Abstract
HFE is a nonclassical class I major histocompatibility complex (MHC) molecule that is mutated in the autosomal recessive iron overload disease hereditary hemochromatosis. There is evidence linking HFE with reduced iron uptake by the transferrin receptor (TfR). Using a panel of HFE and TfR monoclonal antibodies to examine human HFE (hHFE)-expressing cell lines, we demonstrate the expression of stable and fully glycosylated TfR-free and TfR-associated hHFE/β2m complexes. We show that both the stability and assembly of hHFE complexes can be modified by the human cytomegalovirus (HCMV) viral protein US2, known to interfere with the expression of classical class I MHC molecules. HCMV US2, but not US11, targets HFE molecules for degradation by the proteasome. Whether this interference with the regulation of iron metabolism by a viral protein is a means of potentiating viral replication remains to be determined. The reduced expression of classical class I MHC and HFE complexes provides the virus with an efficient tool for altering cellular metabolism and escaping certain immune responses.
The iron overload disease hereditary hemochromatosis (HH) is one of the most common genetic disorders, affecting 1 in 300 Caucasians (21). The disease is characterized by inappropriate control of intestinal iron absorption, resulting in excessive accumulation of iron in organs such as the liver, heart, and pancreas and eventually leading to multiorgan dysfunction (1). The gene that is mutated in patients with HH encodes HFE, a glycoprotein resembling class I major histocompatibility complex (MHC) molecules in sequence and in three-dimensional structure (9, 17). A mutation in the HFE protein (Cys282Tyr) that prevents assembly of the HFE heavy chain with β2 microglobulin (β2m) and transport of HFE complexes to the cell surface (41) is responsible for most HH (9). Not surprisingly, β2m knockout mice (27, 31), as well as HFE knockout mice (47), suffer from iron overload similar to that seen in HH patients.
A direct link between HFE and iron metabolism was provided by experiments showing that HFE associates with the transferrin receptor (TfR) in HFE-transfected cells (10), in human placentas (22), and in the cryptal cells of the intestine (40) and that this association plays a key role in the regulation of iron uptake. The results of several studies have suggested a role for HFE in downregulating Tf-mediated iron uptake (10, 11, 18, 28, 29). These results include data demonstrating that (i) recombinant HFE reduces the affinity of the TfR for holotransferrin, (ii) HFE can compete with Tf for binding to TfR, and (iii) HFE reduces the endocytosis rate of HFE/TfR/Tf complexes. However, these data do not preclude the possibility that HFE complexed with other proteins may affect, directly or indirectly, other types of iron transport systems or immune responses (6, 8, 25, 26, 30, 36). By virtue of being a class I MHC molecule, HFE complexes might be modulated by viral antigens and might thus manifest another target for virus manipulation of cellular proteins. Several viral proteins are well known to manipulate antigen presentation by classical class I MHC molecules (38); adenovirus E3/19K retains class I molecules in the endoplasmic reticulum (19) and binds to TAP (transporter associated with antigen presentation) (3), human cytomegalovirus (HCMV) US2 and US11 target class I heavy chains for degradation (37, 43), UL18 is a class I homologue (2), human immunodeficiency virus Nef causes rapid endocytosis of cell surface MHC class I molecules (24), and herpes simplex virus ICP47 inhibits peptide transport through the TAP channel (12, 14, 44).
In the present study, we analyze the effects of viral proteins on the assembly, trafficking, and expression of TfR-free and TfR-associated human HFE (hHFE) complexes. We demonstrate clearly that HCMV US2 targets HFE for degradation by the proteasome. This is the first manifestation of an effect of US2 on the expression of a class I MHC molecule that is not involved in antigen presentation, which might suggest that this protein has a novel pathway that intervenes in iron metabolism.
Assembly and trafficking of TfR-free and TfR-associated hHFE complexes.
