Abstract
In this report, three Mamu-A*01+ rhesus macaques were examined to compare the emergence of simian immunodeficiency virus (SIV)-specific CD8+ T cells in the intestines and blood in early SIV infection using a major histocompatibility complex class I tetramer complexed with the Gag181–189 peptide. Fourteen days after intravenous inoculation with SIVmac251, large numbers of SIV Gag181–189-specific CD8+ T cells were detected in the intestinal mucosa (3.1 to 11.5% of CD3+ CD8+ lymphocytes) as well as in the blood (3.1 to 13.4%) of all three macaques. By 21 days postinoculation, levels of tetramer-binding cells had dropped in both the intestines and blood. At day 63, however, levels of SIV Gag181–189-specific CD8+ T cells in the intestines had rebounded in all three macaques to levels that were higher (8.6 to 18.7%) than those at day 21. In contrast, percentages of tetramer-binding cells in the peripheral blood remained comparatively stable (2.5 to 4.5%) at this time point. In summary, SIV Gag181–189-specific CD8+ T cells appeared in both the intestinal mucosa and peripheral blood at a comparable rate and magnitude in primary SIV infection. Given that the intestine is a major site of early viral replication as well as the site where most of the total body lymphocyte pool resides, these data indicate that it is also an early and important site of development of antiviral immune responses.
Increasing evidence indicates that CD8+ cytotoxic T lymphocytes (CTL) play a major role in the control of human immunodeficiency virus (HIV) infection (4, 15, 19, 24). Detection of simian immunodeficiency virus (SIV)- or HIV-specific CTL responses in the blood in early infection often correlates with a reduction in viral loads, despite the absence of a detectable neutralizing antibody response (4, 15, 21, 25). In addition, there is a significant inverse correlation between the number of HIV-specific CD8+ T cells and the viral load in the peripheral blood in untreated patients (24). Finally, depletion of CD8+ T cells in macaques results in increases in viremia in both acute and chronic SIV infection during the period of maximal depletion (12, 29).
HIV- or SIV-specific cytotoxic T cells have been demonstrated in a number of different tissues, including peripheral blood, bronchiolar lavage lymphocytes, lymph nodes, spleen, skin, liver, cerebrospinal fluid, thymus, bone marrow, intestine, and vaginal mucosa (4, 5, 10, 11, 15, 18, 20, 23, 27, 30, 31, 39). To date, however, most of the data on CTL development in primary infection has been generated using either chromium release assays or limiting dilution analyses (20, 23, 39). Since both of these methods require in vitro stimulation, these techniques yield relatively imprecise estimations of the actual number of virus-specific cells that exist in vivo (38). Recently, a new approach to identify virus-specific CD8+ T cells has been developed that utilizes soluble class I major histocompatibility complex (MHC)-peptide tetrameric complexes, which permit the direct visualization and quantification of antigen-specific T cells immediately ex vivo (3).
We and others have recently demonstrated that the intestinal tract is a major site of early SIV replication and the major site of CD4+ T cell loss in primary SIV infection (13, 32, 35). Rapid and profound CD4+ T-cell depletion occurs specifically within the intestinal tract within 14 days of SIV infection, despite relatively stable CD4+ T-cell counts in peripheral tissues. Coinciding with this CD4+ T-cell loss, increased numbers of activated CD8+ T cells appear in the intestinal tract in primary SIV infection (28, 37). However, the extent to which SIV-specific CD8+ T cells contribute to this increase in intestinal CD8+ T cells is not known. Analysis of the emergence of SIV-specific CD8+ T cells using MHC class I tetramers in primary SIV infection has demonstrated larger numbers of SIV-specific CD8+ T cells in the blood than those in the lymph nodes (17). However, little information is presently available on the kinetics of SIV-specific CD8+ T-cell responses in gastrointestinal tissues during primary infection with SIV.
