Abstract
Introduction
Contradictory claims about the efficacy of several medicinal plants to promote glycemic control in patients with type 2 diabetes mellitus (T2DM) have been explained by divergences in the administration form and by extrapolation of data obtained from healthy individuals. It is not known whether the antidiabetic effects of traditional herbal medicines are influenced by gelatin capsules. This randomized crossover trial aimed to evaluate the acute effect of a single dose of raw cinnamon consumed orally either dissolved in water as a beverage or as ordinary hard gelatin capsules on postprandial hyperglycemia (>140 mg/dL; >7.8 mmol/L) in T2DM patients elicited by a nutritionally-balanced meal providing 50 g of complex carbohydrates.
Methods
Fasting T2DM patients (n = 19) randomly ingested a standardized meal in five experimental sessions, one alone (Control) and the other after prior intake of 3 or 6 g of crude cinnamon in the form of hard gelatin capsules or powder dissolved in water. Blood glucose was measured at fasting and at 0.25, 0.5, 0.75, 1, 1.5 and 2 hours postprandially. After each breakfast, its palatability scores for visual appeal, smell and pleasantness of taste were assessed, as well as the taste intensity sweetness, saltiness, bitterness, sourness and creaminess.
Results
The intake of raw cinnamon dissolved in water, independently of the dose, decreased the meal-induced large glucose spike (peak-rise of +87 mg/dL and Δ1-hour glycemia of +79 mg/dL) and the hyperglycemic blood glucose peak. When cinnamon was taken as capsules, these anti-hyperglycemic effects were lost or significantly diminished. Raw cinnamon intake did not change time-to-peak or the 2-h post-meal glycaemia, but flattened the glycemic curve (lower iAUC) without changing the shape that is typical of T2DM patients.
Conclusions
This cinnamon’s antihyperglycemic action confirms its acarbose-like property to inhibit the activities of the carbohydrate-digesting enzymes α-amylases/α-glucosidases, which is in accordance with its exceptionally high content of raw insoluble fiber. The efficacy of using raw cinnamon as a diabetes treatment strategy seems to require its intake at a specific time before/concomitantly the main hyperglycemic daily meals. Trial registration: Registro Brasileiro de Ensaios Clínicos (ReBEC), number RBR-98tx28b.
Introduction
Several researchers have been investigating the antidiabetic properties of different medicinal plants through diverse approaches [1, 2]. However, the statements in the conclusions of the studies are frequently contradictory. While some have concluded that a herb lowers fasting blood glucose [3–12] and HbA1c level [13–16], others came to divergent conclusions [17–26].
These contradictions are normally associated with misinterpretations of experimental data [27–29], such as the simple extrapolation to the humans based on data from animals taking large doses of the compound [1, 30, 31] or from in vitro assays [29, 31–34]. In humans, the effect of a plant is known to be changed by its administration form [1, 22, 35–40]. For example, medicinal plants are commonly consumed through swallowing hard gelatin capsules [41–47]; however, this formulation may decrease the bioavailability of bioactive molecules in relation to beverage forms [48–54]. Spices and herbs are often consumed inside a meal that undergo heating; however, some of their properties become decreased in relation to their native/raw state [55–57] and toxins/carcinogens may be formed [58–67]. Moreover, the effect of a natural product may be also different between healthy humans and patients with type 2 diabetes mellitus (T2DM) [68]. The post-meal glycemic curve of these patients has an extremely particular shape in relation to healthy people, namely a much larger post-meal blood glucose rise, higher peak levels and abnormal elevated values of 2-h post-meal blood glucose level [69–71]. Considering that abrupt high glucose ‘spikes’ are more deleterious than steady high levels of blood glucose [72–74] and that larger values of 2-hour post-meal blood glucose are associated with more severe diabetic complications [69, 75–77], evaluations of the antidiabetic potential of herbs may lead to imprecise conclusions if the particularities of the T2DM patients are not taken into account [30, 78, 79]. Some of the debatable claims about therapeutic benefits of natural products are attributed to the poor justification for experiments, i.e., not based on published papers and all available relevant data of the literature [29, 41, 80, 81].
To our knowledge, there is no study that investigated the influence of the common hard gelatin capsules on the effects of medicinal plants with antidiabetic properties. Among herbal remedies known as antidiabetic, cinnamon is one of the safest [82–85], a highly available and worldwide consumed spice [86–91], is used in Ayurvedic [79, 92–94], Traditional Chinese [1, 95, 96] and Japanese (Kampo) Medicine [1, 97–100], and is the most investigated in glucose tolerance studies [101–103]. Moreover, no study assessed its influence on the postprandial hyperglycemia (>140 mg/dL; >7.8 mmol/L) in T2DM patients elicited by a real-life common meal [104, 105]. Therefore, the aim of the present study was to investigate the acute effect of a single dose of raw cinnamon powder consumed orally either dissolved in water as a beverage or as ordinary hard gelatin capsules on postprandial hyperglycaemia in patients with T2DM elicited by a balanced breakfast providing complex carbohydrates.
Methods
Study design
In this randomized crossover clinical trial, participants ingested a standardized meal on five separate test days. During the test day, each patient ingested the meal either alone (Control) or after prior intake of 3 or 6 g of raw cinnamon in the form of hard gelatin capsules or in the form of powder dissolved in water. After a washout period, each participant underwent another experimental session. The sequence in which these five procedures were administered to each participant was defined in random order (simple randomization through Excel spreadsheet). The primary outcome was postprandial blood glucose concentrations. Secondary outcomes were palatability markers. The study flow diagram is shown in Fig 1.
Fig 1. Study flowchart diagram of the participants (following CONSORT).
T2DM men after eating a standardized meal alone (Control) or after prior ingestion of 3 g of raw cinnamon in capsules (3gCaps), 6 g of raw cinnamon in capsules (6gCaps), 3 g of raw cinnamon powder dissolved in water (3gPowder), or 6 g of raw cinnamon powder (6gPowder).
The protocol was approved by the Research Ethics Committee of the Foundation for Teaching and Research in Health Sciences (FEPECS/SES, for its abbreviation in Portuguese) on 4th June 2019 (decision number 3367200). The prior approval of this project (doctoral research) was a requirement of our PhD program to start the recruitment of volunteers, which occurred between 11 July 2019 and 15 October 2019. The approved trial protocol may be accessed as supplementary material in the supporting information section (S1 and S2 Protocols). Then, we conducted the study according the Declaration of Helsinki and in line with the CONSORT (Consolidated Standards of Reporting Trials) (S1 Checklist and S1 Fig). All participants were informed about the objectives of the study and provided written informed consent. Follow-up was conducted between 22 July 2019 and 28 October 2019. Although the requirements neither of the Ethics Committee nor of the PhD program did not include the registration of the protocol, this clinical trial was retrospectively registered on the publicly accessible registry https://ensaiosclinicos.gov.br on 12 November 2021 (ReBEC: RBR-98tx28b). Registration was not performed in advance because the authors thought the ethical clearance was sufficient. The authors confirm that all ongoing and related trials for this intervention are registered.
Participants
Volunteers were recruited by advertisements using posters in public health centers in Brasília (Brazil). Eligibility criteria were: male patients with T2DM, 30–60 years of age, consistent breakfast consumption (ingestion of ≥100 kcal within 2 hours after awakening on ≥4 days per week), willingness to eat all meals, absence of allergy to all foods used in the study, and no self-reported sleep disorders. The diagnosis of T2DM was based on the standards of the American Diabetes Association [106]: fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) or 2-h postprandial plasma glucose ≥200 mg/dL (11.1 mmol/L) during a 75 g oral glucose tolerance test (OGTT) or HbA1c ≥6.5% (48 mmol/mol) or a random plasma glucose ≥200 mg/dL (11.1 mmol/L). We did not include women in the study to avoid confounding factors derived from hormone-elicited changes in carbohydrate metabolism [107, 108].
Exclusion criteria were: use of exogenous insulin, diabetes-related health complications, gastrointestinal disorders or irregular intestinal rhythm (diarrhea or constipation), smoking, and post-meal blood glucose peak of less than 140 mg/dL (7.8 mmol/L). The cut-off value of 140 mg/dL was based on the fact that healthy subjects usually have euglycemic values below this limit [109–111] and because this threshold is commonly used to define a glycemic response as ‘postprandial hyperglycemia’ [106, 112].
Sample size was determined using G*Power software (Dusseldorf University, Germany) [113–115] with a statistical power of 95%, alpha error of 5% (two-tails), and the glucose levels reported by de Carvalho et al. (135.8 ± 20.7 mg/dL) [116], which resulted in a sample size of 19 participants (crossover design). To compensate for possible dropouts, we were added three extra subjects and recruited a total of 22 volunteers.
Clinical assessments
During the initial screening visit, participants completed questionnaires about the study’s criteria, diabetes diagnosis, current medications use, health conditions, eating habits and sleeping routine. Body composition and anthropometric data were also assessed. Then, the subjects reported to a clinical laboratory (07:00–8:30 AM) after an overnight fasting (8–12 h) and abstained from their diabetes medication to collect 3 mL of venous blood in a vacutainer tube with no anticoagulant or preservative. Insulin level was measured by electrochemiluminescence (ADVIA, model Centaur, Siemens Healthcare Diagnostics S.A., Brazil) and glucose was determined by the glucose oxidase method (ADVIA, 208 model 2400, Siemens Healthcare Diagnostics S.A., Brazil). Glycated hemoglobin (HbA1c) was quantified by turbidimetric inhibition immunoassay (COBAS, Roche Diagnostics, Brazil) [117]. The homeostatic model assessment-insulin resistance (HOMA-IR) and HOMA-β were calculated to evaluate insulin resistance and pancreatic β cell function, respectively [118].
Anthropometry
Height was measured using a stadiometer (Balmak®) fixed to the wall measuring to the nearest 0.1 cm. Weight was assessed using an electronic scale (Ramuza®) with a precision of 50 g. Body fat was determined by bioelectrical impedance (InBody 570, Seoul, South Korea). The circumference of the waist was measured with a precision of 0.1 cm at the midway between the lowest rib and the iliac crest [119, 120].
Meals
The standardized meal offered during each experimental session was a nutritionally-balanced breakfast showing an energy and macronutrient composition that followed the recommendations of the Dietary Guidelines for Americans for [121]. The breakfast provided 50.0 g available carbohydrate (59%), 10.7 g fat (29%), 10.0 g protein (12%) and 2.4 g of fiber (40 g of toast, 40 g of cheese and 200 mL of peach juice), totaling 337 kcal. The amount of 50 g of carbohydrates is commonly consumed in the breakfast in North America [122] and in other studies that investigated the effect of cinnamon on the glycemic response [123–126].
Cinnamon treatment
Ground powder of Chinese cinnamon, obtained from the dried inner bark of the tree Cinnamomum cassia, also known as aromaticum/aromaticaum [127], was purchased in packages of 50 g sealed by the manufacturer (Kitano, São Bernardo do Campo, Brazil), all from the same batch (Batch: F19BRPP032). The encapsulation was carried out at a compounding pharmacy (Pharmacotécnica, Brasília, Brazil) using a semi-automatic encapsulator to pack 600 mg of powder into ordinary hard gelatin capsules size 00 from the supplier GEMINI (Batch: 190370) [128, 129]. The cinnamon to be taken in the powder form was weighed on a high-precision scale and separated into small plastic cups with a lid. The doses of 3 and 6 g of crude cinnamon contained, respectively, 4.68 and 9.36 kcal (156 kcal/100 g), 0.10 and 0.19 g of protein (3.21%), 0.06 and 0.12 g of lipids (1.98%), 0.94 and 1.88 g of carbohydrates (31.31%), 1.50 and 3.01 g of total fiber (50.11%), 1.46 and 2.91 g of insoluble fiber (48.56%); and 0.05 and 0.09 g of soluble fiber (1.55%) (Table 1).
Table 1. Nutrient content of cinnamon cassia powder in 100 g (%), 3 g and 6 g portions.
| 100 g | 3 g | 6 g | |
|---|---|---|---|
| Energy (kcal) | 156 | 4.68 | 9.36 |
| Protein (g) | 3.21 | 0.10 | 0.19 |
| Lipids (g) | 1.98 | 0.06 | 0.12 |
| Carbohydrate (g) | 31.31 | 0.94 | 1.88 |
| Total fiber (g) | 50.11 | 1.50 | 3.01 |
| Insoluble fiber (g) | 48.56 | 1.46 | 2.91 |
| Insoluble fiber (% of total fiber) | 96.91 | . | . |
| Soluble fiber (g) | 1.55 | 0.05 | 0.09 |
| Soluble fiber (% of total fiber) | 3.09 | . | . |
Data calculated from Araújo et al. [130].
The dose of 3 g of raw cinnamon was chosen because the intake of the dose of 1 g (capsules of C. cassia) does not influence the post-meal incremental area under the curve (iAUC) in healthy subjects [131], but the dose of 3 g does [132] and because it may be taken twice a day without surpassing the maximum recommended dose of 6 g per day [7, 133]. The dose of 6 g was also tested considering that the metabolic effects of cinnamon have shown to be dose-dependent [15, 134, 135] and because its acute intake has been demonstrated to be safe in studies under laboratory conditions [123, 136].
The choice of Cinnamomum cassia was based on the following information: (a) the precise content of nutrients, insoluble and soluble fiber of other varieties is not available, while C. cassia constitution was determined by accurate food chemical analyses (Table 1) [130]; (b) has the ability to inhibit the carbohydrate-digesting enzyme α-glucosidase [137–140] more intensely than Ceylon cinnamon (C. zeylanicum) [131, 141]; (c) is a stronger α-amylase inhibitor than other three varieties: Indonesian (C. burmanii), Vietnamese (C. loureirii), and Ceylon (C. zeylanicum) [141] (d) is highly recommended for administration in glycemic and lipid profiles studies [133]; and (e) is the most common commercial cinnamon variety [142] studied for treating TD2M [21, 133, 143–145]. Moreover, the safety of cinnamon cassia long-term intake has been evidenced by its property to improve liver function in animals [146–152] and the lack of reports of toxicity [82–85, 97, 147, 153, 154], even in a dose of 12 g per day taken by subjects for 12 weeks [153] and the presence of compound potentially hepatotoxic such as coumarin [155–159].
We chose the raw form of cinnamon because baking, frying or cooking foods for ≥10 minutes alters nutrient content and functional properties of vegetables: (a) decreasing the concentration of insoluble fiber [160–165]; (b) decreasing the content of several flavonoids and phenolic compounds [57, 166–168]; (c) decreasing the inhibitory activity on the carbohydrate-hydrolyzing enzymes α-amylase and α-glucosidase [55–57, 169, 170]; and (d) increasing starch hydrolysis rate [171–173]. Heating plants also produces the known toxic/carcinogenic polycyclic aromatic hydrocarbons, heterocyclic amines [58–64, 174] and advanced glycation endproducts [65–67].
Experimental protocol
After refraining from alcohol and exercise for 24 h and fasting for 8–12 h, 19 patients with T2DM underwent five morning experimental sessions (at 7:00–9:00 AM) separated by a washout period of 3–10 d. Each patient randomly consumed a breakfast either alone (Control) or immediately after prior ingestion of 3 or 6 g of raw cinnamon powder mixed with 150 mL water (3gPowder and 6gPowder groups) or prior ingestion of 5 or 10 capsules (600 mg each) with 150 mL water (3gCaps and 6gCaps groups). The maximum time allowed to ingest cinnamon and the standard meal was 15 minutes. Glycemia was measured at fasting (time 0) and postprandially at 15, 30, 45, 60, 90, and 120 min. After approximately 15 min of finishing each breakfast, each participant completed a visual analog scale (VAS) in order to rate palatability scores for visual appeal, smell and pleasantness of taste were assessed, as well as the taste intensity sweetness, saltiness, bitterness, sourness and creaminess [175]. Due to the property of the material, it was impossible for the participants to be blinded. Blinding was performed during the statistical assessment of the outcomes. Patients were requested to withhold their morning medications until the end of the procedures. During the period of participation in the research, the participants were instructed to keep their usual diet and daily physical activities. In addition, they were asked not to consume cinnamon, even for culinary purposes, during their participation in the research.
Blood glucose
Glycemic response was measured following Wolever’s recommendations and procedures to achieve the highest sensitivity–through capillary finger-stick blood samples [176]. Blood glucose concentration was measured using a glucometer [177–180] (Accu-Chek Active, Roche, Brazil) [181–184] that meets ISO 15197 accuracy criteria (r = 0.998; hexokinase method) [185]. The same approach has been used in studies that evaluated the effect of a single dose of cinnamon on postprandial blood glucose [123, 132, 136, 186], as well as in other similar recent works [183, 187–189].
Calculations
Glucose peak rise, also named as “glucose spikes” [73, 190–193], was defined as the maximum amplitude of glucose excursion, i.e., the maximum blood glucose concentration during the 120-minute test minus the fasting concentration [194, 195]. Time-to-peak was the time taken to reach the maximum measured blood glucose [196, 197]. The variation in glucose concentration (Δ blood glucose) was calculated as the post-meal blood glucose level at the different times throughout the glycemic curve minus fasting concentration [195]. Taking into account the association between higher levels of the 2-hour post-meal blood glucose in T2DM patients with complications and with delayed time-to-peak [69, 75, 76, 198], we also compared the 2-hour post-meal blood glucose among groups. The iAUC for glycemic response was calculated using the trapezoidal method, excluding the values below the baseline [199].
Statistical analysis
Data were tested for normality by the Shapiro-Wilk test and homoscedasticity by Levene’s test. The effect of raw cinnamon on the following parameters was evaluated using one-way repeated measures ANOVA with post hoc Bonferroni test: mean glucose peak rise, Δ1-hour post-meal blood glucose, 1-h post-meal blood glucose, Δ2-hour blood glucose, 2-h post-meal blood glucose and 2-h iAUC. A non-parametric repeated-measures Friedman test was used to compare data on time-to-glucose-peak and palatability. To examine the effects of the different forms and doses of cinnamon at different time points of the post-meal glycemic curve, we performed two-way repeated measures ANOVA using Bonferroni’s post hoc test for multiple comparisons. Data are presented as means with standard error or medians with 25th and 75th percentiles (interquartile range). All analyses were conducted using the Statistical Package for the Social Sciences (SPSS) software (version 21.0). P < 0.05 was considered significant.
Results
Initially, ninety-three men responded to the announcement and completed the screening visit. Of those, seventy-one were excluded and twenty-two participants met all eligibility criteria and were enrolled in the study. After clinical assessments and anthropometric measurements, three dropouts occurred due to research abandonment (n = 1), change in working hours (n = 1), and change of place residence (n = 1). Finally, nineteen participants completed the full study protocol (Fig 1).
Participants’ characteristics
The patients’ characteristics are summarized in Table 2. All patients were using oral hypoglycemic medications: nine (47%) took only one medication (eight used metformin and one used gliclazide), five (26%) took metformin plus glibenclamide, two (10%) took metformin plus vildagliptin, two (11%) took metformin-glibenclamide combination plus dapagliflozin, and one subject (5%) took metformin-glibenclamide plus pioglitazone. None of the subjects reported regular use of cinnamon and all of them did not remember the last time they consumed the herb.
Table 2. Baseline characteristics of the nineteen participants that completed the study.
| Variable | Median (25th-75th) | Range |
|---|---|---|
| Age (years) | 56 (47.5–57.0) | (38–59) |
| Height (cm) | 172.0 (168.3–176.2) | (154.0–192.0) |
| Weight (kg) | 89 (81.55–104.99) | (67.60–128.40) |
| Body mass index (kg/m²) | 30.44 (27.15–36.37) | (23.10–41.00) |
| Body fat percentage | 34.50 (23.60–36.45) | (15.60–46.60) |
| Waist circumference (cm) | 106.5 (100.5–117.25) | (85.0–132.0) |
| Months of diabetes duration | 49 (30.5–87) | (6–300) |
| Fasting venous glucose (mg/dL) | 124.0 (110.5–138.5) | (81–191) |
| Fasting insulin (μUI/mL) | 12.80 (11.50–19.00) | (4.00–33.68) |
| HbA1c percentage | 7.0 (6.3–7.6) | (5.6–8.3) |
| HOMA-IR | 4.20 (3.00–6.67) | (1.20–9.70) |
| HOMA-β | 89.40 (55.65–130.05) | (23.70–676.64) |
HbA1c: Glycated hemoglobin; HOMA-IR: Homeostasis model assessment of insulin resistance; HOMA-β: Homeostasis model assessment of beta-cell function.
All participants showed blood glucose levels of at least 150 mg/dL (8.3 mmol/L) at 1-hour post-meal and at peak, confirming that the chosen standardized meal and the selected patients were appropriate to induce a glycemic response that exceeds the normal euglycemic level of 140 mg/dL (7.8 mmol/L) and mimics the postprandial hyperglycemia typically experienced by T2DM patients.
Post-meal blood glucose spikes (peak rise and Δ1hour)
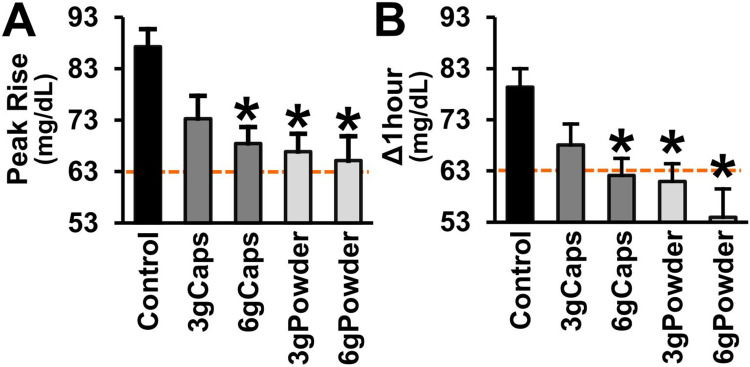
After the intake of the standardized meal, the fasted patients with T2DM showed a mean peak rise (glucose spike) of +87 mg/dL and a mean post-meal Δ1-hour blood glucose of +79 mg/dL (control group). These values of maximum amplitude of glucose excursion surpass the highest mean blood glucose rise of +63 mg/dL (+3.5 mmol/L) achieved by healthy individuals after ingesting 50 g of carbohydrate from 27 tested foods [194], as indicated in Fig 2 (orange lines).
Fig 2. Post-meal blood glucose peak rise and Δ1hour of T2DM patients without or with raw cinnamon.
(A) Post-meal mean blood glucose peak rise (mg/dL) and (B) mean difference between 1-hour post-meal blood glucose and fasting blood glucose (Δ1hour) of T2DM men after eating a standardized meal alone (Control) or after prior ingestion of 3 g of raw cinnamon in capsules (3gCaps), 6 g of raw cinnamon in capsules (6gCaps), 3 g of raw cinnamon powder dissolved in water (3gPowder), or 6 g of raw cinnamon powder (6gPowder). The line at the value of +63 mg/dL (+3.5 mmol/L) indicates the maximum mean blood glucose rise achieved by healthy individuals after ingesting 50 g of carbohydrate from 27 tested foods [194]. *p ≤ 0.013 in relation to control.
The acute ingestion of raw cinnamon markedly decreased blood glucose peak rise (one-way RM-ANOVA [F(4,72) = 8.260; p < 0.001]) (Fig 2A). The post hoc multiple comparisons tests (Bonferroni-adjusted) showed that the mean peak rise of the T2DM patients (+87 mg/dL) was significantly reduced by the intake of the dose of 3 g of raw cinnamon in the form powder (-23%; p = 0.003), but not by this dose taken as capsules (p = 0.139) (Fig 2A). The mean peak rise was decreased by the dose of 6 g in both forms, capsules (-22%; p = 0.013) and powder (-25%; p = 0.001) (Fig 2A). There was no difference in mean peak rise between the four cinnamon groups (p > 0.05). The groups 3gCaps, 6gCaps, 3gPowder and 6gPowder showed a mean peak rise of +73 mg/dL, +68 mg/dL, +67 mg/dL and +65 mg/dL, respectively (Fig 2A).
The post-meal level of Δ1-hour blood glucose (+79 mg/dL) was also decreased by the acute intake of raw cinnamon (one-way RM-ANOVA [F(4,72) = 8.947; p < 0.001]) (Fig 2B). The post hoc multiple comparisons tests (Bonferroni-adjusted) showed that the post-meal mean Δ1-hour blood glucose of the fasted T2DM patients after breakfast was decreased by the dose of 3 g of raw cinnamon in the form powder (-22%; p < 0.001), but not by capsules (p = 0.083) (Fig 2B). The Δ1-hour blood glucose was reduced by 6 g of raw cinnamon in both forms, capsules (-23%; p = 0.006) and powder (-32%; p = 0.003). There was no difference in mean Δ1-hour blood glucose between the four cinnamon groups (p > 0.05). The decreases in the mean Δ1-hour blood glucose provided by raw cinnamon were sufficient to achieve values that are proximate to those (+63 mg/dL or +3.5 mmol/L) of healthy individuals (Fig 2). The groups 3gCaps, 6gCaps, 3gPowder and 6gPowder showed a mean Δ1-hour blood glucose of +68 mg/dL, +62 mg/dL, +61 mg/dL and +54 mg/dL, respectively (Fig 2B).
Post-meal hyperglycemia
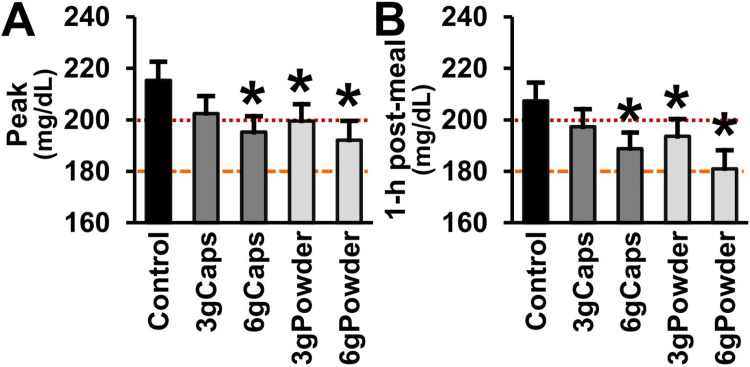
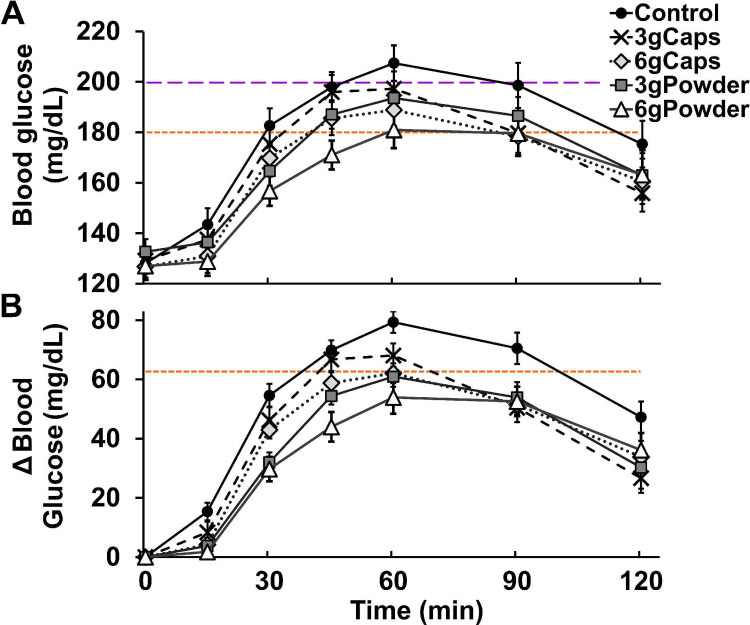
The mean of the highest blood glucose level and the 1-h post-meal mean blood glucose level of the fasted T2DM patients (control) after eating the breakfast were hyperglycemic (higher than 140 mg/dL; >7.8 mmol/L): 215 mg/dL and 207 mg/dL, respectively. As indicated in Fig 3, these values are higher than the renal threshold of 180 mg/dL (10 mmol/L) up to which glucose reabsorption is preserved at physiological rates [200–202] and insulin therapy is not necessary [203, 204]. They are also higher than the cutoff level of 200 mg/dL (11.1 mmol/L) used to diagnose T2DM [205] and strongly associated with metabolic disturbances [206].
Fig 3. Post-meal blood glucose peak and 1-h post-meal of T2DM patients without or with raw cinnamon.
(A) Post-meal mean blood glucose peak (mg/dL) and (B) mean 1-hour post-meal blood glucose (mg/dL) of T2DM men after eating a standardized meal alone (Control) or after prior ingestion of 3 g of raw cinnamon in capsules (3gCaps), 6 g of raw cinnamon in capsules (6gCaps), 3 g of raw cinnamon powder dissolved in water (3gPowder), or 6 g of raw cinnamon powder (6gPowder). The line at the value of 180 mg/dL (10 mmol/L) indicates the level up to which renal glucose reabsorption is preserved at physiological rates [200–202] and insulin therapy is not yet necessary [203, 204]. The line at the value of 200 mg/dL (11.1 mmol/L) indicates the 1-h post-meal threshold used to diagnose T2DM [205] and strongly associated with metabolic disturbances [206]. *p ≤ 0.017 in relation to control.
The acute ingestion of raw cinnamon decreased the post-meal hyperglycemic peak (one-way RM-ANOVA [F(4,72) = 5.288; p < 0.001]) (Fig 3A). The post hoc multiple comparisons tests (Bonferroni-adjusted) showed that the mean blood glucose peak of the T2DM patients (215 mg/dL) was significantly reduced by the intake of the dose of 3 g of raw cinnamon in the form powder (-9%; p = 0.009), but not by this dose taken in the form of capsules (p = 0.644) (Fig 3A). The mean blood glucose peak was also decreased by the dose of 6 g in both forms, capsules (-6%; p = 0.016) and powder (-13%; p = 0.003) (Fig 3A). The groups 3gCaps, 6gCaps, 3gPowder and 6gPowder showed a mean peak of 202 mg/dL, 195 mg/dL, 200 mg/dL and 192 mg/dL, respectively (Fig 3A).
The hyperglycemic level of the 1-hour post-meal blood glucose (207 mg/dL) was also decreased by the acute intake of raw cinnamon (one-way RM-ANOVA [F(4,72) = 6.763; p < 0.001]) (Fig 3B). The post hoc multiple comparisons tests (Bonferroni-adjusted) showed that the mean blood glucose level of the T2DM patients at one hour after breakfast was significantly decreased by the dose of 3 g of raw cinnamon in the form powder (-9%; p = 0.017), but not by capsules (p = 0.935) (Fig 3B). The level of 1-h post-meal blood glucose was reduced by 6 g of raw cinnamon in both forms, capsules (-6%; p = 0.013) and powder (-13%; p = 0.001). However, there was no difference in the mean peak and the level of 1-h post-meal blood glucose between the four cinnamon groups (p > 0.05). The groups 3gCaps, 6gCaps, 3gPowder and 6gPowder showed a mean 1-hour post-meal blood glucose of 197 mg/dL, 189 mg/dL, 194 mg/dL and 181 mg/dL, respectively (Fig 3B).
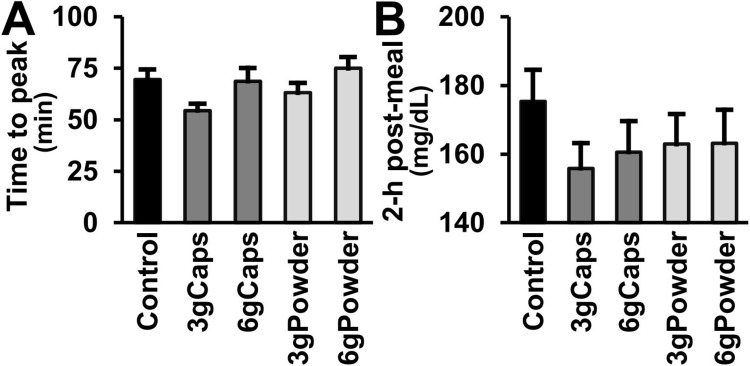
Time to blood glucose peak
The level of blood glucose in the patients with T2DM reached its peak at a mean time of 69.5 minutes after eating breakfast (Control) (Fig 4A). The occurrence of the peak at this time, which is greatly later than the normal time-to-peak of healthy individuals, is an additional marker of insulin resistance in our volunteers [69–71]. In all tests, the highest mean glycemic levels were observed at the 1-hour post-meal time. None of the doses or forms of cinnamon intake changed the time-to-peak in relation to the standard meal alone (Friedman test; p ≥ 0.159).
Fig 4. Post-meal time to blood glucose peak and 2-h post-meal of T2DM patients without or with raw cinnamon.
(A) Post-meal mean time (min) to reach blood glucose peak and (B) mean 2-hour post-meal blood glucose (mg/dL) of T2DM men after eating a standardized meal alone (Control) or after prior ingestion of 3 g of raw cinnamon in capsules (3gCaps), 6 g of raw cinnamon in capsules (6gCaps), 3 g of raw cinnamon powder dissolved in water (3gPowder), or 6 g of raw cinnamon powder (6gPowder).
