Abstract
Background
In KEYNOTE-522 (NCT03036488), neoadjuvant pembrolizumab plus chemotherapy and then adjuvant pembrolizumab significantly improved pathological complete response and event-free survival vs neoadjuvant chemotherapy in early-stage triple-negative breast cancer (TNBC). We report patient-reported outcomes (PROs) from KEYNOTE-522.
Methods
Patients were randomized 2:1 to neoadjuvant pembrolizumab 200 mg or placebo every 3 weeks, plus 4 cycles of paclitaxel plus carboplatin and then 4 cycles of doxorubicin (or epirubicin) plus cyclophosphamide. After surgery, patients received adjuvant pembrolizumab or placebo for up to 9 cycles. European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 (EORTC QLQ-C30) and EORTC Breast Cancer-Specific Quality of Life Questionnaire (EORTC QLQ-BR23) were prespecified secondary objectives. Between-group differences in least squares (LS) mean change from baseline (day 1 of cycle 1 in both neoadjuvant and adjuvant phases) to the prespecified latest time point with at least 60% completion and at least 80% compliance were assessed using a longitudinal model (no alpha error assigned).
Results
Week 21 (neoadjuvant phase) and week 24 (adjuvant phase) were the latest time points at which completion/compliance rates were ≥60%/80%. In the neoadjuvant phase, between-group differences (pembrolizumab plus chemotherapy [n = 762] vs placebo plus chemotherapy [n = 383]) in LS mean change from baseline to week 21 in QLQ-C30 global health status/quality of life (GHS/QoL), emotional functioning, and physical functioning were −1.04 (95% confidence interval = −3.46 to 1.38), −0.69 (95% CI = −3.13 to 1.75), and −2.85 (95% CI = −5.11 to −0.60), respectively. In the adjuvant phase, between-group differences (pembrolizumab [n = 539] vs placebo [n = 308]) in LS mean change from baseline to week 24 were −0.41 (95% CI = −2.60 to 1.77), −0.60 (95% CI = −2.99 to 1.79), and −1.57 (95% CI = −3.36 to 0.21).
Conclusions
No substantial differences in PRO assessments were observed between neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab vs neoadjuvant placebo plus chemotherapy in early-stage TNBC.
Trial registration
ClinicalTrials.gov, NCT03036488.
Patients with early-stage triple-negative breast cancer (TNBC) have poorer survival outcomes than those with other types of early-stage breast cancer (1,2). Additionally, TNBC is an aggressive tumor type with a high rate of disease recurrence. In a real-world study, nearly 50% of patients with early-stage TNBC experienced recurrence (locoregional or distant) despite treatment with chemotherapy in the neoadjuvant and adjuvant settings; median time to first recurrence (locoregional or metastatic) was approximately 4.5 years (3). Strategies to potentially improve outcomes for patients with early-stage TNBC, including the addition of immunotherapies (eg, programmed cell death protein 1 [PD-1] or programmed cell death ligand 1 [PD-L1] inhibitors) to chemotherapy and the use of targeted drugs (eg, poly[ADP-ribose] polymerase inhibitors), have been studied over the past few years, with some being approved for clinical use (4).
The addition of the anti−PD-1 antibody pembrolizumab or the anti−PD-L1 antibodies atezolizumab or durvalumab to neoadjuvant chemotherapy demonstrated manageable safety and clinical benefit in several studies of patients with high-risk, early-stage TNBC (5-10). In the phase 3 KEYNOTE-522 study, 1174 patients were randomized (2:1) across 181 sites to receive neoadjuvant pembrolizumab plus chemotherapy followed by surgery and then adjuvant pembrolizumab or neoadjuvant placebo plus chemotherapy followed by surgery and then adjuvant placebo (7,8). Treatment with pembrolizumab resulted in clinically meaningful and statistically significant improvements in the dual primary endpoints of pathological complete response (64.8% vs 51.2%; P < .001) and event-free survival (hazard ratio = 0.63 [95% confidence interval = 0.48 − 0.82]; P < .001) in this patient population. Efficacy was observed regardless of tumor PD-L1 expression status as measured by PD-L1 IHC 22C3 pharmDx (7,8). The incidence and severity of adverse events were consistent with the known safety profiles of each treatment component (8). The most common treatment-related adverse events of grade 3 or greater throughout the study were neutropenia (34.5% with pembrolizumab plus chemotherapy vs 33.4% with chemotherapy alone), decreased neutrophil count (18.6% vs 23.1%), anemia (18.0% vs 14.9%), and increased alanine aminotransferase (5.5% vs 2.3%) (8). The results from the KEYNOTE-522 study led to approval of the regimen in many countries (eg, United States, 27 European Union member states, United Kingdom, some Asian countries) and its recommendation as a standard-of-care treatment for patients with high-risk, early-stage TNBC in the National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO), and European Society for Medical Oncology (ESMO) guidelines (11-13).
Patients with cancer and their oncology clinicians report that quality of life is an important priority in making treatment decisions (14). Breast cancer treatments, including chemotherapy, may increase symptom burden and negatively affect patient health-related quality of life (HRQoL) (15-17). Here, we report the prespecified secondary and exploratory patient-reported outcome (PRO) results from the KEYNOTE-522 study.
Methods
Methods for the global, phase 3, randomized, double-blind, placebo-controlled KEYNOTE-522 study (ClinicalTrials.gov, NCT03036488) (18) were previously published in detail (7,8) and are briefly summarized here. The study protocol was approved by an independent institutional review board or ethics committee before being initiated at each study site.
Patients
Eligible patients were at least 18 years of age; had centrally confirmed TNBC as defined by the American Society of Clinical Oncology–College of American Pathologists guidelines (19,20); had newly diagnosed, previously untreated, locally advanced disease (T1c N1–N2 or T2–T4 N0–N2); had an Eastern Cooperative Oncology Group performance status of 0 or 1; provided a biopsy from the primary tumor for PD-L1 assessment at a central laboratory using PD-L1 IHC 22C3 pharmDx (Agilent Technologies) with PD-L1 expression characterized according to the combined positive score (CPS); and provided written informed consent to participate.
