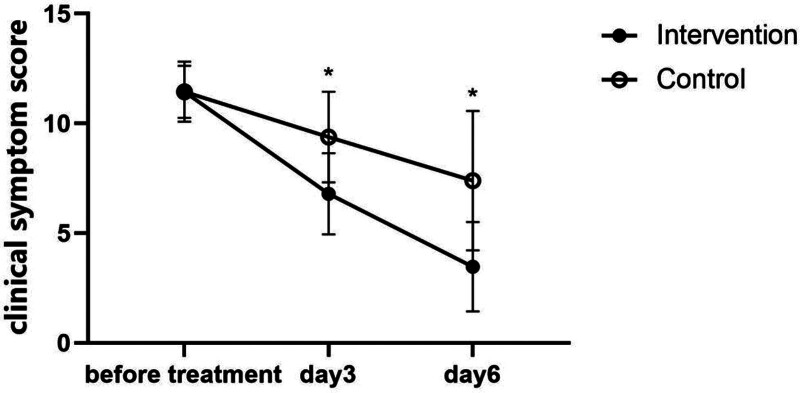
Meanwhile, 2 × 3 ANOVA revealed significant main effects of group (F = 43.42, P < .001) and time (F = 675.31, P = .000), as well as a significant group–time interaction (F = 74.11, P = .000). Furthermore, simple effects analysis demonstrated significant decreases in the clinical symptoms score in both the intervention (F = 1080.47, P < .001) and control groups (F = 151.243, P < .001). These results are presented in Figure 5.
Figure 5.
Comparison of the clinical symptom score between the groups before and during treatment. Data are presented as the mean and standard error. *Significant decreases in the clinical symptoms score in both the intervention and control groups over time (P < .005).
3.2. Laboratory outcomes
3.2.1. Changes in the white blood cell count
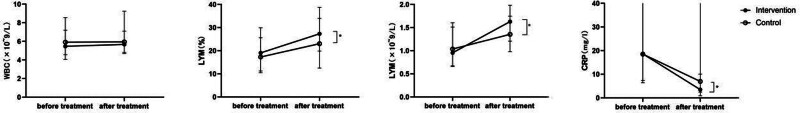
Before the intervention, the median white blood cell (WBC) count was 5.47 (IQR = 4.06–7.20) in the intervention group, compared to 5.91 (IQR = 4.57–8.54) in the control group (P = .267). After 6 days of treatment, the median WBC count increased slightly to 5.67 (IQR = 4.67–7.10) in the intervention group but remained largely unchanged at 5.95 (IQR = 4.82–9.25) in the control group. No significant difference was observed between the 2 groups (P = .067). These results are presented in Table 3 and Figure 6.
Table 3.
WBC counts, LYM, LYMP%, and CRP levels before and after treatment.
| Outcomes | Groups | Before treatment | After treatment | P |
|---|---|---|---|---|
| WBC (×109/L) | Intervention | 5.47 (4.06–7.20) |
5.67 (4.67–7.10) |
.193 |
| Control | 5.91 (4.57–8.54) |
5.95 (4.82–9.25) |
.853 | |
| LYM (×109/L) | Intervention | 0.96 (0.67–1.60) |
1.63 (1.20–1.98) |
.000* |
| Control | 1.03 (0.66–1.51) |
1.36 (0.98–1.75) |
.458 | |
| LYM% | Intervention | 19.05 (10.53–29.93) |
27.3 (19.8–38.7) |
.000* |
| Control | 17.3 (11.23–25.60) |
23.00 (12.48–33.98) |
.353 | |
| CRP (mg/L) | Intervention | 18.6 (7.37–47.03) |
3.49 (0.93–10.05) | .000* |
| Control | 18.5 (6.43–78.15) |
6.95 (2.45–41.37) |
.095 |
P < .05.
Figure 6.
Comparison of WBC counts, LYM, LYM%, and CRP levels before and after treatment between the groups. Data are presented as the median and IQR. *Statistically significant difference between the 2 groups (P < .05).
3.2.2. Changes in the lymphocyte count and LYM%
Before treatment in the intervention group, the median lymphocyte (LYM) was 0.96 (IQR = 0.67–1.60) × 109/L, and LYM% was 19.05% (IQR = 10.53–29.93). In the control group, the median LYM was 1.03 (IQR = 0.66–1.51), and LYM% was 17.3% (IQR = 11.23–25.60). No significant difference was observed between the 2 groups (P = .706).