2-h post-meal blood glucose
In line with the delay in the time-to-peak of T2DM patients, the mean blood glucose level at two hours (175 mg/dL) after the intake of the standardized meal containing 50 g of complex carbohydrate was still higher (+47 mg/dL) than fasting (Fig 4B). The mean 2-h post-meal of 175 mg/dL surpassed the threshold of 166 mg/dL (9.2 mmol/L) used after a post-load of 75 g of glucose to diagnose impaired glucose tolerance (IGT) and impaired fasting blood glucose (IFG) [207]. In contrast, all cinnamon groups showed mean levels of 2-h post-meal blood glucose lower than this mark: the groups 3gCaps, 6gCaps, 3gPowder, and 6gPowder showed a mean 2-h post-meal blood glucose of 156 mg/dL, 161 mg/dL, 163 mg/dL, and 163 mg/dL, respectively (Fig 4B). However, there was no significant difference in the mean 2-h post-meal blood glucose among groups (RM-ANOVA; [F(4,72) = 2.476; p = 0.052]).
Effects of dose and capsule
The two-way ANOVA with repeated measures showed that the form (capsule or powder dissolved in water) that T2DM patients used to intake raw cinnamon exerted a significant effect (interaction between the factors ‘form’ and ‘time’) on their blood glucose levels throughout 120 minutes [F(2.187, 196.796) = 3.972; p = 0.017]. Post-hoc analysis showed that the mean blood glucose level was significantly decreased by raw cinnamon ingested in the form of powder at 30 min (p = 0.013) at 45 min (p = 0.042). The effect of capsule did not change blood glucose levels (p ≥ 0.159) although it did not differ from the effect of powder (p ≥ 0.168). The dose (3 g or 6 g) of raw cinnamon did not exerte a significant effect on blood glucose levels at the different times, since the interaction between ‘dose’ and ‘time’ did not reach statistical significance (p = 0.127) as well as between ‘form’, ‘dose’ and ‘time’ (p = 0.993).
The post-meal values of Δ blood glucose after the fasted T2DM patients ingested breakfast were significantly changed along the glycemic curve (interaction between the factors ‘form’ and ‘time’) by the form used to intake raw cinnamon [F(2.717, 244.498) = 3.307; p = 0.025]. The levels of Δ blood glucose were significantly decreased by the intake of raw cinnamon in the form of powder at 15 min (p = 0.001), at 30 min (p < 0.001), at 45 min (p < 0.001), at 60 min (p < 0.001), and at 90 min (p = 0.013) and in the form of capsules at 15 min (p = 0.020), 60 min (p = 0.016), and 90 min (p = 0.004). The decreases in Δ blood glucose levels caused by the form of powder were significantly greater than by the form of capsule at 30 min (p = 0.002) and at 45 min (p = 0.003). The effect of the interaction between ‘dose’ and ‘time’ (p = 0.204) and of the interaction between ‘dose’, ‘form’, and ‘time’ on Δ blood glucose levels was not significant (p = 0.978).
Glycemic response curve and iAUC
The glycemic response curve of the participants in the present study showed a shape after eating breakfast (Control) that is typical of patients with T2DM. The Fig 5 shows that, in contrast to the glycemic curve of healthy individuals, the time to blood glucose peak occurred more than 100% later in our T2DM participants [69–71]. Their blood glucose level after peak also showed a slight decay (opposing to the normal rapid drop) and the level of the 2-h post-meal blood glucose was still elevated at a hyperglycemic level (>140 mg//dL) [69–71]. The intake of powder of raw cinnamon dissolved in water flattened the glycemic curve without changing the shape, as evidenced by the above reported results of time-to-peak and 2-h post-meal blood glucose.
Fig 5. Post-meal glycemic curve of T2DM patients without or with raw cinnamon.
(A) Mean blood glucose (mg/dL) and (B) mean Δ blood glucose (difference between post-meal blood glucose and fasting blood glucose) of T2DM men throughout 120 minutes after eating a standardized meal alone (Control) or after prior ingestion of 3 g of raw cinnamon in capsules (3gCaps), 6 g of raw cinnamon in capsules (6gCaps), 3 g of raw cinnamon powder dissolved in water (3gPowder), or 6 g of raw cinnamon powder (6gPowder). The line at the value of 180 mg/dL (10 mmol/L) indicates the level up to which renal glucose reabsorption is preserved at physiological rates [200–202] and insulin therapy is not yet necessary [203, 204]. The line at the value of 200 mg/dL (11.1 mmol/L) indicates the 1-h post-meal threshold used to diagnose T2DM [205] and strongly associated with metabolic disturbances [206]. The line at the value of 63 mg/dL (3.5 mmol/L) indicates the maximum mean blood glucose rise achieved by healthy individuals after ingesting 50 g of carbohydrate from 27 tested foods [194]. The post-meal blood glucose levels along the glycemic curve were significantly decreased by raw cinnamon ingested in the form of powder (p ≤ 0.042), independently of the dose (p > 0.05), but not in the form of capsule (p ≥ 0.159) (two-way ANOVA with repeated measures). The levels of post-meal Δ blood glucose along the curve were significantly decreased by raw cinnamon ingested in the form of powder (p ≤ 0.013) and in the form of capsule (p ≤ 0.020), independently of the dose (p > 0.05), and the decreases caused by the form of powder were significantly stronger than by the form of capsule (p ≤ 0.003).
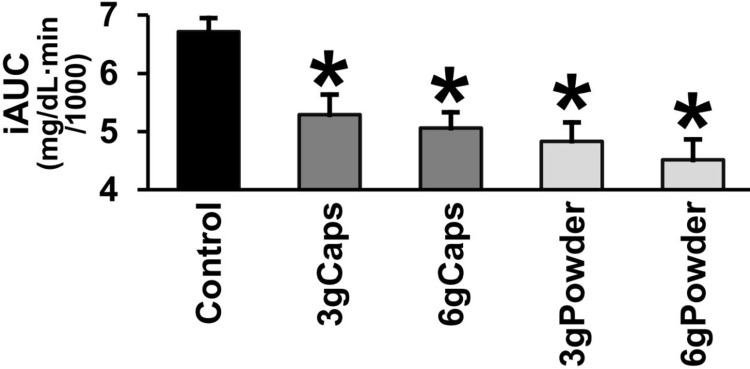
As a consequence of this general flattening, raw cinnamon caused a marked decrease in iAUC (Fig 6). The iAUC of the patients with T2DM after the intake of the breakfast was significantly decreased by the acute ingestion of raw cinnamon (one-way RM-ANOVA [F(4,72) = 14.216; p < 0.001]). The mean iAUC was lower in all raw cinnamon groups in comparison to control: 3gCaps (-21%; p = 0.012), 6gCaps (-25%; p < 0.001), 3gPowder (-28%; p < 0.001), and 6gPowder (-33%; p < 0.001) (Fig 6).
Fig 6. Post-meal incremental area under the glycemic response curve (iAUC) of T2DM patients without or with raw cinnamon.
Post-meal incremental area under the glycemic response curve (iAUC) of T2DM men after eating a standardized meal alone (Control) or after prior ingestion of 3 g of raw cinnamon in capsules (3gCaps), 6 g of raw cinnamon in capsules (6gCaps), 3 g of raw cinnamon powder dissolved in water (3gPowder), or 6 g of raw cinnamon powder (6gPowder). *p ≤ 0.012 in relation to control.
Markers of palatability and taste intensity
The prior ingestion of 3 or 6 g of raw cinnamon in the form of capsules or powder dissolved in water did not change any of the rates of palatability or taste intensity of the standardized meal. No significant differences between groups (p ≥ 0.80) were observed: visual appeal, smell, pleasantness of taste, sweetness, saltiness, bitterness, sourness and creaminess (Table 3).
Table 3. Taste perception ratings (median and 25th -75th interquartile) of T2DM men after eating breakfast without (control) or after prior ingestion of raw cinnamon in the forms/doses of 3 g capsules, 3 g powder, 6 g capsules or 6 g powder.
| Control | 3gCaps | 6gCaps | 3gPowder | 6gPowder | ||
|---|---|---|---|---|---|---|
| PALATABILITY | Visual appeal | 8.00 | 7.80 | 7.40 | 7.80 | 7.30 |
| (6.65–9.45) | (7.05–8.90) | (6.60–9.45) | (6.75–9.25) | (6.85–9.35) | ||
| Smell | 7.80 | 7.30 | 7.90 | 7.30 | 6.90 | |
| (6.65–9.10) | (6.50–8.85) | (6.20–9.45) | (6.65–8.90) | (5.60–8.70) | ||
| Pleasantness of taste | 7.50 | 8.00 | 7.00 | 7.50 | 7.50 | |
| (6.85–8.85) | (6.75–9.20) | (6.40–8.55) | (6.40–9.00) | (5.80–9.25) | ||
| TASTE INTENSITY | Sweetness | 5.30 | 5.20 | 5.00 | 5.30 | 5.40 |
| (3.85–6.95) | (2.95–6.10) | (3.15–6.70) | (2.70–6.90) | (2.45–7.15) | ||
| Saltiness | 2.10 | 4.00 | 3.30 | 2.30 | 4.20 | |
| (1.35–5.20) | (1.75–5.25) | (1.60–4.85) | (1.55–5.20) | (1.75–5.25) | ||
| Bitterness | 1.80 | 1.90 | 2.10 | 2.30 | 2.20 | |
| (0.50–3.20) | (0.65–4.55) | (0.95–3.55) | (0.35–7.80) | (1.50–6.70) | ||
| Sourness | 1.80 | 2.20 | 2.70 | 2.40 | 2.40 | |
| (0.75–3.15) | (1.20–4.30) | (1.55–3.90) | (0.90–4.50) | (1.75–4.70) | ||
| Creaminess | 7.80 | 7.30 | 7.70 | 7.40 | 7.10 | |
| (6.25–9.30) | (6.35–8.95) | (5.90–9.10) | (6.55–8.85) | (6.40–8.50) |
Discussion
The present study investigated the effect of a single dose of raw cinnamon on the blood glucose increase of patients with T2DM after the intake of a nutritionally balanced meal containing complex carbohydrates. After a breakfast providing 50 g carbohydrates, all participants showed post-meal blood glucose levels that exceeded normal euglycemic values and achieved the postprandial hyperglycemia commonly experienced by T2DM patients. The ingestion of raw cinnamon powder dissolved in water, independently of the dose, decreased the meal-induced large glucose spike (peak-rise and Δ1-hour blood glucose) and the hyperglycemic blood glucose peak. When the herb was taken in the form of capsules, these anti-hyperglycemic effects were lost or significantly diminished. The intake of raw cinnamon did not change time-to-peak or the 2-h post-meal blood glucose, but flattened the glycemic curve without changing the shape that is typical of T2DM patients, as evidenced by the decrease in iAUC. Lastly, another new finding was that the palatability of a standard meal is not affected by previous intake of cinnamon dissolved in water.
Acute effect of raw cinnamon on post-meal glucose spikes
Before this study, research has exclusively used non-diabetic populations to investigate the acute effect of cinnamon on the post-meal blood glucose elevation in a real-life situation (i.e., in response to the ingestion of complex carbohydrates) [123, 131, 136, 208, 209]. These studies reported glucose excursions that are within the normal range, with the highest mean Δ of approximately +45–62 mg/dL [210], which do not reach harmful levels [211]. In contrast, abrupt hyperglycemic ‘spikes’ observed in T2DM patients are highly deleterious and even more damaging than steady hyperglycemia [212–216]. In the present study, a balanced meal providing 50 g of carbohydrates caused mean values of peak rise of 87 mg/dL and Δ60-0min blood glucose of +79 mg/dL in patients with T2DM, values that are close to those from T2DM patients eating controlled meals, e.g., +79 mg/dL and +75 mg/dL [217]. These numbers exceed, by far, normal values of healthy individuals after eating breakfasts [210] and food such as white bread [23, 199, 218–221], white rice [222], brown rice [223], banana [224], and glucose solution [188, 225]. Hence, the present study is the first to assess the potential of raw cinnamon to acutely reduce the large glycemic response that T2DM patients show after a regular meal. A single dose of raw cinnamon taken by T2DM patients caused decreases of 22–32% in the mean post-meal peak rise and the 1-hour post-meal Δ60-0min blood glucose. One mechanism that underlie the deleterious effects of glucose spikes is the formation of free radicals and other reactive oxygen species (ROS) associated with the drastic shifts in glucose availability [226–232]. Indeed, there are a number of similarities between abrupt hyperglycemic excursions and episodes of ischemia/reperfusion, which are associated with extensive oxidative damage [229, 233–238]. Blood glucose spikes also promote an overproduction of ROS [239], oxidative stress [72, 240–244], and glucotoxicity [245–247]. Therefore, the post-meal hyperglycemic glucose spikes from our patients were down to the non-harmful physiological spikes seen in healthy humans [211] by simply drinking a glass of water with raw cinnamon before breakfast.
According to the American Diabetes Association, a higher ‘glycemic burden’ is suggested to explain higher levels of metabolic products of glucose-induced non-enzymatic reactions in T2DM patients [106] that may be monitored by measuring glycated albumin [248–251], fructosamine [248, 252–254], glycated β-lipoprotein [248, 255], glycated LDL [256–258], glycated HDL [259], and/or glycosylated hemoglobin [119, 260]. In accordance to this, the suppression in post-meal spikes for some weeks indirectly increases insulin sensitivity [193, 261–267]. Therefore, it is plausible to expect that the suppression of the post-meal glucose peak rises in patients with T2DM by raw cinnamon may decrease the levels of these markers. In the same way, considering that interventions which decrease glucose spikes diminish oxidative stress [242, 243] by inhibiting the overproduction of ROS [240, 241, 268], the addition of raw cinnamon in the diet of patients with T2DM, taken before the main meals, may also reduce the levels of the ROS-modified particule/biomolecule oxidized LDL [269–271] and carbamylated albumin [272]. Still, these speculations warrant further research.
Acute effect of raw cinnamon on post-meal hyperglycemia
At a first glance, the decreases in the blood glucose peak caused by the intake of raw cinnamon in the form of powder (-32%) observed in the present study seem similar to those observed in other studies with participants without diabetes; however, post-meal euglycemic peaks of healthy subjects, within the physiological range, i.e., <140 mg/dL (7.8 mmol/L) [111, 210], do not have deleterious effects [211, 273]. On the other hand, postprandial hyperglycemic levels as high as 155 mg/dL (8.6 mmol/L) at 1-h are associated with T2DM-related complications, which worsen as the degree of hyperglycemia increases [200, 274–276]. In the present study, each participant showed blood glucose levels of at least 150 mg/dL (8.3 mmol/L) at 1-hour post-meal and at peak, confirming that all of them experienced postprandial hyperglycemia [112]. The values of 207 mg/dL at 1-hour post-meal and 211 mg/dL at peak blood glucose level measured in the present study are classified as ‘very high’ hyperglycemia (>160 mg/dL) [109] and are very similar to those reported previously for T2DM patients [277]. Consequently, the decreases that were observed in the hyperglycemic post-meal blood glucose peaks in patients with T2DM caused by raw cinnamon powder is another new finding of the present study.
Meal-induced time-to-glucose peak and 2-h post-meal blood glucose
The intake of raw cinnamon before breakfast did not change the post-meal time-to-glucose-peak. This lack of effect greatly differs from the delays of up to 65 min in the blood glucose rise caused by fat [278] and viscous soluble fibers, such as guar gum [279], psyllium [280], and oat beta-glucans [197, 281, 282]. Considering that this delay is due to their property to slow gastric emptying and/or to prolong gastrointestinal transit time, the absence of effect of cinnamon on time-to-peak indicates that this spice would not retard gastric emptying or slow intestinal transit time. This is supported by the observations that insoluble fibers do not slow gastric emptying or prolong gastrointestinal transit time [283–285] and more than half of the weight of raw cinnamon is actually dietary fiber, which is made up of almost exclusively (97% of total fibre content) water insoluble fiber [130]. In contrast to the postulated gastric emptying effect of cinnamon [23, 145, 286], the decrease in post-meal blood glucose curve caused by raw cinnamon is very similar to those caused by amylase/glycosidase inhibitors, which do not prolong the gastrointestinal transit time [277, 283–285, 287].
The idea that cinnamon would delay digestion comes from studies with animals that received cinnamaldehyde; however, the dose used (250 mg/kg) [288] is impossible to achieve by the ingestion of natural cinnamon [289]. The concentration of cinnamaldehyde in powdered cinnamon cassia is estimated between 1.8 mg/g [290] and 57 mg/g of powder [289]. Actually, in rats, cinnamon has an opposite effect. It promotes peristaltic propulsion and accelerates both gastric emptying and reduces gastrointestinal transit time, showing a laxative effect against constipation [82]. The lack of change in the post-meal time-to-peak by raw cinnamon ingestion corroborates the findings that gastrointestinal transit time in humans is not altered by cinnamon [124] or is only slightly reduced (-7%) [123]. Since longer time to reach blood glucose peak is associated with more diabetes-related markers [70, 291], the absence of effect of cinnamon on this parameter may be beneficial.
Two hours after the intake of a balanced meal, our patients with T2DM showed higher blood glucose means than fasting levels. This response is very similar to that of diabetic patients of the majority of the studies investigating post-meal glycemic curves [277, 292]. Among our five experimental groups, those that did not ingest cinnamon (control) showed the highest 2-h post-meal mean of absolute of 175 mg/dL, exceeding the recommended postprandial threshold of 160 mg/dL (8.89 mmol/L) for optimal glycemic control [194, 276, 293, 294]. Larger values of 2-hour post-meal blood glucose are associated with more severe diabetic complications [69, 75, 76] and delays in the time-to-glucose-peak [71]. In healthy individuals, the level of postprandial blood glucose normally returns to fasting values, even after a load of 75 g of glucose in solution [225]. Here, the prior intake of raw cinnamon resulted in 2-hour post-meal blood glucose means that were lower or near the aforementioned threshold of 160 mg/dL. Therefore, raw cinnamon improved also this diabetes-associated parameter.
Effects of hard gelatin capsules
The decrease in the meal-induced large blood glucose peak-rise caused by the ingestion of raw cinnamon powder dissolved in water was lost when this herb was taken in the form of capsules. This result contradicts the assumption that common hard gelatin capsules would be promptly dissolved after being swallowed [295–297] and indicates that the high use of this formulation to administrate medicinal plants [41–45] might reduce the desired benefits of their consumption. However, our results confirm some findings of the few articles in the literature that directly compared the absorption/pharmacokinetics of some drugs between these two formulations: capsules versus solution [298, 299]. For example, the maximal blood concentration of an anti-allergic medication taken diluted in water is decreased by 33% when taken through capsules [300]. Among studies that investigated molecules of natural origin, one showed that the absorption of caffeine in the form of chewing gum is decreased when it is taken through capsules [50] and that the bioavailability of polyphenols and other bioactive molecules through the consumption of tea may also be lowered when the herb is ingested as capsules [51–53, 155, 301, 302]. Moreover, the metabolic change caused by red pepper ingested orally is stronger than consumed in capsule form [303–305]. These data may be explained by the fact that rupture and disintegration of the shell of standard gelatin capsules are processes required to release their bioactive compounds [43, 128, 129, 306–309]. In accordance to this, it was shown that the disintegration of capsules containing herbal products (Ginkgo leaf) may fail [310]. To our knowledge, the present study is the first that realized a direct comparison between the formulations of capsule versus solution to deliver a plant powder and to obtain its anti-hyperglycemic effect. The results showed that the anti-hyperglycemic effect of raw cinnamon powder is significantly weakened when the plant is ingested through ordinary hard gelatin capsules formulation.
Acute effect of raw cinnamon on iAUC
In previous studies that showed that the intake of cinnamon or its extract [209] decreases the post-meal glycemic response, the participants ingested meals showing medium [123] and high [131, 136] glycemic index [194, 311], which produced iAUCs that are normally observed in a real-world post-breakfast circumstance [173, 312]. In contrast, only a slight (but still significant) decrease of 7% in the iAUC was reported after consuming a meal with a very low glycemic index [132].
Herein, the intake of the dose of 3 g of raw cinnamon decreased the mean post-meal 1-hour blood glucose only in the form of powder. The dose of 6 g diminished the control level of 207 mg/dL to significantly lower levels, reaching 181 mg/dL. Another noteworthy effect of raw cinnamon ingestion was the reduction in iAUC>180mg/dL. Recently developed approaches measure specifically the iAUC above the threshold 180 mg/dL (iAUC>180mg/dL) (associated with metabolic disturbances and mortality) [251, 313] and the time spent in the range of 181–250 mg/dL (TIR181-250) through continuous glucose monitoring (CGM) [204]. Here, the mean iAUC>180mg/dL of our T2DM patients (as may be seen in the glycemic curve) approached 0 in patients that had ingested 6 g of raw cinnamon mixed with water. Similarly, the use of CGM by our T2DM patients during the post-meal hours [314] revealed the disappearance of the TIR181-250 by the prior intake of raw cinnamon. This acute antihyperglycemic effect exerted by raw cinnamon indicates its usefulness for T2DM patients to follow the recommendation of avoiding post-meal blood glucose levels of 180 mg/dL (10 mmol/L) [203, 204, 315]. Theoretically, the availability of this antihyperglycemic herb to diabetic patients could contribute to rapid improvements in glycemic control since partial and complete remission are achieved when they are asked to monitor their plasma glucose and to avoid ‘anormal’ levels higher than 140 mg/dL (7.8 mmol/L) [316].
These data were produced during experimental human studies that followed the methods recommended to investigate glycemic response, such as the use of the standard portion size of 50 g carbohydrate in a controlled laboratory environment [317], allowing rigorous control of variables and confounding factors [318, 319]. These tightly controlled conditions confers more precision, accuracy, and degree of sensitivity in relation to free-living interventional studies [320–323] that investigate the chronic effects of long-term dietary modifications [324–328]. Taken together with previous research, our results demonstrated that, after common meals, the acute intake of raw cinnamon suppresses not only the postprandial normal iAUC in healthy subjects, but also the hyperglycemic iAUC in T2DM patients.
Mechanistic considerations
As discussed above, slowing gastric emptying is unlikely the mechanism associated with the observed antidiabetic property of raw cinnamon in this study. One common postulated mechanism of action of cinnamon is an increase in peripheral glucose uptake through increased insulin sensitivity [2, 15, 37, 38, 84, 104, 291, 329–349]. This explanation is based on data from in vitro studies using isolated cells, which showed that cinnamon induced changes in the expression of insulin-sensitive glucose transporter type 4 (GLUT-4), peroxisome proliferator-activated receptors (PPARs), glycogen-associated protein kinase B (Akt) signaling pathway and/or adenosine monophosphate-activated protein kinase (AMPK) [26, 35, 91, 142, 340, 347, 350–358]. However, a single oral dose of cinnamon given to rats [359, 360] and humans does not decrease steady blood glucose concentrations [124, 236], and there are no reports of hypoglycemia in humans with the use of any type of cinnamon [361], contrasting insulin sensitizers. Differently from cinnamon, a single dose of the following molecules and plants (known to act by increasing insulin sensitivity) cause a rapid drop in blood glucose level: pioglitazone [362–365]; metformin [142, 366–369]; glibenclamide [142, 370]; and extracts of Black tea (Camellia sinensis) and of Peniocereus greggii [142, 366]. The molecular changes involved in these hypoglycemic effects occur very rapidly [362, 363, 371, 372], for example, a large activation of AMPK in tissues of normal rats is observed 30 min after a single dose of thiazolidinedione [373]. Cinnamon also does not cause any acute change in the glycemic response induced by a glucose load during an OGTT in rats [374] and in mice [142]. In humans, several studies showed that the high iAUC elicited by the ingestion of glucose solution is not affected by ingestion of different cinnamon varieties: C. cassia [375–377], C. burmannii [186, 378], and C. verum [379]. In one of these studies, the intake of cinnamon by patients with T2DM, whose mean blood glucose peak reached 327 mg/dL (18.19 mmol/L), had no effect on their post-load hyperglycemia [378]. Only one research group observed a small attenuation (-7%) in the post-glucose load iAUC in humans caused by cinnamon intake [380, 381]. The absence of effect of cinnamon on the OGTT’s glycemic curves also refutes another proposed mechanism: the inhibition of intestinal glucose absorption transporters (SGLT1 and GLUT2) [2, 38, 290, 360, 382–384]. In contrast to cinnamon, a single dose of SGLT1-inhibiting drugs (e.g., canagliflozin) and extracts from plants such as Guava (Psidium Guajava) and Salvia polystachya clearly cause an acute suppression in the OGTT’s iAUC [385, 386]. Only in vitro experiments demonstrated that high concentrations of cinnamon could diminish the absorption of glucose [387–389]. These data support that a direct influence of cinnamon on tissue’s insulin sensitivity or on intestinal absorption is not responsible for its antidiabetic property.
To support the claim that the cinnamon’s antidiabetic effect comes from its ability to enhance insulin sensitivity, some authors cite the molecular modifications (e.g., GLUT-4, PPARs, Akt and AMPK) that are observed with the long-term consumption of this plant [47, 147, 350, 352, 360, 390–397]; however, these changes are the same indirect outcomes of other interventions lasting two or more weeks that reduce glucotoxicity through decreasing glucose excursions [126, 193, 264–266, 398–404] and calorie restriction [267, 405–408]. Besides increasing insulin sensitivity, the simple inhibition for some weeks of carbohydrate digestion in the gastrointestinal tract, without direct systemic actions [409–413], cause increase in AMPK level in tissues [267, 414] and GLUT4 protein and glucose transport [415]. These responses are in accordance with pleiotropic effects of suppressing post-meal glucose spikes [192, 267, 401, 413, 414, 416–426]. Moreover, the markers of insulin sensitivity after the beginning of cinnamon consumption increase only gradually [360, 427], showing the same time-dependent effect of inhibitors of carbohydrate digestion [261, 267, 423, 428–432]. Another distinguishing feature of cinnamon is that the administration of its extract for up to 15 weeks does not cause any effect in glucose tolerance and in GLUT4 level in normal mice and rats, differing from diabetic animals [350, 352, 392]. These long-term effects from cinnamon use contrast the rapid effects of insulin sensitizers and support the explanation that this herbal medicine promotes insulin sensitivity indirectly in a similar manner to compounds whose main property is to attenuate postprandial blood glucose response [265, 266, 398–401].
The absence of effect of cinnamon on glucose solution-induced hyperglycemia greatly differs from the robust decreases (21%-46%) in the glycemic responses observed in humans who had consumed complex carbohydrates in a balanced meal. A difference between these two conditions is that the glycemic response artificially elicited by a glucose solution skips the crucial step of digestion of complex carbohydrates of a normal diet [433–435], allowing the adequate evaluation of glucose metabolism without the strong influence of digestion in the postprandial hyperglycemia [399, 400, 436]. These contrasting results are the same observed with the intake of acarbose [142, 374], which inhibits carbohydrate-hydrolyzing enzymes (alpha-amylases and alpha-glucosidases) with minimal systemic absorption [409–413]; this antihyperglycemic agent does not cause any change in the glycemic response promoted by glucose solution, but greatly decreases the increase in glycemia induced by complex carbohydrates [366, 367, 437, 438]. This similarity indicates that the main mechanism by which cinnamon would exert an antidiabetic effect is by its acarbose-like property to inhibit the activity of carbohydrate-hydrolyzing enzymes.
Inhibitory activity on the carbohydrate-hydrolyzing enzymes
Several pieces of evidence point towards the inhibition of carbohydrate-hydrolyzing enzymes as the mechanism underlying the antihyperglycemic effect of cinnamon. The notorious suppression of meal-induced hyperglycemia (present study and other studies) is the same observed in oral sucrose/starch tolerance tests (OSTT). While the ingestion of cinnamon caused a strong decrease in the iAUC during OSTT experiments with rats [374] and mice [142], no change occurred in the iAUC during OGTT [142, 374]. In fact, all cinnamon varieties possess this property of inhibiting carbohydrate digestion [439]. Animal and in vitro studies have shown that cinnamon inhibits the activity of pancreatic amylase [140, 141, 170, 440–443], salivary amylase [444, 445] and intestinal glucosidases [138, 139, 141, 356, 374], more specifically of the disaccharidases sucrase and maltase [137, 374, 440, 446, 447]. Cinnamomum cassia and C. burmanii are more potent inhibitors of glucosidase activity than acarbose and C. zeylanicum [141], whose mode of inhibition is similar to acarbose [209, 374]. In line with this, cinnamon cassia generally exerts a more pronounced inhibition of carbohydrate digestion in vitro than other medicinal plants [198, 448–451]. Considering that cinnamon’s inhibitory properties are dose-dependent [137, 138, 351, 374, 441, 452], our finding that the glucose peak rise was inhibited by capsules containing raw cinnamon only the large amount of 6 g classified as a high dose (≥6 g/day) [84], but not by the medium dose of 3 g [15], corroborates these data and indicates that a high dose of encapsulated raw cinnamon cassia may be necessary to promote the effect of a medium dose mixed with water. Another finding from the present study that may help to understand the cinnamon’s antidiabetic mechanisms is that the degree of the raw cinnamon’s suppression of the post-meal hyperglycemia observed in our patients was similar to that seen in healthy individuals [131, 136] despite the fact that T2DM patients show lower amylase activity than healthy subjects [453–455]. This indicates that the antihyperglycemic effect of raw cinnamon in T2DM patients could be exerted mainly through the inhibition of alpha-glucosidase activity.
The inhibitory property of raw cinnamon is in agreement with the expected effect of its main constituent, insoluble fiber [130], which inhibits alpha-amylase and alpha-glycosidase in both forms, purified [456–458] or naturally in food [459–463]. This may explain the inverse relationship between the intake of insoluble fiber in the diet and the risk of diabetes [464] and metabolic syndrome [465]. The dose of insoluble fiber given in the present study through raw cinnamon (2.9 g) was sufficient to inhibit glycemic responses in T2DM patients, since it corresponds to more than half of the doses known to exert a post-meal antihyperglycemic effect in patients with T2DM [68, 466]. Studies on the effect of insoluble fiber often use wheat bran, which has insoluble fiber as its main constituent and a nutrient content that is very similar to cinnamon [460]. In agreement with this, the addition of insoluble fiber through wheat bran (a cinnamon-similar food) to the diet promoted glycemic control [461, 467], as well as in the form of whole grain and cereal fiber [459, 462, 468–470]. The actual comparison between the effects of isolated insoluble fiber (cellulose) and cinnamon has been performed in one study, where diabetes-related parameters were monitored for 12 weeks. Cellulose taken as one capsule of 700 mg each morning and another each evening promoted improvement on insulin sensitivity, contrasting the lack of significant effect of capsules containing 500 mg of cinnamon (~243 mg of insoluble fiber) plus 200 mg of cellulose (a total of ~443 mg of insoluble fiber). This result demonstrated that a more pronounced insulin sensitivity was achieved with the ingestion of the capsules containing a higher dose of insoluble fiber (700 mg vs ~443 mg) [153]. As other fibers, cellulose inhibits the activity of carbohydrate-hydrolyzing enzymes [456–458]. The contribution of other macronutrients to the anti-hyperglycemic effect of cinnamon is likely negligible given that the amounts of them are not enough to elicit changes in the postprandial glycemic response, such as protein [471, 472], fat [472, 473], and soluble fiber [474, 475]. The presence of higher amounts of macronutrients in other food that are also rich in insoluble fiber may explain why their lowering-effect of the postprandial glycemic response in T2DM patients is less impressive than cinnamon, e.g., a dose of raw flaxseed providing 3.5 g of insoluble fibre [476]. In conclusion, it is very plausible that the cinnamon’s inhibitory activity is mainly due to its exceptionally high content of insoluble fiber. If this is true, considering that the glycemic response to starch and sucrose is decreased also by cinnamon’s extract [142, 208, 374], which does not contain the bulk of its fiber content, the inhibitory activity should be exerted by some specific insoluble fibers. In fact, some types of insoluble fibers (hemicelluloses) show higher inhibitory activity on α-glucosidase/α-amylase when they are in a bound (feruloylated) form [477], which is the main raw wheat bran phenolic compound, found largely attached (90%) to arabinoxylans [478].
Alternatively, or in combination, there are other molecules or classes of molecules that might contribute to the effect of cinnamon in suppressing the post-meal hyperglycemic spikes in our T2DM patients. These included several volatile molecules and other compounds present in small quantities in the plant [479–482] that are known to inhibit carbohydrate-digesting enzymes, such as cinnamic acid [481, 483–485], cinnamaldehyde [290, 486, 487], procyanidins [139, 481, 488], proanthocyanidin [488–492], eugenol [156, 493–495], safrole [448, 496], coumarin [497–499], benzyl benzoate [482, 500–502], cadinene [503, 504], cinnamyl acetate [94, 482, 505], cinnamyl alcohol [481, 497, 506, 507], kaempferol [508–512], quercetin [343, 509, 513–517], quercetrin [389, 508, 518, 519], protocatechuic acid [520–522], vanillin [521, 523], gallic acid [521, 524, 525], β- and α-pinene [526, 527], chlorogenic acid [480, 528, 529], and benzaldehyde [482, 530–532]. However, the current data do not allow to affirm that the amount of these potential inhibitors in high doses of cinnamon (≥6 g) actually alter digestion and post-meal glycemic responses. Furthermore, some of these bioactive compounds are labile and may be oxidized and lose their functional properties when exposed to air [533], especially with a high surface area to volume ratio of powder preparations.