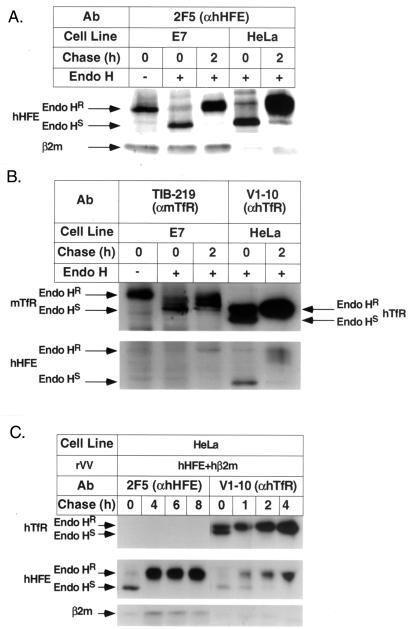
To analyze the expression of hHFE complexes and to study whether the expression of these complexes is affected by viral proteins, we generated (i) a panel of monoclonal antibodies (MAbs) directed against hHFE by the syngeneic immunization of mice with TAP-deficient cells (34, 35) stably transfected with the hHFE gene and (ii) recombinant vaccinia viruses (5) expressing the hHFE gene (rVV hHFE). E7 (a murine TAP1KO cell line that was generated by the transformation of embryonal TAP1KO cells with the E1 region of adenovirus 5) and HeLa cells were infected with rVV hHFE (10 PFU/cell for 1 h at 37°C), and the cells were diluted in culture medium and incubated for an additional 4 h), metabolically labeled with [35S]Met, chased as indicated in the figures, lysed (0.5% Triton X-100 in lysis buffer), and immunoprecipitated with the relevant Abs. Immunoprecipitation with anti-hHFE (2F5) revealed that this MAb recognizes an epitope present on hHFE/β2m heterodimers synthesized in either E7 or HeLa cells (Fig. 1A). TfR-associated hHFE complexes were assembled in HeLa cells (as indicated by the coimmunoprecipitation of TfR-associated hHFE complexes by the anti-human TfR [anti-hTfR] MAb V1-10) but not in E7 cells (as indicated by the fact that anti-mouse TfR [anti-mTfR] antibodies [TIB-219] did not immunoprecipitate TfR-associated hHFE complexes in the mouse cells) (Fig. 1B). These results were supported by the results of Western blot analysis. Identical data were obtained with other anti-mTfR Abs or with Abs directed against mouse β2m (data not shown), suggesting that mTfR does not interact efficiently with hHFE. The observation that MAb 2F5 (as well as another MAb, 8C10; data not shown) did not coimmunoprecipitate TfR-associated hHFE complexes in HeLa cells (Figs. 1C) implies that the epitope recognized by this MAb is altered or masked by its association with hTfR. To prove that the 2F5 and 8C10 MAbs do not recognize TfR-associated hHFE complexes (rather than causing disassociation of such complexes), sequential exhaustive immunoprecipitations with 2F5 or 8C10 Abs followed by immunoprecipitations with anti-TfR Abs were performed. TfR-associated hHFE complexes could still be immunoprecipitated, indicating that they were not disassociated by exposure to anti-hHFE MAbs (see Fig. 2 to 4). Figure 1 also shows that hHFE complexes in mouse and human cell lines acquired endo-β-N-acetylglucoaminidase H (endo H) resistance within 2 h (Fig. 1A and B) and that hHFE complexes were also stable following longer chase periods (Fig. 1C). The data indicate that TfR-free complexes exit the endoplasmic reticulum in mouse and human cells and that these complexes are as stable as the TfR-associated hHFE complexes. Assuming that TfR-associated HFE complexes in human cells are internalized rapidly, as is the TfR, the stable expression of these complexes following a 4-h chase (Fig. 1C) suggests that the TfR-associated HFE complexes do not dissociate instantaneously in the endosomes.
FIG. 1.
Assembly and trafficking of hHFE complexes. E7 (mouse TAP1KO) (A and B) and HeLa cells (A to C) were infected with rVV hHFE (A and B) or rVV hHFE and rVV hβ2m (C), followed by metabolic labeling and chasing as indicated. The [35S]Met-labeled lysates were immunoprecipitated with the anti-HFE MAb 2F5 (A and C) and anti-TfR MAbs (B and C). The immunoprecipitates were untreated or treated with endo H as indicated, followed by fractionation on sodium dodecyl sulfate–13% (A and C) and 8% (B) polyacrylamide gel electrophoresis (SDS-PAGE). Endo HR, resistance to endo H; Endo HS, sensitivity to endo H.
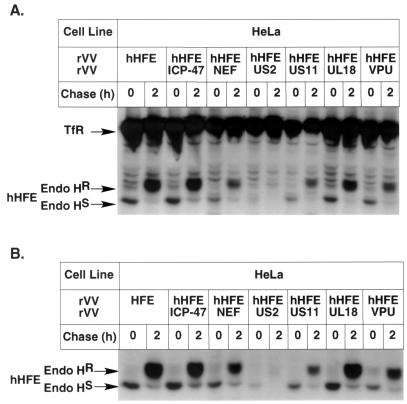
FIG. 2.