Study design and treatment
Patients were stratified before randomization according to nodal status (positive vs negative), tumor size (T1/T2 vs T3/T4), and carboplatin regimen (every 3 weeks vs weekly). Patients were randomly assigned 2:1 to receive neoadjuvant pembrolizumab 200 mg or placebo every 3 weeks, together with chemotherapy. Randomization was performed using a block method (block size of 6) and a central interactive voice-response system with an integrated Web-response system. Neoadjuvant chemotherapy comprised carboplatin area under the curve (AUC) 5 (maximum dose, 750 mg) every 3 weeks or AUC 1.5 (maximum dose, 225 mg) every week plus paclitaxel 80 mg/m2 every week for 4 cycles (12 weeks), followed by doxorubicin 60 mg/m2 or epirubicin 90 mg/m2 every 3 weeks plus cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles (12 weeks). Patients then underwent definitive surgery and began adjuvant treatment with pembrolizumab 200 mg or placebo every 3 weeks for up to 9 cycles (27 weeks). All study medication was administered intravenously. Treatment was continued for the maximum number of cycles for each component or until disease progression, recurrence, or unacceptable toxicity.
Endpoints
The dual primary endpoints of the study were pathological complete response (ypT0/Tis ypN0) and investigator-assessed event-free survival in the intention-to-treat population. PROs measured with the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 (EORTC QLQ-C30) and the EORTC Breast Cancer-Specific Quality of Life Questionnaire (EORTC QLQ-BR23) were prespecified secondary objectives, and those measured with the EuroQol 5-Dimension Questionnaire visual analog scale (EQ-5D VAS) were exploratory objectives.
Assessments
Questionnaires were available in the local language or languages (eg, Dutch, French, and German for Belgium; German, French, and Italian for Switzerland) for all countries included in the study. PROs were administered (EQ-5D first, QLQ-C30 second, and QLQ-BR23 third) by trained study site personnel and completed electronically by the patients themselves. PROs were assessed before treatment on day 1 of cycles 1, 5, and 8 during the neoadjuvant phase; on day 1 of cycles 1, 5, and 9 during the adjuvant phase; and then every 12 months for 2 years or until disease progression or recurrence, whichever was earlier.
The QLQ-C30 is a self-reported, 30-item, cancer-specific instrument that assesses 15 domains: 5 functional scales (physical, role, emotional, cognitive, and social functioning), 9 symptom scales or single items (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties), and global health status/quality of life (GHS/QoL) (21, 22). The QLQ-BR23 is a breast-specific module that includes 23 items composed of 4 functional scales (body image, sexual functioning, sexual enjoyment, and future perspective) and 4 symptom scales (systemic therapy side effects, breast symptoms, arm symptoms, and upset by hair loss) (23). Scores range from 0 to 100 for the QLQ-C30 and QLQ-BR23; a higher score for GHS/QoL or functional scales represents a higher (better) QoL or level of functioning, respectively, whereas a higher score for symptom scales or items represents a worse level of symptoms (22). EQ-5D is a standardized measure of health status consisting of 2 separate elements, utility score and VAS. EQ-5D VAS scores range from 0 (“worst imaginable health state”) to 100 (“best imaginable health state”) (24).
Statistical analysis
PROs were analyzed for the PRO full analysis set, defined as all randomized patients who had at least 1 PRO assessment and received ≥1 study treatment. PROs were also analyzed for the subgroup of patients with PD-L1 CPS ≥1 tumors. The prespecified primary analysis time point for PROs was the latest time at which there was a ≥60% completion rate and a ≥80% compliance rate. Completion rate was defined as the number of patients who completed at least 1 score/item at each time point over the number of patients in the PRO full analysis set population. Compliance rate was defined as the number of patients who completed at least 1 score/item at each time point over the number of patients expected to complete at each time point, excluding those missing by design (eg, due to death, discontinuation, translations unavailable, or visit not scheduled).
Between-group differences in least-squares (LS) mean change from baseline (neoadjuvant baseline in neoadjuvant phase, adjuvant baseline in adjuvant phase) in PROs at the primary analysis time point were assessed using a constrained longitudinal data analysis model (25). No alpha error was assigned. PRO score was the response variable in the model, and treatment by time point interaction and stratification stratum were covariates. The model implicitly treated missing data as missing at random. Only PRO data up to the primary analysis time point were included. Some scores were imputed as per the EORTC Scoring Manual.
Results
Patients
Between March 2017 and September 2018, 1174 patients were randomly assigned to the pembrolizumab plus chemotherapy arm (n = 784) or the placebo plus chemotherapy arm (n = 390). At the March 23, 2021, data cutoff, the median duration of follow-up was 39.1 (range = 30.0–48.0) months. As reported previously (7,8), patient demographics and baseline disease characteristics were similar between the 2 treatment groups.
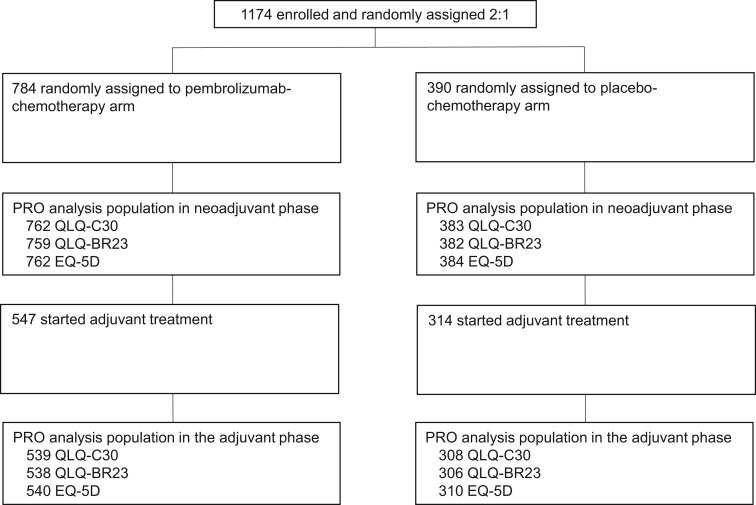
The PRO full analysis set included 1145 patients for QLQ-C30, 1141 patients for QLQ-BR23, and 1146 patients for EQ-5D in the neoadjuvant phase and 847, 844, and 850 patients, respectively, in the adjuvant phase (Figure 1). In both treatment groups, completion and compliance rates for QLQ-C30, QLQ-BR23, and EQ-5D were greater than 90% at baseline in the neoadjuvant and adjuvant phases (Table 1). Week 21 in the neoadjuvant phase (calculated from day 1 of cycle 1 of the neoadjuvant phase and corresponding to day 1 of cycle 5 of the neoadjuvant phase) and week 24 in the adjuvant phase (calculated from day 1 of cycle 1 of the adjuvant phase and corresponding to day 1 of cycle 6 of the adjuvant phase) were selected for analysis because these were the latest time points at which completion and compliance rates were at least 60% and at least 80%, respectively. Completion and compliance rates among patients with PD-L1 CPS ≥1 are shown in Supplementary Table 1 (available online).