Implications
Our findings confirmed the postulates that raw cinnamon cassia inhibits post-meal glucose excursions not only within the harmless euglycemic range (up to ~140–160 mg/dL) but also in the deleterious hyperglycemic levels, reinforcing its potential use as a diabetes treatment strategy [534–536] by inhibiting carbohydrate digestion [344, 367, 400, 410, 438, 484, 490, 537–550]. We chose to use a meal with the healthiest proportion of macronutrients for patients with T2DM [121] in order to have methodological conditions mimicking a real-life context. Therefore, the antihyperglycemic effect observed in the present study could be directly applied in clinical practice. Furthermore, the ingestion of cinnamon dissolved in water did not change markers of taste intensity or palatability of a balanced meal, characteristics that facilitate acceptance of this food intervention and indicate that the consumption of cinnamon is a dietetic management executable by diabetic patients [551]. In addition, cinnamon powder at a dose of 3 g exerted a more pronounced effect in lowering the glucose peak rise than the same dose in capsules, corroborating the findings that capsules decrease the bioavailability of some molecules [299]. Therefore, raw cinnamon encapsulation is not necessary, which increases accessibility to the benefits of this spice and likely improves treatment adherence.
There are several advantages of the use of cinnamon for glycemic control. In addition to its recognized safety [83–85] and widespread use [84, 341, 552, 553], it does not cause flatulence [440, 554–556], diarrhea [381, 557, 558] or dyspepsia [559], as well as common adverse effects of antihyperglycemic drugs [22, 545, 560–562]. Due to its acarbose-like action, raw cinnamon may act as calorie restriction mimetics [536, 545, 563–565], what is in accordance with its effect in promoting loss of visceral fat, waist and weight [88, 131, 133, 135, 331, 342, 534, 566–572]. Moreover, the present findings support that the addition of a moderate dose of raw cinnamon cassia into the main meals is a small and easily applicable strategy that diabetic patients may implement to improve their glucose control, without drastically modifying diet or the food palatability. Still, care should be taken in such implementation, since our findings stem from the specific use of raw cinnamon.
Three decades ago, a study showed that “pressure cooking destroyed the amylase inhibitory activity in sorghum varieties” [169]. In general, heating raw vegetable-derived food by frying, baking and cooking/boiling blunts the inhibitory activity of insoluble fibers on carbohydrate-hydrolyzing enzymes [573]. For example, boiling (98°C) raw pumpkin leaves (Momordica balsamina L.) for only 15 minutes is enough to decrease their inhibitory action on α-amylase and α-glucosidase activities [574]. Similarly, the alpha-amylase inhibitory activity of the extract of commercial cinnamon prepared with boiling or decoction is greatly lower than extracts obtained by four other extraction methods [170, 575]. Part of this effect of heating is due to the decrease in the content of insoluble fiber [165], phenolic molecules [576–578] and volatile compounds [579], and the inactivation/denaturation of bioactive proteins in food [580–583]. Cooking also reduces the content of phytate [584–589], an antidiabetic nutraceutical that inhibits α-amylase that was classified as an antinutrient in the past [590–600]. As a consequence, the antihyperglycemic effect of different foods is suppressed when they are used as ingredient in recipes that involve cooking/boiling or baking [166, 169, 173, 583]. Recent data indicated that cinnamnon extracts prepared with longer exposition to heat cause lower decreases in blood glucose of diabetic animals than extracts made with shorter heating cycles [144]. For these reasons, our findings cannot be readily extrapolated for situations in which cinnamon is not used in its raw form.
In the long term, previous studies on the use of inhibitors of carbohydrate-hydrolyzing enzymes indicate that raw cinnamon might also enhance insulin sensitivity of T2DM patients [265, 398–400]. This outcome greatly depends on key variables and is expected to occur only if: (a) the timing of ingestion is synchronized with the main hyperglycemic meals and not with small snacks [436, 601, 602]; (b) the dose taken matches the amount of carbohydrate sufficiently to attenuate the post-meal glucotoxicity [266, 603]; (c) the ingestion regime decreases the mean amplitude of glycemic excursions (MAGE) [436]; and (d) duration of treatment should be long enough [15, 554, 604]. The lack of control for these factors might explain why some interventional studies promoted glycemic control [361, 568, 570, 605–620] while others did not [153, 379, 380, 555, 571, 621–627]. There are yet other knowledges gaps about the use of cinnamon with the aim to improve diabetes treatment. Long-term intervention trials with well-controlled variables could clarify questions [628, 629] such as the effect of splitting the daily dose of this herb with meals and of the precise time (minutes) prior a meal its intake is more efficient in inhibiting postprandial glucose excursion.
Limitations
The patients with T2DM evaluated in the present study did not show micro nor macrovascular complications and were not using exogenous insulin; therefore, the results may not be replicated in patients at further advanced stages of T2DM. Only male T2DM patients were included in the protocol to avoid alterations in the glucose metabolism caused by hormonal fluctuations [107] and a postulated abortifacient potential [630], which precludes stating that the effects observed in this work can be reproduced in the female population. Furthermore, we did not quantify the concentration of bioactive compounds in raw cinnamon cassia powder, which can vary greatly between different samples [156, 289, 505, 631, 632] and interferes with the ability to inhibit glucosidase [633]. We did not equalize the fiber content between the control and experimental groups, making plausible that the strong effect of raw cinnamon cassia on postprandial hyperglycemia in T2DM could be exerted mainly by its insoluble fibers and not by any bioactive molecules. Another aspect is that the use of cinnamon powder prevented us from doing a blind experiment that prevented the volunteers from knowing what they were ingesting. The very short-term exposition of our patients to cinnamon did not allow to evaluate whether plant-derived food would promote glycemic control by changing gut microbiota [634, 635]. Finally, our results may not be replicated with milk ingestion, as milk decreases the bioaccessibility of cinnamaldehyde [343] and cinnamon cassia has a low inhibitory effect on lactase [446].
Conclusion
The present study showed that the intake of a single dose of 3 g of raw cinnamon in the form of powder dissolved in water before a meal containing complex carbohydrates suppressed the glucose spike and the postprandial hyperglycemia in T2DM patients. The intake of raw cinnamon flattened the glycemic curve without changing the shape that is typical of T2DM patients. When the herb was taken in the form of capsules, these anti-hyperglycemic effects were lost or significantly diminished. The cinnamon’s anti-diabetic action confirms its acarbose-like property to inhibit the activities of the carbohydrate-digesting enzymes α-amylases/α-glucosidases, which is in accordance with its exceptionally high content of raw insoluble fiber. Our results reinforce the potential use of raw cinnamon as a diabetes treatment strategy by reducing postprandial hyperglycemia and the consequent and various complications of this condition.
Supporting information
Protocol of the study project approved by the ethics committee in original language.
(DOCX)
Protocol of the study project approved by the ethics committee translated into English.
(DOCX)
(TIF)
(DOCX)
Acknowledgments
We thank the local health system (SES/DF) for supporting research, and Dr. Gabriela Cristina de Souza Camargo (Instituto de Neurociências de Brasília–INCB) and Herbert Andreas Welker for suggestions and comments on the manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was partially supported by the Núcleo de Apoio à Pesquisa (NAP) of the Instituto Laboratório Sabin – Medicina Diagnóstica (https://www.sabin.com.br/envie-seu-projeto/; Award Number: 957963981-72; Recipient: F.D.M.). Sabin measured baseline levels of fasting blood glucose, insulin, HbA1c, HOMA-IR and HOMA-β of patients before experimental sessions, but did not have any additional role in other data collection and analysis, study design, decision to publish, or preparation of the manuscript. This study was also partially supported by Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF; https://www.fap.df.gov.br/; Award Number: 244/2022; Recipient: F.D.M.) by providing publication fee, but had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Furman BL, Candasamy M, Bhattamisra SK, Veettil SK. Reduction of blood glucose by plant extracts and their use in the treatment of diabetes mellitus; discrepancies in effectiveness between animal and human studies. J Ethnopharmacol. 2020;247: 112264. doi: 10.1016/j.jep.2019.112264 [DOI] [PubMed] [Google Scholar]
- 2.Przeor M. Some Common Medicinal Plants with Antidiabetic Activity, Known and Available in Europe (A Mini-Review). Pharmaceuticals (Basel). 2022;15. doi: 10.3390/ph15010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: Meta-analysis. J Med Food. 2011;14: 884–9. doi: 10.1089/jmf.2010.0180 [DOI] [PubMed] [Google Scholar]
- 4.Allen RW, Schwartzman E, Baker WL, Coleman CI, Phung OJ. Cinnamon use in type 2 diabetes: An updated systematic review and meta-analysis. Ann Fam Med. 2013;11: 452–9. doi: 10.1370/afm.1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwak JS, Park M young, Kwon O. Effect of cassia cinnamon intake on improvement of the glycemic response: An updated meta-analysis: Focus on preparation of dehydrated powder and water extract. J Nutr Heal. 2017;50: 437. doi: 10.4163/jnh.2017.50.5.437 [DOI] [Google Scholar]
- 6.Deyno S, Eneyew K, Seyfe S, Tuyiringire N, Peter EL, Muluye RA, et al. Efficacy and safety of cinnamon in type 2 diabetes mellitus and pre-diabetes patients: A meta-analysis and meta-regression. Diabetes Res Clin Pract. 2019;156: 107815. doi: 10.1016/j.diabres.2019.107815 [DOI] [PubMed] [Google Scholar]
- 7.Namazi N, Khodamoradi K, Khamechi SP, Heshmati J, Ayati MH, Larijani B. The impact of cinnamon on anthropometric indices and glycemic status in patients with type 2 diabetes: A systematic review and meta-analysis of clinical trials. Complement Ther Med. 2019;43: 92–101. doi: 10.1016/j.ctim.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 8.Silva AGS, Gonçalves LC, Silva PAN da, Carneiro LC, Sousa JAS e, Ataídes FS, et al. Lipid profile and blood glucose in patients with Diabetes Mellitus treated with Cinnamon ‐ Systematic review and meta-analysis with randomized clinical research. Res Soc Dev. 2021;10: e45910918203. doi: 10.33448/rsd-v10i9.18203 [DOI] [Google Scholar]
- 9.Hekmat-Ardakani A, Morshed-Behbahani B, Rahimi-Ardabili H, Ayati MH, Namazi N. The effects of dietary supplements and natural products targeting glucose levels: an overview. Crit Rev Food Sci Nutr. 2022; 1–30. doi: 10.1080/10408398.2022.2028716 [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Sharma SK, Mudgal SK, Gaur R, Agarwal R, Singh H, et al. Comparative effectiveness of six herbs in the management of glycemic status of type 2 diabetes mellitus patients: {A} systematic review and network meta-analysis of randomized controlled trials. Diabetes \& Metab Syndr. 2023;17: 102826. doi: 10.1016/j.dsx.2023.102826 [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q, Lei X, Fu S, Li Z, Chen Y, Long C, et al. Efficacy of cinnamon supplementation on glycolipid metabolism in T2DM diabetes: A meta-analysis and systematic review. Frontiers in physiology. Switzerland; 2022. p. 960580. doi: 10.3389/fphys.2022.960580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romeo GR, Lee J, Mulla CM, Noh Y, Holden C, Lee B-C. Influence of cinnamon on glycemic control in individuals with prediabetes: A randomized controlled trial. J Endocr Soc. 2020;4: bvaa094. doi: 10.1210/jendso/bvaa094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akilen R, Tsiami A, Devendra D, Robinson N. Cinnamon in glycaemic control: Systematic review and meta analysis. Clin Nutr. 2012;31: 609–15. doi: 10.1016/j.clnu.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 14.Zarezadeh M, Musazadeh V, Foroumandi E, Keramati M, Ostadrahimi A, Mekary RA. The effect of cinnamon supplementation on glycemic control in patients with type 2 diabetes or with polycystic ovary syndrome: an umbrella meta-analysis on interventional meta-analyses. Diabetol \& Metab Syndr. 2023;15: 127. doi: 10.1186/s13098-023-01057-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutbi EH, Sohouli MH, Fatahi S, Lari A, Shidfar F, Aljhdali MM, et al. The beneficial effects of cinnamon among patients with metabolic diseases: A systematic review and dose-response meta-analysis of randomized-controlled trials. Crit Rev Food Sci Nutr. 2022;62: 6113–6131. doi: 10.1080/10408398.2021.1896473 [DOI] [PubMed] [Google Scholar]
- 16.Moridpour AH, Kavyani Z, Khosravi S, Farmani E, Daneshvar M, Musazadeh V, et al. The effect of cinnamon supplementation on glycemic control in patients with type 2 diabetes mellitus: An updated systematic review and dose-response meta-analysis of randomized controlled trials. Phytother Res. 2024;38: 117–130. doi: 10.1002/ptr.8026 [DOI] [PubMed] [Google Scholar]
- 17.Kirkham S, Akilen R, Sharma S, Tsiami A. The potential of cinnamon to reduce blood glucose levels in patients with type 2 diabetes and insulin resistance. Diabetes Obes Metab. 2009;11: 1100–13. doi: 10.1111/j.1463-1326.2009.01094.x [DOI] [PubMed] [Google Scholar]
- 18.Suksomboon N, Poolsup N, Boonkaew S, Suthisisang CC. Meta-analysis of the effect of herbal supplement on glycemic control in type 2 diabetes. J Ethnopharmacol. 2011;137: 1328–33. doi: 10.1016/j.jep.2011.07.059 [DOI] [PubMed] [Google Scholar]
- 19.Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane database Syst Rev. 2012; CD007170. doi: 10.1002/14651858.CD007170.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costello RB, Dwyer JT, Saldanha L, Bailey RL, Merkel J, Wambogo E. Do cinnamon supplements have a role in glycemic control in type 2 diabetes? A narrative review. J Acad Nutr Diet. 2016;116: 1794–1802. doi: 10.1016/j.jand.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sierra-Puente D, Abadi-Alfie S, Arakanchi-Altaled K, Bogard-Brondo M, Garcia-Lascurain M, Gutierrez-Salmean G. Cinammon (Cinnamomum Spp.) and type 2 diabetes mellitus. Curr Top Nutraceutical Res. 2020;18: 247–255. doi: 10.37290/ctnr2641-452X.18:247–255 [DOI] [Google Scholar]
- 22.Atta M, Jafari S, Moore K. Complementary and alternative medicine: A review on the effects of ginger, cinnamon and camellia sinensis leaf tea in diabetes. J Diabetes Mellit. 2019;09: 126–136. doi: 10.4236/jdm.2019.93012 [DOI] [Google Scholar]
- 23.Papakonstantinou E, Xaidara M, Siopi V, Giannoglou M, Katsaros G, Theodorou G, et al. Effects of Spaghetti Differing in Soluble Fiber and Protein Content on Glycemic Responses in Humans: A Randomized Clinical Trial in Healthy Subjects. Int J Environ Res Public Health. 2022;19. doi: 10.3390/ijerph19053001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandal A, Sharma S, Rani R, Ranjan S, Kant R, Mirza A. Impact of Cassia Bark Consumption on Glucose and Lipid Control in Type 2 Diabetes: An Updated Systematic Review and Meta-Analysis. Cureus. 2021;13: e16376. doi: 10.7759/cureus.16376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu T, Lu K, Cao X, Xia H, Wang S, Sun G, et al. The {Effect} of {Cinnamon} on {Glycolipid} {Metabolism}: {A} {Dose}-{Response} {Meta}-{Analysis} of {Randomized} {Controlled} {Trials}. Nutrients. 2023;15. doi: 10.3390/nu15132983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khaafi M, Tayarani-Najaran Z, Javadi B. Cinnamaldehyde as a promising dietary phytochemical against metabolic syndrome: {A} systematic review. Mini Rev Med Chem. 2023. doi: 10.2174/1389557523666230725113446 [DOI] [PubMed] [Google Scholar]
- 27.Brainina K, Stozhko N, Vidrevich M. Antioxidants: Terminology, Methods, and Future Considerations. Antioxidants (Basel, Switzerland). 2019;8. doi: 10.3390/antiox8080297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reis CEG. Discussion of “Whey protein supplementation and its potentially adverse effects on health: a systematic review” ‐ Unsubstantiated claims of adverse effects of whey protein supplementation on human kidney and liver function. Appl Physiol Nutr Metab = Physiol Appl Nutr Metab. 2021;46: 90–91. doi: 10.1139/apnm-2020-0674 [DOI] [PubMed] [Google Scholar]
- 29.Heinrich M, Appendino G, Efferth T, Fürst R, Izzo AA, Kayser O, et al. Best practice in research–Overcoming common challenges in phytopharmacological research. J Ethnopharmacol. 2020;246: 112230. doi: 10.1016/j.jep.2019.112230 [DOI] [PubMed] [Google Scholar]
- 30.Krawczyk M, Burzynska-Pedziwiatr I, Wozniak LA, Bukowiecka-Matusiak M. Evidence from a Systematic Review and Meta-Analysis Pointing to the Antidiabetic Effect of Polyphenol-Rich Plant Extracts from Gymnema montanum, Momordica charantia and Moringa oleifera. Curr Issues Mol Biol. 2022;44: 699–717. doi: 10.3390/cimb44020049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan S-P, Tan EN-Y, Lim Q-Y, Nafiah MA. Phyllanthus acidus (L.) Skeels: A review of its traditional uses, phytochemistry, and pharmacological properties. J Ethnopharmacol. 2020;253: 112610. doi: 10.1016/j.jep.2020.112610 [DOI] [PubMed] [Google Scholar]
- 32.Piotrowicz Z, Tabisz Ł, Waligórska M, Pankiewicz R, Łęska B. Phenol-rich alternatives for Rosa x damascena Mill. Efficient phytochemical profiling using different extraction methods and colorimetric assays. Sci Rep. 2021;11: 23883. doi: 10.1038/s41598-021-03337-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dirar AI, Devkota HP. Ethnopharmacological uses, phytochemistry and pharmacological activities of Guiera senegalensis J.F. Gmel. (Combretaceae). J Ethnopharmacol. 2021;267: 113433. doi: 10.1016/j.jep.2020.113433 [DOI] [PubMed] [Google Scholar]
- 34.Tomou E-M, Lytra K, Rallis S, Tzakos AG, Skaltsa H. An updated review of genus Cistus L. since 2014: traditional uses, phytochemistry, and pharmacological properties. Phytochem Rev. 2022;21: 2049–2087. doi: 10.1007/s11101-022-09827-y [DOI] [Google Scholar]
- 35.Silva ML, Bernardo MA, Singh J, de Mesquita MF. Cinnamon as a Complementary Therapeutic Approach for Dysglycemia and Dyslipidemia Control in Type 2 Diabetes Mellitus and Its Molecular Mechanism of Action: A Review. Nutrients. 2022;14. doi: 10.3390/nu14132773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barreto G, Loureiro LMR, Reis CEG, Saunders B. Effects of caffeine chewing gum supplementation on exercise performance: A systematic review and meta-analysis. Eur J Sport Sci. 2022; 1–12. doi: 10.1080/17461391.2022.2049885 [DOI] [PubMed] [Google Scholar]
- 37.Wijenayaka G, Bulugahapitiya VP, Jayasinghe S. Cinnamon, a promising herbal plant for combatting diabetes and Its anti-diabetes mechanisms. Ceylon J Sci. 2022;51: 335–346. doi: 10.4038/cjs.v51i4.8051 [DOI] [Google Scholar]
- 38.Nuffer W, Tall Bull S, Bakhach H, Nuffer M. Sweetly Improving Sugars? Reviewing Cinnamon’s Effects on Blood Glucose. J Med Food. 2022. doi: 10.1089/jmf.2022.0073 [DOI] [PubMed] [Google Scholar]
- 39.Otunola GA. Culinary Spices in Food and Medicine: An Overview of Syzygium aromaticum (L.) Merr. and L. M. Perry [Myrtaceae]. Front Pharmacol. 2022;12. doi: 10.3389/fphar.2021.793200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heinrich M, Jalil B, Abdel-Tawab M, Echeverria J, Kulić Ž, McGaw LJ, et al. Best Practice in the chemical characterisation of extracts used in pharmacological and toxicological research-The ConPhyMP-Guidelines. Front Pharmacol. 2022;13: 953205. doi: 10.3389/fphar.2022.953205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmad R, AlLehaibi LH, AlSuwaidan HN, Alghiryafi AF, Almubarak LS, AlKhalifah KN, et al. Evaluation of clinical trials for natural products used in diabetes: An evidence-based systemic literature review. Medicine (Baltimore). 2021;100: e25641. doi: 10.1097/MD.0000000000025641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jang H-H, Lee J, Lee S-H, Lee Y-M. Effects of Capsicum annuum supplementation on the components of metabolic syndrome: a systematic review and meta-analysis. Sci Rep. 2020;10: 1–11. doi: 10.1038/s41598-020-77983-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butkevičiūtė A, Liaudanskas M, Ramanauskienė K, Janulis V. Biopharmaceutical Evaluation of Capsules with Lyophilized Apple Powder. Molecules. 2021;26. doi: 10.3390/molecules26041095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jezerska L, Prokes R, Gelnar D, Zegzulka J. Hard gelatine capsules: DEM supported experimental study of particle arrangement effect on properties and vibrational transport behaviour. Powder Technol. 2022; 117525. doi: 10.1016/j.powtec.2022.117525 [DOI] [Google Scholar]
- 45.Mudrić J, Arsenijević J, Maksimović Z, Ibrić S, Gopčević K, Đuriš J. Tablet and capsule formulations incorporating high doses of a dry optimized herbal extract: The case of Satureja kitaibelii. J Drug Deliv Sci Technol. 2021;66: 102776. doi: 10.1016/j.jddst.2021.102776 [DOI] [Google Scholar]
- 46.Hameed A, Adamska-Patruno E, Godzien J, Czajkowski P, Miksza U, Pietrowska K, et al. The Beneficial Effect of Cinnamon and Red Capsicum Intake on Postprandial Changes in Plasma Metabolites Evoked by a High-Carbohydrate Meal in Men with Overweight/Obesity. Nutrients. 2022;14. doi: 10.3390/nu14204305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandal A, Sharma SK, Yadav SRM, Mirza AA, Thakur MS, Jachak S, et al. Efficacy of {Young} {Cinnamomum} zeylanicum {Blume} {Bark} on {Hyperglycemia} and {PTPase} {Activity} in {Type} 2 {Diabetes}. Cureus. 2023;15: e35023. doi: 10.7759/cureus.35023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agrosi M, Mischiatti S, Harrasser PC, Savio D. Oral bioavailability of active principles from herbal products in humans. A study on Hypericum perforatum extracts using the soft gelatin capsule technology. Phytomedicine. 2000;7: 455–462. doi: 10.1016/S0944-7113(00)80029-X [DOI] [PubMed] [Google Scholar]
- 49.Lown M, Fuller R, Lightowler H, Fraser A, Gallagher A, Stuart B, et al. Mulberry-extract improves glucose tolerance and decreases insulin concentrations in normoglycaemic adults: Results of a randomised double-blind placebo-controlled study. PLoS One. 2017;12: e0172239. doi: 10.1371/journal.pone.0172239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamimori GH, Karyekar CS, Otterstetter R, Cox DS, Balkin TJ, Belenky GL, et al. The rate of absorption and relative bioavailability of caffeine administered in chewing gum versus capsules to normal healthy volunteers. Int J Pharm. 2002;234: 159–167. doi: 10.1016/s0378-5173(01)00958-9 [DOI] [PubMed] [Google Scholar]
- 51.Henning SM, Niu Y, Lee NH, Thames GD, Minutti RR, Wang H, et al. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am J Clin Nutr. 2004;80: 1558–1564. doi: 10.1093/ajcn/80.6.1558 [DOI] [PubMed] [Google Scholar]
- 52.Silva Figueiredo P, Inada AC, Ribeiro Fernandes M, Granja Arakaki D, Freitas K de C, Avellaneda Guimarães R de C, et al. An Overview of Novel Dietary Supplements and Food Ingredients in Patients with Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease. Molecules. 2018;23. doi: 10.3390/molecules23040877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, et al. Scientific opinion on the safety of green tea catechins. EFSA journal Eur Food Saf Auth. 2018;16: e05239. doi: 10.2903/j.efsa.2018.5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brand-Miller JC, Atkinson FS, Gahler RJ, Kacinik V, Lyon MR, Wood S. Effects of PGX, a novel functional fibre, on acute and delayed postprandial glycaemia. Eur J Clin Nutr. 2010;64: 1488–1493. doi: 10.1038/ejcn.2010.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adefegha SA, Olasehinde TA, Oboh G. Pasting alters glycemic index, antioxidant activities, and starch-hydrolyzing enzyme inhibitory properties of whole wheat flour. Food Sci Nutr. 2018;6: 1591–1600. doi: 10.1002/fsn3.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gélinas P, McKinnon C, Gagnon F. Inhibitory activity towards human α-amylase in cereal foods. LWT. 2018;93: 268–273. doi: 10.1016/j.lwt.2018.03.049 [DOI] [Google Scholar]
- 57.Jimenez-Garcia SN, Vazquez-Cruz MA, Ramirez-Gomez XS, Beltran-Campos V, Contreras-Medina LM, Garcia-Trejo JF, et al. Changes in the Content of Phenolic Compounds and Biological Activity in Traditional Mexican Herbal Infusions with Different Drying Methods. Molecules. 2020;25. doi: 10.3390/molecules25071601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu W, Yang Z, Shi L, Cui Z, Li Y. Degradation of β-Carbolines Harman and Norharman in Edible Oils during Heating. Molecules. 2021;26. doi: 10.3390/molecules26227018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res. 1999;443: 139–147. doi: 10.1016/s1383-5742(99)00016-2 [DOI] [PubMed] [Google Scholar]
- 60.de Vos RH, van Dokkum W, Schouten A, de Jong-Berkhout P. Polycyclic aromatic hydrocarbons in Dutch total diet samples (1984–1986). Food Chem Toxicol an Int J Publ Br Ind Biol Res Assoc. 1990;28: 263–268. doi: 10.1016/0278-6915(90)90038-o [DOI] [PubMed] [Google Scholar]
- 61.Ko J-H, Das G, Kim J-E, Shin H-S. Study on formation of nitrated polycyclic aromatic hydrocarbons from different roasting condition in coffee. J Food Sci Technol. 2018;55: 3991–4000. doi: 10.1007/s13197-018-3324-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.See SW, Balasubramanian R. Chemical characteristics of fine particles emitted from different gas cooking methods. Atmos Environ. 2008;42: 8852–8862. doi: 10.1016/j.atmosenv.2008.09.011 [DOI] [Google Scholar]
- 63.Jimenez A, Adisa A, Woodham C, Saleh M. Determination of polycyclic aromatic hydrocarbons in roasted coffee. J Environ Sci Heal Part B, Pestic food Contam Agric wastes. 2014;49: 828–835. doi: 10.1080/03601234.2014.938552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pedreschi F. Frying of Potatoes: Physical, Chemical, and Microstructural Changes. Dry Technol. 2012;30: 707–725. doi: 10.1080/07373937.2012.663845 [DOI] [Google Scholar]
- 65.Manig F, Hellwig M, Pietz F, Henle T. Quantitation of free glycation compounds in saliva. PLoS One. 2019;14: e0220208. doi: 10.1371/journal.pone.0220208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110: 911–16.e12. doi: 10.1016/j.jada.2010.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Twarda-Clapa A, Olczak A, Białkowska AM, Koziołkiewicz M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells. 2022;11. doi: 10.3390/cells11081312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leone A, Bertoli S, Di Lello S, Bassoli A, Ravasenghi S, Borgonovo G, et al. Effect of Moringa oleifera Leaf Powder on Postprandial Blood Glucose Response: In Vivo Study on Saharawi People Living in Refugee Camps. Nutrients. 2018;10. doi: 10.3390/nu10101494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang Y, Cui S, Zhang R, Zhao XX, Yao L, OuYang R, et al. Shift of Glucose Peak Time During Oral Glucose Tolerance Test is Associated with Changes in Insulin Secretion and Insulin Sensitivity After Therapy with Antidiabetic Drugs in Patients with Type 2 Diabetes. Diabetes Ther Res Treat Educ diabetes Relat Disord. 2021;12: 2437–2450. doi: 10.1007/s13300-021-01107-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.La Grasta Sabolić L, Požgaj Šepec M, Cigrovski Berković M, Stipančić G. Time to the Peak, Shape of the Curve and Combination of These Glucose Response Characteristics During Oral Glucose Tolerance Test as Indicators of Early Beta-cell Dysfunction in Obese Adolescents. J Clin Res Pediatr Endocrinol. 2021;13: 160–169. doi: 10.4274/jcrpe.galenos.2020.2020.0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang X, Zhao X, Zhou R, Gu Y, Zhu X, Tang Z, et al. Delay in glucose peak time during the oral glucose tolerance test as an indicator of insulin resistance and insulin secretion in type 2 diabetes patients. J Diabetes Investig. 2018;9: 1288–1295. doi: 10.1111/jdi.12834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57: 1349–1354. doi: 10.2337/db08-0063 [DOI] [PubMed] [Google Scholar]
- 73.Ceriello A, Esposito K, Piconi L, Ihnat M, Thorpe J, Testa R, et al. Glucose “peak” and glucose “spike”: Impact on endothelial function and oxidative stress. Diabetes Res Clin Pract. 2008;82: 262–267. doi: 10.1016/j.diabres.2008.07.015 [DOI] [PubMed] [Google Scholar]
- 74.Chattopadhyay S, George A, John J, Sathyapalan T. Postload glucose spike but not fasting glucose determines prognosis after myocardial infarction in patients without known or newly diagnosed diabetes. J Diabetes. 2021;13: 191–199. doi: 10.1111/1753-0407.13111 [DOI] [PubMed] [Google Scholar]
- 75.Kim JY, Tfayli H, Bacha F, Lee S, Michaliszyn SF, Yousuf S, et al. β-cell function, incretin response, and insulin sensitivity of glucose and fat metabolism in obese youth: Relationship to OGTT-time-to-glucose-peak. Pediatr Diabetes. 2020;21: 18–27. doi: 10.1111/pedi.12940 [DOI] [PubMed] [Google Scholar]