Viral proteins prevent the expression of hHFE complexes. HeLa cells were coinfected with rVV hHFE and a panel of rVV expressing viral proteins (rVV VPU [16], rVV US2 [33, 39], rVV ICP47 [14], rVV US11 [33], and rVV Nef), followed by metabolic labeling and a 2-h chase. The labeled lysates were immunoprecipitated sequentially with anti-hHFE MAb 2F5 (B) and anti-TfR MAb V1-10 (A), treated with endo H, and fractionated by SDS-PAGE. Endo HR, resistance to endo H; Endo HS, sensitivity to endo H.
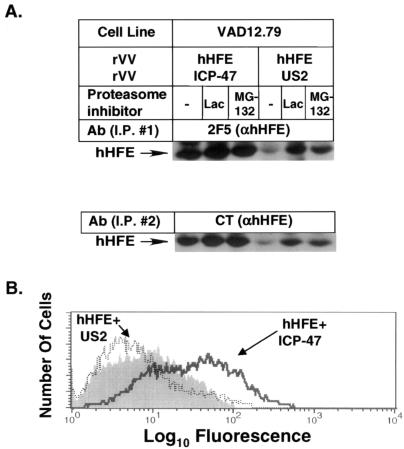
FIG. 4.
HCMV US2 targets hHFE for proteasome-dependent degradation in mouse cells and prevents their cell surface expression. VAD12.79 cells were coinfected with rVV hHFE and rVV ICP47 or rVV US2 and labeled with [35S]Met (A). The infected cells were incubated during starvation and labeling with or without the proteasome inhibitors lactacystin (Lac) and MG-132 as described for Fig. 3. The labeled lysates were immunoprecipitated sequentially with anti-hHFE MAb 2F5 and anti-HFE(CT), followed by fractionation by SDS-PAGE. The rVV-infected cells were analyzed by FACS analysis with anti-hHFE MAb 2F5 (B). I.P., immunoprecipitation.
Viral proteins prevent the expression of hHFE complexes.
The ability of particular viral proteins to interfere with antigen presentation by downregulating the expression of classical class I MHC complexes is well documented (38). Figure 2 demonstrates that viral proteins also interfere with the expression of HFE complexes. HeLa cells were coinfected with rVV hHFE and with a panel of rVV expressing different viral proteins. Cell lysates were sequentially immunoprecipitated with anti-hHFE (2F5) for the detection of hHFE/β2m complexes (Fig. 2B) and with anti-hTfR antibodies for the detection of TfR-associated HFE complexes (Fig. 2A). A separate immunoprecipitation with MAb TW2.3, which recognizes a 25-kDa VV protein (45), verified that levels of expression of VV proteins in the individual infections were identical (data not shown). The data demonstrate that HCMV US2 caused a complete elimination of both types of complexes. The effect was specific for HFE, since the level of free TfR remained unchanged. Other viral proteins showed a marginal effect or no effect. In order to further verify whether US2 and US11, both of which are expressed by the same virus and cause rapid degradation of class I MHC molecules, affect differentially the expression of hHFE, the effects of these proteins on hHFE expression were further studied.
HCMV US2 but not US11 targets hHFE for rapid proteasome-mediated degradation.