Figure 1.
Patient disposition. EQ-5D = EuroQol 5-Dimension Questionnaire; PRO = patient-reported outcome; QLQ-BR23 = European Organisation for Research and Treatment of Cancer Breast Cancer–Specific Quality of Life Questionnaire; QLQ-C30 = European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30.
Table 1.
Completion and compliance rates for patient-reported outcome questionnaires
| QLQ-C30 |
QLQ-BR23 |
EQ-5D |
||||
|---|---|---|---|---|---|---|
| Neoadjuvant phase | Pembrolizumab + Chemotherapy n = 762 | Placebo + Chemotherapy n = 383 | Pembrolizumab + Chemotherapy n = 759 | Placebo + Chemotherapy n= 382 | Pembrolizumab + Chemotherapy n = 762 | Placebo + Chemotherapy n = 384 |
| Baseline | ||||||
| Completion, n (%)a | 701 (92.0) | 366 (95.6) | 695 (91.6) | 361 (94.5) | 707 (92.8) | 369 (96.1) |
| Compliance, n/N (%)b | 701/762 (92.0) | 366/382 (95.8) | 695/759 (91.6) | 361/381 (94.8) | 707/762 (92.8) | 369/383 (96.3) |
| Week 12 | ||||||
| Completion, n (%)a | 648 (85.0) | 329 (85.9) | 644 (84.8) | 328 (85.9) | 657 (86.2) | 336 (87.5) |
| Compliance, n/N (%)b | 648/711 (91.1) | 329/363 (90.6) | 644/709 (90.8) | 328/362 (90.6) | 657/711 (92.4) | 336/365 (92.1) |
| Week 21 | ||||||
| Completion, n (%)a | 615 (80.7) | 309 (80.7) | 611 (80.5) | 307 (80.4) | 616 (80.8) | 311 (81.0) |
| Compliance, n/N (%)b | 615/688 (89.4) | 309/349 (88.5) | 611/686 (89.1) | 307/348 (88.2) | 616/688 (89.5) | 311/350 (88.9) |
| Adjuvant phase | Pembrolizumab n = 539 | Placebo n = 308 | Pembrolizumab n = 538 | Placebo n = 306 | Pembrolizumab n = 540 | Placebo n = 310 |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| Completion, n (%)a | 489 (90.7) | 283 (91.9) | 487 (90.5) | 282 (92.2) | 495 (91.7) | 285 (91.9) |
| Compliance, n/N (%)b | 489/539 (90.7) | 283/308 (91.9) | 487/538 (90.5) | 282/306 (92.2) | 495/540 (91.7) | 285/310 (91.9) |
| Week 12 | ||||||
| Completion, n (%)a | 485 (90.0) | 269 (87.3) | 483 (89.8) | 267 (87.3) | 485 (89.8) | 274 (88.4) |
| Compliance, n/N (%)b | 485/527 (92.0) | 269/302 (89.1) | 483/526 (91.8) | 267/300 (89.0) | 485/528 (91.9) | 274/304 (90.1) |
| Week 24 | ||||||
| Completion, n (%)a | 444 (82.4) | 249 (80.8) | 442 (82.2) | 247 (80.7) | 444 (82.2) | 249 (80.3) |
| Compliance, n/N (%)b | 444/484 (91.7) | 249/282 (88.3) | 442/483 (91.5) | 247/280 (88.2) | 444/485 (91.5) | 249/284 (87.7) |
EQ-5D = EuroQol 5-Dimension Questionnaire; PRO = patient-reported outcome; QLQ-BR23 = European Organisation for Research and Treatment of Cancer Breast Cancer–Specific Quality of Life Questionnaire; QLQ-C30 = European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30.
Completion rate: number of patients who completed at least 1 score/item over the number of patients in the PRO full analysis set population at each time point.
Compliance rate: number of patients who completed at least 1 score/item over the number of patients expected to complete at each time point excluding those missing by design (eg, due to death, discontinuation, translation unavailable).
Patient-reported outcomes
Results for the PRO full analysis set are described in the text. Results for the PD-L1 CPS ≥1 population were similar (see Supplementary Table 2, Supplementary Figures 1 and 2, available online for details).