- 76.Lim WXJ, Chepulis L, von Hurst P, Gammon CS, Page RA. An Acute, Placebo-Controlled, Single-Blind, Crossover, Dose-Response, Exploratory Study to Assess the Effects of New Zealand Pine Bark Extract (Enzogenol(®)) on Glycaemic Responses in Healthy Participants. Nutrients. 2020;12. doi: 10.3390/nu12020497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Obura M, Beulens JWJ, Slieker R, Koopman ADM, Hoekstra T, Nijpels G, et al. Post-load glucose subgroups and associated metabolic traits in individuals with type 2 diabetes: An IMI-DIRECT study. PLoS One. 2020;15: e0242360. doi: 10.1371/journal.pone.0242360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blahova J, Martiniakova M, Babikova M, Kovacova V, Mondockova V, Omelka R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals (Basel). 2021;14. doi: 10.3390/ph14080806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chattopadhyay K, Wang H, Kaur J, Nalbant G, Almaqhawi A, Kundakci B, et al. Effectiveness and Safety of Ayurvedic Medicines in Type 2 Diabetes Mellitus Management: A Systematic Review and Meta-Analysis. Frontiers in pharmacology. Switzerland; 2022. p. 821810. doi: 10.3389/fphar.2022.821810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rifai N, Annesley TM, Berg JP, Brugnara C, Delvin E, Lamb EJ, et al. An appeal to medical journal editors: the need for a full description of laboratory methods and specimen handling in clinical study reports. Clinical chemistry and laboratory medicine. Germany; 2012. pp. 411–413. doi: 10.1515/cclm-2011-0904 [DOI] [PubMed] [Google Scholar]
- 81.Schroter S, Black N, Evans S, Godlee F, Osorio L, Smith R. What errors do peer reviewers detect, and does training improve their ability to detect them? J R Soc Med. 2008;101: 507–514. doi: 10.1258/jrsm.2008.080062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sebai H, Rtibi K, Selmi S, Jridi M, Balti R, Marzouki L. Modulating and opposite actions of two aqueous extracts prepared from Cinnamomum cassia L. bark and Quercus ilex L. on the gastrointestinal tract in rats. RSC Adv. 2019;9: 21695–21706. doi: 10.1039/c9ra02429h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gu D-T, Tung T-H, Jiesisibieke ZL, Chien C-W, Liu W-Y. Safety of Cinnamon: An Umbrella Review of Meta-Analyses and Systematic Reviews of Randomized Clinical Trials. Front Pharmacol. 2021;12: 790901. doi: 10.3389/fphar.2021.790901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharifi-Rad J, Dey A, Koirala N, Shaheen S, El Omari N, Salehi B, et al. Cinnamomum Species: Bridging Phytochemistry Knowledge, Pharmacological Properties and Toxicological Safety for Health Benefits. Front Pharmacol. 2021;12: 600139. doi: 10.3389/fphar.2021.600139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mousavi SM, Jayedi A, Bagheri A, Zargarzadeh N, Wong A, Persad E, et al. What is the influence of cinnamon supplementation on liver enzymes? A systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2021;35: 5634–5646. doi: 10.1002/ptr.7200 [DOI] [PubMed] [Google Scholar]
- 86.Nguyen L, Duong LT, Mentreddy RS. The U.S. import demand for spices and herbs by differentiated sources. J Appl Res Med Aromat Plants. 2019;12: 13–20. doi: 10.1016/j.jarmap.2018.12.001 [DOI] [Google Scholar]
- 87.Ali A, Wu H, Ponnampalam EN, Cottrell JJ, Dunshea FR, Suleria HAR. Comprehensive Profiling of Most Widely Used Spices for Their Phenolic Compounds through LC-ESI-QTOF-MS(2) and Their Antioxidant Potential. Antioxidants (Basel, Switzerland). 2021;10. doi: 10.3390/antiox10050721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deekshith C, Jois M, Radcliffe J, Thomas J. Effects of culinary herbs and spices on obesity: A systematic literature review of clinical trials. J Funct Foods. 2021;81: 104449. doi: 10.1016/j.jff.2021.104449 [DOI] [Google Scholar]
- 89.Javaid R, Javed G, Aslam M, Siddiqui A, Javaid R, Ahmed F. Darchini (Cinnamomum Zeylanicum J. Presl)-A Potent Unani Herb With Its Descriptive Parameters Of Pharmacognosy And Pharmacology-A Review. J Pharm Negat Results. 2022; 4138–4147. doi: 10.47750/pnr.2022.13.S09.513 [DOI] [Google Scholar]
- 90.Liu Y, An T, Wan D, Yu B, Fan Y, Pei X. Targets and Mechanism Used by Cinnamaldehyde, the Main Active Ingredient in Cinnamon, in the Treatment of Breast Cancer. Frontiers in pharmacology. Beijing University of Chinese Medicine Third Affiliated Hospital, Beijing, China.; 2020. p. 582719. doi: 10.3389/fphar.2020.582719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kawatra P, Rajagopalan R. Cinnamon: Mystic powers of a minute ingredient. Pharmacognosy Res. 2015;7: S1–6. doi: 10.4103/0974-8490.157990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meena AK, Narasimhaji C V, Rekha P, Velvizhi D, Ilavarasan R. Comparative Preliminary Phytochemical and HPTLC Fingerprint profile Studies of two Cinnamon Species Commonly used in ASU Formulations. Asian J Res Chem. 2018;11: 344–350. doi: 10.5958/0974-4150.2018.00062.7 [DOI] [Google Scholar]
- 93.Ranasinghe P, Galappaththy P. Health benefits of Ceylon cinnamon (Cinnamomum zeylanicum): a summary of the current evidence. Ceylon Med J. 2016;61: 1–5. doi: 10.4038/cmj.v61i1.8251 [DOI] [PubMed] [Google Scholar]
- 94.Tepe AS, Ozaslan M. Anti-Alzheimer, anti-diabetic, skin-whitening, and antioxidant activities of the essential oil of Cinnamomum zeylanicum. Ind Crops Prod. 2020;145: 112069. doi: 10.1016/j.indcrop.2019.112069 [DOI] [Google Scholar]
- 95.Liu J, Feng W, Peng C. A Song of Ice and Fire: Cold and Hot Properties of Traditional Chinese Medicines. Front Pharmacol. 2020;11: 598744. doi: 10.3389/fphar.2020.598744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pereira ASP, Banegas-Luna AJ, Peña-García J, Pérez-Sánchez H, Apostolides Z. Evaluation of the Anti-Diabetic Activity of Some Common Herbs and Spices: Providing New Insights with Inverse Virtual Screening. Molecules. 2019;24. doi: 10.3390/molecules24224030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iwata N, Kainuma M, Kobayashi D, Kubota T, Sugawara N, Uchida A, et al. The Relation between Hepatotoxicity and the Total Coumarin Intake from Traditional Japanese Medicines Containing Cinnamon Bark. Front Pharmacol. 2016;7: 174. doi: 10.3389/fphar.2016.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takayama S, Arita R, Kikuchi A, Ohsawa M, Kaneko S, Ishii T. Clinical Practice Guidelines and Evidence for the Efficacy of Traditional Japanese Herbal Medicine (Kampo) in Treating Geriatric Patients. Front Nutr. 2018;5: 66. doi: 10.3389/fnut.2018.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hosogi S, Ohsawa M, Kato I, Kuwahara A, Inui T, Inui A, et al. Improvement of Diabetes Mellitus Symptoms by Intake of Ninjin’yoeito. Front Nutr. 2018;5: 112. doi: 10.3389/fnut.2018.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miyano K, Nonaka M, Uzu M, Ohshima K, Uezono Y. Multifunctional Actions of Ninjinyoeito, a Japanese Kampo Medicine: Accumulated Scientific Evidence Based on Experiments With Cells and Animal Models, and Clinical Studies. Front Nutr. 2018;5: 93. doi: 10.3389/fnut.2018.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rakhi NK, Tuwani R, Mukherjee J, Bagler G. Data-driven analysis of biomedical literature suggests broad-spectrum benefits of culinary herbs and spices. PLoS One. 2018;13: e0198030. doi: 10.1371/journal.pone.0198030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alzahrani AS, Price MJ, Greenfield SM, Paudyal V. Global prevalence and types of complementary and alternative medicines use amongst adults with diabetes: systematic review and meta-analysis. Eur J Clin Pharmacol. 2021;77: 1259–1274. doi: 10.1007/s00228-021-03097-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mackonochie M, Rodriguez-Mateos A, Mills S, Rolfe V. A Scoping Review of the Clinical Evidence for the Health Benefits of Culinary Doses of Herbs and Spices for the Prevention and Treatment of Metabolic Syndrome. Nutrients. 2023;15. doi: 10.3390/nu15234867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stevens N, Allred K. Antidiabetic Potential of Volatile Cinnamon Oil: A Review and Exploration of Mechanisms Using In Silico Molecular Docking Simulations. Molecules. 2022;27. doi: 10.3390/molecules27030853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Skurk Anja; Grünerbel, Arthur; Kabisch, Stefan; Keuthage, Winfried; Kronsbein, Peter; Müssig, Karsten; et al. Dietary recommendations for persons with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2022;130: S151–S184. doi: 10.1055/a-1624-5095 [DOI] [PubMed]
- 106.American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45: S17–S38. doi: 10.2337/dc22-S002 [DOI] [PubMed] [Google Scholar]
- 107.Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav. 2018;187: 20–23. doi: 10.1016/j.physbeh.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moore JM, Vinoskey C, Salmons H, Hooshmand S, Kressler J. Sex differences in the acute effect of stair-climbing on postprandial blood glucose levels: A randomized controlled trial. Metab open. 2022;15: 100200. doi: 10.1016/j.metop.2022.100200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Biradar RA, Singh DP, Thakur H, Halli SS. Gender differences in the risk factors for high and very high blood glucose levels: A study of Kerala. Diabetes Metab Syndr. 2020;14: 627–636. doi: 10.1016/j.dsx.2020.05.001 [DOI] [PubMed] [Google Scholar]
- 110.Monnier L, Colette C, Owens D. Application of medium-term metrics for assessing glucose homoeostasis: Usefulness, strengths and weaknesses. Diabetes Metab. 2021;47: 101173. doi: 10.1016/j.diabet.2020.06.004 [DOI] [PubMed] [Google Scholar]
- 111.Pazos-Couselo M, Portos-Regueiro C, González-Rodríguez M, Manuel García-Lopez J, Alonso-Sampredro M, Rodríguez-González R, et al. Aging of glucose profiles in an adult population without diabetes. Diabetes Res Clin Pract. 2022;188: 109929. doi: 10.1016/j.diabres.2022.109929 [DOI] [PubMed] [Google Scholar]
- 112.Nakayama Y, Ono K, Okagawa J, Urabe J, Yamau R, Ishikawa A. Home-Based High-Intensity Interval Exercise Improves the Postprandial Glucose Response in Young Adults with Postprandial Hyperglycemia. Int J Environ Res Public Health. 2022;19. doi: 10.3390/ijerph19074227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saito Y, Kajiyama S, Nitta A, Miyawaki T, Matsumoto S, Ozasa N, et al. Eating Fast Has a Significant Impact on Glycemic Excursion in Healthy Women: Randomized Controlled Cross-Over Trial. Nutrients. 2020;12. doi: 10.3390/nu12092767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Serdar CC, Cihan M, Yücel D, Serdar MA. Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem medica. 2021;31: 10502. doi: 10.11613/BM.2021.010502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kang H. Sample size determination and power analysis using the G*Power software. J Educ Eval Health Prof. 2021;18: 17. doi: 10.3352/jeehp.2021.18.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.de Carvalho CM, de Paula TP, Viana L V, Machado VM, de Almeida JC, Azevedo MJ. Plasma glucose and insulin responses after consumption of breakfasts with different sources of soluble fiber in type 2 diabetes patients: A randomized crossover clinical trial. Am J Clin Nutr. 2017; ajcn157263. doi: 10.3945/ajcn.117.157263 [DOI] [PubMed] [Google Scholar]
- 117.Gilani M, Aamir M, Akram A, Haroon ZH, Ijaz A, Khadim MT. Comparison of turbidimetric inhibition immunoassay, high-performance liquid chromatography, and capillary electrophoresis methods for glycated hemoglobin determination. Lab Med. 2020;51: 579–584. doi: 10.1093/labmed/lmaa010 [DOI] [PubMed] [Google Scholar]
- 118.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28: 412–9. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 119.Kasujja FX, Mayega RW, Daivadanam M, Kiracho EE, Kusolo R, Nuwaha F. Glycated haemoglobin and fasting plasma glucose tests in the screening of outpatients for diabetes and abnormal glucose regulation in Uganda: A diagnostic accuracy study. PLoS One. 2022;17: e0272515. doi: 10.1371/journal.pone.0272515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Song DK, Hong YS, Sung Y-A, Lee H. Waist circumference and mortality or cardiovascular events in a general Korean population. PLoS One. 2022;17: e0267597. doi: 10.1371/journal.pone.0267597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Department of Agriculture U.S. and Department of Health U.S. and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Ed. 2020. [Google Scholar]
- 122.Barr SI, Vatanparast H, Smith J. Breakfast in Canada: Prevalence of Consumption, Contribution to Nutrient and Food Group Intakes, and Variability across Tertiles of Daily Diet Quality. A Study from the International Breakfast Research Initiative. Nutrients. 2018;10. doi: 10.3390/nu10080985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hlebowicz J, Darwiche G, Björgell O, Almér L-O. Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. Am J Clin Nutr. 2007;85: 1552–6. doi: 10.1093/ajcn/85.6.1552 [DOI] [PubMed] [Google Scholar]
- 124.Hlebowicz J, Hlebowicz A, Lindstedt S, Björgell O, Höglund P, Holst JJ, et al. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. Am J Clin Nutr. 2009;89: 815–21. doi: 10.3945/ajcn.2008.26807 [DOI] [PubMed] [Google Scholar]
- 125.Markey O, McClean CM, Medlow P, Davison GW, Trinick TR, Duly E, et al. Effect of cinnamon on gastric emptying, arterial stiffness, postprandial lipemia, glycemia, and appetite responses to high-fat breakfast. Cardiovasc Diabetol. 2011;10: 78. doi: 10.1186/1475-2840-10-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Z, Wang J, Hu J, Chen Y, Dong B, Wang Y. A comparative study of acarbose, vildagliptin and saxagliptin intended for better efficacy and safety on type 2 diabetes mellitus treatment. Life Sci. 2021;274: 119069. doi: 10.1016/j.lfs.2021.119069 [DOI] [PubMed] [Google Scholar]
- 127.Chen P, Sun J, Ford P. Differentiation of the four major species of cinnamons (C. burmannii, C. verum, C. cassia, and C. loureiroi) using a flow injection mass spectrometric (FIMS) fingerprinting method. J Agric Food Chem. 2014;62: 2516–2521. doi: 10.1021/jf405580c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fauzi MARD, Pudjiastuti P, Wibowo AC, Hendradi E. Preparation, Properties and Potential of Carrageenan-Based Hard Capsules for Replacing Gelatine: A Review. Polymers (Basel). 2021;13. doi: 10.3390/polym13162666 [DOI] [PMC free article] [PubMed]
- 129.Rump A, Weiss FN, Schulz L, Kromrey M-L, Scheuch E, Tzvetkov M V, et al. The Effect of Capsule-in-Capsule Combinations on In Vivo Disintegration in Human Volunteers: A Combined Imaging and Salivary Tracer Study. Pharmaceutics. 2021;13. doi: 10.3390/pharmaceutics13122002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Araújo EM de, Menezes HC de, Tomazini JM. Fibras solúveis e insolúveis de verduras, tubérculos e canela para uso em nutrição clínica. Ciência e Tecnol Aliment. 2009;29: 401–406. doi: 10.1590/S0101-20612009000200027 [DOI] [Google Scholar]
- 131.Aldayel TS. Health benefits of cinnamon supplement: in vitro and in vivo. Ph.D. Dissertation. Park SF, Gallagher AM, Brown JE, Lanham-New SA, Surrey U of, editors. University of Surrey. 2016. Available: https://openresearch.surrey.ac.uk/esploro/outputs/doctoral/Health-benefits-of-cinnamon-supplement—in-vitro-and-in-vivo/99516070802346
- 132.Moncada MM, Bernardo MA, Silva ML, Jorge A, Pereira P, Brito J, et al. Effect of cinnamon powder addition to a Portuguese custard tart (Pastel de Nata) on healthy adults’ postprandial glycemia. World Heart J. 2017;9. [Google Scholar]
- 133.Santos HO, da Silva GAR. To what extent does cinnamon administration improve the glycemic and lipid profiles? Clin Nutr ESPEN. 2018;27: 1–9. doi: 10.1016/j.clnesp.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 134.De Silva DAM, Jeewanthi RKC, Rajapaksha RHN, Weddagala WMTB, Hirotsu N, Shimizu B-I, et al. Clean vs dirty labels: Transparency and authenticity of the labels of Ceylon cinnamon. PloS one. Department of Agribusiness Management, Faculty of Agricultural Sciences, Sabaragamuwa University of Sri Lanka, Belihuloya, Sabaragamuwa Province, Sri Lanka.; 2021. p. e0260474. doi: 10.1371/journal.pone.0260474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mousavi SM, Rahmani J, Kord-Varkaneh H, Sheikhi A, Larijani B, Esmaillzadeh A. Cinnamon supplementation positively affects obesity: A systematic review and dose-response meta-analysis of randomized controlled trials. Clin Nutr. 2020;39: 123–133. doi: 10.1016/j.clnu.2019.02.017 [DOI] [PubMed] [Google Scholar]
- 136.Magistrelli A, Chezem JC. Effect of ground cinnamon on postprandial blood glucose concentration in normal-weight and obese adults. J Acad Nutr Diet. 2012;112: 1806–9. doi: 10.1016/j.jand.2012.07.037 [DOI] [PubMed] [Google Scholar]
- 137.Kang BH, Racicot K, Pilkenton SJ, Apostolidis E. Evaluation of the In vitro Anti-hyperglycemic Effect of Cinnamomum cassia Derived Phenolic Phytochemicals, via Carbohydrate Hydrolyzing Enzyme Inhibition. Plant Foods Hum Nutr. 2014;69: 155–160. doi: 10.1007/s11130-014-0415-z [DOI] [PubMed] [Google Scholar]
- 138.Vijayakumar K, Prasanna B, Rengarajan RL, Rathinam A, Velayuthaprabhu S, Vijaya Anand A. Anti-diabetic and hypolipidemic effects of Cinnamon cassia bark extracts: An in vitro, in vivo, and in silico approach. Arch Physiol Biochem. 2020; 1–11. doi: 10.1080/13813455.2020.1822415 [DOI] [PubMed] [Google Scholar]
- 139.Ercan P, El SN. Inhibitory effects of bioaccessible anthocyanins and procyanidins from apple, red grape, cinnamon on α-amylase, α-glucosidase and lipase. Int J Vitam Nutr Res. 2021;91: 16–24. doi: 10.1024/0300-9831/a000652 [DOI] [PubMed] [Google Scholar]
- 140.Li Y, Xu R, Xiu H, Feng J, Jin Park H, Prabhakar H, et al. Effect of cinnamon on starch hydrolysis of rice pudding: Comparing static and dynamic in vitro digestion models. Food Res Int. 2022;161: 111813. doi: 10.1016/j.foodres.2022.111813 [DOI] [PubMed] [Google Scholar]
- 141.Hayward NJ, McDougall GJ, Farag S, Allwood JW, Austin C, Campbell F, et al. Cinnamon shows antidiabetic properties that are species-specific: Effects on enzyme activity inhibition and starch digestion. Plant Foods Hum Nutr. 2019;74: 544–552. doi: 10.1007/s11130-019-00760-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Weng L, Chen T-H, Huang L, Lai D, Kang N, Fu Y-S, et al. A nutraceutical combination of cinnamon, purple onion, and tea linked with key enzymes on treatment of type 2 diabetes. J Food Biochem. 2021;45: e13971. doi: 10.1111/jfbc.13971 [DOI] [PubMed] [Google Scholar]
- 143.Choudhury H, Pandey M, Hua CK, Mun CS, Jing JK, Kong L, et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J Tradit Complement Med. 2018;8: 361–376. doi: 10.1016/j.jtcme.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wariyapperuma WANM, Jayawardena B, Thammitiyagodage MG, Karunakaran R, Kumara WGSS. Hypoglycemic and anti-lipidemic properties of Cinnamomum zeylanicum (” Sri Wijaya” accession) water-soluble nutraceutical in streptozotocin-induced diabetic and healthy wistar rats. Pharmacogn Mag. 2021;17: 188. doi: 10.4103/pm.pm_334_20 [DOI]
- 145.Shinjyo N, Waddell G, Green J. A tale of two cinnamons: a comparative review of the clinical evidence of Cinnamomum verum and C. cassia as diabetes interventions. J Herb Med. 2020;21: 100342. doi: 10.1016/j.hermed.2020.100342 [DOI] [Google Scholar]
- 146.Vijayakumar K, Rengarajan RL, Suganthi N, Prasanna B, Velayuthaprabhu S, Shenbagam M, et al. Acute toxicity studies and protective effects of Cinnamon cassia bark extract in streptozotocin-induced diabetic rats. Drug Chem Toxicol. 2022;45: 2086–2096. doi: 10.1080/01480545.2021.1907908 [DOI] [PubMed] [Google Scholar]
- 147.Sheng X, Zhang Y, Gong Z, Huang C, Zang YQ. Improved Insulin Resistance and Lipid Metabolism by Cinnamon Extract through Activation of Peroxisome Proliferator-Activated Receptors. PPAR Res. 2008;2008: 581348. doi: 10.1155/2008/581348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shakeel M, Jabeen F, Iqbal R, Chaudhry AS, Zafar S, Ali M, et al. Assessment of Titanium Dioxide Nanoparticles (TiO(2)-NPs) Induced Hepatotoxicity and Ameliorative Effects of Cinnamomum cassia in Sprague-Dawley Rats. Biol Trace Elem Res. 2018;182: 57–69. doi: 10.1007/s12011-017-1074-3 [DOI] [PubMed] [Google Scholar]
- 149.Okwuosa C, Azubuike N, Chigozie OP. Evaluation of Changes in Biochemical and Haematological Parameters of Albino Rats Following Subacute Oral Administration of Cinnamomum cassia (Cinnamon) Extract. Annu Res Rev Biol. 2021;36: 91–99. doi: 10.9734/ARRB/2021/v36i630393 [DOI] [Google Scholar]
- 150.Mohamed RE, El-Said M, Arief M. Phytochemical Screening of Cinnamon Cassia and Its Protective Effects Against Hepatotoxicity Induced By Difenoconazole in Male Albino Rats. Research Square; 2021. doi: 10.21203/rs.3.rs-173661/v1 [DOI] [Google Scholar]
- 151.Adli DEH, Brahmi M, Kahloula K, Arabi W, Bouzouira B, Talatizi M, et al. The therapeutic effect of Cinnamomum cassia essential oil against hepatotoxicity induced by co-exposure to lead and manganese in developing Wistar rats. J Drug Deliv Ther. 2020;10. doi: 10.22270/jddt.v10i5.4361 [DOI] [Google Scholar]
- 152.Iqbal S, Jabeen F, Peng C, Ijaz MU, Chaudhry AS. Cinnamomum cassia ameliorates Ni-NPs-induced liver and kidney damage in male Sprague Dawley rats. Hum Exp Toxicol. 2020;39: 1565–1581. doi: 10.1177/0960327120930125 [DOI] [PubMed] [Google Scholar]
- 153.Wickenberg J, Lindstedt S, Nilsson J, Hlebowicz J. Cassia cinnamon does not change the insulin sensitivity or the liver enzymes in subjects with impaired glucose tolerance. Nutr J. 2014;13: 96. doi: 10.1186/1475-2891-13-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shekarchizadeh-Esfahani P, Heydarpour F, Izadi F, Jalili C. The effect of cinnamon supplementation on liver enzymes in adults: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2021;58: 102699. doi: 10.1016/j.ctim.2021.102699 [DOI] [PubMed] [Google Scholar]
- 155.Abraham K, Pfister M, Wöhrlin F, Lampen A. Relative bioavailability of coumarin from cinnamon and cinnamon-containing foods compared to isolated coumarin: a four-way crossover study in human volunteers. Mol Nutr Food Res. 2011;55: 644–653. doi: 10.1002/mnfr.201000394 [DOI] [PubMed] [Google Scholar]
- 156.Woehrlin F, Fry H, Abraham K, Preiss-Weigert A. Quantification of flavoring constituents in cinnamon: High variation of coumarin in cassia bark from the German retail market and in authentic samples from indonesia. J Agric Food Chem. 2010;58: 10568–75. doi: 10.1021/jf102112p [DOI] [PubMed] [Google Scholar]
- 157.Yun J-W, You J-R, Kim Y-S, Kim S-H, Cho E-Y, Yoon J-H, et al. In vitro and in vivo safety studies of cinnamon extract (Cinnamomum cassia) on general and genetic toxicology. Regul Toxicol Pharmacol. 2018;95: 115–123. doi: 10.1016/j.yrtph.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 158.Heghes SC, Vostinaru O, Mogosan C, Miere D, Iuga CA, Filip L. Safety Profile of Nutraceuticals Rich in Coumarins: An Update. Front Pharmacol. 2022;13: 803338. doi: 10.3389/fphar.2022.803338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dinesh R, Leela NK, Zachariah TJ, Anandaraj M. Controversies surrounding coumarin in cassia: the good, the bad and the not so ugly. Curr Sci. 2015;108: 482–484. Available: http://www.jstor.org/stable/24216590 [Google Scholar]
- 160.Roye C, Henrion M, Chanvrier H, De Roeck K, De Bondt Y, Liberloo I, et al. Extrusion-cooking modifies physicochemical and nutrition-related properties of wheat bran. Foods (Basel, Switzerland). 2020;9. doi: 10.3390/foods9060738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rashid S, Rakha A, Anjum FM, Ahmed W, Sohail M. Effects of extrusion cooking on the dietary fibre content and Water Solubility Index of wheat bran extrudates. Int J Food Sci Technol. 2015;50: 1533–1537. doi: 10.1111/ijfs.12798 [DOI] [Google Scholar]
- 162.Khanum F, Siddalinga Swamy M, Sudarshana Krishna KR, Santhanam K, Viswanathan KR. Dietary fiber content of commonly fresh and cooked vegetables consumed in India. Plant Foods Hum Nutr. 2000;55: 207–218. doi: 10.1023/a:1008155732404 [DOI] [PubMed] [Google Scholar]
- 163.AlGeffari MA, Almogbel ES, Alhomaidan HT, El-Mergawi R, Barrimah IA. Glycemic indices, glycemic load and glycemic response for seventeen varieties of dates grown in Saudi Arabia. Ann Saudi Med. 2016;36: 397–403. doi: 10.5144/0256-4947.2016.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Menga V, Amato M, Phillips TD, Angelino D, Morreale F, Fares C. Gluten-free pasta incorporating chia (Salvia hispanica L.) as thickening agent: An approach to naturally improve the nutritional profile and the in vitro carbohydrate digestibility. Food Chem. 2017;221: 1954–1961. doi: 10.1016/j.foodchem.2016.11.151 [DOI] [PubMed] [Google Scholar]
- 165.Kulathunga J, Simsek S. Dietary fiber variation in ancient and modern wheat species: Einkorn, emmer, spelt and hard red spring wheat. J Cereal Sci. 2022;104: 103420. doi: 10.1016/j.jcs.2022.103420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Irondi EA, Akintunde JK, Agboola SO, Boligon AA, Athayde ML. Blanching influences the phenolics composition, antioxidant activity, and inhibitory effect of Adansonia digitata leaves extract on α-amylase, α-glucosidase, and aldose reductase. Food Sci Nutr. 2017;5: 233–242. doi: 10.1002/fsn3.386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Francisco M, Velasco P, Moreno DA, García-Viguera C, Cartea ME. Cooking methods of Brassica rapa affect the preservation of glucosinolates, phenolics and vitamin C. Food Res Int. 2010;43: 1455–1463. doi: 10.1016/j.foodres.2010.04.024 [DOI] [Google Scholar]
- 168.Perla V, Holm DG, Jayanty SS. Effects of cooking methods on polyphenols, pigments and antioxidant activity in potato tubers. LWT ‐ Food Sci Technol. 2012;45: 161–171. doi: 10.1016/j.lwt.2011.08.005 [DOI] [Google Scholar]
- 169.Mulimani VH, Supriya D. Effect of heat treatments on alpha-amylase inhibitor activity in sorghum (Sorghum bicolour L.). Plant Foods Hum Nutr. 1993;44: 181–186. doi: 10.1007/BF01088383 [DOI] [PubMed] [Google Scholar]
- 170.Wariyapperuma WANM, Kannangara S, Wijayasinghe YS, Subramanium S, Jayawardena B. In vitro anti-diabetic effects and phytochemical profiling of novel varieties of Cinnamomum zeylanicum (L.) extracts. PeerJ. 2020;8: e10070. doi: 10.7717/peerj.10070 [DOI] [PMC free article] [PubMed]
- 171.Adedayo BC, Adebayo AA, Nwanna EE, Oboh G. Effect of cooking on glycemic index, antioxidant activities, α-amylase, and α-glucosidase inhibitory properties of two rice varieties. Food Sci Nutr. 2018;6: 2301–2307. doi: 10.1002/fsn3.806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Abhilasha A, Kaur L, Monro J, Hardacre A, Singh J. Intact, kibbled, and cut wheat grains:Physico‐chemical, microstructural characteristics and gastro‐small intestinal digestion in vitro. Starch ‐ Stärke. 2021;73: 2000267. doi: 10.1002/star.202000267 [DOI] [Google Scholar]
- 173.Zhao W, Ye T, Fan Z, Wu Y, Liu A, Lu X. Yam paste in glycemic preloads curbs peak glycemia of rice meals in apparent healthy subjects. Asia Pac J Clin Nutr. 2021;30: 436–445. doi: 10.6133/apjcn.202109_30(3).0010 [DOI] [PubMed] [Google Scholar]
- 174.Rodríguez-Ayala M, Sandoval-Insausti H, Bayán-Bravo A, Banegas JR, Donat-Vargas C, Ortolá R, et al. Cooking Methods and Their Relationship with Anthropometrics and Cardiovascular Risk Factors among Older Spanish Adults. Nutrients. 2022;14. doi: 10.3390/nu14163426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Melson CE, Nepocatych S, Madzima TA. The effects of whey and soy liquid breakfast on appetite response, energy metabolism, and subsequent energy intake. Nutrition. 2019;61: 179–186. doi: 10.1016/j.nut.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 176.Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, et al. Glycaemic index methodology. Nutr Res Rev. 2005;18: 145–171. doi: 10.1079/NRR2005100 [DOI] [PubMed] [Google Scholar]
- 177.Flavel M, Jois M, Kitchen B. Potential contributions of the methodology to the variability of glycaemic index of foods. World J Diabetes. 2021;12: 108–123. doi: 10.4239/wjd.v12.i2.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Barclay AW, Brand-Miller JC, Wolever TMS. Glycemic index, glycemic load, and glycemic response are not the same. Diabetes care. United States; 2005. pp. 1839–1840. doi: 10.2337/diacare.28.7.1839 [DOI] [PubMed] [Google Scholar]
- 179.Wolever T, Jenkins A, Clinthorne J, Heimowitz C. Equivalent Glycemic Load (EGL) of Atkins Chocolate Peanut Butter Bar and Atkins Chocolate Ready-to-Drink Shake in People With Type 2 Diabetes. Current Developments in Nutrition. 2022. p. 459. doi: 10.1093/cdn/nzac057.025 [DOI] [Google Scholar]
- 180.Kado S, Murakami T, Aoki A, Nagase T, Katsura Y, Noritake M, et al. Effect of acarbose on postprandial lipid metabolism in type 2 diabetes mellitus. Diabetes Res Clin Pract. 1998;41: 49–55. doi: 10.1016/s0168-8227(98)00062-x [DOI] [PubMed] [Google Scholar]
- 181.Bellissimo N, Fansabedian T, Wong VCH, Totosy de Zepetnek JO, Brett NR, Schwartz A, et al. Effect of Increasing the Dietary Protein Content of Breakfast on Subjective Appetite, Short-Term Food Intake and Diet-Induced Thermogenesis in Children. Nutrients. 2020;12. doi: 10.3390/nu12103025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ampofo D, Agbenorhevi JK, Firempong CK, Adu-Kwarteng E. Glycemic index of different varieties of yam as influenced by boiling, frying and roasting. Food Sci Nutr. 2021;9: 1106–1111. doi: 10.1002/fsn3.2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pandolfo A, Messina B, Russo G. Evaluation of Glycemic Index of Six Different Samples of Commercial and Experimental Pasta Differing in Wheat Varieties and Production Processes. Foods (Basel, Switzerland). 2021;10. doi: 10.3390/foods10092221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Haini N, Jau-Shya L, Mohd Rosli RG, Mamat H. Effects of high-amylose maize starch on the glycemic index of Chinese steamed buns (CSB). Heliyon. 2022;8: e09375. doi: 10.1016/j.heliyon.2022.e09375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Freckmann G, Schmid C, Baumstark A, Pleus S, Link M, Haug C. System accuracy evaluation of 43 blood glucose monitoring systems for self-monitoring of blood glucose according to DIN EN ISO 15197. J Diabetes Sci Technol. 2012;6: 1060–75. doi: 10.1177/193229681200600510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bernardo MA, Silva ML, Santos E, Moncada MM, Brito J, Proenca L, et al. Effect of Cinnamon Tea on Postprandial Glucose Concentration. J Diabetes Res. 2015;2015: 913651. doi: 10.1155/2015/913651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lu J-Y, Peng J-H, Ma X-J, Zhang Y-N, Zhu W, He X-X, et al. Metabolic perturbations of post-load hyperglycemia vs. fasting hyperglycemia. Acta Pharmacol Sin. 2019;40: 216–221. doi: 10.1038/s41401-018-0018-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rita K, Bernardo MA, Silva ML, Brito J, Mesquita MF, Pintão AM, et al. Adansonia digitata L. (Baobab Fruit) Effect on Postprandial Glycemia in Healthy Adults: A Randomized Controlled Trial. Nutrients. 2022;14. doi: 10.3390/nu14020398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Feise NK, Johnston CS. Commercial Vinegar Tablets Do Not Display the Same Physiological Benefits for Managing Postprandial Glucose Concentrations as Liquid Vinegar. J Nutr Metab. 2020;2020: 9098739. doi: 10.1155/2020/9098739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ceriello A. The emerging role of post-prandial hyperglycaemic spikes in the pathogenesis of diabetic complications. Diabet Med. 1998;15: 188–193. doi: [DOI] [PubMed] [Google Scholar]