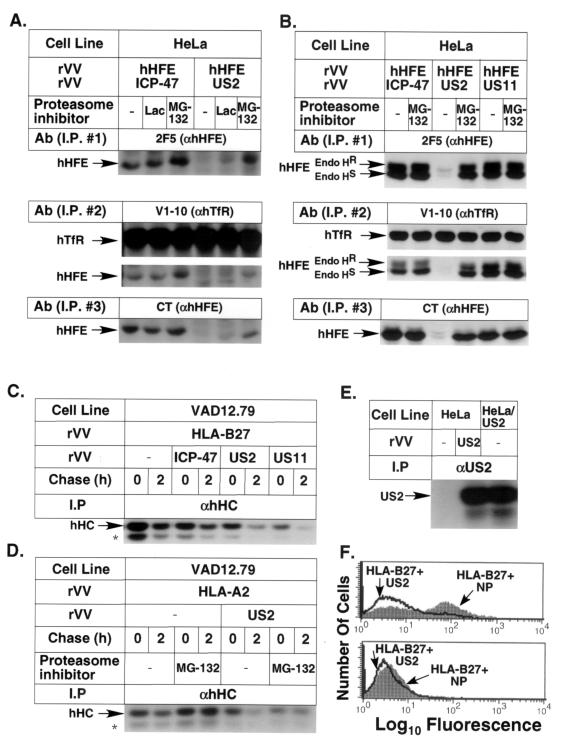
Since the most dramatic effect on the expression of HFE complexes was observed with HCMV US2, this protein was analyzed in more detail. To determine whether HCMV US2 targets HFE for proteasomal degradation, HeLa cells were coinfected with rVV hHFE and either rVV ICP47, rVV US2, or rVV US11, followed by metabolic labeling and immunoprecipitation with the relevant antibodies. A proteasome inhibitor(s) (lactacystin and/or MG-132) was added to the culture medium following infection and was present throughout the experiment. The lysates were immunoprecipitated sequentially with anti-hHFE (2F5), anti-hTfR (V1-10), and the polyclonal anti-hHFE Ab anti-hHFE(CT) [prepared by immunization of rabbits with the peptide (C)RKRQGSRGAMGHYVLAERE, which corresponds to the sequence of the cytoplasmic tail of hHFE]. The last antibody preferentially immunoprecipitates free HFE chains. Figure 3A and B show that the expression of HCMV US2 but not US11 or HSV ICP47 resulted in the elimination of both TfR-free and TfR-associated hHFE complexes as well as of free hHFE chains. The expression of both hHFE complexes and hHFE-free heavy chains was stabilized following incubation with proteasome inhibitors. The effect of MG-132 was usually more pronounced than that of lactacystin. hHFE remained glycosylated in the presence of US2 and proteasome inhibitors. The two glycosylated forms are clearly seen in Fig. 3B. To verify the expression of rVV US2 as well as its known effect on the expression of classical class I MHC molecules, mouse and human cells were infected with rVV US2, rVV ICP47, rVV US11, or rVV influenza virus nucleoprotein (FluNP) (Fig. 3C to F) and rVV HLA-B27 (Fig. 3C and F) or rVV HLA-A2 (Fig. 3D). Figure 3C demonstrates that both HCMV US11 and US2 but not ICP47 interfered with the expression of HLA-B27 heavy chains as expected (it is already known that ICP47 interferes with TAP function only in human cells). Figure 3D demonstrates that US2 interfered with the expression of HLA-A2 heavy chains and that this expression can be recovered by treatment of the cells with MG-132. In these assays we could not detect the deglycosylated form of class I MHC molecules in the presence of HCMV US2 or US11 and MG-132, probably due to the assay conditions (42). Figure 3E verifies the expression of US2 in rVV US2-infected cells (by immunoprecipitation of labeled lysates from infected cells compared to that of cells stably transfected with the US2 gene and control cells, using polyclonal Abs directed against HCMV US2). Figure 3F (top) shows that rVV US2 but not rVV FluNP reduced the cell surface expression of HLA-B27 in human cell line 45. The specific TAP-dependent expression of HLA-B27 in rVV-B27-infected cells was further proven by the lack of expression of these complexes in cell line .174, which lacks TAP expression (Fig. 3F, bottom).
FIG. 3.
HCMV US2, but not HCMV US11, targets hHFE for proteasome-dependent degradation. HeLa (A, B, and E), VAD12.79 (C and D), .45 (F, top), and .174 (F, bottom) cells were infected with either rVV hHFE (A and B), rVV HLA-B27 (C and F), or rVV HLA-A2 (D) and either rVV ICP47 (A to C), rVV US2 (A to F), VV US11 (B and C), or rVV FluNP (NP) (F) in the combinations marked in the figure. US2-transfected HeLa cells were used in panel E as a positive control. The cells were labeled with [35S]Met and chased as indicated. The infected cells in panels A, B, and D were incubated during starvation and labeling with or without the proteasome inhibitors (Calbiochem-Novabiochem) lactacystin (Lac; 15 μM) and MG-132 (30 μM). (A and B) The labeled lysates were immunoprecipitated sequentially with anti-hHFE MAb 2F5, anti-TfR MAb V1-10, and anti-HFE(CT); treated with endo H (B); and fractionated by SDS-PAGE. (C and D) The labeled lysates were immunoprecipitated with anti-human class I MHC heavy chains (αhHC). ∗, unidentified band. (E) Polyclonal antibodies directed against a US2-derived peptide (33) were used for immunoprecipitation of US2 from labeled lysates of rVV US2-infected and US2-transfected HeLa cells. (F) FACS analysis of rVV HLA-B27-and rVV US2- or rVV FluNP-infected .45 cells (top) and .174 cells (TAP-deficient, bottom). I.P., immunoprecipitation; Endo HR, resistance to endo H; Endo HS, sensitivity to endo H.