In this study we utilized tetrameric complexes of the Mamu-A*01 class I molecule and the SIV Gag181–189 peptide (2) to identify SIV-specific CD8+ T-cell responses in rhesus macaques acutely infected with SIVmac251. Three adult male macaques expressing the Mamu-A*01 class I allele were prospectively examined at 0, 7, 14, 21, and 63 days after infection, thus allowing a detailed comparison of the kinetics of SIV-specific CD8+ T cells in peripheral blood and intestinal mucosa. Macaques were selected by screening for the presence of the Mamu-A*01 allele by PCR with sequence-specific primers as previously described (14).
Animals were intravenously inoculated with SIVmac251 (50 ng of p27) on day 0. Endoscope-guided intestinal biopsies and peripheral blood mononuclear cells (PBMC) were collected before inoculation and at 0, 7, 14, 21, 35, and 63 days postinoculation (p.i.). To collect adequate numbers of intestinal lymphocytes for analyses, between 12 and 15 pinch biopsies (approximately 1 mm3 each) of intestinal mucosa (duodenum and upper jejunum) were obtained at each time point using an Olympus endoscope and small biopsy forceps. These intestinal pinch biopsies were well tolerated in macaques, and no adverse events occurred as a result of these biopsies. Lymphocytes were isolated from intestinal biopsies as previously described (36, 37).
For detection of SIV-specific CD8+ T cells by flow cytometry, intestinal cells and PBMC were simultaneously stained with CD3-fluorescein isothiocyanate (FITC) (clone SP34; PharMingen), CD8-peridinin chlorophyll protein (PerCP) (Leu-2a; Becton Dickinson), and the tetrameric complex coupled to allophycocyanin (APC). To determine CD4 counts, parallel samples were stained with CD3-FITC, CD8-PerCP, and CD4-APC (Becton Dickinson). Data on chemokine receptor expression by CD4+ T cells on these animals has previously been reported (36). These cells were stained at 4°C in the dark for 30 min, washed, and fixed overnight in 2% paraformaldehyde. Cells were acquired with a FACS Calibur flow cytometer (Becton Dickinson) and analyzed with Cell Quest software (Becton Dickinson). In general, a total of 200,000 events were acquired, and analysis of tetramer staining cells was carried out on CD3+ CD8+ gated lymphocytes.
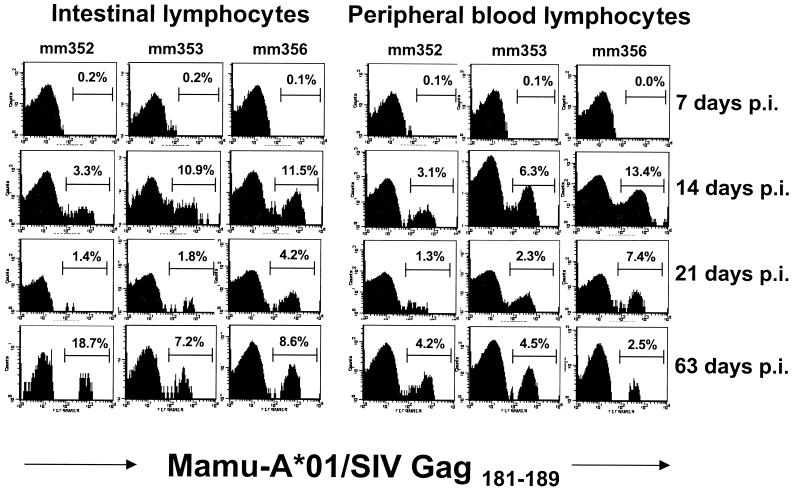
No significant SIV Gag181–189-specific CD8+ T-cell response was detected in the peripheral blood or intestine of any of the macaques at day 7 p.i. However, in all three macaques relatively large percentages (between 3.1 to 13.4% of CD3+ CD8+ lymphocytes) of SIV Gag181–189-specific CD8+ T cells were detected in both the intestinal mucosa and in the peripheral blood by 14 days p.i. (Fig. 1). Although the percentages were slightly higher for the intestines than for the PBMC in 2 out of 3 of the macaques at day 14, the percentages of intestinal SIV Gag181–189-specific CD8+ T cells closely reflected those in the peripheral blood at this time point. However, by day 21 p.i., levels of tetramer-binding cells had markedly decreased in both the blood and intestine compared to those at day 14 in all three animals (Fig. 1).