- 191.Ceriello A. Cardiovascular effects of acute hyperglycaemia: pathophysiological underpinnings. Diabetes Vasc Dis Res. 2008;5: 260–268. doi: 10.3132/dvdr.2008.038 [DOI] [PubMed] [Google Scholar]
- 192.Harrison DE, Strong R, Alavez S, Astle CM, DiGiovanni J, Fernandez E, et al. Acarbose improves health and lifespan in aging HET3 mice. Aging Cell. 2019;18: e12898. doi: 10.1111/acel.12898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang RR, Lv ZM, Dan YP, Chen KY, Zhang C. Effects of acarbose and siglitine on blood glucose fluctuation and islet β-cell function in patients with type 2 diabetes mellitus. J Biol Regul Homeost Agents. 2019;33: 365–374. [PubMed] [Google Scholar]
- 194.Brand-Miller JC, Stockmann K, Atkinson F, Petocz P, Denyer G. Glycemic index, postprandial glycemia, and the shape of the curve in healthy subjects: analysis of a database of more than 1,000 foods. Am J Clin Nutr. 2009;89: 97–105. doi: 10.3945/ajcn.2008.26354 [DOI] [PubMed] [Google Scholar]
- 195.Wolever TMS, Jenkins AL, Prudence K, Johnson J, Duss R, Chu Y, et al. Effect of adding oat bran to instant oatmeal on glycaemic response in humans ‐ a study to establish the minimum effective dose of oat β-glucan. Food Funct. 2018;9: 1692–1700. doi: 10.1039/c7fo01768e [DOI] [PubMed] [Google Scholar]
- 196.Noronha JC, Braunstein CR, Glenn AJ, Khan TA, Viguiliouk E, Noseworthy R, et al. The effect of small doses of fructose and allulose on postprandial glucose metabolism in type 2 diabetes: A double-blind, randomized, controlled, acute feeding, equivalence trial. Diabetes Obes Metab. 2018;20: 2361–2370. doi: 10.1111/dom.13374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wolever TMS, Mattila O, Rosa-Sibakov N, Tosh SM, Jenkins AL, Ezatagha A, et al. Effect of Varying Molecular Weight of Oat β-Glucan Taken just before Eating on Postprandial Glycemic Response in Healthy Humans. Nutrients. 2020;12. doi: 10.3390/nu12082275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li Y, Li Z, Wang R, Mi B, Jiang T, Lu M, et al. Alleviating the Hydrolysis of Carbohydrates, Tangzhiqing (TZQ) Decreased the Postprandial Glycemia in Healthy Volunteers: An Eight-Period Crossover Study. Evid Based Complement Alternat Med. 2020;2020: 8138195. doi: 10.1155/2020/8138195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shah P, Wolever TM, Jenkins AL, Ezatagha A, Campbell J, Zurbau A, et al. Acute glycemic and insulin response of FossenceTM alone, or when substituted or added to a carbohydrate challenge: A three-phase, acute, randomized, cross-over, double blind clinical trial. Heliyon. 2021;7: e06805. doi: 10.1016/j.heliyon.2021.e06805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bergman M, Abdul-Ghani M, DeFronzo RA, Manco M, Sesti G, Fiorentino TV, et al. Review of methods for detecting glycemic disorders. Diabetes Res Clin Pract. 2020;165: 108233. doi: 10.1016/j.diabres.2020.108233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cui S-S, Duan L-J, Li J-F, Qin Y-Z, Bao S-Q, Jiang X. The Factors Influencing the Renal Glucose Threshold in Patients with Newly Diagnosed Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2021;14: 4497–4503. doi: 10.2147/DMSO.S336791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hieshima K, Sugiyama S, Yoshida A, Kurinami N, Suzuki T, Ijima H, et al. Elevation of the renal threshold for glucose is associated with insulin resistance and higher glycated hemoglobin levels. J Diabetes Investig. 2020;11: 617–625. doi: 10.1111/jdi.13191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.American Diabetes Association Professional Practice Committee. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45: S83–S96. doi: 10.2337/dc22-S006 [DOI] [PubMed] [Google Scholar]
- 204.American Diabetes Association Professional Practice Committee. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin Diabetes. 2022;40: 10–38. doi: 10.2337/cd22-as01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gyberg V, De Bacquer D, Kotseva K, De Backer G, Schnell O, Tuomilehto J, et al. Time-saving screening for diabetes in patients with coronary artery disease: a report from EUROASPIRE IV. BMJ Open. 2016;6: e013835. doi: 10.1136/bmjopen-2016-013835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Selvin E, Rawlings A, Lutsey P, Maruthur N, Pankow JS, Steffes M, et al. Association of 1,5-Anhydroglucitol With Cardiovascular Disease and Mortality. Diabetes. 2016;65: 201–208. doi: 10.2337/db15-0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hemmingsen B, Gimenez-Perez G, Mauricio D, Roqué I Figuls M, Metzendorf M-I, Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane database Syst Rev. 2017;12: CD003054. doi: 10.1002/14651858.CD003054.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Faqih AM, Al-Nawaiseh FY. The immediate glycemic response to four herbal teas in healthy adults. Jordan Med J. 2006;40. [Google Scholar]
- 209.Beejmohun V, Peytavy-Izard M, Mignon C, Muscente-Paque D, Deplanque X, Ripoll C, et al. Acute effect of Ceylon cinnamon extract on postprandial glycemia: Alpha-amylase inhibition, starch tolerance test in rats, and randomized crossover clinical trial in healthy volunteers. BMC Complement Altern Med. 2014;14: 351. doi: 10.1186/1472-6882-14-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.DuBose SN, Li Z, Sherr JL, Beck RW, Tamborlane W V, Shah VN. Effect of Exercise and Meals on Continuous Glucose Monitor Data in Healthy Individuals Without Diabetes. J Diabetes Sci Technol. 2021;15: 593–599. doi: 10.1177/1932296820905904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Horton WB, Jahn LA, Hartline LM, Aylor KW, Patrie JT, Barrett EJ. Acute hyperglycaemia enhances both vascular endothelial function and cardiac and skeletal muscle microvascular function in healthy humans. J Physiol. 2022;600: 949–962. doi: 10.1113/JP281286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23: 1830–1834. doi: 10.2337/diacare.23.12.1830 [DOI] [PubMed] [Google Scholar]
- 213.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol J-P, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295: 1681–1687. doi: 10.1001/jama.295.14.1681 [DOI] [PubMed] [Google Scholar]
- 214.Hanssen NMJ, Kraakman MJ, Flynn MC, Nagareddy PR, Schalkwijk CG, Murphy AJ. Postprandial Glucose Spikes, an Important Contributor to Cardiovascular Disease in Diabetes? Front Cardiovasc Med. 2020;7: 570553. doi: 10.3389/fcvm.2020.570553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cheng P-C, Kao C-H. Postprandial plasma glucose excursion is associated with an atherogenic lipid profile in individuals with type 2 diabetes mellitus: A cross-sectional study. PLoS One. 2021;16: e0258771. doi: 10.1371/journal.pone.0258771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mo Y, Wang C, Lu J, Shen Y, Chen L, Zhang L, et al. Impact of short-term glycemic variability on risk of all-cause mortality in type 2 diabetes patients with well-controlled glucose profile by continuous glucose monitoring: A prospective cohort study. Diabetes Res Clin Pract. 2022;189: 109940. doi: 10.1016/j.diabres.2022.109940 [DOI] [PubMed] [Google Scholar]
- 217.Shukla AP, Andono J, Touhamy SH, Casper A, Iliescu RG, Mauer E, et al. Carbohydrate-last meal pattern lowers postprandial glucose and insulin excursions in type 2 diabetes. BMJ open diabetes Res care. 2017;5: e000440. doi: 10.1136/bmjdrc-2017-000440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lanzerstorfer P, Rechenmacher E, Lugmayr O, Stadlbauer V, Höglinger O, Vollmar A, et al. Effects of various commercial whole-grain breads on postprandial blood glucose response and glycemic index in healthy subjects. Austin J Clin Med. 2018;5: 1031. [Google Scholar]
- 219.Östman E, Samigullin A, Heyman-Lindén L, Andersson K, Björck I, Öste R, et al. A novel nutritional supplement containing amino acids and chromium decreases postprandial glucose response in a randomized, double-blind, placebo-controlled study. PLoS One. 2020;15: e0234237. doi: 10.1371/journal.pone.0234237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dos Reis Gallo LR, Reis CEG, Mendonça MA, da Silva VSN, Pacheco MTB, Botelho RBA. Impact of Gluten-Free Sorghum Bread Genotypes on Glycemic and Antioxidant Responses in Healthy Adults. Foods (Basel, Switzerland). 2021;10. doi: 10.3390/foods10102256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zurbau A, Jenkins AL, Jovanovski E, Au-Yeung F, Bateman EA, Brissette C, et al. Acute effect of equicaloric meals varying in glycemic index and glycemic load on arterial stiffness and glycemia in healthy adults: a randomized crossover trial. Eur J Clin Nutr. 2019;73: 79–85. doi: 10.1038/s41430-018-0182-2 [DOI] [PubMed] [Google Scholar]
- 222.Kim JS, Nam K, Chung S-J. Effect of nutrient composition in a mixed meal on the postprandial glycemic response in healthy people: a preliminary study. Nutr Res Pract. 2019;13: 126–133. doi: 10.4162/nrp.2019.13.2.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kazemi F, Danaei G, Farzadfar F, Malik V, Parsaeian M, Pouraram H, et al. Glycemic Index (GI) Values for Major Sources of Dietary Carbohydrates in Iran. Int J Endocrinol Metab. 2020;18: e99793. doi: 10.5812/ijem.99793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kouamé CA, Kouassi NK, N’dri DY, Pereko KKA, Casiraghi MC, Rhedoor AJ, et al. Glycemic Responses, Glycemic Index, and Glycemic Load Values of Some Street Foods Prepared from Plantain (Musa spp., AAB Genome) in Côte d’Ivoire. Foods (Basel, Switzerland). 2017;6. doi: 10.3390/foods6090083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hochkogler CM, Hoi JK, Lieder B, Müller N, Hans J, Widder S, et al. Cinnamyl Isobutyrate Decreases Plasma Glucose Levels and Total Energy Intake from a Standardized Breakfast: A Randomized, Crossover Intervention. Mol Nutr Food Res. 2018;62: e1701038. doi: 10.1002/mnfr.201701038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kato T, Node K. Therapeutic potential of alpha-glucosidase inhibitors to prevent postprandial endothelial dysfunction. Int Heart J. 2014;55: 386–390. doi: 10.1536/ihj.14-194 [DOI] [PubMed] [Google Scholar]
- 227.Nusca A, Tuccinardi D, Albano M, Cavallaro C, Ricottini E, Manfrini S, et al. Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes Metab Res Rev. 2018;34: e3047. doi: 10.1002/dmrr.3047 [DOI] [PubMed] [Google Scholar]
- 228.Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol an Off J Polish Physiol Soc. 2019;70. doi: 10.26402/jpp.2019.6.01 [DOI] [PubMed] [Google Scholar]
- 229.Dambrova M, Zuurbier CJ, Borutaite V, Liepinsh E, Makrecka-Kuka M. Energy substrate metabolism and mitochondrial oxidative stress in cardiac ischemia/reperfusion injury. Free Radic Biol Med. 2021;165: 24–37. doi: 10.1016/j.freeradbiomed.2021.01.036 [DOI] [PubMed] [Google Scholar]
- 230.Tretter V, Hochreiter B, Zach ML, Krenn K, Klein KU. Understanding Cellular Redox Homeostasis: A Challenge for Precision Medicine. Int J Mol Sci. 2021;23. doi: 10.3390/ijms23010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Soares MJ, Müller MJ, Boeing H, Maffeis C, Misra A, Muscogiuri G, et al. Conflict of interest in nutrition research: an editorial perspective. European journal of clinical nutrition. England; 2019. pp. 1213–1215. doi: 10.1038/s41430-019-0488-8 [DOI] [PubMed] [Google Scholar]
- 232.Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt PIHJ. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J. 2016;473: 4527–4550. doi: 10.1042/BCJ20160503C [DOI] [PubMed] [Google Scholar]
- 233.Zhang Y, Yuan D, Yao W, Zhu Q, Liu Y, Huang F, et al. Hyperglycemia Aggravates Hepatic Ischemia Reperfusion Injury by Inducing Chronic Oxidative Stress and Inflammation. Oxid Med Cell Longev. 2016;2016: 3919627. doi: 10.1155/2016/3919627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kawahito K, Sato H, Kadosaki M, Egawa A, Misawa Y. Spike in glucose levels after reperfusion during aortic surgery: assessment by continuous blood glucose monitoring using artificial endocrine pancreas. Gen Thorac Cardiovasc Surg. 2018;66: 150–154. doi: 10.1007/s11748-017-0872-z [DOI] [PubMed] [Google Scholar]
- 235.Ryou M-G, Mallet RT. An In Vitro Oxygen-Glucose Deprivation Model for Studying Ischemia-Reperfusion Injury of Neuronal Cells. Methods Mol Biol. 2018;1717: 229–235. doi: 10.1007/978-1-4939-7526-6_18 [DOI] [PubMed] [Google Scholar]
- 236.Wang J, Wang S, Yang J, Henning SM, Ezzat-Zadeh Z, Woo S-L, et al. Acute Effects of Cinnamon Spice on Post-prandial Glucose and Insulin in Normal Weight and Overweight/Obese Subjects: A Pilot Study. Front Nutr. 2020;7: 619782. doi: 10.3389/fnut.2020.619782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yang T, Wang D, Qu Y, Wang Y, Feng Y, Yang Y, et al. N-hydroxy-N’-(4-butyl-2-methylphenyl)-formamidine attenuates oxygen-glucose deprivation and reoxygenation-induced cerebral ischemia-reperfusion injury via regulation of microRNAs. J Integr Neurosci. 2020;19: 303–311. doi: 10.31083/j.jin.2020.02.1236 [DOI] [PubMed] [Google Scholar]
- 238.Frantz S, Calvillo L, Tillmanns J, Elbing I, Dienesch C, Bischoff H, et al. Repetitive postprandial hyperglycemia increases cardiac ischemia/reperfusion injury: prevention by the alpha-glucosidase inhibitor acarbose. FASEB J Off Publ Fed Am Soc Exp Biol. 2005;19: 591–593. doi: 10.1096/fj.04-2459fje [DOI] [PubMed] [Google Scholar]
- 239.Ogiso K, Shayo SC, Kawade S, Hashiguchi H, Deguchi T, Nishio Y. Repeated glucose spikes and insulin resistance synergistically deteriorate endothelial function and bardoxolone methyl ameliorates endothelial dysfunction. PLoS One. 2022;17: e0263080. doi: 10.1371/journal.pone.0263080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ceriello A, Quagliaro L, Catone B, Pascon R, Piazzola M, Bais B, et al. Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes Care. 2002;25: 1439–1443. doi: 10.2337/diacare.25.8.1439 [DOI] [PubMed] [Google Scholar]
- 241.Ceriello A. Oxidative stress and diabetes-associated complications. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2006;12 Suppl 1: 60–62. doi: 10.4158/EP.12.S1.60 [DOI] [PubMed] [Google Scholar]
- 242.Li F-F, Fu L-Y, Xu X-H, Su X-F, Wu J-D, Ye L, et al. Analysis of the add-on effect of α-glucosidase inhibitor, acarbose in insulin therapy: A pilot study. Biomed reports. 2016;5: 461–466. doi: 10.3892/br.2016.744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Li F-F, Gao G, Li Q, Zhu H-H, Su X-F, Wu J-D, et al. Influence of Dapagliflozin on Glycemic Variations in Patients with Newly Diagnosed Type 2 Diabetes Mellitus. J Diabetes Res. 2016;2016: 5347262. doi: 10.1155/2016/5347262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Papachristoforou E, Lambadiari V, Maratou E, Makrilakis K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J Diabetes Res. 2020;2020: 7489795. doi: 10.1155/2020/7489795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Schlotterer A, Kukudov G, Bozorgmehr F, Hutter H, Du X, Oikonomou D, et al. C. elegans as model for the study of high glucose- mediated life span reduction. Diabetes. 2009;58: 2450–2456. doi: 10.2337/db09-0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Alcántar-Fernández J, Navarro RE, Salazar-Martínez AM, Pérez-Andrade ME, Miranda-Ríos J. Caenorhabditis elegans respond to high-glucose diets through a network of stress-responsive transcription factors. PLoS One. 2018;13: e0199888. doi: 10.1371/journal.pone.0199888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Alcántar-Fernández J, González-Maciel A, Reynoso-Robles R, Pérez Andrade ME, Hernández-Vázquez A de J, Velázquez-Arellano A, et al. High-glucose diets induce mitochondrial dysfunction in Caenorhabditis elegans. PLoS One. 2019;14: e0226652. doi: 10.1371/journal.pone.0226652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kalaria TR, Sirajwala HB, Gohel MG. Serum fructosamine, serum glycated albumin and serum glycated β-lipoprotein in type 2 diabetes mellitus patients with and without microvascular complications. J Diabetes Metab Disord. 2016;15: 53. doi: 10.1186/s40200-016-0276-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Shin A, Vazmitsel Y, Connolly S, Kabytaev K. Comprehensive profiling and kinetic studies of glycated lysine residues in human serum albumin. Anal Bioanal Chem. 2022;414: 4861–4875. doi: 10.1007/s00216-022-04108-1 [DOI] [PubMed] [Google Scholar]
- 250.Siddiqui K, George TP, Nawaz SS, Yaslam M, Almogbel E, Al-Rubeaan K. Significance of glycated LDL in different stages of diabetic nephropathy. Diabetes Metab Syndr. 2019;13: 548–552. doi: 10.1016/j.dsx.2018.11.023 [DOI] [PubMed] [Google Scholar]
- 251.Hassanein M, Shafi T. Assessment of glycemia in chronic kidney disease. BMC Med. 2022;20: 117. doi: 10.1186/s12916-022-02316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lee J-E. Alternative biomarkers for assessing glycemic control in diabetes: fructosamine, glycated albumin, and 1,5-anhydroglucitol. Ann Pediatr Endocrinol Metab. 2015;20: 74–78. doi: 10.6065/apem.2015.20.2.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hutchins AM, Brown BD, Cunnane SC, Domitrovich SG, Adams ER, Bobowiec CE. Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: a randomized study. Nutr Res. 2013;33: 367–375. doi: 10.1016/j.nutres.2013.02.012 [DOI] [PubMed] [Google Scholar]
- 254.Chan CL, Pyle L, Kelsey MM, Newnes L, Baumgartner A, Zeitler PS, et al. Alternate glycemic markers reflect glycemic variability in continuous glucose monitoring in youth with prediabetes and type 2 diabetes. Pediatr Diabetes. 2017;18: 629–636. doi: 10.1111/pedi.12475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Iqbal Z, Bashir B, Adam S, Ho JH, Dhage S, Azmi S, et al. Glycated apolipoprotein B decreases after bariatric surgery in people with and without diabetes: A potential contribution to reduction in cardiovascular risk. Atherosclerosis. 2022;346: 10–17. doi: 10.1016/j.atherosclerosis.2022.01.005 [DOI] [PubMed] [Google Scholar]
- 256.Alique M, Luna C, Carracedo J, Ramírez R. LDL biochemical modifications: a link between atherosclerosis and aging. Food Nutr Res. 2015;59: 29240. doi: 10.3402/fnr.v59.29240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kim J-Y, Lee JW, Lee JS, Jang DS, Shim SH. Inhibitory effects of compounds isolated from roots of Cynanchum wilfordii on oxidation and glycation of human low-density lipoprotein (LDL). J Funct Foods. 2019;59: 281–290. doi: 10.1016/j.jff.2019.05.045 [DOI] [Google Scholar]
- 258.Salehi N, Walters M. When and What to Eat? A Scoping Review of Health Outcomes of Fasting in Conjunction with a Low-Carbohydrate Diet. Br J Nutr. 2022; 1–41. doi: 10.1017/S0007114522001854 [DOI] [PubMed] [Google Scholar]
- 259.Al Saudi RM, Kasabri V, Naffa R, Bulatova N, Bustanji Y. Glycated LDL-C and glycated HDL-C in association with adiposity, blood and atherogenicity indices in metabolic syndrome patients with and without prediabetes. Ther Adv Endocrinol Metab. 2018;9: 311–323. doi: 10.1177/2042018818788198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Foreman YD, Brouwers MCGJ, Berendschot TTJM, van Dongen MCJM, Eussen SJPM, van Greevenbroek MMJ, et al. The oral glucose tolerance test-derived incremental glucose peak is associated with greater arterial stiffness and maladaptive arterial remodeling: The Maastricht Study. Cardiovasc Diabetol. 2019;18: 152. doi: 10.1186/s12933-019-0950-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wang N, Zhang J-P, Xing X-Y, Yang Z-J, Zhang B, Wang X, et al. MARCH: factors associated with weight loss in patients with newly diagnosed type 2 diabetes treated with acarbose or metformin. Arch Med Sci. 2019;15: 309–320. doi: 10.5114/aoms.2018.75255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Rachmani R, Bar-Dayan Y, Ronen Z, Levi Z, Slavachevsky I, Ravid M. The effect of acarbose on insulin resistance in obese hypertensive subjects with normal glucose tolerance: A randomized controlled study. Diabetes Obes Metab. 2004;6: 63–8. doi: 10.1111/j.1463-1326.2004.00317.x [DOI] [PubMed] [Google Scholar]
- 263.Wang X, Xu T, Liu R, Wu G, Gu L, Zhang Y, et al. High-Fiber Diet or Combined With Acarbose Alleviates Heterogeneous Phenotypes of Polycystic Ovary Syndrome by Regulating Gut Microbiota. Front Endocrinol (Lausanne). 2021;12: 806331. doi: 10.3389/fendo.2021.806331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Delgado H, Lehmann T, Bobbioni-Harsch E, Ybarra J, Golay A. Acarbose improves indirectly both insulin resistance and secretion in obese type 2 diabetic patients. Diabetes Metab. 2002;28: 195–200. [PubMed] [Google Scholar]
- 265.Meneilly GS, Ryan EA, Radziuk J, Lau DC, Yale JF, Morais J, et al. Effect of acarbose on insulin sensitivity in elderly patients with diabetes. Diabetes Care. 2000;23: 1162–7. doi: 10.2337/diacare.23.8.1162 [DOI] [PubMed] [Google Scholar]
- 266.Chiasson JL, Josse RG, Leiter LA, Mihic M, Nathan DM, Palmason C, et al. The effect of acarbose on insulin sensitivity in subjects with impaired glucose tolerance. Diabetes Care. 1996;19: 1190–3. doi: 10.2337/diacare.19.11.1190 [DOI] [PubMed] [Google Scholar]
- 267.Dodds SG, Parihar M, Javors M, Nie J, Musi N, Dave Sharp Z, et al. Acarbose improved survival for Apc(+/Min) mice. Aging Cell. 2020;19: e13088. doi: 10.1111/acel.13088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ceriello A. Controlling oxidative stress as a novel molecular approach to protecting the vascular wall in diabetes. Curr Opin Lipidol. 2006;17: 510–518. doi: 10.1097/01.mol.0000245256.17764.fb [DOI] [PubMed] [Google Scholar]
- 269.Vlassara H. The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab Res Rev. 2001;17: 436–443. doi: 10.1002/dmrr.233 [DOI] [PubMed] [Google Scholar]
- 270.Reverri EJ, Randolph JM, Steinberg FM, Kappagoda CT, Edirisinghe I, Burton-Freeman BM. Black Beans, Fiber, and Antioxidant Capacity Pilot Study: Examination of Whole Foods vs. Functional Components on Postprandial Metabolic, Oxidative Stress, and Inflammation in Adults with Metabolic Syndrome. Nutrients. 2015;7: 6139–6154. doi: 10.3390/nu7085273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.van Leeuwen EM, Emri E, Merle BMJ, Colijn JM, Kersten E, Cougnard-Gregoire A, et al. A new perspective on lipid research in age-related macular degeneration. Prog Retin Eye Res. 2018;67: 56–86. doi: 10.1016/j.preteyeres.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 272.Yadav SPS, Sandoval RM, Zhao J, Huang Y, Wang E, Kumar S, et al. Mechanism of how carbamylation reduces albumin binding to FcRn contributing to increased vascular clearance. Am J Physiol Renal Physiol. 2021;320: F114–F129. doi: 10.1152/ajprenal.00428.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Jagannathan R, Neves JS, Dorcely B, Chung ST, Tamura K, Rhee M, et al. The Oral Glucose Tolerance Test: 100 Years Later. Diabetes Metab Syndr Obes. 2020;13: 3787–3805. doi: 10.2147/DMSO.S246062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bergman M, Manco M, Sesti G, Dankner R, Pareek M, Jagannathan R, et al. Petition to replace current OGTT criteria for diagnosing prediabetes with the 1-hour post-load plasma glucose≥155mg/dl (8.6mmol/L). Diabetes Res Clin Pract. 2018;146: 18–33. doi: 10.1016/j.diabres.2018.09.017 [DOI] [PubMed] [Google Scholar]
- 275.The Bergman M. 1-Hour Plasma Glucose: Common Link Across the Glycemic Spectrum. Front Endocrinol (Lausanne). 2021;12: 752329. doi: 10.3389/fendo.2021.752329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chawla R, Mukherjee JJ, Chawla M, Kanungo A, Shunmugavelu MS, Das AK. Expert Group Recommendations on the Effective Use of Bolus Insulin in the Management of Type 2 Diabetes Mellitus. Med Sci (Basel, Switzerland). 2021;9. doi: 10.3390/medsci9020038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Xiong Q, Li Z, Nie R, Meng X, Yang X-J. Comparison of the Effects of a Bean-Based and a White Rice-Based Breakfast Diet on Postprandial Glucose and Insulin Levels in Chinese Patients with Type 2 Diabetes. Med Sci Monit Int Med J Exp Clin Res. 2021;27: e930349. doi: 10.12659/MSM.930349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gentilcore D, Chaikomin R, Jones KL, Russo A, Feinle-Bisset C, Wishart JM, et al. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J Clin Endocrinol Metab. 2006;91: 2062–2067. doi: 10.1210/jc.2005-2644 [DOI] [PubMed] [Google Scholar]
- 279.Salleh SN, Fairus AAH, Zahary MN, Bhaskar Raj N, Mhd Jalil AM. Unravelling the Effects of Soluble Dietary Fibre Supplementation on Energy Intake and Perceived Satiety in Healthy Adults: Evidence from Systematic Review and Meta-Analysis of Randomised-Controlled Trials. Foods (Basel, Switzerland). 2019;8. doi: 10.3390/foods8010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.McRorie JWJ, Gibb RD, Sloan KJ, McKeown NM. Psyllium: The Gel-Forming Nonfermented Isolated Fiber That Delivers Multiple Fiber-Related Health Benefits. Nutr Today. 2021;56. doi: 10.1097/NT.0000000000000489 [DOI] [Google Scholar]
- 281.Wolever TMS, Johnson J, Jenkins AL, Campbell JC, Ezatagha A, Chu Y. Impact of oat processing on glycaemic and insulinaemic responses in healthy humans: a randomised clinical trial. Br J Nutr. 2019;121: 1264–1270. doi: 10.1017/S0007114519000370 [DOI] [PubMed] [Google Scholar]
- 282.Zurbau A, Noronha JC, Khan TA, Sievenpiper JL, Wolever TMS. The effect of oat β-glucan on postprandial blood glucose and insulin responses: a systematic review and meta-analysis. Eur J Clin Nutr. 2021;75: 1540–1554. doi: 10.1038/s41430-021-00875-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Huang Y-L, Tsai Y-H, Chow C-J. Water-insoluble fiber-rich fraction from pineapple peel improves intestinal function in hamsters: evidence from cecal and fecal indicators. Nutr Res. 2014;34: 346–354. doi: 10.1016/j.nutres.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 284.Müller M, Canfora EE, Blaak EE. Gastrointestinal transit time, glucose homeostasis and metabolic health: Modulation by dietary fibers. Nutrients. 2018;10. doi: 10.3390/nu10030275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Timm D, Willis H, Thomas W, Sanders L, Boileau T, Slavin J. The use of a wireless motility device (SmartPill®) for the measurement of gastrointestinal transit time after a dietary fibre intervention. Br J Nutr. 2011;105: 1337–1342. doi: 10.1017/S0007114510004988 [DOI] [PubMed] [Google Scholar]
- 286.Heshmati J, Sepidarkish M, Morvaridzadeh M, Farsi F, Tripathi N, Razavi M, et al. The effect of cinnamon supplementation on glycemic control in women with polycystic ovary syndrome: A systematic review and meta-analysis. J Food Biochem. 2021;45: e13543. doi: 10.1111/jfbc.13543 [DOI] [PubMed] [Google Scholar]
- 287.Wachters-Hagedoorn RE, Priebe MG, Heimweg JAJ, Heiner AM, Elzinga H, Stellaard F, et al. Low-dose acarbose does not delay digestion of starch but reduces its bioavailability. Diabet Med. 2007;24: 600–606. doi: 10.1111/j.1464-5491.2007.02115.x [DOI] [PubMed] [Google Scholar]
- 288.Camacho S, Michlig S, de Senarclens-Bezençon C, Meylan J, Meystre J, Pezzoli M, et al. Anti-obesity and anti-hyperglycemic effects of cinnamaldehyde via altered ghrelin secretion and functional impact on food intake and gastric emptying. Sci Rep. 2015;5: 7919. doi: 10.1038/srep07919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.He Z-D, Qiao C-F, Han Q-B, Cheng C-L, Xu H-X, Jiang R-W, et al. Authentication and quantitative analysis on the chemical profile of cassia bark (cortex cinnamomi) by high-pressure liquid chromatography. J Agric Food Chem. 2005;53: 2424–8. doi: 10.1021/jf048116s [DOI] [PubMed] [Google Scholar]
- 290.Van Hul M, Geurts L, Plovier H, Druart C, Everard A, Ståhlman M, et al. Reduced obesity, diabetes, and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am J Physiol Endocrinol Metab. 2018;314: E334–E352. doi: 10.1152/ajpendo.00107.2017 [DOI] [PubMed] [Google Scholar]
- 291.Zhu R, Liu H, Liu C, Wang L, Ma R, Chen B, et al. Cinnamaldehyde in diabetes: A review of pharmacology, pharmacokinetics and safety. Pharmacol Res. 2017;122: 78–89. doi: 10.1016/j.phrs.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 292.Mustad VA, Hegazi RA, Hustead DS, Budiman ES, Rueda R, Maki K, et al. Use of a diabetes-specific nutritional shake to replace a daily breakfast and afternoon snack improves glycemic responses assessed by continuous glucose monitoring in people with type 2 diabetes: a randomized clinical pilot study. BMJ open diabetes Res care. 2020;8. doi: 10.1136/bmjdrc-2020-001258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Alyass A, Almgren P, Akerlund M, Dushoff J, Isomaa B, Nilsson P, et al. Modelling of OGTT curve identifies 1 h plasma glucose level as a strong predictor of incident type 2 diabetes: results from two prospective cohorts. Diabetologia. 2015;58: 87–97. doi: 10.1007/s00125-014-3390-x [DOI] [PubMed] [Google Scholar]
- 294.Organization WH. Diagnosis and management of type 2 diabetes (HEARTS-D). World Heal Organ Geneva, Switz. 2020. [Google Scholar]
- 295.Glube N, Moos L von, Duchateau G. Capsule shell material impacts the in vitro disintegration and dissolution behaviour of a green tea extract. Results Pharma Sci. 2013;3: 1–6. doi: 10.1016/j.rinphs.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Markl D, Zeitler JA. A Review of Disintegration Mechanisms and Measurement Techniques. Pharm Res. 2017;34: 890–917. doi: 10.1007/s11095-017-2129-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Fülöpová N, Pavloková S, DeBono I, Vetchý D, Franc A. Development and Comparison of Various Coated Hard Capsules Suitable for Enteric Administration to Small Patient Cohorts. Pharmaceutics. 2022;14. doi: 10.3390/pharmaceutics14081577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Drew RH, Weller S, Gallis HA, Walmer KA, Bartlett JA, Blum MR. Bioequivalence assessment of zidovudine (Retrovir) syrup, solution, and capsule formulations in patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1989;33: 1801–1803. doi: 10.1128/AAC.33.10.1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Bende G, Biswal S, Bhad P, Chen Y, Salunke A, Winter S, et al. Relative bioavailability of diclofenac potassium from softgel capsule versus powder for oral solution and immediate-release tablet formulation. Clin Pharmacol drug Dev. 2016;5: 76–82. doi: 10.1002/cpdd.215 [DOI] [PubMed] [Google Scholar]