After 6 days of treatment, the median LYM and LYM% were 1.63 (IQR = 1.20–1.98) × 109/L and 27.3% (IQR = 19.8–38.7), respectively, in the intervention group. In the control group, the median LYM was 1.36 (IQR = 0.98–1.75) × 109/L, and LYM% was 23.00% (IQR = 12.48–33.98). Although the difference within the intervention group was statistically significant for both LYM and LYM% (P < .001), no statistically significant difference was observed between the 2 groups (P = .123 for LYM and P = .071 for %LYM). These results are presented in Table 3 and Figure 6.
3.2.3. Changes in CRP levels
Before treatment, the median CRP level was 18.6 mg/L (IQR = 7.37–47.03) in the intervention group, versus 18.5 mg/L (IQR = 6.43–78.15) in the control group (P = .211). After 6 days of treatment, the median CRP level was 3.49 mg/L (IQR = 0.93–10.05) in the intervention group, compared to 6.95 mg/L (IQR = 2.45–41.37) in the control group. The difference between before and after treatment was significant in the intervention group (P < .001), and a significant difference was detected between the 2 groups after treatment (P = .011). These results are presented in Table 3 and Figure 6.
3.3. Side effects
We observed no occurrences of hematoma at the press needle insertion site among participants in the intervention group. No cases of acushock or infection were reported at the press needle insertion sites. No subjects withdrew from the trial because of pain or discomfort from press needles.
4. Discussion
Recently, there has been a notable increase in research on TCM treatments for COVID-19, such as acupuncture. Most of these studies concentrated on acupuncture techniques such as de qi or electroacupuncture. This is 1 of the first studies to examine the effect of press needle acupuncture on COVID-19.
The study’s results supported its aims by highlighting the potential benefits of adjunctive press needle acupuncture together with pharmacologic treatment for patients experiencing mild-to-moderate COVID-19 symptoms. The results revealed that patients in the intervention group had significantly better clinical outcomes, including a shorter symptom duration and a lower deterioration rate, than those in the control group. Laboratory test results, such as a higher LYM and lower CRP level in the intervention group, suggested that treatment with press needles can affect immune function.
Acupuncture, which is a cornerstone of TCM, has historically displayed efficacy in managing acute respiratory infections and alleviating respiratory symptoms. Since the beginning of the COVID-19 pandemic, acupuncture has been widely acknowledged for its supportive role in treating respiratory problems associated with COVID-19. Chinese authorities have also issued various guidelines highlighting its potential benefits. Clinical studies and reviews have reported that acupuncture can effectively alleviate various COVID-19 symptoms, such as dysosmia, cough, fever, and fatigue, ultimately contributing to shortened hospital stays and improved outcomes for patients.[8,9]
Our study demonstrated a reduction in COVID-19 clinical symptom scores, including scores for pharyngalgia, cough with phlegm, fever, headache, muscle soreness, nasal congestion, runny nose, weakness, and changes in bowel habits, aligning with previous findings that acupuncture effectively mitigates these symptoms in patients with COVID-19.[9] Acupuncture notably improved or eliminated dysosmia, dry throat, cough, diarrhea, fever, nausea, and vomiting. Research has indicated that acupuncture can significantly decrease hospital stay, ICU admission, and mortality rates among patients with COVID-19,[10] with 1 trial of 93 hospitalized patients reporting a reduced duration of hospitalization compared to standard care.[11] COVID-19 can lead to lung inflammation and poor lung oxygenation. The Shenzhen TCM medical team used acupuncture methods such as Huiyang, abdominal, and balance acupuncture to ease symptoms, enhance blood oxygen levels, reduce the need for oxygen supplementation, and aid in recovery, thereby preventing disease progression.[12] Wang and colleagues[13] utilized the key acupoints Dazhui (GV14), Fengchi (GB20), Gongzhi (GB40), and Hegu (GB40) to improve respiratory symptoms and alleviate associated non-respiratory symptoms such as insomnia, anorexia, and fatigue. Their approach highlighted acupuncture’s effectiveness in hastening recovery and shortening hospital stay, in addition to being safe and well accepted by patients. In another study, Gong et al[14] targeted the acupoints Lique, Hegu, Neiguan, Quchi, Zusanli, and Taichong to relieve symptoms such as chest tightness, fatigue, palpitation, anxiety, poor appetite, and insomnia.
Our study demonstrated similar outcomes in terms of a decreased symptom duration and a decreased risk of deterioration compared to the control group. The results demonstrated that press needle acupuncture can be equally effective as traditional acupuncture in the treatment of COVID-19.