To determine whether US2 targeting of hHFE for degradation depends upon species-specific factors, the same experiments were performed with a mouse cell line. Figure 4A shows that HCMV US2 caused degradation of hHFE in the mouse cell line VAD12.79 and that this degradation was inhibited by proteasome inhibitors. Since TfR-free HFE complexes can easily be detected on the surfaces of mouse cells, the rVV-infected cells were also analyzed by fluorescence-activated cell sorter (FACS) analysis. Figure 4B demonstrates that US2 expression resulted in the complete reduction of cell surface expression of hHFE/β2m complexes.
Summary.
HFE is a class I-like MHC glycoprotein that is involved in iron metabolism (9). It is commonly accepted that the high-affinity binding of HFE to the TfR (17) leads to reduced intracellular iron stores. In addition to directly mediating iron metabolism within cells, it has been suggested that HFE, being an MHC class I-like molecule, might be the ligand for specific γδ lymphocytes in the intestines of mammalian species and communicate the body's iron status to T cells, which would then use cytokines as feedback modulators to achieve iron homeostasis (8, 30). Viruses have evolved a variety of means for interfering with immune responses. One of the most common is interference with class I MHC-mediated antigen presentation in order to evade cellular immune responses (38). Since this interference often involves direct interaction with class I heavy chains and since HFE is closely related to these antigen-presenting molecules, we postulated a broader scope of action.
We analyzed the effect of the expression of several viral proteins known to manipulate antigen presentation by classical class I MHC molecules on TfR-free and TfR-associated hHFE complexes. Coinfection of rVV expressing these proteins with rVV hHFE demonstrated that HCMV US2 but not HCMV US11 or other viral proteins prevents the expression of TfR-free and TfR-associated HFE complexes, as well as of free HFE heavy chains, by targeting HFE for rapid proteasome-mediated degradation. In the presence of proteasome inhibitors, the HFE remains glycosylated, as was described for class II MHC molecules (37), and both free heavy chains and conformed HFE complexes are stabilized. The data also demonstrate that this process does not depend on any additional species-specific factors since it occurs in both human and mouse cells.
Available data show that HCMV US2 targets HLA-A and HLA-B locus products but not HLA-C and HLA-G locus products for proteasome-dependent degradation (33). The fact that US2 also targets class II MHC HLA-DR and HLA-DM complexes for degradation, despite their very low homology to HLA-A and HLA-B locus products, raised the possibility that US2 is specifically selected for its ability to block class I and class II MHC presentation pathways (37). Hence, MHC molecules that are localized in specific tissues and might have other functions such as those of HLA-G (expressed in the trophoblasts and providing protection from NK-mediated activity [23]) and HLA-C (known to have reduced surface stability [46]) might be somehow resistant to this effect. However, the data presented in this paper show that US2 has a more dynamic effect. It targets for degradation a nonclassical class I MHC molecule that regulates iron metabolism and does not play any direct role in the classical pathway of antigen presentation. Thus, the virus employs the same protein for altering two functions that might interfere with its survival and efficient replication. On the one hand, it interferes with classical antiviral immune responses, and on the other hand, it induces cellular iron uptake to support its growth. The fact that iron supports viral replication and chronic infections is known for several systems. Hepatic iron concentration has consistently been observed as being directly correlated with the response to interferon therapy in the treatment of chronic hepatitis C virus infection (7). Moreover, treatment of patients with hepatitis C and iron overload with iron chelators improved their response to interferon therapy (4). In vitro studies demonstrated that iron enhances hepatitis C virus replication in cultured human hepatocytes (15). Replication of human immunodeficiency virus type 1 can be influenced by iron, as demonstrated by the fact that iron chelators inhibit virus replication (13, 32). Iron chelators also inhibit CMV infection and CMV-induced pathogenic changes (20). Thus, increasing the cellular iron pool by downregulating HFE expression might promote the persistence of viruses. Hopefully, the discovery of novel mechanisms utilized by viruses to alter cell metabolism and function will ultimately lead to the development of more-efficient tools for overcoming such viral infections.
Acknowledgments
The research was supported by the German-Israel Foundation (GIF), EC contract no. QLG1-CT-1999-00665, the Israel Ministry of Health, the Ela Kodesz Institute for Research on Cancer Development Prevention, and the Mozelsio Fund for Pediatric Cancer Research. Sayeh Vahdati Ben-Arieh is the recipient of an ICRETT fellowship (International Union Against Cancer [UICC], Geneva, Switzerland).