- 300.Grahnén A, Lönnebo A, Beck O, Eckernäs SA, Dahlström B, Lindström B. Pharmacokinetics of ketotifen after oral administration to healthy male subjects. Biopharm Drug Dispos. 1992;13: 255–262. doi: 10.1002/bdd.2510130404 [DOI] [PubMed] [Google Scholar]
- 301.Venier S, Grgic J, Mikulic P. Caffeinated Gel Ingestion Enhances Jump Performance, Muscle Strength, and Power in Trained Men. Nutrients. 2019;11. doi: 10.3390/nu11040937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Grgic J, Sabol F, Venier S, Tallis J, Schoenfeld BJ, Coso J Del, et al. Caffeine Supplementation for Powerlifting Competitions: An Evidence-Based Approach. J Hum Kinet. 2019;68: 37–48. doi: 10.2478/hukin-2019-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Juturu V. Capsaicinoids Modulating Cardiometabolic Syndrome Risk Factors: Current Perspectives. J Nutr Metab. 2016;2016: 4986937. doi: 10.1155/2016/4986937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Opara EI, Chohan M. Culinary herbs and spices: their bioactive properties, the contribution of polyphenols and the challenges in deducing their true health benefits. Int J Mol Sci. 2014;15: 19183–19202. doi: 10.3390/ijms151019183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Janssens PLHR, Hursel R, Westerterp-Plantenga MS. Capsaicin increases sensation of fullness in energy balance, and decreases desire to eat after dinner in negative energy balance. Appetite. 2014;77: 44–49. doi: 10.1016/j.appet.2014.02.018 [DOI] [PubMed] [Google Scholar]
- 306.Jones BE, Basit AW, Tuleu C. The disintegration behaviour of capsules in fed subjects: A comparison of hypromellose (carrageenan) capsules and standard gelatin capsules. Int J Pharm. 2012;424: 40–43. doi: 10.1016/j.ijpharm.2011.12.034 [DOI] [PubMed] [Google Scholar]
- 307.Duconseille A, Astruc T, Quintana N, Meersman F, Sante-Lhoutellier V. Gelatin structure and composition linked to hard capsule dissolution: A review. Food Hydrocoll. 2015;43: 360–376. doi: 10.1016/j.foodhyd.2014.06.006 [DOI] [Google Scholar]
- 308.Gullapalli RP, Mazzitelli CL. Gelatin and Non-Gelatin Capsule Dosage Forms. J Pharm Sci. 2017;106: 1453–1465. doi: 10.1016/j.xphs.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 309.Sager M, Schick P, Mischek M, Schulze C, Hasan M, Kromrey M-L, et al. Comparison of In Vitro and In Vivo Results Using the GastroDuo and the Salivary Tracer Technique: Immediate Release Dosage Forms under Fasting Conditions. Pharmaceutics. 2019;11. doi: 10.3390/pharmaceutics11120659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Sato-Masumoto N, Masada S, Takahashi S, Terasaki S, Yokota Y, Hakamatsuka T, et al. Disintegration Test of Health Food Products Containing Ginkgo Biloba L. or Vitex Agnus-Castus L. in the Japanese Market. Med (Basel, Switzerland). 2015;2: 47–54. doi: 10.3390/medicines2020047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Tan VMH, Wu T, Henry CJ, Lee YS. Glycaemic and insulin responses, glycaemic index and insulinaemic index values of rice between three Asian ethnic groups. Br J Nutr. 2015;113: 1228–1236. doi: 10.1017/S0007114515000586 [DOI] [PubMed] [Google Scholar]
- 312.Souptik B, Ashutosh S, Namino G, Casey C, Arianna L, Wendy B, et al. The northeast glucose drift: Stratification of post-breakfast dysglycemia among predominantly Hispanic/Latino adults at-risk or with type 2 diabetes. EClinicalMedicine. 2022;43: 101241. doi: 10.1016/j.eclinm.2021.101241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Naraba H, Goto T, Shirakawa T, Sonoo T, Kanda N, Nakano H, et al. Time in blood glucose range 70 to 180 mg/dL and survival rate in critically ill patients: A retrospective cohort study. PLoS One. 2021;16: e0252158. doi: 10.1371/journal.pone.0252158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Jackson SL, Safo SE, Staimez LR, Olson DE, Narayan KM V, Long Q, et al. Glucose challenge test screening for prediabetes and early diabetes. Diabet Med. 2017;34: 716–724. doi: 10.1111/dme.13270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Klonoff DC, Wang J, Rodbard D, Kohn MA, Li C, Liepmann D, et al. A Glycemia Risk Index (GRI) of Hypoglycemia and Hyperglycemia for Continuous Glucose Monitoring Validated by Clinician Ratings. J Diabetes Sci Technol. 2022; 19322968221085270. doi: 10.1177/19322968221085273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Haschka SJ, Gar C, Potzel AL, Sacco V, Kern-Matschilles S, Benz I, et al. A Normalized Real-Life Glucose Profile After Diet-Induced Remission of Type 2 Diabetes: A Pilot Trial. Cureus. 2022;14: e23916. doi: 10.7759/cureus.23916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Wolever TMS, Brand-Miller JC, Abernethy J, Astrup A, Atkinson F, Axelsen M, et al. Measuring the glycemic index of foods: interlaboratory study. Am J Clin Nutr. 2008;87: 247S–257S. doi: 10.1093/ajcn/87.1.247S [DOI] [PubMed] [Google Scholar]
- 318.Mattes RD, Hollis J, Hayes D, Stunkard AJ. Appetite: measurement and manipulation misgivings. J Am Diet Assoc. 2005;105: S87–97. doi: 10.1016/j.jada.2005.02.029 [DOI] [PubMed] [Google Scholar]
- 319.Benelam B. Satiatio, satiety and their effects on eating behaviour. Nutr Bull. 2009;34: 126–173. doi: 10.1111/j.1467-3010.2009.01753.x [DOI] [Google Scholar]
- 320.Stubbs RJ, Johnstone AM, O’Reilly LM, Poppitt SD. Methodological issues relating to the measurement of food, energy and nutrient intake in human laboratory-based studies. Proc Nutr Soc. 1998;57: 357–372. doi: 10.1079/pns19980053 [DOI] [PubMed] [Google Scholar]
- 321.Livingstone MBE, Robson PJ, Welch RW, Burns AA, Burrows MS, McCormack C. Methodological issues in the assessment of satiety. Näringsforskning. 2000;44: 98–103. doi: 10.3402/fnr.v44i0.1776 [DOI] [Google Scholar]
- 322.Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, et al. Appetite control: methodological aspects of the evaluation of foods. Obes Rev an Off J Int Assoc Study Obes. 2010;11: 251–270. doi: 10.1111/j.1467-789X.2010.00714.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Gough T, Haynes A, Clarke K, Hansell A, Kaimkhani M, Price B, et al. Out of the lab and into the wild: The influence of portion size on food intake in laboratory vs. real-world settings. Appetite. 2021;162: 105160. doi: 10.1016/j.appet.2021.105160 [DOI] [PubMed] [Google Scholar]
- 324.Kristensen M, Pelletier X, Ross AB, Thielecke F. A High Rate of Non-Compliance Confounds the Study of Whole Grains and Weight Maintenance in a Randomised Intervention Trial-The Case for Greater Use of Dietary Biomarkers in Nutrition Intervention Studies. Nutrients. 2017;9. doi: 10.3390/nu9010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Jones JM, García CG, Braun HJ. Perspective: Whole and Refined Grains and Health-Evidence Supporting “Make Half Your Grains Whole”. Adv Nutr. 2020;11: 492–506. doi: 10.1093/advances/nmz114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sadeghi O, Sadeghian M, Rahmani S, Maleki V, Larijani B, Esmaillzadeh A. Whole-Grain Consumption Does Not Affect Obesity Measures: An Updated Systematic Review and Meta-analysis of Randomized Clinical Trials. Adv Nutr. 2020;11: 280–292. doi: 10.1093/advances/nmz076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Grammatikopoulou MG, Nigdelis MP, Theodoridis X, Gkiouras K, Tranidou A, Papamitsou T, et al. How fragile are Mediterranean diet interventions? A research-on-research study of randomised controlled trials. BMJ Nutr Prev Heal. 2021;4: 115–131. doi: 10.1136/bmjnph-2020-000188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Dashti HS, Scheer FAJL, Saxena R, Garaulet M. Timing of Food Intake: Identifying Contributing Factors to Design Effective Interventions. Adv Nutr. 2019;10: 606–620. doi: 10.1093/advances/nmy131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Singh N, Rao AS, Nandal A, Kumar S, Yadav SS, Ganaie SA, et al. Phytochemical and pharmacological review of Cinnamomum verum J. Presl-a versatile spice used in food and nutrition. Food Chem. 2021;338: 127773. doi: 10.1016/j.foodchem.2020.127773 [DOI] [PubMed] [Google Scholar]
- 330.Alam F, Shafique Z, Amjad ST, Bin Asad MHH. Enzymes inhibitors from natural sources with antidiabetic activity: A review. Phytother Res. 2019;33: 41–54. doi: 10.1002/ptr.6211 [DOI] [PubMed] [Google Scholar]
- 331.Miah MA, Himel MH, Sujan KM, Mustari A, Haque MI. Protective effects of cinnamon powder against hyperlipidemia and hepatotoxicity in butter fed female albino mice. Saudi J Biol Sci. 2022;29: 3069–3074. doi: 10.1016/j.sjbs.2022.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Alam S, Sarker MMR, Sultana TN, Chowdhury MNR, Rashid MA, Chaity NI, et al. Antidiabetic Phytochemicals From Medicinal Plants: Prospective Candidates for New Drug Discovery and Development. Front Endocrinol (Lausanne). 2022;13: 800714. doi: 10.3389/fendo.2022.800714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Barzkar F, Baradaran HR, Khamseh ME, Vesal Azad R, Koohpayehzadeh J, Moradi Y. Medicinal plants in the adjunctive treatment of patients with type-1 diabetes: a systematic review of randomized clinical trials. J Diabetes Metab Disord. 2020;19: 1917–1929. doi: 10.1007/s40200-020-00633-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Rahman MM, Islam MR, Shohag S, Hossain ME, Rahaman MS, Islam F, et al. The Multifunctional Role of Herbal Products in the Management of Diabetes and Obesity: A Comprehensive Review. Molecules. 2022;27. doi: 10.3390/molecules27051713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Saad B, Kmail A, Haq SZH. Anti-Diabesity Middle Eastern Medicinal Plants and Their Action Mechanisms. Evid Based Complement Alternat Med. 2022;2022: 2276094. doi: 10.1155/2022/2276094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Kahksha, Alam O, Naaz S, Sharma V, Manaithiya A, Khan J, et al. Recent developments made in the assessment of the antidiabetic potential of gymnema species ‐ From 2016 to 2020. J Ethnopharmacol. 2022;286: 114908. doi: 10.1016/j.jep.2021.114908 [DOI] [PubMed]
- 337.Medagama AB. The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Nutr J. 2015;14: 108. doi: 10.1186/s12937-015-0098-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Jacob B, Narendhirakannan RT. Role of medicinal plants in the management of diabetes mellitus: a review. 3 Biotech. 2019;9: 4. doi: 10.1007/s13205-018-1528-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Dou L, Zheng Y, Li L, Gui X, Chen Y, Yu M, et al. The effect of cinnamon on polycystic ovary syndrome in a mouse model. Reprod Biol Endocrinol. 2018;16: 99. doi: 10.1186/s12958-018-0418-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Omale S, Amagon KI, Johnson TO, Bremner SK, Gould GW. A systematic analysis of anti-diabetic medicinal plants from cells to clinical trials. PeerJ. 2023;11: e14639. doi: 10.7717/peerj.14639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Rao PV, Gan SH. Cinnamon: A multifaceted medicinal plant. Evid Based Complement Alternat Med. 2014;2014: 642942. doi: 10.1155/2014/642942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Yazdanpanah Z, Azadi-Yazdi M, Hooshmandi H, Ramezani-Jolfaie N, Salehi-Abargouei A. Effects of cinnamon supplementation on body weight and composition in adults: A systematic review and meta-analysis of controlled clinical trials. Phytother Res. 2020;34: 448–463. doi: 10.1002/ptr.6539 [DOI] [PubMed] [Google Scholar]
- 343.Helal A, Tagliazucchi D, Verzelloni E, Conte A. Bioaccessibility of polyphenols and cinnamaldehyde in cinnamon beverages subjected to in vitro gastro-pancreatic digestion. J Funct Foods. 2014;7: 506–516. doi: 10.1016/j.jff.2014.01.005 [DOI] [Google Scholar]
- 344.Bhatia A, Singh B, Arora R, Arora S. In vitro evaluation of the α-glucosidase inhibitory potential of methanolic extracts of traditionally used antidiabetic plants. BMC Complement Altern Med. 2019;19: 74. doi: 10.1186/s12906-019-2482-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Ghannadiasl F, Bordbar Lomer B. Nutraceutical in the Management of Diabetes Mellitus: A Review. Iran J Diabetes Obes. 2022;14. doi: 10.18502/ijdo.v14i4.11232 [DOI] [Google Scholar]
- 346.Ota A, Ulrih NP. An Overview of Herbal Products and Secondary Metabolites Used for Management of Type Two Diabetes. Front Pharmacol. 2017;8: 436. doi: 10.3389/fphar.2017.00436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Governa P, Baini G, Borgonetti V, Cettolin G, Giachetti D, Magnano AR, et al. Phytotherapy in the Management of Diabetes: A Review. Molecules. 2018;23. doi: 10.3390/molecules23010105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Mohsin SN, Saleem F, Humayun A, Tanweer A, Muddassir A. Prospective {Nutraceutical} {Effects} of {Cinnamon} {Derivatives} {Against} {Insulin} {Resistance} in {Type} {II} {Diabetes} {Mellitus}-{Evidence} {From} the {Literature}. Dose Response. 2023;21: 15593258231200528. doi: 10.1177/15593258231200527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Ansari P, Samia JF, Khan JT, Rafi MR, Rahman MS, Rahman AB, et al. Protective Effects of Medicinal Plant-Based Foods against Diabetes: A Review on Pharmacology, Phytochemistry, and Molecular Mechanisms. Nutrients. 2023;15. doi: 10.3390/nu15143266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Shen Y, Fukushima M, Ito Y, Muraki E, Hosono T, Seki T, et al. Verification of the antidiabetic effects of cinnamon (Cinnamomum zeylanicum) using insulin-uncontrolled type 1 diabetic rats and cultured adipocytes. Biosci Biotechnol Biochem. 2010;74: 2418–2425. doi: 10.1271/bbb.100453 [DOI] [PubMed] [Google Scholar]
- 351.Ismail HF, Hashim Z, Zaidel DNA, Zainol SN, Tap FM, Majid FAA, et al. Triple-action of the standardized antidiabetic polyherbal extract; Synacinn(TM) through upregulation of GLUT(4) and inhibition of DPP(IV), α-amylase, and α-glucosidase activity. Med J Malaysia. 2022;77: 16–22. [PubMed] [Google Scholar]
- 352.Nishikai-Shen T, Hosono-Fukao T, Ariga T, Hosono T, Seki T. Cinnamon extract improves abnormalities in glucose tolerance by decreasing Acyl-CoA synthetase long-chain family 1 expression in adipocytes. Scientific reports. Department of Chemistry and Life Science, College of Bioresource Sciences, Nihon University, Kanagawa, 252–0880, Japan.; 2022. p. 12574. doi: 10.1038/s41598-022-13421-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Li J-E, Futawaka K, Yamamoto H, Kasahara M, Tagami T, Liu T-H, et al. Cinnamaldehyde contributes to insulin sensitivity by activating PPARδ, PPARγ, and RXR. Am J Chin Med. 2015;43: 879–892. doi: 10.1142/S0192415X15500512 [DOI] [PubMed] [Google Scholar]
- 354.Lee SG, Siaw JA, Kang HW. Stimulatory Effects of Cinnamon Extract (Cinnamomum cassia) during the Initiation Stage of 3T3-L1 Adipocyte Differentiation. Foods (Basel, Switzerland). 2016;5. doi: 10.3390/foods5040083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Sartorius T, Peter A, Schulz N, Drescher A, Bergheim I, Machann J, et al. Cinnamon extract improves insulin sensitivity in the brain and lowers liver fat in mouse models of obesity. PLoS One. 2014;9: e92358. doi: 10.1371/journal.pone.0092358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Sriramavaratharajan V, Chellappan DR, Karthi S, Ilamathi M, Murugan R. Multi target interactions of essential oil nanoemulsion of Cinnamomum travancoricum against diabetes mellitus via in vitro, in vivo and in silico approaches. Process Biochem. 2022;118: 190–204. doi: 10.1016/j.procbio.2022.04.031 [DOI] [Google Scholar]
- 357.Yanakiev S. Effects of Cinnamon (Cinnamomum spp.) in Dentistry: A Review. Molecules. 2020;25. doi: 10.3390/molecules25184184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Azimian L, Weerasuriya NM, Munasinghe R, Song S, Lin C-Y, You L. Investigating the effects of {Ceylon} cinnamon water extract on {HepG2} cells for {Type} 2 diabetes therapy. Cell Biochem Funct. 2023;41: 254–267. doi: 10.1002/cbf.3778 [DOI] [PubMed] [Google Scholar]
- 359.Verspohl EJ, Bauer K, Neddermann E. Antidiabetic effect of Cinnamomum cassia and Cinnamomum zeylanicum in vivo and in vitro. Phytother Res. 2005;19: 203–206. doi: 10.1002/ptr.1643 [DOI] [PubMed] [Google Scholar]
- 360.Usman MI, Alhassan AJ, Saיad H. Antihyperglycemic Effects of Aqueous Extracts of Zingiber Officinale, Cinnamonum Zeylanicum and their Combination in Experimental Rats. Int J Biotechnol. 2018;7: 25–30. doi: 10.18488/journal.57.2018.71.25.30 [DOI] [Google Scholar]
- 361.Lira Neto JCG, Damasceno MMC, Ciol MA, de Freitas RWJF, de Araújo MFM, Teixeira CR de S, et al. Efficacy of cinnamon as an adjuvant in reducing the glycemic biomarkers of type 2 diabetes mellitus: A three-month, randomized, triple-blind, placebo-controlled clinical trial. J Am Coll Nutr. 2021; 1–9. doi: 10.1080/07315724.2021.1878967 [DOI] [PubMed] [Google Scholar]
- 362.Shannon CE, Ragavan M, Palavicini JP, Fourcaudot M, Bakewell TM, Valdez IA, et al. Insulin resistance is mechanistically linked to hepatic mitochondrial remodeling in non-alcoholic fatty liver disease. Mol Metab. 2021;45: 101154. doi: 10.1016/j.molmet.2020.101154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Nishimura Y, Inoue Y, Takeuchi H, Oka Y. Acute effects of pioglitazone on glucose metabolism in perfused rat liver. Acta Diabetol. 1997;34: 206–210. doi: 10.1007/s005920050075 [DOI] [PubMed] [Google Scholar]
- 364.Colca JR, McDonald WG, Cavey GS, Cole SL, Holewa DD, Brightwell-Conrad AS, et al. Identification of a mitochondrial target of thiazolidinedione insulin sensitizers (mTOT)—relationship to newly identified mitochondrial pyruvate carrier proteins. PLoS One. 2013;8: e61551. doi: 10.1371/journal.pone.0061551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Lee MK, Olefsky JM. Acute effects of troglitazone on in vivo insulin action in normal rats. Metabolism. 1995;44: 1166–1169. doi: 10.1016/0026-0495(95)90010-1 [DOI] [PubMed] [Google Scholar]
- 366.Muñoz-Gómez RJ, Rivero-Cruz I, Ovalle-Magallanes B, Linares E, Bye R, Tovar AR, et al. Antidiabetic Sterols from Peniocereus greggii Roots. ACS omega. 2022;7: 13144–13154. doi: 10.1021/acsomega.2c00595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Kasabri V, Al-Hallaq EK, Bustanji YK, Abdul-Razzak KK, Abaza IF, Afifi FU. Antiobesity and antihyperglycaemic effects of Adiantum capillus-veneris extracts: in vitro and in vivo evaluations. Pharm Biol. 2017;55: 164–172. doi: 10.1080/13880209.2016.1233567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Bailey CJ, Mynett KJ, Page T. Importance of the intestine as a site of metformin-stimulated glucose utilization. Br J Pharmacol. 1994;112: 671–675. doi: 10.1111/j.1476-5381.1994.tb13128.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Koffert JP, Mikkola K, Virtanen KA, Andersson A-MD, Faxius L, Hällsten K, et al. Metformin treatment significantly enhances intestinal glucose uptake in patients with type 2 diabetes: Results from a randomized clinical trial. Diabetes Res Clin Pract. 2017;131: 208–216. doi: 10.1016/j.diabres.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 370.Ashe S, Hebrok M. Role of Cell-Based Therapies in T2D. Semin Nephrol. 2023;43: 151432. doi: 10.1016/j.semnephrol.2023.151432 [DOI] [PubMed] [Google Scholar]
- 371.Divakaruni AS, Wiley SE, Rogers GW, Andreyev AY, Petrosyan S, Loviscach M, et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc Natl Acad Sci U S A. 2013;110: 5422–5427. doi: 10.1073/pnas.1303360110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Zhang M, Hu M, Alles SRA, Montera MA, Adams I, Santi MD, et al. Peroxisome proliferator-activated receptor gamma agonist ELB00824 suppresses oxaliplatin-induced pain, neuronal hypersensitivity, and oxidative stress. Neuropharmacology. 2022;218: 109233. doi: 10.1016/j.neuropharm.2022.109233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.LeBrasseur NK, Kelly M, Tsao T-S, Farmer SR, Saha AK, Ruderman NB, et al. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab. 2006;291: E175–81. doi: 10.1152/ajpendo.00453.2005 [DOI] [PubMed] [Google Scholar]
- 374.Mohamed Sham Shihabudeen H, Hansi Priscilla D, Thirumurugan K. Cinnamon extract inhibits α-glucosidase activity and dampens postprandial glucose excursion in diabetic rats. Nutr Metab (Lond). 2011;8: 46. doi: 10.1186/1743-7075-8-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Mettler S, Schwarz I, Colombani PC. Additive postprandial blood glucose-attenuating and satiety-enhancing effect of cinnamon and acetic acid. Nutr Res. 2009;29: 723–7. doi: 10.1016/j.nutres.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 376.Gutierrez JL, Cooke M, Lutz R, Rodney B, Kane R, Willoughby D. Cassia cinnamon supplementation before an oral glucose tolerance test in overweight or obese young women. FASEB J. 2010;24. doi: 10.1096/fasebj.24.1_supplement.540.14 [DOI] [Google Scholar]
- 377.Gutierrez JL, Bowden RG, Willoughby DS. Cassia Cinnamon supplementation reduces peak blood glucose responses but does not improve insulin resistance and sensitivity in young, sedentary, obese women. J Diet Suppl. 2016;13: 461–71. doi: 10.3109/19390211.2015.1110222 [DOI] [PubMed] [Google Scholar]
- 378.Rachid AP, Moncada M, Mesquita MF de, Brito J, Bernardo MA, Silva ML. Effect of Aqueous Cinnamon Extract on the Postprandial Glycemia Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Nutrients. 2022;14: 1576. doi: 10.3390/nu14081576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Wickenberg J, Lindstedt S, Berntorp K, Nilsson J, Hlebowicz J. Ceylon cinnamon does not affect postprandial plasma glucose or insulin in subjects with impaired glucose tolerance. Br J Nutr. 2012;107: 1845–1849. doi: 10.1017/S0007114511005113 [DOI] [PubMed] [Google Scholar]
- 380.Solomon TPJ, Blannin AK. Changes in glucose tolerance and insulin sensitivity following 2 weeks of daily cinnamon ingestion in healthy humans. Eur J Appl Physiol. 2009;105: 969–76. doi: 10.1007/s00421-009-0986-9 [DOI] [PubMed] [Google Scholar]
- 381.Solomon TPJ, Blannin AK. Effects of short-term cinnamon ingestion on in vivo glucose tolerance. Diabetes Obes Metab. 2007;9: 895–901. doi: 10.1111/j.1463-1326.2006.00694.x [DOI] [PubMed] [Google Scholar]
- 382.Lin S, Wang Z, Lam K-L, Zeng S, Tan BK, Hu J. Role of intestinal microecology in the regulation of energy metabolism by dietary polyphenols and their metabolites. Food Nutr Res. 2019;63. doi: 10.29219/fnr.v63.1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Heghes SC, Filip L, Vostinaru O, Mogosan C, Miere D, Iuga CA, et al. Essential Oil-Bearing Plants From Balkan Peninsula: Promising Sources for New Drug Candidates for the Prevention and Treatment of Diabetes Mellitus and Dyslipidemia. Front Pharmacol. 2020;11: 989. doi: 10.3389/fphar.2020.00989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Sood A, Kumar B, Singh SK, Prashar P, Gautam A, Gulati M, et al. Flavonoids as Potential Therapeutic Agents for the Management of Diabetic Neuropathy. Curr Pharm Des. 2020;26: 5468–5487. doi: 10.2174/1381612826666200826164322 [DOI] [PubMed] [Google Scholar]
- 385.Ortega R, Valdés M, Alarcón-Aguilar FJ, Fortis-Barrera Á, Barbosa E, Velazquez C, et al. Antihyperglycemic Effects of Salvia polystachya Cav. and Its Terpenoids: α-Glucosidase and SGLT1 Inhibitors. Plants (Basel, Switzerland). 2022;11. doi: 10.3390/plants11050575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Müller U, Stübl F, Schwarzinger B, Sandner G, Iken M, Himmelsbach M, et al. In Vitro and In Vivo Inhibition of Intestinal Glucose Transport by Guava (Psidium Guajava) Extracts. Mol Nutr Food Res. 2018;62: e1701012. doi: 10.1002/mnfr.201701012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Kreydiyyeh SI, Usta J, Copti R. Effect of cinnamon, clove and some of their constituents on the Na(+)-K(+)-ATPase activity and alanine absorption in the rat jejunum. Food Chem Toxicol. 2000;38: 755–62. doi: 10.1016/s0278-6915(00)00073-9 [DOI] [PubMed] [Google Scholar]
- 388.Usta J, Kreydiyyeh S, Barnabe P, Bou-Moughlabay Y, Nakkash-Chmaisse H. Comparative study on the effect of cinnamon and clove extracts and their main components on different types of ATPases. Hum Exp Toxicol. 2003;22: 355–362. doi: 10.1191/0960327103ht379oa [DOI] [PubMed] [Google Scholar]
- 389.Liu Y, Pang D, Xing D, Wang W, Li Q, Liao S, et al. Cinnamon free phenolic extract regulates glucose absorption in intestinal cells by inhibiting glucose transporters. Food Biosci. 2023;52: 102405. doi: 10.1016/j.fbio.2023.102405 [DOI] [Google Scholar]
- 390.Qusti S, El Rabey HA, Balashram SA. The Hypoglycemic and Antioxidant Activity of Cress Seed and Cinnamon on Streptozotocin Induced Diabetes in Male Rats. Evid Based Complement Alternat Med. 2016;2016: 5614564. doi: 10.1155/2016/5614564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Shang C, Lin H, Fang X, Wang Y, Jiang Z, Qu Y, et al. Beneficial effects of cinnamon and its extracts in the management of cardiovascular diseases and diabetes. Food Funct. 2021;12: 12194–12220. doi: 10.1039/d1fo01935j [DOI] [PubMed] [Google Scholar]
- 392.Shen Y, Honma N, Kobayashi K, Jia LN, Hosono T, Shindo K, et al. Cinnamon extract enhances glucose uptake in 3T3-L1 adipocytes and C2C12 myocytes by inducing LKB1-AMP-activated protein kinase signaling. PLoS One. 2014;9: e87894. doi: 10.1371/journal.pone.0087894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Nikzamir A, Palangi A, Kheirollaha A, Tabar H, Malakaskar A, Shahbazian H, et al. Expression of Glucose Transporter 4 (GLUT4) is Increased by Cinnamaldehyde in C2C12 Mouse Muscle Cells. Iran Red Crescent Med J. 2014;16: e13426. doi: 10.5812/ircmj.13426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Anand P, Murali KY, Tandon V, Murthy PS, Chandra R. Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chem Biol Interact. 2010;186: 72–81. doi: 10.1016/j.cbi.2010.03.044 [DOI] [PubMed] [Google Scholar]
- 395.Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res Clin Pract. 2003;62: 139–148. doi: 10.1016/s0168-8227(03)00173-6 [DOI] [PubMed] [Google Scholar]
- 396.Li H, Zhou J, Liu S, Chen X, Qin T, Huang G, et al. Cinnamomum cassia Presl flavonoids prevent hyperglycemia-induced cognitive impairment via inhibiting of AGEs accumulation and oxidative stress. J Funct Foods. 2023;100: 105374. doi: 10.1016/j.jff.2022.105374 [DOI] [Google Scholar]
- 397.Liu Y, Liu F, Xing D, Wang W, Yang Q, Liao S, et al. Effects of Cinnamon Powder on Glucose Metabolism in Diabetic Mice and the Molecular Mechanisms. Foods (Basel, Switzerland). 2023;12. doi: 10.3390/foods12203852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Scheen AJ. Is there a role for alpha-glucosidase inhibitors in the prevention of type 2 diabetes mellitus? Drugs. 2003;63: 933–51. doi: 10.2165/00003495-200363100-00002 [DOI] [PubMed] [Google Scholar]
- 399.Honma K, Jin F, Tonaka R, Sabashi T, Otsuki N, Ichikawa Y, et al. Changes in peripheral inflammation-related gene expression by postprandial glycemic response in healthy Japanese men. Nutrition. 2021;84: 111026. doi: 10.1016/j.nut.2020.111026 [DOI] [PubMed] [Google Scholar]
- 400.Kaur N, Kumar V, Nayak SK, Wadhwa P, Kaur P, Sahu SK. Alpha-amylase as molecular target for treatment of diabetes mellitus: A comprehensive review. Chem Biol Drug Des. 2021;98: 539–560. doi: 10.1111/cbdd.13909 [DOI] [PubMed] [Google Scholar]
- 401.Standl E, Theodorakis MJ, Erbach M, Schnell O, Tuomilehto J. On the potential of acarbose to reduce cardiovascular disease. Cardiovasc Diabetol. 2014;13: 81. doi: 10.1186/1475-2840-13-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Wang G, Liu J, Yang N, Gao X, Fan H, Xu Y, et al. MARCH2: comparative assessment of therapeutic effects of acarbose and metformin in newly diagnosed type 2 diabetes patients. PLoS One. 2014;9: e105698. doi: 10.1371/journal.pone.0105698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Fu J, Liu J, Xu Y, Yang N, Yang W, Wang G. Comparison of therapeutic effects of acarbose and metformin under different β-cell function status in Chinese patients with type 2 diabetes. Endocr J. 2019;66: 443–450. doi: 10.1507/endocrj.EJ18-0466 [DOI] [PubMed] [Google Scholar]
- 404.Yang W, Liu J, Shan Z, Tian H, Zhou Z, Ji Q, et al. Acarbose compared with metformin as initial therapy in patients with newly diagnosed type 2 diabetes: an open-label, non-inferiority randomised trial. lancet Diabetes Endocrinol. 2014;2: 46–55. doi: 10.1016/S2213-8587(13)70021-4 [DOI] [PubMed] [Google Scholar]
- 405.Herrera JJ, Pifer K, Louzon S, Leander D, Fiehn O, Day SM, et al. Early or late-life treatment with acarbose or rapamycin improves physical performance and affects cardiac structure in aging mice. J Gerontol A Biol Sci Med Sci. 2022. doi: 10.1093/gerona/glac221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Sears B, Saha AK. Dietary Activation of AMP-Activated Protein Kinase (AMPK) to Treat Insulin Resistance. In: Infante M, editor. Evolving Concepts in Insulin Resistance. Rijeka: IntechOpen; 2022. doi: 10.5772/intechopen.103787 [DOI] [Google Scholar]
- 407.Madeo F, Carmona-Gutierrez D, Hofer SJ, Kroemer G. Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab. 2019;29: 592–610. doi: 10.1016/j.cmet.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 408.Dong D, Cai G-Y, Ning Y-C, Wang J-C, Lv Y, Hong Q, et al. Alleviation of senescence and epithelial-mesenchymal transition in aging kidney by short-term caloric restriction and caloric restriction mimetics via modulation of AMPK/mTOR signaling. Oncotarget. 2017;8: 16109–16121. doi: 10.18632/oncotarget.14884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Hanefeld M. The role of acarbose in the treatment of non-insulin-dependent diabetes mellitus. J Diabetes Complications. 1998;12: 228–237. doi: 10.1016/s1056-8727(97)00123-2 [DOI] [PubMed] [Google Scholar]