EORTC QLQ-C30
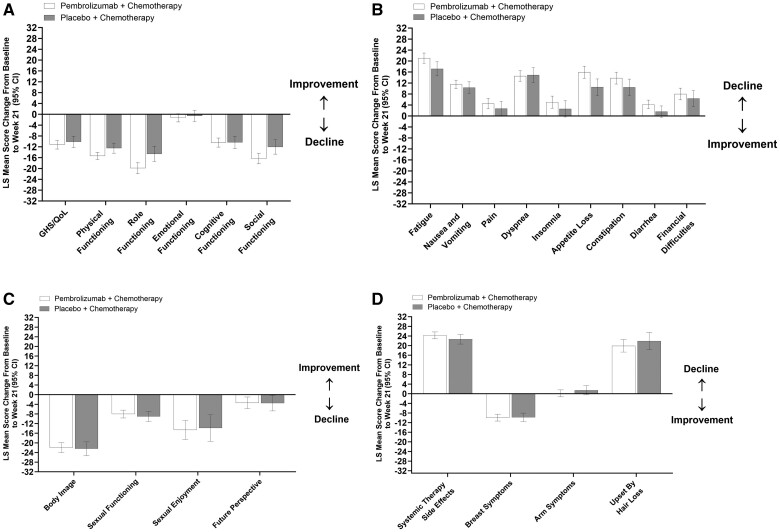
In the neoadjuvant phase, LS mean scores for QLQ-C30 GHS/QoL and physical functioning decreased from baseline to week 21 in both treatment groups, whereas scores for QLQ-C30 emotional functioning were unchanged in both treatment groups (Table 2, Figure 2, A). Between-group differences were −1.04 (95% CI = −3.46 to 1.38), −2.85 (95% CI = −5.11 to −0.60), and −0.69 (95% CI = −3.13 to 1.75), respectively. LS mean scores for QLQ-C30 role functioning, cognitive functioning, and social functioning similarly decreased from baseline to week 21 in both treatment groups (Figure 2, A). LS mean scores for QLQ-C30 symptom scales or items including fatigue, nausea and vomiting, pain, dyspnea, appetite loss, constipation, and financial difficulties similarly increased from baseline to week 21 (indicative of worse symptoms) in both treatment groups (Figure 2, B). Scores for insomnia and diarrhea increased in the pembrolizumab plus chemotherapy group (95% CIs did not include 0) but remained unchanged in the placebo plus chemotherapy group (95% CIs included 0).
Figure 2.
Change from baseline to week 21 in the neoadjuvant phase: A) QLQ-C30 GHS/QoL and functional scales, B) QLQ-C30 symptom scales or items, C) QLQ-BR23 functional scales or items, and D) QLQ-BR23 symptom scales or items. For GHS/QoL score and all functional scales, a higher score denotes better HRQoL or function. For symptoms scales or items, a higher score denotes worse symptoms. CI = confidence interval; GHS/QoL = global health status/quality of life; HRQoL = health-related quality of life; LS = least squares; QLQ-BR23 = European Organisation for Research and Treatment of Cancer Breast Cancer–Specific Quality of Life Questionnaire; QLQ-C30 = European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30.
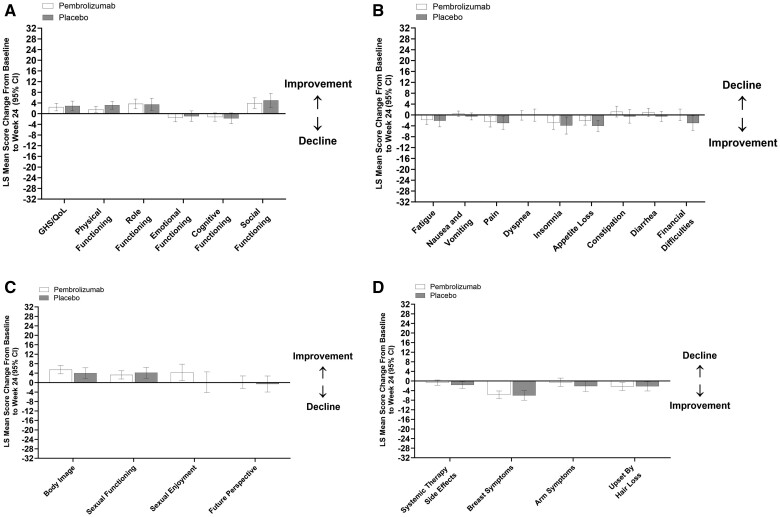
In the adjuvant phase, LS mean scores for QLQ-C30 GHS/QoL and physical functioning increased from baseline to week 24 in both treatment groups, whereas scores for QLQ-C30 emotional functioning decreased in the pembrolizumab group and were unchanged in the placebo group (Table 2, Figure 3, A). Between-group differences were −0.41 (95% CI = −2.60 to 1.77), −1.57 (95% CI = −3.36 to 0.21), and −0.60 (95% CI = −2.99 to 1.79), respectively. LS mean scores for QLQ-C30 role functioning and social functioning similarly increased from baseline to week 24 in both treatment groups, whereas scores for cognitive functioning were unchanged (Figure 3, A). LS mean scores for QLQ-C30 symptom scales or items including pain, insomnia, and appetite loss similarly decreased from baseline to week 24 (indicative of symptom improvement) in both treatment groups (Figure 3, B). Scores for nausea and vomiting, dyspnea, constipation, and diarrhea remained unchanged in both treatment groups (95% CIs included 0), whereas scores for fatigue decreased in the pembrolizumab group (95% CI did not include 0) but were unchanged in the placebo group, and scores of financial difficulties were unchanged in the pembrolizumab group but decreased in the placebo group.
Table 2.