FIG. 1.
Kinetics of SIV Gag181–189-specific CD8+ T cells in the intestines and peripheral blood in acute SIV infection. Histograms of Mamu-A*01/Gag181–189 tetramer fluorescence on CD3+ CD8+ cells in the intestines and peripheral blood of three rhesus macaques in early SIV infection are shown. PBMC and intestinal lymphocytes obtained from endoscopic biopsies of the duodenum and upper jejunum were analyzed by gating on CD3+ CD8+ lymphocytes. The percentage of tetramer-binding cells as a fraction of CD3+ CD8+ T cells is indicated in each panel. Background levels of tetramer binding were ≤0.1% in peripheral blood and were ≤0.2% in intestinal lymphocytes.
Surprisingly, however, SIV Gag181–189-specific CD8+ T-cell responses in the intestine appeared to increase by 63 days p.i. compared to those at day 21. Moreover, SIV Gag181–189-specific CD8+ T-cell levels were significantly higher in the intestines at this time point than in peripheral blood for all three macaques (P < 0.05; Mann Whitney U test, performed using StatView; Abacus Concepts, Berkely, Calif.) (Fig. 1). One animal (Mm352) demonstrated the highest percentage (18.7%) of SIV Gag181–189-specific CD8+ T cells observed in this study at day 63. Interestingly, this was the macaque that had the lowest percentage at day 14 p.i. Intestinal SIV-specific CD8+ T cells in the other two macaques approached peak levels previously attained on day 14 p.i. (Fig. 1). In contrast, levels of SIV Gag181–189-specific CD8+ T cells in Mm353 and Mm356 were lower in PBMC at day 63 than at day 14 p.i., consistent with previous reports that have shown peak levels of tetramer-binding cells at around 13 days p.i. (16).
As expected, marked intestinal CD4+ T-cell depletion was evident in all three animals by 14 days p.i., reaching a nadir by 21 days p.i. (Table 1). The depletion of CD4+ T cells was equally evident in examination of both total intestinal lymphocytes (Table 1) and gated CD3+ T cells (36). In contrast, only minor changes in CD4+ T cells were observed in the blood for these early time points (Table 1). Macaque Mm356 showed a moderate decrease in CD4+ T cells at day 14 p.i., yet by days 21 and 63 they were essentially back to preinfection levels (Table 1).
TABLE 1.