- 410.DiNicolantonio JJ, Bhutani J, O’Keefe JH. Acarbose: Safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Hear. 2015;2: e000327. doi: 10.1136/openhrt-2015-000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Ahr HJ, Boberg M, Krause HP, Maul W, Müller FO, Ploschke HJ, et al. Pharmacokinetics of acarbose. Part I: Absorption, concentration in plasma, metabolism and excretion after single administration of [14C]acarbose to rats, dogs and man. Arzneimittelforschung. 1989;39: 1254–1260. [PubMed] [Google Scholar]
- 412.Balaich J, Estrella M, Wu G, Jeffrey PD, Biswas A, Zhao L, et al. The human microbiome encodes resistance to the antidiabetic drug acarbose. Nature. 2021;600: 110–115. doi: 10.1038/s41586-021-04091-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13: 273–282. doi: 10.1111/acel.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Chan K-C, Yu M-H, Lin M-C, Huang C-N, Chung D-J, Lee Y-J, et al. Pleiotropic effects of acarbose on atherosclerosis development in rabbits are mediated via upregulating AMPK signals. Sci Rep. 2016;6: 38642. doi: 10.1038/srep38642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Dolan PL, Tapscott EB, Peterson RG, Dohm GL. Effects of feeding acarbose on muscle glucose transport and GLUT4 protein in lean and obese diabetic (ZDFGmi-fa) rats. J Nutr Biochem. 1997;8: 322–327. doi: 10.1016/S0955-2863(97)84448-8 [DOI] [Google Scholar]
- 416.Yu S-Y, Kwon Y-I, Lee C, Apostolidis E, Kim Y-C. Antidiabetic effect of chitosan oligosaccharide (GO2KA1) is mediated via inhibition of intestinal alpha-glucosidase and glucose transporters and PPARγ expression. Biofactors. 2017;43: 90–99. doi: 10.1002/biof.1311 [DOI] [PubMed] [Google Scholar]
- 417.Han X, Deng Y, Yu J, Sun Y, Ren G, Cai J, et al. Acarbose Accelerates Wound Healing via Akt/eNOS Signaling in db/db Mice. Oxid Med Cell Longev. 2017;2017: 7809581. doi: 10.1155/2017/7809581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Ledwig D, Müller H, Bischoff H, Eckel J. Early acarbose treatment ameliorates resistance of insulin-regulated GLUT4 trafficking in obese Zucker rats. Eur J Pharmacol. 2002;445: 141–148. doi: 10.1016/s0014-2999(02)01714-4 [DOI] [PubMed] [Google Scholar]
- 419.Rosak C, Nitzsche G, König P, Hofmann U. The effect of the timing and the administration of acarbose on postprandial hyperglycaemia. Diabet Med. 1995;12: 979–84. doi: 10.1111/j.1464-5491.1995.tb00409.x [DOI] [PubMed] [Google Scholar]
- 420.Rosak C, Mertes G. Critical evaluation of the role of acarbose in the treatment of diabetes: patient considerations. Diabetes Metab Syndr Obes. 2012;5: 357–367. doi: 10.2147/DMSO.S28340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Giri B, Dey S, Das T, Sarkar M, Banerjee J, Dash SK. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed Pharmacother. 2018;107: 306–328. doi: 10.1016/j.biopha.2018.07.157 [DOI] [PubMed] [Google Scholar]
- 422.Nakhaee A, Sanjari M. Evaluation of effect of acarbose consumption on weight losing in non-diabetic overweight or obese patients in Kerman. J Res Med Sci Off J Isfahan Univ Med Sci. 2013;18: 391–394. [PMC free article] [PubMed] [Google Scholar]
- 423.Khalili N, Safavipour A. Evaluation of the Effects of Acarbose on Weight and Metabolic, Inflammatory, and Cardiovascular Markers in Patients with Obesity and Overweight. Int J Prev Med. 2020;11: 140. doi: 10.4103/ijpvm.IJPVM_229_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Mo D, Liu S, Ma H, Tian H, Yu H, Zhang X, et al. Effects of acarbose and metformin on the inflammatory state in newly diagnosed type 2 diabetes patients: a one-year randomized clinical study. Drug Des Devel Ther. 2019;13: 2769–2776. doi: 10.2147/DDDT.S208327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Zhang F, Xu S, Tang L, Pan X, Tong N. Acarbose with comparable glucose-lowering but superior weight-loss efficacy to dipeptidyl peptidase-4 inhibitors: A systematic review and network meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne). 2020;11: 288. doi: 10.3389/fendo.2020.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Hsu P-F, Sung S-H, Cheng H-M, Shin S-J, Lin K-D, Chong K, et al. Cardiovascular Benefits of Acarbose vs Sulfonylureas in Patients With Type 2 Diabetes Treated With Metformin. J Clin Endocrinol Metab. 2018;103: 3611–3619. doi: 10.1210/jc.2018-00040 [DOI] [PubMed] [Google Scholar]
- 427.Lee S-C, Xu W-X, Lin L-Y, Yang J-J, Liu C-T. Chemical composition and hypoglycemic and pancreas-protective effect of leaf essential oil from indigenous cinnamon (Cinnamomum osmophloeum Kanehira). J Agric Food Chem. 2013;61: 4905–4913. doi: 10.1021/jf401039z [DOI] [PubMed] [Google Scholar]
- 428.Walter-Sack IE, Ittner-Holland A, Wolfram G, Zoellner N. Effect of acarbose on carbohydrate tolerance during administration of a fibre-free formula diet on healthy subjects. Eur J Clin Pharmacol. 1986;30: 607–614. doi: 10.1007/BF00542422 [DOI] [PubMed] [Google Scholar]
- 429.Santilli F, Formoso G, Sbraccia P, Averna M, Miccoli R, Di Fulvio P, et al. Postprandial hyperglycemia is a determinant of platelet activation in early type 2 diabetes mellitus. J Thromb Haemost. 2010;8: 828–837. doi: 10.1111/j.1538-7836.2010.03742.x [DOI] [PubMed] [Google Scholar]
- 430.Li M, Huang X, Ye H, Chen Y, Yu J, Yang J, et al. Randomized, Double-Blinded, Double-Dummy, Active-Controlled, and Multiple-Dose Clinical Study Comparing the Efficacy and Safety of Mulberry Twig (Ramulus Mori, Sangzhi) Alkaloid Tablet and Acarbose in Individuals with Type 2 Diabetes Mellitus. Evid Based Complement Alternat Med. 2016;2016: 7121356. doi: 10.1155/2016/7121356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Bai Y-H, Shi D-X, Lu H-Y, Yang K-B, Zhao H-H, Lu B-N, et al. Hypoglycemic effects of Tibetan medicine Huidouba in STZ-induced diabetic mice and db/db mice. Chinese Herb Med. 2021;13: 202–209. doi: 10.1016/j.chmed.2021.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Youn J-Y, Park H-Y, Cho K-H. Anti-hyperglycemic activity of Commelina communis L.: inhibition of alpha-glucosidase. Diabetes Res Clin Pract. 2004;66 Suppl 1: S149-55. doi: 10.1016/j.diabres.2003.08.015 [DOI] [PubMed] [Google Scholar]
- 433.Hall SE, Bolton TM, Hetenyi G. The effect of bran on glucose kinetics and plasma insulin in non-insulin-dependent diabetes mellitus. Diabetes Care. 1980;3: 520–5. doi: 10.2337/diacare.3.4.520 [DOI] [PubMed] [Google Scholar]
- 434.Cherbut C, Bruley Des Varannes S, Schnee M, Rival M, Galmiche J-P, Delort-Laval J. Involvement of small intestinal motility in blood glucose response to dietary fibre in man. Br J Nutr. 1994;71: 675–685. doi: 10.1079/bjn19940175 [DOI] [PubMed] [Google Scholar]
- 435.Hamberg O, Rumessen JJ, Gudmand-Høyer E. Blood glucose response to pea fiber: comparisons with sugar beet fiber and wheat bran. Am J Clin Nutr. 1989;50: 324–328. doi: 10.1093/ajcn/50.2.324 [DOI] [PubMed] [Google Scholar]
- 436.Wang J-S, Lee I-T, Lee W-J, Lin S-D, Su S-L, Tu S-T, et al. Glycemic excursions are positively associated with HbA1c reduction from baseline after treatment with acarbose in patients with type 2 diabetes on metformin monotherapy. J Diabetes. 2017;9: 248–255. doi: 10.1111/1753-0407.12406 [DOI] [PubMed] [Google Scholar]
- 437.Joubert PH, Venter HL, Foukaridis GN. The effect of miglitol and acarbose after an oral glucose load: A novel hypoglycaemic mechanism? Br J Clin Pharmacol. 1990;30: 391–6. doi: 10.1111/j.1365-2125.1990.tb03789.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Riyaphan J, Jhong C-H, Lin S-R, Chang C-H, Tsai M-J, Lee D-N, et al. Hypoglycemic Efficacy of Docking Selected Natural Compounds against α-Glucosidase and α-Amylase. Molecules. 2018;23. doi: 10.3390/molecules23092260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Zayapor MN, Abdullah A, Wan Mustapha WA. The antioxidant analysis and α-glucosidase inhibition activities of spices and herbs (22 species) in Asian traditional beverages. J Food Meas Charact. 2021;15: 1703–1718. doi: 10.1007/s11694-020-00766-w [DOI] [Google Scholar]
- 440.Adisakwattana S, Lerdsuwankij O, Poputtachai U, Minipun A, Suparpprom C. Inhibitory activity of cinnamon bark species and their combination effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Plant Foods Hum Nutr. 2011;66: 143–8. doi: 10.1007/s11130-011-0226-4 [DOI] [PubMed] [Google Scholar]
- 441.Chimbetete N, Verghese M, Sunkara R, Walker L. Phytochemical Content, Radical Scavenging Ability & Enzyme Inhibiting Activities of Selected Spices (Cinnamon, Cardamom and Cloves). Food Nutr Sci. 2019;10: 266–275. doi: 10.4236/fns.2019.103020 [DOI] [Google Scholar]
- 442.Morais SR de, Bezerra IN, Souza A de M, Vergara CMAC, Sichieri R. [Eating away from home and biomarkers for chronic noncommunicable diseases in Brazilian adolescents]. Cad Saude Publica. 2021;37: e00219619. doi:10.1590/0102-311X00219619 [DOI] [PubMed]
- 443.How SC, Goh LPW, Johansah N, Matawali A, Gansau JA, How S-E. Medicinal plants in Sabah (North Borneo) exhibit anti-pancreatic lipase, anti-amylase, and antioxidant properties. Acta Sci Technol. 2022;44: 1i+. doi: 10.4025/actascitechnol.v44i1.56879 [DOI] [Google Scholar]
- 444.Takács I, Takács Á, Pósa A, Gyémánt G. HPLC Method for Measurement of Human Salivary α-Amylase Inhibition by Aqueous Plant Extracts. Acta Biol Hung. 2017;68: 127–136. doi: 10.1556/018.68.2017.2.1 [DOI] [PubMed] [Google Scholar]
- 445.Gyémánt G, Lehoczki G, Szabó K, Kandra L. Inhibition studies on α-amylase using isothermal titration calorimetry. 2018;2: 11–16. doi: 10.1515/amylase-2018-0002 [DOI] [Google Scholar]
- 446.Kim SH, Hyun SH, Choung SY. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J Ethnopharmacol. 2006;104: 119–23. doi: 10.1016/j.jep.2005.08.059 [DOI] [PubMed] [Google Scholar]
- 447.Puttaswamy NY, Rupini GD, Ahmed F, Urooj A. In vitro hypoglycemic potential of spices: Cinnamon and Cumi. Pak J Pharm Sci. 2018;31: 2367–2372. [PubMed] [Google Scholar]
- 448.Eid AM, Hawash M. Biological evaluation of Safrole oil and Safrole oil Nanoemulgel as antioxidant, antidiabetic, antibacterial, antifungal and anticancer. BMC Complement Med Ther. 2021;21: 159. doi: 10.1186/s12906-021-03324-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Guerrero-Romero F, Simental-Mendía LE, Guerra Rosas MI, Sayago-Monreal VI, Morales Castro J, Gamboa-Gómez CI. Hypoglycemic and antioxidant effects of green tomato (Physalis ixocarpa Brot.) calyxes’ extracts. J Food Biochem. 2021;45: e13678. doi: 10.1111/jfbc.13678 [DOI] [PubMed] [Google Scholar]
- 450.Miller N, Joubert E. Critical Assessment of In Vitro Screening of α-Glucosidase Inhibitors from Plants with Acarbose as a Reference Standard. Planta Med. 2022;88: 1078–1091. doi: 10.1055/a-1557-7379 [DOI] [PubMed] [Google Scholar]
- 451.Taslimi P, Gulçin İ. Antidiabetic potential: in vitro inhibition effects of some natural phenolic compounds on α-glycosidase and α-amylase enzymes. J Biochem Mol Toxicol. 2017;31. doi: 10.1002/jbt.21956 [DOI] [PubMed] [Google Scholar]
- 452.Ullah N, Amin A, Alamoudi RA, Rasheed SA, Alamoudi RA, Nawaz A, et al. Fabrication and Optimization of Essential-Oil-Loaded Nanoemulsion Using Box-Behnken Design against Staphylococos aureus and Staphylococos epidermidis Isolated from Oral Cavity. Pharmaceutics. Natural Products Research Lab, Gomal Centre of Pharmaceutical Sciences, Faculty of Pharmacy, Gomal University, Dera Ismail Khan 29050, Pakistan.; 2022. p. 1640. doi: 10.3390/pharmaceutics14081640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Kishimoto M, Yamasaki Y, Kubota M, Arai K, Morishima T, Kawamori R, et al. 1,5-Anhydro-D-glucitol evaluates daily glycemic excursions in well-controlled NIDDM. Diabetes Care. 1995;18: 1156–1159. doi: 10.2337/diacare.18.8.1156 [DOI] [PubMed] [Google Scholar]
- 454.Moreira VC, Silva C, Welker AF, da Silva ICR. Visceral Adipose Tissue Influence on Health Problem Development and Its Relationship with Serum Biochemical Parameters in Middle-Aged and Older Adults: A Literature Review. J Aging Res. 2022;2022. doi: 10.1155/2022/8350527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Harano Y, Sakamoto A, Izumi K, Shimizu Y, Hoshi M. Usefulness of maltose for testing glucose tolerance. Am J Clin Nutr. 1977;30: 924–931. doi: 10.1093/ajcn/30.6.924 [DOI] [PubMed] [Google Scholar]
- 456.Dhital S, Gidley MJ, Warren FJ. Inhibition of α-amylase activity by cellulose: Kinetic analysis and nutritional implications. Carbohydr Polym. 2015;123: 305–312. doi: 10.1016/j.carbpol.2015.01.039 [DOI] [PubMed] [Google Scholar]
- 457.Nsor-Atindana J, Goff HD, Saqib MN, Chen M, Liu W, Ma J, et al. Inhibition of α-amylase and amyloglucosidase by nanocrystalline cellulose and spectroscopic analysis of their binding interaction mechanism. Food Hydrocoll. 2019;90: 341–352. doi: 10.1016/j.foodhyd.2018.12.031 [DOI] [Google Scholar]
- 458.Ji N, Liu C, Li M, Sun Q, Xiong L. Interaction of cellulose nanocrystals and amylase: Its influence on enzyme activity and resistant starch content. Food Chem. 2018;245: 481–487. doi: 10.1016/j.foodchem.2017.10.130 [DOI] [PubMed] [Google Scholar]
- 459.Kabisch S, Honsek C, Kemper M, Gerbracht C, Arafat AM, Birkenfeld AL, et al. Dose-dependent effects of insoluble fibre on glucose metabolism: a stratified post hoc analysis of the Optimal Fibre Trial (OptiFiT). Acta Diabetol. 2021;58: 1649–1658. doi: 10.1007/s00592-021-01772-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Arun KB, Dhanya R, Chandran J, Abraham B, Satyan S, Nisha P. A comparative study to elucidate the biological activities of crude extracts from rice bran and wheat bran in cell line models. J Food Sci Technol. 2020;57: 3221–3231. doi: 10.1007/s13197-020-04353-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Elmadbouly MA elmoneim. Effect of wheat bran on anthropometric measures, serum glucose and lipid profile in type 2 diabetes patients. Pakistan J Biol Sci. 2021;24: 345–349. doi: 10.3923/pjbs.2021.345.349 [DOI] [PubMed] [Google Scholar]
- 462.Nazari J, Yadegari N, Khodam S, Almasi-Hashian A, Amini S. Effect of consumption of whole-wheat breads on FBS, HbA1c, and blood lipids in patients with type 2 diabetes. Prev Nutr food Sci. 2021;26: 269–274. doi: 10.3746/pnf.2021.26.3.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Chen H, Xiong M, Bai T, Chen D, Zhang Q, Lin D, et al. Comparative study on the structure, physicochemical, and functional properties of dietary fiber extracts from quinoa and wheat. LWT. 2021;149: 111816. doi: 10.1016/j.lwt.2021.111816 [DOI] [Google Scholar]
- 464.Yao B, Fang H, Xu W, Yan Y, Xu H, Liu Y, et al. Dietary fiber intake and risk of type 2 diabetes: a dose-response analysis of prospective studies. Eur J Epidemiol. 2014;29: 79–88. doi: 10.1007/s10654-013-9876-x [DOI] [PubMed] [Google Scholar]
- 465.Lepping K, Adams-Campbell LL, Hicks J, Mills M, Dash C. Dietary fiber intake and metabolic syndrome in postmenopausal African American women with obesity. PLoS One. 2022;17: e0273911. doi: 10.1371/journal.pone.0273911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Moreira FD. Resposta glicêmica aguda e saciedade após adição alternada de farelo de trigo, farinha de maracujá e pó de algas marinhas no desjejum de diabéticos tipo 2. M.Sc. Thesis. Universidade de Brasíllia. 2011. Available: http://repositorio.unb.br/handle/10482/10090 [Google Scholar]
- 467.Haripriya S, Premakumari S. Effect of wheat bran on diabetic subjects. Indian J Sci Technol. 2010;3: 284–246. doi: 10.17485/ijst/2010/v3i3.8 [DOI] [Google Scholar]
- 468.Weickert MO, Pfeiffer AFH. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J Nutr. 2018;148: 7–12. doi: 10.1093/jn/nxx008 [DOI] [PubMed] [Google Scholar]
- 469.Kabisch S, Meyer NMT, Honsek C, Gerbracht C, Dambeck U, Kemper M, et al. Fasting glucose state determines metabolic response to supplementation with insoluble cereal fibre: a secondary analysis of the Optimal Fibre Trial (OptiFiT). Nutrients. 2019;11. doi: 10.3390/nu11102385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Partula V, Deschasaux M, Druesne-Pecollo N, Latino-Martel P, Desmetz E, Chazelas E, et al. Associations between consumption of dietary fibers and the risk of cardiovascular diseases, cancers, type 2 diabetes, and mortality in the prospective NutriNet-Santé cohort. Am J Clin Nutr. 2020;112: 195–207. doi: 10.1093/ajcn/nqaa063 [DOI] [PubMed] [Google Scholar]
- 471.Meng H, Matthan NR, Ausman LM, Lichtenstein AH. Effect of macronutrients and fiber on postprandial glycemic responses and meal glycemic index and glycemic load value determinations. Am J Clin Nutr. 2017;105: 842–853. doi: 10.3945/ajcn.116.144162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Lan-Pidhainy X, Wolever TMS. The hypoglycemic effect of fat and protein is not attenuated by insulin resistance. Am J Clin Nutr. 2010;91: 98–105. doi: 10.3945/ajcn.2009.28125 [DOI] [PubMed] [Google Scholar]
- 473.Augustin LSA, Kendall CWC, Jenkins DJA, Willett WC, Astrup A, Barclay AW, et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr Metab Cardiovasc Dis. 2015;25: 795–815. doi: 10.1016/j.numecd.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 474.Tubili C, Morviducci L, Nardone MR, Agrigento S, Villani N. Addition of different soluble fiber fractions to oven baked products is not always a successful tool for reducing the Glycemic Index. Nutrition, metabolism, and cardiovascular diseases: NMCD. Netherlands; 2010. pp. e2–3. doi: 10.1016/j.numecd.2009.04.019 [DOI] [PubMed] [Google Scholar]
- 475.Boers HM, MacAulay K, Murray P, Seijen Ten Hoorn J, Hoogenraad A-R, Peters HPF, et al. Efficacy of different fibres and flour mixes in South-Asian flatbreads for reducing post-prandial glucose responses in healthy adults. Eur J Nutr. 2017;56: 2049–2060. doi: 10.1007/s00394-016-1242-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Moreira FD, Reis CEG, Welker AF, Gallassi AD. Acute Flaxseed Intake Reduces Postprandial Glycemia in Subjects with Type 2 Diabetes: A Randomized Crossover Clinical Trial. Nutrients. 2022;14. doi: 10.3390/nu14183736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Malunga LN, Eck P, Beta T. Inhibition of intestinal α-glucosidase and glucose absorption by feruloylated arabinoxylan mono-and oligosaccharides from corn bran and wheat aleurone. J Nutr Metab. 2016;2016: 1932532. doi: 10.1155/2016/1932532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Pazo-Cepeda MV, Aspromonte SG, Alonso E. Extraction of ferulic acid and feruloylated arabinoxylo-oligosaccharides from wheat bran using pressurized hot water. Food Biosci. 2021;44: 101374. doi: 10.1016/j.fbio.2021.101374 [DOI] [Google Scholar]
- 479.Awad AM, Kumar P, Ismail-Fitry MR, Jusoh S, Ab Aziz MF, Sazili AQ. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants (Basel, Switzerland). 2021;10. doi: 10.3390/antiox10091465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Lopes JDS, Lima ABS de, Cangussu RR da C, Silva MV da, Ferrão SPB, Santos LS. Application of spectroscopic techniques and chemometric methods to differentiate between true cinnamon and false cinnamon. Food Chem. 2022;368: 130746. doi: 10.1016/j.foodchem.2021.130746 [DOI] [PubMed] [Google Scholar]
- 481.Liu C, Long H, Wu X, Hou J, Gao L, Yao S, et al. Quantitative and fingerprint analysis of proanthocyanidins and phenylpropanoids in Cinnamomum verum bark, Cinnamomum cassia bark, and Cassia twig by UPLC combined with chemometrics. Eur Food Res Technol. 2021;247: 2687–2698. doi: 10.1007/s00217-021-03795-x [DOI] [Google Scholar]
- 482.Phu HH, Pham Van K, Tran TH, Pham DT. Extraction, Chemical Compositions and Biological Activities of Essential Oils of Cinnamomum verum Cultivated in Vietnam. Processes. 2022. doi: 10.3390/pr10091713 [DOI] [Google Scholar]
- 483.Wu Y, Wang M, Yang T, Qin L, Hu Y, Zhao D, et al. Cinnamic acid ameliorates nonalcoholic fatty liver disease by suppressing hepatic lipogenesis and promoting fatty acid oxidation. Evid Based Complement Alternat Med. 2021;2021: 9561613. doi: 10.1155/2021/9561613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Tolmie M, Bester MJ, Apostolides Z. Inhibition of α-glucosidase and α-amylase by herbal compounds for the treatment of type 2 diabetes: A validation of in silico reverse docking with in vitro enzyme assays. J Diabetes. 2021;13: 779–791. doi: 10.1111/1753-0407.13163 [DOI] [PubMed] [Google Scholar]
- 485.Adisakwattana S, Chantarasinlapin P, Thammarat H, Yibchok-Anun S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal alpha-glucosidase. J Enzyme Inhib Med Chem. 2009;24: 1194–1200. doi: 10.1080/14756360902779326 [DOI] [PubMed] [Google Scholar]
- 486.Okutan L, Kongstad KT, Jäger AK, Staerk D. High-resolution α-amylase assay combined with high-performance liquid chromatography-solid-phase extraction-nuclear magnetic resonance spectroscopy for expedited identification of α-amylase inhibitors: proof of concept and α-amylase inhibitor in cinnamon. J Agric Food Chem. 2014;62: 11465–71. doi: 10.1021/jf5047283 [DOI] [PubMed] [Google Scholar]
- 487.Barre DE, Mizier-Barre KA. The polypharmacy reduction potential of cinnamic acids and some related compounds in pre- and post-onset management of type 2 diabetes mellitus. Endocr Regul. 2020;54: 137–155. doi: 10.2478/enr-2020-0017 [DOI] [PubMed] [Google Scholar]
- 488.Jiao L, Zhang X, Huang L, Gong H, Cheng B, Sun Y, et al. Proanthocyanidins are the major anti-diabetic components of cinnamon water extract. Food Chem Toxicol an Int J Publ Br Ind Biol Res Assoc. 2013;56: 398–405. doi: 10.1016/j.fct.2013.02.049 [DOI] [PubMed] [Google Scholar]
- 489.Jayaprakasha GK, Ohnishi-Kameyama M, Ono H, Yoshida M, Jaganmohan Rao L. Phenolic constituents in the fruits of Cinnamomum zeylanicum and their antioxidant activity. J Agric Food Chem. 2006;54: 1672–1679. doi: 10.1021/jf052736r [DOI] [PubMed] [Google Scholar]
- 490.Siegień J, Buchholz T, Popowski D, Granica S, Osińska E, Melzig MF, et al. Pancreatic lipase and α-amylase inhibitory activity of extracts from selected plant materials after gastrointestinal digestion in vitro. Food Chem. 2021;355: 129414. doi: 10.1016/j.foodchem.2021.129414 [DOI] [PubMed] [Google Scholar]
- 491.Lu M, Yuan B, Zeng M, Chen J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res Int. 2011;44: 530–536. doi: 10.1016/j.foodres.2010.10.055 [DOI] [Google Scholar]
- 492.de Souza VB, Thomazini M, Echalar Barrientos MA, Nalin CM, Ferro-Furtado R, Genovese MI, et al. Functional properties and encapsulation of a proanthocyanidin-rich cinnamon extract (Cinnamomum zeylanicum) by complex coacervation using gelatin and different polysaccharides. Food Hydrocoll. 2018;77: 297–306. doi: 10.1016/j.foodhyd.2017.09.040 [DOI] [Google Scholar]
- 493.Mnafgui K, Kaanich F, Derbali A, Hamden K, Derbali F, Slama S, et al. Inhibition of key enzymes related to diabetes and hypertension by Eugenol in vitro and in alloxan-induced diabetic rats. Arch Physiol Biochem. 2013;119: 225–33. doi: 10.3109/13813455.2013.822521 [DOI] [PubMed] [Google Scholar]
- 494.Singh P, Jayaramaiah RH, Agawane SB, Vannuruswamy G, Korwar AM, Anand A, et al. Potential dual role of eugenol in inhibiting advanced glycation end products in diabetes: Proteomic and mechanistic insights. Sci Rep. 2016;6: 18798. doi: 10.1038/srep18798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Carvalho RPR, Lima GD de A, Machado-Neves M. Effect of eugenol treatment in hyperglycemic murine models: A meta-analysis. Pharmacol Res. 2021;165: 105315. doi: 10.1016/j.phrs.2020.105315 [DOI] [PubMed] [Google Scholar]
- 496.Jayatilaka A, Poole SK, Poole CF, Chichila TMP. Simultaneous micro steam distillation/solvent extraction for the isolation of semivolatile flavor compounds from cinnamon and their separation by series coupled-column gas chromatography. Anal Chim Acta. 1995;302: 147–162. doi: 10.1016/0003-2670(94)00445-R [DOI] [Google Scholar]
- 497.Kongstad KT, Özdemir C, Barzak A, Wubshet SG, Staerk D. Combined use of high-resolution α-glucosidase inhibition profiling and high-performance liquid chromatography-high-resolution mass spectrometry-solid-phase extraction-nuclear magnetic resonance spectroscopy for investigation of antidiabetic principles in. J Agric Food Chem. 2015;63: 2257–63. doi: 10.1021/jf506297k [DOI] [PubMed] [Google Scholar]
- 498.Sumudu Chandana NGAS Morlock GE. Eight different bioactivity profiles of 40 cinnamons by multi-imaging planar chromatography hyphenated with effect-directed assays and high-resolution mass spectrometry. Food Chem. 2021;357: 129135. doi: 10.1016/j.foodchem.2021.129135 [DOI] [PubMed] [Google Scholar]
- 499.Suzuki R, Kasuya Y, Sano A, Tomita J, Maruyama T, Kitamura M. Comparison of various commercially available cinnamon barks using NMR metabolomics and the quantification of coumarin by quantitative NMR methods. J Nat Med. 2022;76: 87–93. doi: 10.1007/s11418-021-01554-6 [DOI] [PubMed] [Google Scholar]
- 500.Miller KG, Poole CF, Pawlowskí TMP. Classification of the botanical origin of cinnamon by solid-phase microextraction and gas chromatography. Chromatographia. 1996;42: 639–646. doi: 10.1007/BF02267695 [DOI] [Google Scholar]
- 501.Sriramavaratharajan V, Murugan R. Chemical profile of leaf essential oil of Cinnamomum walaiwarense and comparison of its antioxidant and hypoglycemic activities with the major constituent benzyl benzoate. Nat Prod Commun. 2018;13: 1934578X1801300. doi: 10.1177/1934578X1801300633 [DOI] [Google Scholar]
- 502.Sriramavaratharajan V, Murugan R. Screening of Chemical Composition, in vitro Antioxidant, α-Amylase and α-Glucosidase Inhibitory Activities of the Leaf Essential Oils of Cinnamomum wightii from Different Populations. Nat Prod Commun. 2018;13: 1934578X1801301132. doi: 10.1177/1934578X1801301132 [DOI] [Google Scholar]
- 503.Thantsin K, Zhang Q, Yang J, Wang Q. Composition of semivolatile compounds of 10 Cinnamomum species from China and Myanmar. Nat Prod Res. 2008;22: 576–83. doi: 10.1080/14786410701592802 [DOI] [PubMed] [Google Scholar]
- 504.Yan D, Wong YF, Shellie RA, Marriott PJ, Whittock SP, Koutoulis A. Assessment of the phytochemical profiles of novel hop (Humulus lupulus L.) cultivars: A potential route to beer crafting. Food Chem. 2019;275: 15–23. doi: 10.1016/j.foodchem.2018.09.082 [DOI] [PubMed] [Google Scholar]
- 505.Wang Y-H, Avula B, Nanayakkara NPD, Zhao J, Khan IA. Cassia cinnamon as a source of coumarin in cinnamon-flavored food and food supplements in the United States. J Agric Food Chem. 2013;61: 4470–6. doi: 10.1021/jf4005862 [DOI] [PubMed] [Google Scholar]
- 506.Cha J, Kim C-T, Kim T-E, Cho Y-J. Optimization of subcritical extraction process for cinnamon (Cinnamomum Cassia Blume) using response surface methodology. Food Sci Biotechnol. 2019;28: 1703–1711. doi: 10.1007/s10068-019-00616-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Wang P, Chi J, Guo H, Wang S-X, Wang J, Xu E-P, et al. Identification of {Differential} {Compositions} of {Aqueous} {Extracts} of {Cinnamomi} {Ramulus} and {Cinnamomi} {Cortex}. Molecules. 2023;28. doi: 10.3390/molecules28052015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Prasad KN, Yang B, Dong X, Jiang G, Zhang H, Xie H, et al. Flavonoid contents and antioxidant activities from Cinnamomum species. Innov Food Sci Emerg Technol. 2009;10: 627–632. doi: 10.1016/j.ifset.2009.05.009 [DOI] [Google Scholar]
- 509.Wang H, Du Y-J, Song H-C. α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem. 2010;123: 6–13. doi: 10.1016/j.foodchem.2010.03.088 [DOI] [Google Scholar]
- 510.Varghese GK, Bose LV, Habtemariam S. Antidiabetic components of Cassia alata leaves: Identification through α-glucosidase inhibition studies. Pharm Biol. 2013;51: 345–9. doi: 10.3109/13880209.2012.729066 [DOI] [PubMed] [Google Scholar]
- 511.Peng X, Zhang G, Liao Y, Gong D. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem. 2016;190: 207–215. doi: 10.1016/j.foodchem.2015.05.088 [DOI] [PubMed] [Google Scholar]
- 512.Sheng Z, Ai B, Zheng L, Zheng X, Xu Z, Shen Y, et al. Inhibitory activities of kaempferol, galangin, carnosic acid and polydatin against glycation and α-amylase and α-glucosidase enzymes. Int J Food Sci Technol. 2018;53: 755–766. doi: 10.1111/ijfs.13579 [DOI] [Google Scholar]
- 513.Jhong C-H, Riyaphan J, Lin S-H, Chia Y-C, Weng C-F. Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. Biofactors. 2015;41: 242–51. doi: 10.1002/biof.1219 [DOI] [PubMed] [Google Scholar]
- 514.Oboh G, Agunloye OM, Adefegha SA, Akinyemi AJ, Ademiluyi AO. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J Basic Clin Physiol Pharmacol. 2015;26: 165–70. doi: 10.1515/jbcpp-2013-0141 [DOI] [PubMed] [Google Scholar]
- 515.Meng Y, Su A, Yuan S, Zhao H, Tan S, Hu C, et al. Evaluation of total flavonoids, myricetin, and quercetin from hovenia dulcis thunb. As inhibitors of α-amylase and α-glucosidase. Plant Foods Hum Nutr. 2016;71: 444–449. doi: 10.1007/s11130-016-0581-2 [DOI] [PubMed] [Google Scholar]
- 516.Su J, Tang Z. Effects of (-)-epigallocatechin gallate and quercetin on the activity and structure of α-amylase. Trop J Pharm Res. 2019;18: 585–590. doi: 10.4314/tjpr.v18i3.20 [DOI] [Google Scholar]
- 517.Nyambe-Silavwe H, Villa-Rodriguez JA, Ifie I, Holmes M, Aydin E, Jensen JM, et al. Inhibition of human α-amylase by dietary polyphenols. J Funct Foods. 2015;19: 723–732. doi: 10.1016/j.jff.2015.10.003 [DOI] [Google Scholar]
- 518.Kim JS, Kwon CS, Son KH. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem. 2000;64: 2458–61. doi: 10.1271/bbb.64.2458 [DOI] [PubMed] [Google Scholar]
- 519.Swain A, Hariprasad P. Identification of α-glucosidase inhibitors from Cyperus articulatus L. rhizome extract using HRLC-MS/MS and molecular docking. Asian J Chem. 2020;32: 1235–1242. doi: 10.14233/ajchem.2020.22606 [DOI] [Google Scholar]
- 520.Cen Y, Xiao A, Chen X, Liu L. Isolation of α-amylase inhibitors from Kadsura longipedunculata using a high-speed counter-current chromatography target guided by centrifugal ultrafiltration with LC-MS. Molecules. 2016;21. doi: 10.3390/molecules21091190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Klejdus B, Kováčik J. Quantification of phenols in cinnamon: A special focus on “total phenols” and phenolic acids including DESI-Orbitrap MS detection. Ind Crops Prod. 2016;83: 774–780. doi: 10.1016/j.indcrop.2015.11.060 [DOI] [Google Scholar]
- 522.Aleixandre A, Gil JV, Sineiro J, Rosell CM. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022;372: 131231. doi: 10.1016/j.foodchem.2021.131231 [DOI] [PubMed] [Google Scholar]