According to theories in TCM, the underlying mechanisms of COVID-19 involve blood stasis, heat, dampness, toxicity, and Qi stagnation.[15] Although COVID-19 is considered a single disease entity in modern Western medicine, the disease is categorized into various phases in TCM, including those characterized by cold damp conditions leading to heat accumulation, the presence of damp toxin, and Qi stagnation.[16] The selection of acupoints in the current study was based on differential diagnosis principles derived from our prior research. Specifically, LU7 and LU9 were chosen for their roles in dispelling wind and directing Qi upward; LI4 and LI11 were utilized to eliminate wind, dispel cold, aid in early upper respiratory conditions, and harmonize Qi flow; ST36 was targeted to bolster Qi, enhance resistance to external pathogens, and eliminate cold and dampness[17]; CV4 and CV6 were targeted to harmonize and mobilize Qi, as well as facilitate chest openness; and GV14 was selected to reduce Yang and heat manifestations. A comprehensive review highlighted that LI4 and ST36 are among the acupoints most commonly applied in studies involving patients with COVID-19.[9]
In patients with COVID-19, a factor contributing to lymphocytopenia is the elevation in the levels of pro-inflammatory cytokines such as TNF-α and IL-6.[18,19] TNF-α is known to inhibit hematopoiesis, leading to cytopenia, whereas elevated IL-6 levels have been linked to reduced cytotoxic function in T cells and NK cells.[20] The increases in these cytokines promote granulopoiesis and myelopoiesis but inhibit lymphopoiesis in bone marrow, resulting in increased numbers of monocytes and granulocytes, higher cytokine levels, and fewer lymphocytes.[21,22] CRP, an acute-phase protein, is produced in response to IL-6 in the liver, and it serves as a sensitive indicator of inflammation, infection, and tissue damage. Over half of mild COVID-19 cases involve increased CRP levels. SARS-CoV-2 infection triggers cellular damage, and an inability to control the virus can lead to an overactive inflammatory response, causing further tissue and organ damage.[23] Consequently, high CRP levels in patients with COVID-19 might signal overwhelming inflammatory activity, potentially leading to severe symptoms or death. The risk of developing serious complications in COVID-19 increases with increasing CRP concentrations.[24,25]
Several studies investigating acupuncture’s mechanisms suggested that stimulating specific acupoints can mitigate COVID-19 through anti-inflammatory actions, immune system regulation, organ protection, and anti-bacterial effects.[26–29] Activation of the acupoint LI4 engages the vagus cholinergic pathway, increasing acetylcholine release, which, upon binding to α7nAChR on macrophages, reduces pro-inflammatory cytokine production.[28] Likewise, activating ST36 triggers the vagus medulla adrenal dopamine pathway, leading to dopamine release. This neurotransmitter interacts with type I dopamine receptors, curbing the release of inflammatory cytokines such as IL-6, INF-γ, and TNF-α and enhancing survival rates in patients with sepsis.[28] LI11 and ST36 stimulation also blocks TLR-4 and NF-κB activation, diminishing the secretion of the inflammatory cytokines TNF-α, IL-1β, and IL-6.[28,30] Furthermore, LI4 and ST36 stimulation boosts adrenocorticotropic hormone and cortisol levels in the bloodstream,[28] directly reducing IL-6 production and subsequently lowering CRP and ferritin concentrations.[31,32] Our research observed analogous outcomes with press needle application, noting an increase in LYM and a decrease in CRP levels, thereby underscoring the benefits of press needle therapy in COVID-19 treatment.
In terms of safety, press needle acupuncture is a convenient and less invasive alternative to traditional acupuncture, lowering the danger of exposure to infectious viruses such as SARS-CoV-2 for both acupuncturists and patients. Our findings revealed no adverse events linked to press needle intervention, highlighting its safety and cost-effectiveness.
Nevertheless, this study had several limitations. The sample size was modest, and basic randomization was used without stratification based on comorbidities, which could have introduced bias. The absence of clinical trials on press needle acupuncture for COVID-19 highlights the necessity for additional studies in this field.
5. Conclusions
Our study found that combining press needle acupuncture with pharmacologic treatment improved clinical and laboratory outcomes in hospitalized patients with mild-to-moderate COVID-19 symptoms.
In terms of clinical outcomes, we found that the intervention group had significantly better clinical symptom scores, a lower deterioration rate, and a shorter duration of PCR positivity than the control group.
Furthermore, our laboratory test results illustrated that the combination of press needle intervention and pharmacologic treatment had a positive effect on blood inflammatory indicators, notably CRP and LYM.