We are grateful to C. Enns (Oregon Health Sciences University, Portland), N. Laham, and T. Mushaiew (Tel Aviv University) for fruitful discussions; to Bethany Buschling (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Md.) for excellent technical help; to Z. Eshhar (The Weizmann Institute of Science, Rehovot, Israel) for the V1-10 antibody; and to H. Ploegh (Harvard Medical School, Boston, Mass.) for the anti-US2 antiserum.
REFERENCES
- 1.Anderson G J. Control of iron absorption. J Gastroenterol Hepatol. 1996;11:1030–1032. doi: 10.1111/j.1440-1746.1996.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 2.Beck S, Barrell B G. Human cytomegalovirus encodes a glycoprotein homologous to MHC class-I antigens. Nature. 1988;331:269–272. doi: 10.1038/331269a0. [DOI] [PubMed] [Google Scholar]
- 3.Bennett E M, Bennink J R, Yewdell J W, Brodsky F M. Cutting edge: adenovirus E19 has two mechanisms for affecting class I MHC expression. J Immunol. 1999;162:5049–5052. [PubMed] [Google Scholar]
- 4.Cagnoni C, Corsini F, Pancotti D, Carrara G. Effect of iron depletion on long-term response to interferon in patients with chronic hepatitis C with increased plasma iron without accumulation of liver iron. Ann Ital Med Int. 2000;15:132–138. [PubMed] [Google Scholar]
- 5.Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Sousa M, Reimao R, Porto G, Grady R W, Hilgartner M W, Giardina P. Iron and lymphocytes: reciprocal regulatory interactions. Curr Stud Hematol Blood Transfus. 1991;58:171–177. doi: 10.1159/000419357. [DOI] [PubMed] [Google Scholar]
- 7.Di Bisceglie A M, Bonkovsky H L, Chopra S, Flamm S, Reddy R K, Grace N, Killenberg P, Hunt C, Tamburro C, Tavill A S, Ferguson R, Krawitt E, Banner B, Bacon B R. Iron reduction as an adjuvant to interferon therapy in patients with chronic hepatitis C who have previously not responded to interferon: a multicenter, prospective, randomized, controlled trial. Hepatology. 2000;32:135–138. doi: 10.1053/jhep.2000.8700. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich R, Lemonnier F A. HFE—a novel nonclassical class I molecule that is involved in iron metabolism. Immunity. 2000;13:585–588. doi: 10.1016/s1074-7613(00)00058-3. [DOI] [PubMed] [Google Scholar]
- 9.Feder J N, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy D A, Basava A, Dormishian F, Domingo R, Jr, Ellis M C, Fullan A, Hinton L M, Jones N L, Kimmel B E, Kronmal G S, Lauer P, Lee V K, Loeb D B, Mapa F A, McClelland E, Meyer N C, Mintier G A, Moeller N, Moore T, Morikang E, Wolff R K. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Gene. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 10.Feder J N, Penny D M, Irrinki A, Lee V K, Lebron J A, Watson N, Tsuchihashi Z, Sigal E, Bjorkman P J, Schatzman R C. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc Natl Acad Sci USA. 1998;95:1472–1477. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming R E, Migas M C, Zhou X, Jiang J, Britton R S, Brunt E M, Tomatsu S, Waheed A, Bacon B R, Sly W S. Mechanism of increased iron absorption in murine model of hereditary hemochromatosis: increased duodenal expression of the iron transporter DMT1. Proc Natl Acad Sci USA. 1999;96:3143–3148. doi: 10.1073/pnas.96.6.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fruh K, Ahn K, Djaballah H, Sempe P, van Endert P M, Tampe R, Peterson P A, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 13.Georgiou N A, van der Bruggen T, Oudshoorn M, Nottet H S, Marx J J, van Asbeck B S. Inhibition of human immunodeficiency virus type 1 replication in human mononuclear blood cells by the iron chelators deferoxamine, deferiprone, and bleomycin. J Infect Dis. 2000;181:484–490. doi: 10.1086/315223. [DOI] [PubMed] [Google Scholar]
- 14.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 15.Kakizaki S, Takagi H, Horiguchi N, Toyoda M, Takayama H, Nagamine T, Mori M, Kato N. Iron enhances hepatitis C virus replication in cultured human hepatocytes. Liver. 2000;20:125–128. doi: 10.1034/j.1600-0676.2000.020002125.x. [DOI] [PubMed] [Google Scholar]