LS mean score changes from baseline for each PRO in the neoadjuvant and adjuvant treatment phases
| Neoadjuvant phase |
Adjuvant phase |
|||
|---|---|---|---|---|
| PRO instrument | Pembrolizumab + Chemotherapy | Placebo + Chemotherapy | Pembrolizumab | Placebo |
| QLQ-C30 GHS/QoL | ||||
| Mean (SD) at baselinea |
|
|
|
|
| Mean (SD) at week 21 or 24b |
|
|
|
|
| LS mean (95% CI) change from baseline to week 21 or 24a, b |
|
|
|
|
| Difference in LS means (95% CI) | –1.04 (–3.46 to 1.38) | –0.41 (–2.60 to 1.77) | ||
| QLQ-C30 Physical Functioning | ||||
| Mean (SD) at baselinea |
|
|
|
|
| Mean (SD) at week 21 or 24b |
|
|
|
|
| LS mean (95% CI) change from baseline to week 21 or 24a, b |
|
|
|
|
| Difference in LS means (95% CI) | –2.85 (–5.11 to –0.60) | –1.57 (–3.36 to 0.21) | ||
| QLQ-C30 Emotional Functioning | ||||
| Mean (SD) at baselinea |
|
|
|
|
| Mean (SD) at week 21 or 24b |
|
|
|
|
| LS mean (95% CI) change from baseline to week 21 or 24a, b |
|
|
|
|
| Difference in LS means (95% CI) | –0.69 (–3.13 to 1.75) | –0.60 (–2.99 to 1.79) | ||
| QLQ-BR23 Breast Symptoms | ||||
| Mean (SD) at baselinea |
|
|
|
|
| Mean (SD) at week 21 or 24b |
|
|
|
|
| LS mean (95% CI) change from baseline to week 21 or 24a, b |
|
|
|
|
| Difference in LS means (95% CI) | –0.13 (–1.92 to 1.65) | 0.29 (–2.05 to 2.63) | ||
| EQ-5D VAS | ||||
| Mean (SD) at baselinea |
|
|
|
|
| Mean (SD) at week 21 or 24b |
|
|
|
|
| LS mean (95% CI) change from baseline to week 21 or 24a, b |
|
|
|
|
| Difference in LS means (95% CI) | –1.61 (–3.87 to 0.64) | –0.59 (–2.40 to 1.23) | ||
CI = confidence interval; EQ-5D VAS = EuroQol 5-Dimension Questionnaire visual analog scale; GHS/QoL = global health status/quality of life; LS = least squares; PRO = patient-reported outcome; QLQ-BR23 = European Organisation for Research and Treatment of Cancer Breast Cancer–Specific Quality of Life Questionnaire; QLQ-C30 = European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30; SD = standard deviation.
Baseline refers to the baseline time point for the respective phase (neoadjuvant or adjuvant).
The analysis time point was week 21 in the neoadjuvant phase and week 24 in the adjuvant phase.
Figure 3.
Change from baseline to week 24 in the adjuvant phase: A) QLQ-C30 GHS/QoL and functional scales, B) QLQ-C30 symptom scales or items, C) QLQ-BR23 functional scales or items, and D) QLQ-BR23 symptom scales or items. For GHS/QoL score and all functional scales, a higher score denotes better HRQoL or function. For symptoms scales or items, a higher score denotes worse symptoms. CI = confidence interval; GHS/QoL = global health status/quality of life; HRQoL = health-related quality of life; LS = least squares; QLQ-BR23 = European Organisation for Research and Treatment of Cancer Breast Cancer–Specific Quality of Life Questionnaire; QLQ-C30 = European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30.
QLQ-BR23
LS mean score changes from baseline for QLQ-BR23 functional scales and symptom scales or items were similar in both treatment groups in the neoadjuvant (Figure 2, C and D) and adjuvant (Figure 3, C and D) phases.
EQ-5D VAS
As shown in Table 2, LS mean scores for 5Q-5D VAS decreased from baseline to week 21 in both treatment groups in the neoadjuvant phase (between-group difference, −1.61 [95% CI = −3.87 to 0.64]), whereas scores increased from baseline to week 24 in both treatment groups in the adjuvant phase (between-group difference, −0.59 [95% CI = −2.40 to 1.23]).
Discussion
In the KEYNOTE-522 study, completion and compliance rates for all PRO instruments (QLQ-C30, QLQ-BR23, and EQ-5D) were high at baseline (above 90%) and remained above 80% through week 21 of the neoadjuvant phase and week 24 of the adjuvant phase. Treatment with neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab alone did not adversely impact HRQoL compared with neoadjuvant placebo plus chemotherapy followed by adjuvant placebo alone. In both treatment groups, the prespecified QLQ-C30 GHS/QoL and physical functioning scales similarly decreased during the neoadjuvant phase and similarly increased during the adjuvant phase. QLQ-C30 emotional functioning was largely unchanged during both treatment phases. No substantial between-group differences were observed for any of the PRO assessments. Results for patients with PD-L1 CPS ≥1 tumors were similar to those for the overall study population.
In both treatment groups, increases from baseline in QLQ-C30 symptom scales (ie, fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, and diarrhea) were observed during the neoadjuvant phase, whereas no changes or slight decreases were noted during the adjuvant phase. This is consistent with the pattern of treatment-related adverse events that were reported during the study. Treatment-related adverse events for overlapping terms captured by the QLQ-C30 symptom scales occurred more frequently in the neoadjuvant phase than in the adjuvant phase and at rates that were generally similar in both treatment groups during both treatment phases (8,26).
Our findings are consistent with those from the phase 3 IMpassion031 study in which patients with high-risk, early-stage TNBC were treated with neoadjuvant atezolizumab plus chemotherapy followed by adjuvant atezolizumab compared with neoadjuvant placebo plus chemotherapy (27). In that study, a total of 328 patients were included in the analysis of the prespecified secondary objectives of QLQ-C30 GHS/QoL, physical functioning, and role functioning. Baseline values for each of these PRO endpoints were comparable to those in KEYNOTE-522, and a similar pattern of changes occurred during the neoadjuvant and adjuvant phases with no apparent differences between the 2 treatment groups (27). Other studies in which PD-1 or PD-L1 inhibitors were added to neoadjuvant chemotherapy in patients with early-stage TNBC did not report PRO results (5,6,9,28).
The KEYNOTE-522 study has some limitations. It was not powered for PRO endpoints, and the related analyses were not controlled for multiplicity. As expected, completion rates decreased over the course of the study as the number of evaluable patients decreased; however, completion/compliance rates for QLQ-C30, QLQ-BR23, and EQ-5D remained above 60%/80% throughout the study in the neoadjuvant and adjuvant phases. The PRO instruments used in the study were not specifically designed to assess outcomes in patients treated with immunotherapies and therefore may not capture the impact of some toxicities on PROs. Immune checkpoint inhibitors are known to be associated with certain immune-related adverse events, some of which may be severe in nature, highlighting the importance of patient monitoring and management during treatment (29). In the KEYNOTE-522 study (combined neoadjuvant and adjuvant phases), immune-mediated adverse events of grade 3 or greater occurred in 12.9% of patients in the pembrolizumab arm and 1.0% of patients in the placebo arm; the most common grade 3 or greater events were severe skin reaction (4.7% vs 0.3%), hypophysitis (1.3% vs 0%), and adrenal insufficiency (1.0% vs 0%) (8). The proportion of patients who started adjuvant treatment was higher in the placebo arm (80.5%) than in the pembrolizumab arm (69.8%) (8). The results of the adjuvant phase should be interpreted with caution because this was a nonrandomized analysis.