Changes in T-cell subsets in blood and intestines in primary SIV infection
| T-cell type and macaque | Percentage of total lymphocytes at:
|
|||
|---|---|---|---|---|
| Preinfection | Day 14 | Day 21 | Day 63 | |
| Intestinal CD4+ | ||||
| Mm352-96 | 28 | 5 | 5 | 10 |
| Mm353-96 | 38 | 10 | 3 | 5 |
| Mm356-96 | 30 | 7 | 2 | 8 |
| Blood CD4+ | ||||
| Mm352-96 | 38 | 38 | 41 | 39 |
| Mm353-96 | 32 | 32 | 28 | 38 |
| Mm356-96 | 33 | 16 | 30 | 33 |
| Intestinal CD8+ | ||||
| Mm352-96 | 32 | 44 | 56 | 59 |
| Mm353-96 | 47 | 35 | 47 | 40 |
| Mm356-96 | 49 | 55 | 54 | 47 |
| Blood CD8+ | ||||
| Mm352-96 | 44 | 38 | 40 | 47 |
| Mm353-96 | 56 | 46 | 56 | 50 |
| Mm356-96 | 62 | 68 | 55 | 41 |
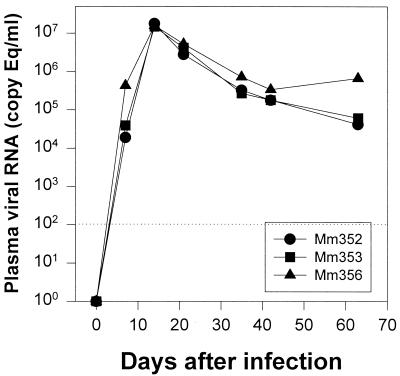
Quantitation of SIV viral RNA levels in plasma were determined by real-time reverse transcriptase-PCR as previously described (34). Only slight differences in viral loads were detected in the three animals after infection. Peak viremia occurred by day 14 in all three macaques and steadily declined thereafter in all three animals until day 63, when Mm356 showed a modest increase in viral load (Fig. 2). Interestingly, this macaque consistently had higher viral loads throughout the course of the study (Fig. 2). Viral loads in all macaques were similar to those previously published for other macaques infected with the same viral strain and route of inoculation (7, 33).
FIG. 2.
Plasma viral loads in macaques in early SIV infection. Quantitative changes in viral loads were assessed at each time point by using real-time PCR. The dotted line indicates the lower limit of detection (200 viral equivalents [Eq]/ml of plasma).
These data reinforce the concept that the intestinal lymphoid tissue is a major target of HIV and SIV infection. Moreover, the observation that relatively equal percentages of SIV-specific CD8+ T cells are found in the peripheral blood and intestines in early infection contrasts with that observed in lymph nodes, where percentages of tetramer-binding cells have been shown to be lower than those in blood in primary infection (16).
The intestinal tract is the largest lymphoid organ in the body (22). It has been estimated that the intestinal epithelium alone contains 60% of the total T cells in the body (9, 22). Therefore, lymphocytes in gastrointestinal lymphoid tissues are, at least numerically, the most significant part of the immune system (22). Thus, the demonstration of equal (day 14) or higher (day 63) percentages of SIV Gag181–189-specific CD8+ T cells in the intestinal mucosa and blood suggests that the majority of HIV- or SIV-specific CD8+ T cells resides in gut-associated lymphoid tissues. This is not entirely surprising, since it is well established that most of the early viral replication and CD4+ T-cell depletion occurs in the intestinal tract in early SIV infection (13, 32, 35).
Although SIV-specific CD8+ T cells developed early in infection in all three animals, marked differences in the magnitude of the CD8+ response were detected among individual animals. Macaque Mm356 consistently had the highest frequency of SIV Gag181–189-specific T cells in both blood and intestines through day 21 p.i. Interestingly, this macaque also had the highest viral load at day 7. Higher early viral loads in the blood of this macaque may have triggered the strong development and persistence of SIV-specific CD8+ T cells at later time points. Mm352 had the lowest frequency of SIV Gag181–189-specific CD8+ T cells until day 63, when large percentages appeared in the intestine. This macaque also had the lowest viral loads at day 7. In all three macaques there was a positive relationship between viral loads and the percentage of SIV-specific CD8+ T cells in the blood in individual macaques. However, the percentages of SIV Gag181–189-specific CD8+ T cells in the intestines were higher at day 63 than at day 21, an increase which occurred coincident with a decrease in the viral load between these time points. Although CD4+ T cells were depleted by 21 days p.i., a slight rebound in CD4+ T cells was observed between days 21 and 63 (Table 1), a change which was associated with an increase in intestinal SIV Gag181–189-specific CD8+ T cells (Fig. 1). Further studies will be necessary to confirm these observations and to elucidate the mechanisms behind regional variations in SIV-specific CD8+ T-cell development.