- 16.Kerkau T, Bacik I, Bennink J R, Yewdell J W, Hunig T, Schimpl A, Schubert U. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J Exp Med. 1997;185:1295–1305. doi: 10.1084/jem.185.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebron J A, Bennett M J, Vaughn D E, Chirino A J, Snow P M, Mintier G A, Feder J N, Bjorkman P J. Crystal structure of the hemochromatosis protein HFE and characterization of its interaction with transferrin receptor. Cell. 1998;93:111–123. doi: 10.1016/s0092-8674(00)81151-4. [DOI] [PubMed] [Google Scholar]
- 18.Lebron J A, West A P, Jr, Bjorkman P J. The hemochromatosis protein HFE competes with transferrin for binding to the transferrin receptor. J Mol Biol. 1999;294:239–245. doi: 10.1006/jmbi.1999.3252. [DOI] [PubMed] [Google Scholar]
- 19.Mahr J A, Gooding L R. Immune evasion by adenoviruses. Immunol Rev. 1999;168:121–130. doi: 10.1111/j.1600-065x.1999.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 20.Martelius T, Scholz M, Krogerus L, Hockerstedt K, Loginov R, Bruggeman C, Cinatl J, Jr, Doerr H W, Lautenschlager I. Antiviral and immunomodulatory effects of desferrioxamine in cytomegalovirus-infected rat liver allografts with rejection. Transplantation. 1999;68:1753–1761. doi: 10.1097/00007890-199912150-00020. [DOI] [PubMed] [Google Scholar]
- 21.Merryweather-Clarke A T, Pointon J J, Shearman J D, Robson K J. Global prevalence of putative haemochromatosis mutations. J Med Genet. 1997;34:275–278. doi: 10.1136/jmg.34.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkkila S, Waheed A, Britton R S, Bacon B R, Zhou X Y, Tomatsu S, Fleming R E, Sly W S. Association of the transferrin receptor in human placenta with HFE, the protein defective in hereditary hemochromatosis. Proc Natl Acad Sci USA. 1997;94:13198–13202. doi: 10.1073/pnas.94.24.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pazmany L, Mandelboim O, Vales-Gomez M, Davis D M, Reyburn H T, Strominger J L. Protection from natural killer cell-mediated lysis by HLA-G expression on target cells. Science. 1996;274:792–795. doi: 10.1126/science.274.5288.792. [DOI] [PubMed] [Google Scholar]
- 24.Piguet V, Schwartz O, Le Gall S, Trono D. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol Rev. 1999;168:51–63. doi: 10.1111/j.1600-065x.1999.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 25.Porto G, Vicente C, Teixeira M A, Martins O, Cabeda J M, Lacerda R, Goncalves C, Fraga J, Macedo G, Silva B M, Alves H, Justica B, de Sousa M. Relative impact of HLA phenotype and CD4-CD8 ratios on the clinical expression of hemochromatosis. Hepatology. 1997;25:397–402. doi: 10.1002/hep.510250223. [DOI] [PubMed] [Google Scholar]
- 26.Reimao R, Porto G, de Sousa M. Stability of CD4/CD8 ratios in man: new correlation between CD4/CD8 profiles and iron overload in idiopathic haemochromatosis patients. C R Acad Sci Ser III. 1991;313:481–487. [PubMed] [Google Scholar]
- 27.Rothenberg B E, Voland J R. beta2 knockout mice develop parenchymal iron overload: a putative role for class I genes of the major histocompatibility complex in iron metabolism. Proc Natl Acad Sci USA. 1996;93:1529–1534. doi: 10.1073/pnas.93.4.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy C N, Penny D M, Feder J N, Enns C A. The hereditary hemochromatosis protein, HFE, specifically regulates transferrin-mediated iron uptake in HeLa cells. J Biol Chem. 1999;274:9022–9028. doi: 10.1074/jbc.274.13.9022. [DOI] [PubMed] [Google Scholar]
- 29.Salter-Cid L, Brunmark A, Li Y, Leturcq D, Peterson P A, Jackson M R, Yang Y. Transferrin receptor is negatively modulated by the hemochromatosis protein HFE: implications for cellular iron homeostasis. Proc Natl Acad Sci USA. 1999;96:5434–5439. doi: 10.1073/pnas.96.10.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salter-Cid L, Peterson P A, Yang Y. The major histocompatibility complex-encoded HFE in iron homeostasis and immune function. Immunol Res. 2000;22:43–59. doi: 10.1385/IR:22:1:43. [DOI] [PubMed] [Google Scholar]