In conclusion, patients with high-risk, early-stage TNBC did not experience substantial differences in any PRO assessments during treatment with neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant placebo plus chemotherapy followed by adjuvant placebo. Taken together with the previously reported efficacy and safety findings from the KEYNOTE-522 study, these PRO results support neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab as an effective standard of care treatment regimen in this setting.
Supplementary Material
Acknowledgments
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, was involved in the conception, design, or planning of the study; analysis of the data; interpretation of the results; drafting and reviewing of the manuscript; and the decision to submit the manuscript for publication. We thank the patients and their families and caregivers for participating in this study, along with all investigators and site personnel. Medical writing assistance was provided by Autumn Kelly, MA, and Michael S. McNamara, MS, of ICON plc (Blue Bell, PA, USA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
A portion of these results have been presented at the European Society for Medical Oncology (ESMO) Congress; September 9-13, 2022; Paris, France; Abstract 135MO.
Contributor Information
Rebecca Dent, National Cancer Center Singapore and Duke-National University of Singapore (NUS) Medical School, Division of Medical Oncology, Singapore, Singapore.
Javier Cortés, Medica Scientia Innovation Research (MEDSIR), Barcelona, Spain; International Breast Cancer Center (IBCC), Pangaea Oncology, Quiron Group, Barcelona, Spain; Universidad Europea de Madrid, Faculty of Biomedical and Health Sciences, Department of Medicine, Madrid, Spain; IOB Madrid, Institute of Oncology, Hospital Beata Maria Ana, Madrid, Spain.
Lajos Pusztai, Yale Cancer Center, Yale University School of Medicine, New Haven, CT, USA.
Heather McArthur, UT Southwestern Medical Center, Dallas, TX, USA.
Sherko Kümmel, Breast Unit, Kliniken Essen-Mitte Evang, Huyssens-Stiftung, Essen, Germany; Department of Gynecology with Breast Center, Charité – Universitätsmedizin Berlin, Berlin, Germany.
Jonas Bergh, Department of Oncology-Pathology, Karolinska Institutet and Breast Cancer Centre, Cancer theme, Karolinska Comprehensive Cancer & University Hospital, Karolinska CCC and Cancer Core Europe, Solna, Sweden.
Carsten Denkert, Institute of Pathology, Philipps University Marburg, Marburg, Germany.
Yeon Hee Park, Hematology-Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea.
Rina Hui, Westmead Breast Cancer Institute, Westmead Hospital and the University of Sydney, Sydney, NSW, Australia; Centre of Cancer Medicine, School of Clinical Medicine, University of Hong Kong, Hong Kong.
Nadia Harbeck, Breast Center, Department of OB&GYN and CCC Munich, LMU University Hospital, Munich, Germany.
Masato Takahashi, Department of Breast Surgery, Hokkaido University Hospital, Sapporo, Japan.
Michael Untch, Breast Cancer Center, Helios Klinikum Berlin-Buch, Berlin, Germany.
Peter A Fasching, University Hospital Erlangen, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany.
Fatima Cardoso, Breast Unit, Champalimaud Clinical Center/Champalimaud Foundation, Lisbon, Portugal.
Amin Haiderali, Center for Observational and Real-World Evidence, Merck & Co., Inc., Rahway, NJ, USA.
Liyi Jia, Biostatistics and Research Division Sciences, Merck & Co., Inc., Rahway, NJ, USA.
Allison Martin Nguyen, Biostatistics and Research Decision Sciences—Epidemiology, Patient-Centered Endpoints & Strategy, Merck & Co., Inc., Rahway, NJ, USA.
Wilbur Pan, Global Clinical Development, Merck & Co., Inc., Rahway, NJ, USA.
Joyce O’Shaughnessy, Baylor University Medical Center, Texas Oncology, US Oncology, Dallas, TX, USA.
Peter Schmid, Centre for Experimental Cancer Medicine, Barts Cancer Institute, Queen Mary University of London, London, UK.
Data availability
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data-sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD either will perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Funding
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Conflicts of interest
R. Dent: Advisory/Consultancy: AstraZeneca, Novartis; Advisory/Consultancy, Travel/Accommodation/Expenses: Eisai, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Pfizer, Roche.
J. Cortés: Honoraria (self), Advisory/Consultancy, Research Grant/Funding (institution), Travel/Accommodation/Expenses: Roche; Honoraria (self), Travel/Accommodation/Expenses: Novartis; Honoraria (self), Advisory/Consultancy: Celgene, Lilly; Honoraria (self), Research Grant/Funding (institution), Travel/Accommodation/Expenses: Eisai, Pfizer; Honoraria (self): Samsung Bioepis; Honoraria (self), Advisory/Consultancy, Research grant/Funding (institution): Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; Advisory/Consultancy: Atenex, Biothera Pharmaceuticals, Cellestia, Erytech, Merus, Polyphor, Seattle Genetics, Servier; Advisory/Consultancy, Research grant/Funding (institution): AstraZeneca; Advisory/Consultancy, Travel/Accommodation/Expenses: Daiichi Sankyo; Research Grant/Funding (institution): Ariad Pharmaceuticals, Baxalta GMBH/Servier Affaires, Bayer Healthcare, Guardian Health, Piqur Therapeutics, Puma C, Queen Mary University of London, Seagen; Shareholder/Stockholder/Stock Options: MedSIR.