Although the numbers of SIV-specific CD8+ T cells reported here are relatively high, these numbers do not fully account for the marked increase in CD8+ T cells observed in HIV-infected patients (26) or SIV-infected macaques (28, 37). However, in this report we only examined the CD8+ T-cell specificity to a single SIV epitope (SIV Gag181–189), and it is clear that CTL recognize a number of different epitopes in SIV infection (1, 6, 8). In fact, analysis of MamuA*01+ macaques infected with SIV has shown that there is a higher frequency of CD8+ T cells specific for the Tat28–35 than for the SIV Gag181–189 epitope in the first 4 to 6 weeks after infection (1). Thus, the magnitude of the SIV-specific CD8+ T-cell response is likely to be several-fold higher than that observed here using tetramers complexed with the Gag181–189 epitope.
In summary, the finding of high frequencies of SIV-specific CD8+ T cells in the intestinal tract early in SIV infection provides additional evidence that the intestinal tract is the major target organ in early HIV and SIV infection.
Acknowledgments
We thank Michael O'Connell for study coordination, Mandy Cromwell for tetramer conjugation, Rhona Glickman and Daniel Shvetz for technical assistance, and Michael Piatak, Jr., for assistance with viral load analysis.
This work was funded in part by National Institutes of Health grants HD36310, RR00168, DK50550, AI45314, and AI49080; by Elizabeth Glaser Pediatric AIDS Foundation grant PG50861; and with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. NO1-CO-56000. R.P.J. and A.A.L. are Elizabeth Glaser Scientists and are supported by the Elizabeth Glaser Pediatric AIDS Foundation.
REFERENCES
- 1.Allen T M, O'Connor D H, Jing P, Dzuris J L, Mothe B R, Vogel T U, Dunphy E, Liebl M E, Emerson C, Wilson N, Kunstman K J, Wang X, Allison D B, Hughes A L, Desrosiers R C, Altman J D, Wolinsky S M, Sette A, Watkins D I. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viremia. Nature. 2000;407:386–390. doi: 10.1038/35030124. [DOI] [PubMed] [Google Scholar]
- 2.Allen T M, Vogel T U, Fuller D H, Mothe B R, Steffen S, Boyson J E, Shipley T, Fuller J, Hanke T, Sette A, Altman J D, Moss B, McMichael A J, Watkins D I. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J Immunol. 2000;164:4968–4978. doi: 10.4049/jimmunol.164.9.4968. [DOI] [PubMed] [Google Scholar]
- 3.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B A, Shaw G M. Antiviral pressure exerted by HIV-1 specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 5.Couedel-Courteille A, Le Grand R, Tulliez M, Guillet J-G, Venet A. Direct ex vivo simian immunodeficiency virus (SIV)-specific cytotoxic T cell activity detected from small intestine intraepithelial lymphocytes of SIV-infected macaques at an advanced stage of infection. J Virol. 1997;71:1052–1057. doi: 10.1128/jvi.71.2.1052-1057.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egan M A, Kuroda M J, Voss G, Schmitz J E, Charini W A, Lord C I, Forman M A, Letvin N L. Use of major histocompatibility complex class I/peptide/beta2M tetramers to quantitate CD8(+) cytotoxic T lymphocytes specific for dominant and nondominant viral epitopes in simian-human immunodeficiency virus-infected rhesus monkeys. J Virol. 1999;73:5466–5472. doi: 10.1128/jvi.73.7.5466-5472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estaquier J, Monceaux V, Cumont M C, Aubertin A M, Hurtrel B, Ameisen J C. Early changes in peripheral blood T cells during primary infection of rhesus macaques with a pathogenic SIV. J Med Primatol. 2000;29:127–135. doi: 10.1034/j.1600-0684.2000.290305.x. [DOI] [PubMed] [Google Scholar]