These results highlight the potential benefits of introducing press needle acupuncture as an adjunct to pharmacologic treatment for managing COVID-19 symptoms in patients with mild-to-moderate symptoms.
Acknowledgments
We thank Medjaden Inc. for its assistance in the preparation of this manuscript.
Author contributions
Conceptualization: Jiawei Yuan, Weizhen Zhang, Beibei Qie, Yuhua Xie, Binbin Zhu, Cheng Chen, Wenwei Qiu, Huanwen Sun, Bin Zhao, Yaqiu Long.
Data curation: Beibei Qie, Wenwei Qiu, Huanwen Sun.
Formal analysis: Jiawei Yuan, Yaqiu Long.
Investigation: Weizhen Zhang, Yuhua Xie, Binbin Zhu, Wenwei Qiu, Huanwen Sun.
Visualization: Beibei Qie, Yuhua Xie, Binbin Zhu, Wenwei Qiu, Huanwen Sun.
Writing – original draft: Cheng Chen, Bin Zhao.
Writing – review & editing: Jiawei Yuan, Weizhen Zhang.
Abbreviations:
- COVID-19
- coronavirus disease 2019
- CRP
- C-reactive protein
- LYM
- Lymphocyte
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- TCM
- traditional Chinese medicine
- WBC
- white blood cell
This study was funded by the President Foundation of Nanfang Hospital Baiyun Branch, Southern Medical University (BYYZ23005).
The study protocol was approved by the Ethics Committee of NanFang Hospital Baiyun Branch of Southern Medical University (approval number: NFBY-Y-2023001-01).
Clinical registration No. ChiCTR2400080931.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Yuan J, Zhang W, Qie B, Xie Y, Zhu B, Chen C, Qiu W, Sun H, Zhao B, Long Y. Utilizing press needle acupuncture to treat mild-to-moderate COVID-19: A single-blind, randomized controlled trial. Medicine 2024;103:40(e39810).
JY, WZ, BZ, and YL contributed equally to this work.
Contributor Information
Jiawei Yuan, Email: yjw46@126.com.
Weizhen Zhang, Email: zhangweizhen157@126.com.
Beibei Qie, Email: 372924706@qq.com.
Yuhua Xie, Email: yuhua2001@126.com.
Binbin Zhu, Email: zubb@qq.com.
Cheng Chen, Email: CC17702067789@outlook.com.
Wenwei Qiu, Email: wienqiu@qq.com.
Huanwen Sun, Email: gzy07zxy@163.com.
Bin Zhao, Email: drzhaobin@126.com.
References
- [1].Dong E, Ratcliff J, Goyea TD, et al. The Johns Hopkins University Center for systems science and engineering COVID-19 dashboard: data collection process, challenges faced, and lessons learned. Lancet Infect Dis. 2022;22:e370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Del Rio C, Malani PN. COVID-19-new insights on a rapidly changing epidemic. JAMA. 2020;323:1339–40. [DOI] [PubMed] [Google Scholar]
- [3].Zhang K. Is traditional Chinese medicine useful in the treatment of COVID-19? Am J Emerg Med. 2020;38:2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].von Trott P, Oei SL, Ramsenthaler C. Acupuncture for breathlessness in advanced diseases: a systematic review and meta-analysis. J Pain Symptom Manage. 2020;59:327–38.e3. [DOI] [PubMed] [Google Scholar]
- [5].China NHCotPsRo. Guideline of diagnosis and treatment for new coronavirus pneumonia (Interim version 9). 2022.
- [6].China NHCotPsRo. Guideline of diagnosis and treatment for new coronavirus pneumonia (Interim version 10). 2023.