L. Pusztai: Honoraria (self), Advisory/Consultancy, Research Grant/Funding (institution), Travel/Accommodation/Expenses: AstraZeneca, Merck & Co., Inc., Rahway, NJ, USA, Seattle Genetics; Honoraria (self), Advisory/Consultancy, Travel/Accommodation/Expenses: Almac, Eisai, Genentech, Immunomedics, Novartis, Pieris, Syndax.
H. McArthur: Consultancy: AstraZeneca, Bristol Myers Squibb, Crown Bioscience, Daiichi-Sankyo, Eli Lilly, Gilead, Pfizer, Puma, Seattle Genetics, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; Research Support: Bristol Myers Squibb, BTG, LLC/AstraZeneca, MedImmune, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
S. Kümmel: Advisory/Consultancy: Agendia, Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Genomic Health, Gilead Sciences, Hologic, Lilly, Novartis, Pfizer, PFM Medical, Roche/Genetech, Seagen, Somatex, Sonoscape, Stryker, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; Travel/Accommodation/Expenses: Daiichi Sankyo, Gilead Sciences, Roche; Uncompensated relationships: WSG Study Group.
J. Bergh: Research Grant/Funding (institution): Amgen, AstraZeneca, Bayer, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Pfizer, Roche, Sanofi Aventis; Chapter Honoraria: from UpToDate to Asklepios Medicin Hb. Stocks: Stratipath AB, Coronis, Asklepios Cancer Research AB.
C. Denkert: Honoraria (self): Novartis, Pfizer, Roche, Teva; Honoraria (self), Advisory/Consultancy: Amgen; Advisory/Consultancy: Daichi Sankyo, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; Licensing/Royalties: VmScope.
Y.H. Park: Consultancy or Advisory Board Member: AstraZeneca, Eisai, Novartis, Pfizer, Roche; Research Funding: Alteogen, AstraZeneca, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Novartis, Pfizer, Roche.
R. Hui: Personal Fees for Advisory Boards: Amgen, AstraZeneca, BMS, Eisai, Eli Lilly, Merck KGaA, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Novartis, Oncosec, Pfizer, Roche, Seagen, Takeda, Zai Lab; Speaker Honoraria: AstraZeneca, Eli Lilly, Janssen, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Novartis, Roche; Study Funding (institution): AstraZeneca, BMS, Corvus, Eisai, Eli Lilly, Janssen, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Novartis, Oncosec, Roche, Seagen.
N. Harbeck: Stock and Other Ownership Interests: West German Study Group; Honoraria: Amgen, AstraZeneca, Daiichi Sankyo, Gilead, Lilly, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi, Seagen, Viatris, Zuelligpharma; Consulting or Advisory Role: Gilead, Roche, Sandoz, Seagen; Research Funding (institution): Lilly, Novartis, Pfizer, Roche/Genentech, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
M. Takahashi: Honoraria (self): AstraZeneca, Lilly, Pfizer; Honoraria (self), Research Grant/Funding (self): Eisai; Research Grant/Funding (self): Kyowa-Hakko Kirin, Nippon Kayaku, Taiho.
M. Untch: Consulting Fees (institution): Abbvie, Amgen, AstraZeneca, Daiichi Sankyo, Gilead, GSK, Lilly, Molecular Health, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Myriad, Novartis, Pfizer, Pierre Fabre, Roche, Menarini Stemline, CD Pharma, Agendia, Seagen; Honoraria Speaker (institution): Abbvie, AstraZeneca, Daiichi Sankyo, Gilead, Lilly, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Myriad, Novartis, Pfizer, Pierre Fabre, Roche, Menarini Stemline, Seagen.
P.A. Fasching: Consulting Fees: Agendia, AstraZeneca, Daiichi Sankyo, Eisai, Hexal, Lilly, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Novartis, Pfizer, Pierre Fabre, Roche, Seagen; Fees for Non-CME Services Received Directly from Commercial Interest or their Agents: Daiichi Sankyo, Lilly, Novartis, Seagen; Research Grant: Biontech, Cepheid.
F. Cardoso: Consultancy/Advisory Role: Amgen, Astellas/Medivation, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, Genentech, GE Oncology, Gilead, GlaxoSmithKline, Iqvia, Macrogenics, Medscape, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Merck & Co., Inc., Rahway, NJ, USA, Merus BV, Mundipharma, Mylan, Novartis, Pfizer, Pierre-Fabre, prIME Oncology, Roche, Samsung Bioepis, Sanofi, Seagen, Teva, Touchime.
A. Haiderali, L. Jia, A.M. Nguyen, and W. Pan: Employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and stockholders in Merck & Co., Inc., Rahway, NJ, USA.
J. O’Shaughnessy: Consulting Fees: AbbVie, Agendia, Amgen, Aptitude Health, AstraZeneca, Bayer, BMS, Celgene, Clovis Oncology, Daiichi Sankyo, Eisai, G1 Therapeutics, Genentech, Gilead Sciences, GRAIL, Halozyme, Heron Therapeutics, Immunomedics, Ipsen Biopharmaceuticals, Lilly, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Myriad, Nektar Therapeutics, Novartis, Pfizer, Pharmacyclics, Pierre Fabre, Prime Oncology, Puma Biotechnology, Roche, Samsung Bioepis, Sanofi, Seagen, Syndax Pharmaceuticals, Synthon, Taiho Oncology, Takeda.
P. Schmid: Consultant/Honoraria: AstraZeneca, Bayer, Boehringer Ingelheim, Celgene, Eisai, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Novartis, Pfizer, Puma, Roche; Grant Funding (institution): Astellas, AstraZeneca, Genentech, Medivation, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Novartis, Oncogenex, Roche.