- 8.Evans D T, Jing P, Allen T M, O'Connor D H, Horton H, Venham J E, Piekarczyk M S, Dzuris J, Dykhuzen M, Mitchen J, Rudersdorf R A, Pauza C D, Settes A, Bontrop R E, MDeMars R, Watkins D I. Definition of five new simian immunodeficincy virus cytotoxic T-lymphocyte epitopes and their restricting major histocompatibility complex class I molecules: evidence for an influence on disease progression. J Virol. 2000;74:7400–7410. doi: 10.1128/jvi.74.16.7400-7410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guy-Grand D, Vassalli P. Gut intraepithelial T lymphocytes. Curr Opin Immunol. 1993;5:247–252. doi: 10.1016/0952-7915(93)90012-h. [DOI] [PubMed] [Google Scholar]
- 10.Hadida F, Parrot A, Kieny M P, Sadat-Sowti B, Mayaud C, Debre P, Autran B. Carboxyl-terminal and central regions of human immunodeficiency virus-1 NEF recognized by cytotoxic T lymphocytes from lymphoid organs. An in vitro limiting dilution analysis. J Clin Investig. 1992;89:53–60. doi: 10.1172/JCI115585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jassoy C, Johnson R P, Navia B A, Worth J, Walker B D. Detection of a vigorous HIV-1-specific cytotoxic T lymphocyte response in cerebrospinal fluid from infected persons with AIDS dementia complex. J Immunol. 1992;149:3113–3119. [PubMed] [Google Scholar]
- 12.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kewenig S, Schneider T, Hohloch K, Lampe-Dreyer K, Ullrich R, Stolte N, Stahl-Hennig C, Kaup F J, Stallmach A, Zeitz M. Rapid mucosal CD4+ T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology. 1999;116:1115–1123. doi: 10.1016/s0016-5085(99)70014-4. [DOI] [PubMed] [Google Scholar]
- 14.Knapp L A, Lehmann E, Piekarczyk M S, Urvater J A, Watkins D I. A high frequency of Mamu-A∗01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens. 1997;50:657–661. doi: 10.1111/j.1399-0039.1997.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 15.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuroda M J, Schmitz J E, Charini W A, Nickerson C E, Lifton M A, Lord C I, Forman M A, Letvin N L. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J Immunol. 1999;162:5127–5133. [PubMed] [Google Scholar]
- 17.Kuroda M J, Schmitz J E, Charini W A, Nickerson C E, Lord C I, Forman M A, Letvin N L. Comparative analysis of cytotoxic T lymphocytes in lymph nodes and peripheral blood of simian immunodeficiency virus-infected rhesus monkeys. J Virol. 1999;73:1573–1579. doi: 10.1128/jvi.73.2.1573-1579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuroda M J, Schmitz J E, Seth A, Veazey R S, Nickerson C E, Lifton M A, Dailey P J, Forman M A, Racz P, Tenner-Racz K, Letvin N L. Simian immunodeficiency virus-specific cytotoxic T lymphocytes and cell-associated viral RNA levels in distinct lymphoid compartments of SIVmac-infected monkeys. Blood. 2000;96:1474–1479. [PubMed] [Google Scholar]
- 19.Letvin N L. Progress in the development of an HIV-1 vaccine. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 20.Lohman B L, Miller C J, McChesney M B. Antiviral cytotoxic T lymphocytes in vaginal mucosa of simian immunodeficiency virus-infected rhesus macaques. J Immunol. 1995;155:5855–5860. [PMC free article] [PubMed] [Google Scholar]
- 21.Matano T, Shibata R, Siemon C, Connors M, Lane H C, Martin M A. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mowat A M, Viney J L. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145–166. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 23.Murphey-Corb M, Wilson L A, Trichel A M, Roberts D E, Xu K, Woodson B, Blanchard J. Selective induction of protective MHC class I-restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J Immunol. 1999;162:540–549. [PubMed] [Google Scholar]