- [7].Wang C, Liu B, Liu Y, He L, Li H, Liu J. Analysis on the concepts related to adverse events and adverse reactions of acupuncture. Zhongguo Zhen Jiu. 2018;38:87–90. [DOI] [PubMed] [Google Scholar]
- [8].Liu B, Wang H, Zhou ZY, Chang XR, Zhang W, Liu BY. Analysis on the theory and clinical ideas of acupuncture and moxibustion for the prevention and treatment of coronavirus disease 2019. Zhongguo Zhen Jiu. 2020;40:571–5. [DOI] [PubMed] [Google Scholar]
- [9].Chen C, Zhan J, Wen H, et al. Current state of research about acupuncture for the treatment of COVID-19: a scoping review. Integr Med Res. 2021;10:100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pan WX, Fan AY, Chen S, Alemi SF. Acupuncture modulates immunity in sepsis: toward a science-based protocol. Auton Neurosci. 2021;232:102793. [DOI] [PubMed] [Google Scholar]
- [11].Alipour R, Jamalimoghadamsiahkali S, Karimi M, et al. Acupuncture or cupping plus standard care versus standard care in moderate to severe COVID-19 patients: an assessor-blinded, randomized, controlled trial. Integr Med Res. 2022;11:100898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu Y, Li Y, Xu M, et al. Summary of the experience of Shenzhen traditional Chinese medicine team supporting Hubei in treating COVID-19 in Leishenshan hospital. J Pract Tradit Chin Intern Med. 2021;35:83–5. [Google Scholar]
- [13].Wang YZ, Li B, Wang LP, et al. Acupuncture as adjuvant therapy for 32 cases of coronavirus disease 2019. Zhongguo Zhen Jiu. 2022;42:634–8. [DOI] [PubMed] [Google Scholar]
- [14].Gong Y, Shi X, Zhang Y, et al. To explore the clinical application and practice of acupuncture therapy in coronavirus disease 2019. Chin Acupunct Moxibustion. 2021;41:142–4. [Google Scholar]
- [15].Zhang K, Li Y, Tang Q. Acupuncture for breathlessness in advanced diseases: methodological issues. J Pain Symptom Manage. 2020;59:e3–4. [DOI] [PubMed] [Google Scholar]
- [16].Leung EL, Pan HD, Huang YF, et al. The scientific foundation of Chinese herbal medicine against COVID-19. Engineering (Beijing). 2020;6:1099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Su Y, Qin W, Wu L, et al. A review of Chinese medicine for the treatment of psoriasis: principles, methods and analysis. Chin Med. 2021;16:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luo XH, Zhu Y, Mao J, Du RC. T cell immunobiology and cytokine storm of COVID-19. Scand J Immunol. 2021;93:e12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Covid-19 Multi-omics Blood ATlas (COMBAT) Consortium. A blood atlas of COVID-19 defines hallmarks of disease severity and specificity. Cell. 2022;185:916–38.e958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Krämer B, Knoll R, Bonaguro L, et al. Early IFN-α signatures and persistent dysfunction are distinguishing features of NK cells in severe COVID-19. Immunity. 2021;54:2650–69.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fathi N, Rezaei N. Lymphopenia in COVID-19: therapeutic opportunities. Cell Biol Int. 2020;44:1792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chang H, Li J. “Lymphocyte * Neutrophil” count decreased in SARS-CoV-2 omicron patients in Shanghai with no significant change in CRP and SAA. J Clin Lab Anal. 2022;36:e24671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Karimi A, Shobeiri P, Kulasinghe A, Rezaei N. Novel systemic inflammation markers to predict COVID-19 prognosis. Front Immunol. 2021;12:741061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Smilowitz NR, Kunichoff D, Berger JS, et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. 2021;42:2270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dai Y, Yue Y, Li J, Wang H. Mechanism of moxibustion in preventing and treating novel coronavirus disease 2019 (in Chinese). Chin J Ethnomed Ethnopharm. 202;29:88–89,99. [Google Scholar]
- [27].Li X, Lu H, Hu H, et al. Possible mechanism of acupuncture and moxibustion in prevention and treatment of COVID-19 (in Chinese). Negative. 2020;11:9–12. [Google Scholar]
- [28].He W, Shi X-S, Zhang Z-Y, et al. Discussion on the effect pathways of preventing and treating coronavirus disease 2019 by acupuncture and moxibustion from the regulation of immune inflammatory response (in Chinese). Chin Acupunct Moxibustion. 2020;40:799–802,809. [DOI] [PubMed] [Google Scholar]
- [29].Zhang Y, Qiao L, Fan C, Guan X. Theoretical discussion on the application of moxibustion in the prophylaxis and treatment in corona virus disease 2019 (in Chinese). J Liaoning Univ Tradit Chin Med. 2020;22:174–7. [Google Scholar]
- [30].Lan L, Tao J, Chen A, et al. Electroacupuncture exerts anti-inflammatory effects in cerebral ischemia-reperfusion injured rats via suppression of the TLR4/NF-κB pathway. Int J Mol Med. 2013;31:75–80. [DOI] [PubMed] [Google Scholar]
- [31].Ismail LA, Ibrahim AA, Abdel-Latif GA, et al. Effect of acupuncture on body weight reduction and inflammatory mediators in egyptian obese patients. Open Access Maced J Med Sci. 2015;3:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128–36.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]