Author contributions
Rebecca Dent, MD, MSc (Conceptualization; Formal analysis; Writing—Original draft; Writing—Review & editing) Javier Cortes, MD (Conceptualization; Writing—Original draft; Writing—Review & editing) Lajos Pusztai, MD (Conceptualization; Writing—Original draft) Heather McArthur, MD (Formal analysis; Writing—Original draft) Sherko Kümmel, MD (Conceptualization; Formal analysis; Writing—Original draft; Writing—Review & editing) Jonas Bergh, MD (Data curation; Writing—Original draft) Carsten Denkert, MD (Conceptualization; Formal analysis; Writing—Original draft) Yeon-Hee Park, MD (Data curation; Writing—Original draft; Writing—Review & editing) Rina Hui, MBBS, PhD (Data curation; Writing—Original draft; Writing—Review & editing) Nadia Harbeck, MD, PhD (Data curation; Writing—Original draft; Writing—Review & editing) Masato Takahashi, MD (Data curation; Writing—Review & editing) Michael Untch, MD (Conceptualization; Data curation; Writing—Review & editing) Peter A. Fasching, MD (Data curation; Writing—Review & editing) Fatima Cardoso, MD (Data curation; Writing—Review & editing) Amin Haiderali, MD (Conceptualization; Data curation; Writing—Original draft; Writing—Review & editing) Liyi Jia, PhD (Conceptualization; Data curation; Writing—Original draft; Writing—Review & editing) Allison Martin Nguyen, MS (Formal analysis; Writing—Review & editing) Wilbur Pan, MD, PhD (Conceptualization; Data curation; Formal analysis; Writing—Original draft; Writing—Review & editing) Joyce O’Shaughnessy, MD (Data curation; Writing—Review & editing) Peter Schmid, MD (Formal analysis; Writing—Original draft; Writing—Review & editing)
References
- 1. Howlader N, Cronin KA, Kurian AW, Andridge R.. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(6):619-626. [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Institute. Cancer Stat Facts: Female Breast Cancer Subtypes. https://seer.cancer.gov/statfacts/html/breast-subtypes.html. Accessed December 13, 2022.
- 3. Haiderali A, Rhodes WC, Gautam S, et al. Real-world treatment patterns and effectiveness outcomes in patients with early-stage triple-negative breast cancer. Future Oncol. 2021;17(29):3819-3831. [DOI] [PubMed] [Google Scholar]
- 4. Howard FM, Pearson AT, Nanda R.. Clinical trials of immunotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2022;195(1):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmid P, Salgado R, Park YH, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. 2020;31(5):569-581. [DOI] [PubMed] [Google Scholar]
- 6. Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020;6(5):676-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmid P, Cortes J, Pusztai L, et al. ; KEYNOTE-522 Investigators. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810-821. [DOI] [PubMed] [Google Scholar]
- 8. Schmid P, Cortes J, Dent R, et al. ; KEYNOTE-522 Investigators. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556-567. [DOI] [PubMed] [Google Scholar]
- 9. Loibl S, Schneeweiss A, Huober J, et al. ; GBG and AGO-B. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann Oncol. 2022;33(11):1149-1158. [DOI] [PubMed] [Google Scholar]
- 10. Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396(10257):1090-1100. [DOI] [PubMed] [Google Scholar]
- 11. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer. Version 3.2023. https://www.nccn.org/. Accessed March 17, 2023.
- 12. Korde LA, Somerfield MR, Hershman DL; Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer Guideline Expert Panel. Use of immune checkpoint inhibitor pembrolizumab in the treatment of high-risk, early-stage triple-negative breast cancer: ASCO guideline rapid recommendation update. J Clin Oncol. 2022;40(15):1696-1698. [DOI] [PubMed] [Google Scholar]
- 13.Loibl S, André F, Bachelot T, et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;35(2):159-182. [DOI] [PubMed] [Google Scholar]
- 14. Williams CP, Miller-Sonet E, Nipp RD, Kamal AH, Love S, Rocque GB.. Importance of quality-of-life priorities and preferences surrounding treatment decision making in patients with cancer and oncology clinicians. Cancer. 2020;126(15):3534-3541. [DOI] [PubMed] [Google Scholar]
- 15. Hamer J, McDonald R, Zhang L, et al. Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Support Care Cancer. 2017;25(2):409-419. [DOI] [PubMed] [Google Scholar]
- 16. Mokhtari-Hessari P, Montazeri A.. Health-related quality of life in breast cancer patients: review of reviews from 2008 to 2018. Health Qual Life Outcomes. 2020;18(1):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di Meglio A, Havas J, Gbenou AS, et al. Dynamics of long-term patient-reported quality of life and health behaviors after adjuvant breast cancer chemotherapy. J Clin Oncol. 2022;40(27):3190-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. ClinicalTrials.gov. Study of Pembrolizumab (MK-3475) Plus Chemotherapy vs Placebo Plus Chemotherapy as Neoadjuvant Therapy and Pembrolizumab vs Placebo as Adjuvant Therapy in Participants With Triple Negative Breast Cancer (TNBC) (MK-3475-522/KEYNOTE-522)( NCT03036488). https://www.clinicaltrials.gov/study/NCT03036488. Accessed January 19, 2024.
- 19. Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S.. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6(4):195-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997-4013. [DOI] [PubMed] [Google Scholar]
- 21. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376. [DOI] [PubMed] [Google Scholar]
- 22. Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer. 2012;48(11):1713-1721. [DOI] [PubMed] [Google Scholar]
- 23. Sprangers MA, Groenvold M, Arraras JI, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14(10):2756-2768. [DOI] [PubMed] [Google Scholar]
- 24. Pickard AS, Neary MP, Cella D.. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liang K, Zeger S.. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankhyā B. 2000;62:134-148. [Google Scholar]
- 26. Schmid P, Cortes J, Dent R, et al. KEYNOTE-522: Phase 3 study of neoadjuvant pembrolizumab + chemotherapy versus placebo + chemotherapy, followed by adjuvant pembrolizumab versus placebo for early-stage triple-negative breast cancer. Paper presented at European Society for Medical Oncology Virtual Plenary; July 15, 2021; Virtual.
- 27. Barrios CH, Saji S, Harbeck N, et al. Patient-reported outcomes from a randomized trial of neoadjuvant atezolizumab-chemotherapy in early triple-negative breast cancer. NPJ Breast Cancer. 2022;8(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gianni L, Huang CS, Egle D, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann Oncol. 2022;33(5):534-543. [DOI] [PubMed] [Google Scholar]
- 29. Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9(6):e002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data-sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD either will perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.