- 24.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 25.Pantaleo G, Demarest J F, Schacker T, Vaccarezza M, Cohen O J, Daucher M, Graziosi C, Schnittman S S, Quinn T C, Shaw G M, Perrin L, Tambussi G, Lazzarin A, Sekaly R P, Soudeyns H, Corey L, Fauci A S. The qualitative nature of the primary immune response to HIV infection is a prognosticator of disease progression independent of the initial level of plasma viremia. Proc Natl Acad Sci USA. 1997;94:254–258. doi: 10.1073/pnas.94.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pantaleo G, Demarest J F, Soudeyns H, Graziosi C, Denis F, Adelsberger J W, Borrow P, Saag M S, Shaw G M, Sekaly R P. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- 27.Plata F, Autran B, Martins L P, Wain-Hobson S, Raphael M, Mayaud C, Denis M, Guillon J M, Debre P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987;328:348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- 28.Sasseville V G, Du Z, Chalifoux L V, Pauley D R, Young H Y, Sehgal P K, Desrosiers R C, Lackner A A. Induction of lymphocyte proliferation and severe gastrointestinal disease in macaques by a nef gene variant of SIVmac239. Am J Pathol. 1996;149:163–175. [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz J E, Kuroda M J, Veazey R S, Seth A, Taylor W M, Nickerson C E, Lifton M A, Dailey P J, Forman M A, Racz P, Tenner-Racz K, Letvin N L. Simian immunodeficiency virus (SIV)-specific CTL are present in large numbers in livers of SIV-infected rhesus monkeys. J Immunol. 2000;164:6015–6019. doi: 10.4049/jimmunol.164.11.6015. [DOI] [PubMed] [Google Scholar]
- 31.Sethi K K, Naher H, Stroehmann I. Phenotypic heterogeneity of cerebrospinal fluid-derived HIV-specific and HLA-restricted cytotoxic T-cell clones. Nature. 1988;335:178–1781. doi: 10.1038/335178a0. [DOI] [PubMed] [Google Scholar]
- 32.Smit-McBride Z, Mattapallil J J, McChesney M, Ferrick D, Dandekar S. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J Virol. 1998;72:6646–6656. doi: 10.1128/jvi.72.8.6646-6656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith S M, Holland B, Russo C, Dailey P J, Marx P A, Connor R I. Retrospective analysis of viral load and SIV antibody responses in rhesus macaques infected with pathogenic SIV: predictive value for disease progression. AIDS Res Hum Retrovir. 1999;15:1691–1701. doi: 10.1089/088922299309739. [DOI] [PubMed] [Google Scholar]
- 34.Suryanarayana K, Wiltrout T A, Vasquez G M, Hirsch V M, Lifson J D. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res Hum Retrovir. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 35.Veazey R S, DeMaria M, Chalifoux L V, Shvetz D E, Pauley D R, Knight H L, Rosenzweig M, Johnson R P, Desrosiers R C, Lackner A A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 36.Veazey R S, Mansfield K G, Tham I C, Carville A C, Shvetz D E, Forand A E, Lackner A A. Dynamics of CCR5 expression by CD4+ T cells in lymphoid tissues during simian immunodeficiency virus infection. J Virol. 2000;74:11001–11007. doi: 10.1128/jvi.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veazey R S, Tham I C, Mansfield K G, DeMaria M, Forand A E, Shvetz D E, Chalifoux L V, Sehgal P K, Lackner A A. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson J D, Ogg G S, Allen R L, Davis C, Shaunak S, Downie J, Dyer W, Workman C, Sullivan S, McMichael A J, Rowland-Jones S L. Direct visualization of HIV-1-specific cytotoxic T lymphocytes during primary infection. AIDS. 2000;14:225–233. doi: 10.1097/00002030-200002180-00003. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto H, Ringler D J, Miller M D, Yasutomi Y, Hasunuma T, Letvin N L. Simian immunodeficiency virus-specific cytotoxic T lymphocytes are present in the AIDS-associated skin rash in rhesus monkeys. J Immunol. 1992;149:728–734. [PubMed] [Google Scholar]