- 31.Santos M, Schilham M W, Rademakers L H, Marx J J, de Sousa M, Clevers H. Defective iron homeostasis in beta 2-microglobulin knockout mice recapitulates hereditary hemochromatosis in man. J Exp Med. 1996;184:1975–1985. doi: 10.1084/jem.184.5.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savarino A, Pescarmona G P, Boelaert J R. Iron metabolism and HIV infection: reciprocal interactions with potentially harmful consequences? Cell Biochem Funct. 1999;17:279–287. doi: 10.1002/(SICI)1099-0844(199912)17:4<279::AID-CBF833>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 33.Schust D J, Tortorella D, Seebach J, Phan C, Ploegh H L. Trophoblast class I major histocompatibility complex (MHC) products are resistant to rapid degradation imposed by the human cytomegalovirus (HCMV) gene products US2 and US11. J Exp Med. 1998;188:497–503. doi: 10.1084/jem.188.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shemesh J, Ehrlich R. Aberrant biosynthesis and transport of class I major histocompatibility complex molecules in cells transformed with highly oncogenic human adenoviruses. J Biol Chem. 1993;268:15704–15711. [PubMed] [Google Scholar]
- 35.Shemesh J, Rotem-Yehudar R, Ehrlich R. Transcriptional and posttranscriptional regulation of class I major histocompatibility complex genes following transformation with human adenoviruses. J Virol. 1991;65:5544–5548. doi: 10.1128/jvi.65.10.5544-5548.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ten Elshof A E, Brittenham G M, Chorney K A, Page M J, Gerhard G, Cable E E, Chorney M J. Gamma delta intraepithelial lymphocytes drive tumor necrosis factor-alpha responsiveness to intestinal iron challenge: relevance to hemochromatosis. Immunol Rev. 1999;167:223–232. doi: 10.1111/j.1600-065x.1999.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 37.Tomazin R, Boname J, Hegde N R, Lewinsohn D M, Altschuler Y, Jones T R, Cresswell P, Nelson J A, Riddell S R, Johnson D C. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat Med. 1999;5:1039–1043. doi: 10.1038/12478. [DOI] [PubMed] [Google Scholar]
- 38.Tortorella D, Gewurz B E, Furman M H, Schust D J, Ploegh H L. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 39.Tortorella D, Story C M, Huppa J B, Wiertz E J, Jones T R, Bacik I, Bennink J R, Yewdell J W, Ploegh H L. Dislocation of type I membrane proteins from the ER to the cytosol is sensitive to changes in redox potential. J Cell Biol. 1998;142:365–376. doi: 10.1083/jcb.142.2.365. . (Erratum, 145:642, 1999.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waheed A, Parkkila S, Saarnio J, Fleming R E, Zhou X Y, Tomatsu S, Britton R S, Bacon B R, Sly W S. Association of HFE protein with transferrin receptor in crypt enterocytes of human duodenum. Proc Natl Acad Sci USA. 1999;96:1579–1584. doi: 10.1073/pnas.96.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waheed A, Parkkila S, Zhou X Y, Tomatsu S, Tsuchihashi Z, Feder J N, Schatzman R C, Britton R S, Bacon B R, Sly W S. Hereditary hemochromatosis: effects of C282Y and H63D mutations on association with β2-microglobulin, intracellular processing, and cell surface expression of the HFE protein in COS-7 cells. Proc Natl Acad Sci USA. 1997;94:12384–12389. doi: 10.1073/pnas.94.23.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiertz E J, Jones T R, Sun L, Bogyo M, Geuze H J, Ploegh H L. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 43.Wiertz E J, Tortorella D, Bogyo M, Yu J, Mothes W, Jones T R, Rapoport T A, Ploegh H L. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 44.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 45.Yuwen H, Cox J H, Yewdell J W, Bennink J R, Moss B. Nuclear localization of a double-stranded RNA-binding protein encoded by the vaccinia virus E3L gene. Virology. 1993;195:732–744. doi: 10.1006/viro.1993.1424. [DOI] [PubMed] [Google Scholar]
- 46.Zemmour J, Parham P. Distinctive polymorphism at the HLA-C locus: implications for the expression of HLA-C. J Exp Med. 1992;176:937–950. doi: 10.1084/jem.176.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou X Y, Tomatsu S, Fleming R E, Parkkila S, Waheed A, Jiang J, Fei Y, Brunt E M, Ruddy D A, Prass C E, Schatzman R C, O'Neill R, Britton R S, Bacon B R, Sly W S. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proc Natl Acad Sci USA. 1998;95:2492–2497. doi: 10.1073/pnas.95.5.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]