- 523.Nanok K, Sansenya S. Combination effects of rice extract and five aromatic compounds against α-glucosidase, α-amylase and tyrosinase. J Biosci Bioeng. 2021;132: 9–17. doi: 10.1016/j.jbiosc.2021.02.003 [DOI] [PubMed] [Google Scholar]
- 524.Lu Q, Chen C, Zhao S, Ge F, Liu D. Investigation of the interaction between gallic acid and α-amylase by spectroscopy. Int J Food Prop. 2016;19: 2481–2494. doi: 10.1080/10942912.2015.1059345 [DOI] [Google Scholar]
- 525.Xue N, He B, Jia Y, Yang C, Wang J, Li M. The mechanism of binding with the α-glucosidase in vitro and the evaluation on hypoglycemic effect in vivo: Cocrystals involving synergism of gallic acid and conformer. Eur J Pharm Biopharm. 2020;156: 64–74. doi: 10.1016/j.ejpb.2020.08.024 [DOI] [PubMed] [Google Scholar]
- 526.De-Montijo-Prieto S, Razola-Díaz MDC, Gómez-Caravaca AM, Guerra-Hernandez EJ, Jiménez-Valera M, Garcia-Villanova B, et al. Essential Oils from Fruit and Vegetables, Aromatic Herbs, and Spices: Composition, Antioxidant, and Antimicrobial Activities. Biology (Basel). 2021;10. doi: 10.3390/biology10111091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Dalli M, Daoudi NE, Abrigach F, Azizi S-E, Bnouham M, Kim B, et al. In vitro α-amylase and hemoglobin glycation inhibitory potential of Nigella sativa essential oil, and molecular docking studies of its principal components. Front Pharmacol. 2022;13: 1036129. doi: 10.3389/fphar.2022.1036129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Zuñiga LY, Aceves-de la Mora MCA, González-Ortiz M, Ramos-Núñez JL, Martínez-Abundis E. Effect of Chlorogenic Acid Administration on Glycemic Control, Insulin Secretion, and Insulin Sensitivity in Patients with Impaired Glucose Tolerance. J Med Food. 2018;21: 469–473. doi: 10.1089/jmf.2017.0110 [DOI] [PubMed] [Google Scholar]
- 529.Ribeiro-Santos R, Andrade M, Madella D, Martinazzo AP, de Aquino Garcia Moura L, de Melo NR, et al. Revisiting an ancient spice with medicinal purposes: Cinnamon. Trends Food Sci Technol. 2017;62: 154–169. doi: 10.1016/j.tifs.2017.02.011 [DOI] [Google Scholar]
- 530.Zhao J-Q, Wang Y-M, Yang Y-L, Zeng Y, Mei L-J, Shi Y-P, et al. Antioxidants and α-glucosidase inhibitors from “Liucha” (young leaves and shoots of Sibiraea laevigata). Food Chem. 2017;230: 117–124. doi: 10.1016/j.foodchem.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 531.Yu T, Yao H, Qi S, Wang J. GC-MS analysis of volatiles in cinnamon essential oil extracted by different methods. Grasas y Aceites. 2020;71: 372. doi: 10.3989/gya.0462191 [DOI] [Google Scholar]
- 532.Plumeriastuti H, Budiastuti, Effendi MH, Budiarto. Identification of bioactive compound of the essential oils of Cinnamomum burmannii from several areas in Indonesia by gas chromatography-mass spectrometry method for antidiabetic potential. Natl J Physiol Pharm Pharmacol. 2019;9: 279–283. doi: 10.5455/njppp.2019.9.1236702022019 [DOI] [Google Scholar]
- 533.Vasconcelos NG, Croda J, Simionatto S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb Pathog. 2018;120: 198–203. doi: 10.1016/j.micpath.2018.04.036 [DOI] [PubMed] [Google Scholar]
- 534.Zare R, Nadjarzadeh A, Zarshenas MM, Shams M, Heydari M. Efficacy of cinnamon in patients with type II diabetes mellitus: A randomized controlled clinical trial. Clin Nutr. 2019;38: 549–556. doi: 10.1016/j.clnu.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 535.Keramati M, Musazadeh V, Malekahmadi M, Jamilian P, Jamilian P, Ghoreishi Z, et al. Cinnamon, an effective anti-obesity agent: Evidence from an umbrella meta-analysis. J Food Biochem. 2022; e14166. doi: 10.1111/jfbc.14166 [DOI] [PubMed] [Google Scholar]
- 536.Smith DLJ, Orlandella RM, Allison DB, Norian LA. Diabetes medications as potential calorie restriction mimetics-a focus on the alpha-glucosidase inhibitor acarbose. GeroScience. 2021;43: 1123–1133. doi: 10.1007/s11357-020-00278-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Kamruzzaman M, Horowitz M, Jones KL, Marathe CS. Gut-based strategies to reduce postprandial glycaemia in type 2 diabetes. Front Endocrinol (Lausanne). 2021;12: 661877. doi: 10.3389/fendo.2021.661877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Khan H, Amin S, Tewari D, Nabavi SM, Atanasov AG. Plant-derived Glycosides with α-Glucosidase Inhibitory Activity: Current Standing and Future Prospects. Endocr Metab Immune Disord Drug Targets. 2019;19: 391–401. doi: 10.2174/1871530319666181128104831 [DOI] [PubMed] [Google Scholar]
- 539.Sharma A, Chawla R, Kaur J, Madaan R. An Overview of Phytotherapy Used in the Management of Type II Diabetes. Curr Diabetes Rev. 2022;18: e170621194148. doi: 10.2174/1573399817666210617154535 [DOI] [PubMed] [Google Scholar]
- 540.Maduke T, Qureshi B, Goite Y, Gandhi K, Bofarrag F, Liu L, et al. Monitoring the Use of Telemonitor: A Resident-run Quality Improvement Initiative Decreases Inappropriate Use of Telemonitor in a Community Hospital. Cureus. 2019;11: e6263. doi: 10.7759/cureus.6263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Peter EL, Nagendrappa PB, Hilonga S, Tuyiringire N, Ashuro E, Kaligirwa A, et al. Pharmacological reflection of plants traditionally used to manage diabetes mellitus in Tanzania. J Ethnopharmacol. 2021;269: 113715. doi: 10.1016/j.jep.2020.113715 [DOI] [PubMed] [Google Scholar]
- 542.Sun L, Miao M. Dietary polyphenols modulate starch digestion and glycaemic level: a review. Crit Rev Food Sci Nutr. 2020;60: 541–555. doi: 10.1080/10408398.2018.1544883 [DOI] [PubMed] [Google Scholar]
- 543.Papoutsis K, Zhang J, Bowyer MC, Brunton N, Gibney ER, Lyng J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021;338: 128119. doi: 10.1016/j.foodchem.2020.128119 [DOI] [PubMed] [Google Scholar]
- 544.Proença C, Ribeiro D, Freitas M, Fernandes E. Flavonoids as potential agents in the management of type 2 diabetes through the modulation of α-amylase and α-glucosidase activity: a review. Crit Rev Food Sci Nutr. 2022;62: 3137–3207. doi: 10.1080/10408398.2020.1862755 [DOI] [PubMed] [Google Scholar]
- 545.Dirir AM, Daou M, Yousef AF, Yousef LF. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem Rev. 2022;21: 1049–1079. doi: 10.1007/s11101-021-09773-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Assefa ST, Yang E-Y, Chae S-Y, Song M, Lee J, Cho M-C, et al. Alpha Glucosidase Inhibitory Activities of Plants with Focus on Common Vegetables. Plants (Basel, Switzerland). 2019;9. doi: 10.3390/plants9010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Kifle ZD, Abdelwuhab M, Melak AD, Genet G, Meseret T, Adugna M. Pharmacological evaluation of medicinal plants with antidiabetic activities in Ethiopia: A review. Metab open. 2022;13: 100174. doi: 10.1016/j.metop.2022.100174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Mohd Bukhari DA, Siddiqui MJ, Shamsudin SH, Rahman MM, So’ad SZM. α-Glucosidase Inhibitory Activity of Selected Malaysian Plants. J Pharm Bioallied Sci. 2017;9: 164–170. doi: 10.4103/jpbs.JPBS_35_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Green HHA-HHA-MNA-MSA-AA-HA-EA- IR. Fruitful decade of fungal metabolites as anti-diabetic agents from 2010 to 2019: emphasis on α-glucosidase inhibitors. Phytochem Rev. 2021;v. 20: 145–179–2021 v.20 no.1. doi: 10.1007/s11101-020-09733-1 [DOI] [Google Scholar]
- 550.Rehman NU, Shah M, Ullah S, Khan M, Khan A, Ullah O, et al. Enzymes Inhibition and Antioxidant Potential of Medicinal Plants Growing in Oman. Biomed Res Int. 2022;2022: 7880387. doi: 10.1155/2022/7880387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Diez-Garcia RW, de Castro IRR. Culinary as an object of study and intervention in the field of Food and Nutrition. Cien Saude Colet. 2011;16: 91–8. doi: 10.1590/s1413-81232011000100013 [DOI] [PubMed] [Google Scholar]
- 552.Muhammad DRA, Dewettinck K. Cinnamon and its derivatives as potential ingredient in functional food—A review. Int J Food Prop. 2017; 1–27. doi: 10.1080/10942912.2017.1369102 [DOI] [Google Scholar]
- 553.Alqathama A, Alluhiabi G, Baghdadi H, Aljahani L, Khan O, Jabal S, et al. Herbal medicine from the perspective of type II diabetic patients and physicians: What is the relationship? BMC Complement Med Ther. 2020;20: 65. doi: 10.1186/s12906-020-2854-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26: 3215–8. doi: 10.2337/diacare.26.12.3215 [DOI] [PubMed] [Google Scholar]
- 555.Hajimonfarednejad M, Ostovar M, Raee MJ, Hashempur MH, Mayer JG, Heydari M. Cinnamon: A systematic review of adverse events. Clin Nutr. 2019;38: 594–602. doi: 10.1016/j.clnu.2018.03.013 [DOI] [PubMed] [Google Scholar]
- 556.Zelicha H, Yang J, Henning SM, Huang J, Lee R-P, Thames G, et al. Effect of cinnamon spice on continuously monitored glycemic response in adults with prediabetes: a 4-week randomized controlled crossover trial. Am J Clin Nutr. 2024. doi: 10.1016/j.ajcnut.2024.01.008 [DOI] [PubMed] [Google Scholar]
- 557.Chen M-R, Zhao J, Fu S-F, Yu J-Q, Zhang X, Zhang Q-Y, et al. Clinical practice of Chinese medicine navel therapy for chronic diarrhea: A literature review. J Gastroenterol Hepatol. 2019;34: 643–649. doi: 10.1111/jgh.14549 [DOI] [PubMed] [Google Scholar]
- 558.Nk R, Tuwani R, Garg N, Mukherjee J, Bagler G. SpiceRx: an integrated resource for the health impacts of culinary spices and herbs. bioRxiv. 2018; 273599. doi: 10.1101/273599 [DOI] [Google Scholar]
- 559.Zobeiri M, Parvizi F, Shahpiri Z, Heydarpour F, Pourfarzam M, Memarzadeh MR, et al. Evaluation of the effectiveness of cinnamon oil soft capsule in patients with functional dyspepsia: A randomized double-blind placebo-controlled clinical trial. Evid Based Complement Alternat Med. 2021;2021: 6634115. doi: 10.1155/2021/6634115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Shapiro K, Gong WC. Use of herbal products for diabetes by Latinos. J Am Pharm Assoc (Washington, DC 1996). 2002;42: 278–9. doi: 10.1331/108658002763508542 [DOI] [PubMed] [Google Scholar]
- 561.Medagama AB, Bandara R. The use of complementary and alternative medicines (CAMs) in the treatment of diabetes mellitus: Is continued use safe and effective? Nutr J. 2014;13: 102. doi: 10.1186/1475-2891-13-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Uuh Narvaez JJ, Segura Campos MR. Combination therapy of bioactive compounds with acarbose: A proposal to control hyperglycemia in type 2 diabetes. J Food Biochem. 2022;46: e14268. doi: 10.1111/jfbc.14268 [DOI] [PubMed] [Google Scholar]
- 563.Udani J, Tan O, Molina J. Systematic Review and Meta-Analysis of a Proprietary Alpha-Amylase Inhibitor from White Bean (Phaseolus vulgaris L.) on Weight and Fat Loss in Humans. Foods (Basel, Switzerland). 2018;7. doi: 10.3390/foods7040063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Pignatti C, D’Adamo S, Stefanelli C, Flamigni F, Cetrullo S. Nutrients and pathways that regulate health span and life span. Geriatrics. 2020;5: 95. doi: 10.3390/geriatrics5040095 [DOI] [PMC free article] [PubMed]
- 565.Ingram DK, Roth GS. Calorie restriction mimetics: can you have your cake and eat it, too? Ageing Res Rev. 2015;20: 46–62. doi: 10.1016/j.arr.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 566.Alsoodeeri FN, Alqabbani HM, Aldossari NM. Effects of Cinnamon (Cinnamomum cassia) Consumption on Serum Lipid Profiles in Albino Rats. J Lipids. 2020;2020: 8469830. doi: 10.1155/2020/8469830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Heydarpour F, Hemati N, Hadi A, Moradi S, Mohammadi E, Farzaei MH. Effects of cinnamon on controlling metabolic parameters of polycystic ovary syndrome: A systematic review and meta-analysis. J Ethnopharmacol. 2020;254: 112741. doi: 10.1016/j.jep.2020.112741 [DOI] [PubMed] [Google Scholar]
- 568.Gupta Jain S, Puri S, Misra A, Gulati S, Mani K. Effect of oral cinnamon intervention on metabolic profile and body composition of Asian Indians with metabolic syndrome: a randomized double -blind control trial. Lipids Health Dis. 2017;16: 113. doi: 10.1186/s12944-017-0504-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Sarmadi B, Musazadeh V, Dehghan P, Karimi E. The effect of cinnamon consumption on lipid profile, oxidative stress, and inflammation biomarkers in adults: {An} umbrella meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2023;33: 1821–1835. doi: 10.1016/j.numecd.2023.03.010 [DOI] [PubMed] [Google Scholar]
- 570.Roussel A-M, Hininger I, Benaraba R, Ziegenfuss TN, Anderson RA. Antioxidant effects of a cinnamon extract in people with impaired fasting glucose that are overweight or obese. J Am Coll Nutr. 2009;28: 16–21. doi: 10.1080/07315724.2009.10719756 [DOI] [PubMed] [Google Scholar]
- 571.Vafa M, Mohammadi F, Shidfar F, Sormaghi MS, Heidari I, Golestan B, et al. Effects of cinnamon consumption on glycemic status, lipid profile and body composition in type 2 diabetic patients. Int J Prev Med. 2012;3: 531–536. [PMC free article] [PubMed] [Google Scholar]
- 572.Borzoei A, Rafraf M, Asghari-Jafarabadi M. Cinnamon improves metabolic factors without detectable effects on adiponectin in women with polycystic ovary syndrome. Asia Pac J Clin Nutr. 2018;27: 556–563. doi: 10.6133/apjcn.062017.13 [DOI] [PubMed] [Google Scholar]
- 573.Saboo B, Misra A, Kalra S, Mohan V, Aravind SR, Joshi S, et al. Role and importance of high fiber in diabetes management in India. Diabetes Metab Syndr. 2022;16: 102480. doi: 10.1016/j.dsx.2022.102480 [DOI] [PubMed] [Google Scholar]
- 574.Mashiane P, Manhivi VE, Shoko T, Slabbert RM, Sultanbawa Y, Sivakumar D. Cooking African Pumpkin Leaves (Momordicabalsamina L.) by Stir-Frying Improved Bioactivity and Bioaccessibility of Metabolites-Metabolomic and Chemometric Approaches. Foods (Basel, Switzerland). 2021;10. doi: 10.3390/foods10112890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Fernando IT, Perera KI, Athauda SBP, Sivakanesan R, Kumar NS, Jayasinghe L. Heat stability of the in vitro inhibitory effect of spices on lipase, amylase, and glucosidase enzymes. Food Sci & Nutr. 2019;7: 425–432. doi: 10.1002/fsn3.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Gunathilake KDPP, Ranaweera KKDS, Rupasinghe HPV. Effect of Different Cooking Methods on Polyphenols, Carotenoids and Antioxidant Activities of Selected Edible Leaves. Antioxidants (Basel, Switzerland). 2018;7. doi: 10.3390/antiox7090117 [DOI] [PMC free article] [PubMed]
- 577.Ayua EO, Nkhata SG, Namaumbo SJ, Kamau EH, Ngoma TN, Aduol KO. Polyphenolic inhibition of enterocytic starch digestion enzymes and glucose transporters for managing type 2 diabetes may be reduced in food systems. Heliyon. 2021;7: e06245. doi: 10.1016/j.heliyon.2021.e06245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Kamau EH, Nkhata SG, Ayua EO. Extrusion and nixtamalization conditions influence the magnitude of change in the nutrients and bioactive components of cereals and legumes. Food Sci Nutr. 2020;8: 1753–1765. doi: 10.1002/fsn3.1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Wieczorek MN, Jeleń HH. Volatile Compounds of Selected Raw and Cooked Brassica Vegetables. Molecules. 2019;24. doi: 10.3390/molecules24030391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Rekha MR, Padmaja G. Alpha-amylase inhibitor changes during processing of sweet potato and taro tubers. Plant Foods Hum Nutr. 2002;57: 285–294. doi: 10.1023/a:1021837115267 [DOI] [PubMed] [Google Scholar]
- 581.Mehrabadi M, Bandani AR, Saadati F. Inhibition of Sunn pest, Eurygaster integriceps, α-amylases by α-amylase inhibitors (T-αAI) from Triticale. J Insect Sci. 2010;10: 179. doi: 10.1673/031.010.14139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Choi WC, Parr T, Lim YS. The impact of four processing methods on trypsin-, chymotrypsin- and alpha-amylase inhibitors present in underutilised legumes. J Food Sci Technol. 2019;56: 281–289. doi: 10.1007/s13197-018-3488-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Peddio S, Padiglia A, Cannea FB, Crnjar R, Zam W, Sharifi-Rad J, et al. Common bean (Phaseolus vulgaris L.) α-amylase inhibitors as safe nutraceutical strategy against diabetes and obesity: An update review. Phytother Res. 2022;36: 2803–2823. doi: 10.1002/ptr.7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Managa MG, Shai J, Thi Phan AD, Sultanbawa Y, Sivakumar D. Impact of Household Cooking Techniques on African Nightshade and Chinese Cabbage on Phenolic Compounds, Antinutrients, in vitro Antioxidant, and β-Glucosidase Activity. Front Nutr. 2020;7: 580550. doi: 10.3389/fnut.2020.580550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Augustin LSA, Chiavaroli L, Campbell J, Ezatagha A, Jenkins AL, Esfahani A, et al. Post-prandial glucose and insulin responses of hummus alone or combined with a carbohydrate food: a dose-response study. Nutr J. 2016;15: 13. doi: 10.1186/s12937-016-0129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Natesh N, Abbey, Lord, Asiedu S. An Overview of Nutritional and Antinutritional Factors in Green Leafy Vegetables. 2017;1: 3–9. doi: 10.15406/hij.2017.01.00011 [DOI] [Google Scholar]
- 587.Mashitoa FM, Manhivi V, Slabbert RM, Shai JL, Sivakumar D. Changes in antinutrients, phenolics, antioxidant activities and in vitro α-glucosidase inhibitory activity in pumpkin leaves (Cucurbita moschata) during different domestic cooking methods. Food Sci Biotechnol. 2021;30: 793–800. doi: 10.1007/s10068-021-00916-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Petroski W, Minich DM. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients. 2020;12. doi: 10.3390/nu12102929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Feizollahi E, Mirmahdi RS, Zoghi A, Zijlstra RT, Roopesh MS, Vasanthan T. Review of the beneficial and anti-nutritional qualities of phytic acid, and procedures for removing it from food products. Food Res Int. 2021;143: 110284. doi: 10.1016/j.foodres.2021.110284 [DOI] [PubMed] [Google Scholar]
- 590.Pujol A, Sanchis P, Grases F, Masmiquel L. Phytate Intake, Health and Disease: “Let Thy Food Be Thy Medicine and Medicine Be Thy Food” Antioxidants. 2023;12. doi: 10.3390/antiox12010146 [DOI] [PMC free article] [PubMed]
- 591.Jimenez-Pulido IJ, Daniel R, Perez J, Martínez-Villaluenga C, De Luis D, Martín Diana AB. Impact of Protein Content on the Antioxidants, Anti-Inflammatory Properties and Glycemic Index of Wheat and Wheat Bran. Foods (Basel, Switzerland). 2022;11. doi: 10.3390/foods11142049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Thompson S V, Winham DM, Hutchins AM. Bean and rice meals reduce postprandial glycemic response in adults with type 2 diabetes: a cross-over study. Nutr J. 2012;11: 23. doi: 10.1186/1475-2891-11-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Winham DM, Nikl RR, Hutchins AM, Martin RL, Campbell CG. Dietitians vary by counseling status in bean promotion with type 2 diabetes clients: A pilot study. Food Sci Nutr. 2020;8: 2839–2847. doi: 10.1002/fsn3.1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Sanchis P, Rivera R, Berga F, Fortuny R, Adrover M, Costa-Bauza A, et al. Phytate Decreases Formation of Advanced Glycation End-Products in Patients with Type II Diabetes: Randomized Crossover Trial. Sci Rep. 2018;8: 9619. doi: 10.1038/s41598-018-27853-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Kunyanga CN, Imungi JK, Okoth MW, Biesalski HK, Vadivel V. Antioxidant and type 2 diabetes related functional properties of phytic acid extract from Kenyan local food ingredients: effects of traditional processing methods. Ecol Food Nutr. 2011;50: 452–471. doi: 10.1080/03670244.2011.604588 [DOI] [PubMed] [Google Scholar]
- 596.Abdulwaliyu I, Arekemase SO, Adudu JA, Batari ML, Egbule MN, Okoduwa SIR. Investigation of the medicinal significance of phytic acid as an indispensable anti-nutrient in diseases. Clin Nutr Exp. 2019;28: 42–61. doi: 10.1016/j.yclnex.2019.10.002 [DOI] [Google Scholar]
- 597.Kumar A, Sahu C, Panda PA, Biswal M, Sah RP, Lal MK, et al. Phytic acid content may affect starch digestibility and glycemic index value of rice (Oryza sativa L.). J Sci Food Agric. 2020;100: 1598–1607. doi: 10.1002/jsfa.10168 [DOI] [PubMed] [Google Scholar]
- 598.Pereira C, Lourenço VM, Menezes R, Brites C. Rice Compounds with Impact on Diabetes Control. Foods (Basel, Switzerland). 2021;10. doi: 10.3390/foods10091992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Nath H, Samtiya M, Dhewa T. Beneficial attributes and adverse effects of major plant-based foods anti-nutrients on health: A review. Hum Nutr Metab. 2022;28: 200147. doi: 10.1016/j.hnm.2022.200147 [DOI] [Google Scholar]
- 600.Omoruyi FO, Stennett D, Foster S, Dilworth L. New Frontiers for the Use of IP6 and Inositol Combination in Treating Diabetes Mellitus: A Review. Molecules. 2020;25. doi: 10.3390/molecules25071720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Aoki K, Terauchi Y. Effect of acarbose therapy once or twice a day on glycemic control in japanese patients with type 2 diabetes. Rinsho yakuri/Japanese J Clin Pharmacol Ther. 2012;43: 17–20. doi: 10.3999/jscpt.43.17 [DOI] [Google Scholar]
- 602.Moelands SV, Lucassen PL, Akkermans RP, De Grauw WJ, Van de Laar FA. Alpha-glucosidase inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane database Syst Rev. 2018;12: CD005061. doi: 10.1002/14651858.CD005061.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Saisho Y, Tanaka K, Abe T, Shimada A, Kawai T, Itoh H. Glycated albumin to glycated hemoglobin ratio reflects postprandial glucose excursion and relates to beta cell function in both type 1 and type 2 diabetes. Diabetol Int. 2011;2: 146–153. doi: 10.1007/s13340-011-0035-x [DOI] [Google Scholar]
- 604.Kizilaslan N, Erdem NZ. The effect of different amounts of cinnamon consumption on blood glucose in healthy adult individuals. Int J food Sci. 2019;2019: 4138534. doi: 10.1155/2019/4138534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Tayebi SM, Nouri AH, Tartibian B, Ahmadabadi S, Basereh A, Jamhiri I. Effects of swimming training in hot and cold temperatures combined with cinnamon supplementation on HbA1C levels, TBC1D1, and TBC1D4 in diabetic rats. Nutr Diabetes. 2024;14: 1. doi: 10.1038/s41387-023-00256-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Sahib AS. Anti-diabetic and antioxidant effect of cinnamon in poorly controlled type-2 diabetic Iraqi patients: A randomized, placebo-controlled clinical trial. J Intercult Ethnopharmacol. 2016;5: 108–113. doi: 10.5455/jice.20160217044511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Lira Neto JCG, Araújo MFM de, Araújo AVEC, Figueira JNR, Maranhão TA, Damasceno MMC. Effectiveness of cinnamon in the reduction of lipid levels in people with diabetes: a randomized clinical trial. Rev Gauch Enferm. 2023;44: e20230051. doi: 10.1590/1983-1447.2023.20230051.en [DOI] [PubMed] [Google Scholar]
- 608.Azimi P, Ghiasvand R, Feizi A, Hariri M, Abbasi B. Effects of cinnamon, cardamom, saffron, and ginger consumption on markers of glycemic control, lipid profile, oxidative stress, and inflammation in type 2 diabetes patients. Rev Diabet Stud. 2014;11: 258–66. doi: 10.1900/RDS.2014.11.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Askari F, Rashidkhani B, Hekmatdoost A. Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutr Res. 2014;34: 143–148. doi: 10.1016/j.nutres.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 610.Sengsuk C, Sanguanwong S, Tangvarasittichai O, Tangvarasittichai S. Effect of cinnamon supplementation on glucose, lipids levels, glomerular filtration rate, and blood pressure of subjects with type 2 diabetes mellitus. Diabetol Int. 2016;7: 124–132. doi: 10.1007/s13340-015-0218-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth DO, et al. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest. 2006;36: 340–4. doi: 10.1111/j.1365-2362.2006.01629.x [DOI] [PubMed] [Google Scholar]
- 612.Lu T, Sheng H, Wu J, Cheng Y, Zhu J, Chen Y. Cinnamon extract improves fasting blood glucose and glycosylated hemoglobin level in Chinese patients with type 2 diabetes. Nutr Res. 2012;32: 408–12. doi: 10.1016/j.nutres.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 613.Akilen R, Tsiami A, Devendra D, Robinson N. Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic Type 2 diabetic patients in the UK: A randomized, placebo-controlled, double-blind clinical trial. Diabet Med. 2010;27: 1159–67. doi: 10.1111/j.1464-5491.2010.03079.x [DOI] [PubMed] [Google Scholar]
- 614.Crawford P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: a randomized, controlled trial. J Am Board Fam Med. 2009;22: 507–512. doi: 10.3122/jabfm.2009.05.080093 [DOI] [PubMed] [Google Scholar]
- 615.Anderson RA, Zhan Z, Luo R, Guo X, Guo Q, Zhou J, et al. Cinnamon extract lowers glucose, insulin and cholesterol in people with elevated serum glucose. J Tradit Complement Med. 2015;6: 332–336. doi: 10.1016/j.jtcme.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Wang JG, Anderson RA, Graham GM, Chu MC, Sauer M V, Guarnaccia MM, et al. The effect of cinnamon extract on insulin resistance parameters in polycystic ovary syndrome: A pilot study. Fertil Steril. 2007;88: 240–3. doi: 10.1016/j.fertnstert.2006.11.082 [DOI] [PubMed] [Google Scholar]
- 617.Soni R, Bhatnagar V. Effect of cinnamon (Cinnamomum cassia) intervention on blood glucose of middle aged adult male with Non Insulin Dependent Diabetes Mellitus (NIDDM). Stud Ethno-Medicine. 2009;3: 141–144. doi: 10.1080/09735070.2009.11886352 [DOI] [Google Scholar]
- 618.Ziegenfuss TN, Hofheins JE, Mendel RW, Landis J, Anderson RA. Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. J Int Soc Sports Nutr. 2006;3: 45–53. doi: 10.1186/1550-2783-3-2-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Zare R, Shams M, Heydari M, Najarzadeh A, Zarshenas M. Analysis of the efficacy of cinnamon for patients with diabetes mellitus type II based on traditional Persian medicine syndrome differentiation: a randomized controlled trial. Shiraz E-Medical J. 2020;21. [Google Scholar]
- 620.Hendre AS, Sontakke A V, Patil SR, Phatak RS. Effect of cinnamon supplementation on fasting blood glucose and insulin resistance in patients with type 2 diabetes. Pravara Med Rev. 2019;11. [Google Scholar]
- 621.Talaei B, Amouzegar A, Sahranavard S, Hedayati M, Mirmiran P, Azizi F. Effects of cinnamon consumption on glycemic indicators, advanced glycation end products, and antioxidant status in type 2 diabetic patients. Nutrients. 2017;9. doi: 10.3390/nu9090991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Mirfeizi M, Mehdizadeh Tourzani Z, Mirfeizi SZ, Asghari Jafarabadi M, Rezvani HR, Afzali M. Controlling type 2 diabetes mellitus with herbal medicines: A triple-blind randomized clinical trial of efficacy and safety. J Diabetes. 2016;8: 647–656. doi: 10.1111/1753-0407.12342 [DOI] [PubMed] [Google Scholar]
- 623.Blevins SM, Leyva MJ, Brown J, Wright J, Scofield RH, Aston CE. Effect of cinnamon on glucose and lipid levels in non insulin-dependent type 2 diabetes. Diabetes Care. 2007;30: 2236–2237. doi: 10.2337/dc07-0098 [DOI] [PubMed] [Google Scholar]
- 624.Suppapitiporn S, Kanpaksi N, Suppapitiporn S. The effect of cinnamon cassia powder in type 2 diabetes mellitus. J Med Assoc Thai. 2006;89 Suppl 3: S200-5. [PubMed] [Google Scholar]
- 625.Vanschoonbeek K, Thomassen BJW, Senden JM, Wodzig WKWH, van Loon LJC. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J Nutr. 2006;136: 977–80. doi: 10.1093/jn/136.4.977 [DOI] [PubMed] [Google Scholar]
- 626.Wainstein J, Stern N, Heller S, Boaz M. Dietary cinnamon supplementation and changes in systolic blood pressure in subjects with type 2 diabetes. J Med Food. 2011;14: 1505–1510. doi: 10.1089/jmf.2010.0300 [DOI] [PubMed] [Google Scholar]
- 627.Hasanzade F, Toliat M, Emami SA, Emamimoghaadam Z. The Effect of Cinnamon on Glucose of Type II Diabetes Patients. J Tradit Complement Med. 2013;3: 171–174. doi: 10.4103/2225-4110.114900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Ioannidis JP. We need more randomized trials in nutrition-preferably large, long-term, and with negative results. The American journal of clinical nutrition. United States; 2016. pp. 1385–1386. doi: 10.3945/ajcn.116.136085 [DOI] [PubMed] [Google Scholar]
- 629.Hall KD. Challenges of human nutrition research. Science. 2020;367: 1298–1300. doi: 10.1126/science.aba3807 [DOI] [PubMed] [Google Scholar]
- 630.Chamorro-Cevallos G, Mojica-Villegas MA, García-Martínez Y, Pérez-Gutiérrez S, Madrigal-Santillán E, Vargas-Mendoza N, et al. A Complete Review of Mexican Plants with Teratogenic Effects. Plants (Basel, Switzerland). 2022;11. doi: 10.3390/plants11131675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Ford PW, Harmon AD, Tucker AO, Sasser M, Jackoway G, Albornoz G, et al. Cinnamon ‐ differentiation of four species by linking classical botany to an automated chromatographic authentication system. J AOAC Int. 2019;102: 363–368. doi: 10.5740/jaoacint.18-0343 [DOI] [PubMed] [Google Scholar]
- 632.Lungarini S, Aureli F, Coni E. Coumarin and cinnamaldehyde in cinnamon marketed in Italy: A natural chemical hazard? Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008;25: 1297–305. doi: 10.1080/02652030802105274 [DOI] [PubMed] [Google Scholar]
- 633.Tisnadjaja D, Irawan H, Ekawati N, Bustanussalam B, Simanjuntak P. Potency of Cinnamomum burmannii as antioxidant and α glucosidase inhibitor and their relation to trans-cinamaldehyde and coumarin contents. J Fitofarmaka Indones. 2020;7: 20–25. doi: 10.33096/jffi.v7i3.639 [DOI] [Google Scholar]
- 634.De Natale C, Annuzzi G, Bozzetto L, Mazzarella R, Costabile G, Ciano O, et al. Effects of a plant-based high-carbohydrate/high-fiber diet versus high-monounsaturated fat/low-carbohydrate diet on postprandial lipids in type 2 diabetic patients. Diabetes Care. 2009;32: 2168–2173. doi: 10.2337/dc09-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Costabile G, Vitale M, Della Pepa G, Cipriano P, Vetrani C, Testa R, et al. A wheat aleurone-rich diet improves oxidative stress but does not influence glucose metabolism in overweight/obese individuals: Results from a randomized controlled trial. Nutr Metab Cardiovasc Dis. 2022;32: 715–726. doi: 10.1016/j.numecd.2021.12.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol of the study project approved by the ethics committee in original language.
(DOCX)
Protocol of the study project approved by the ethics committee translated into English.
(DOCX)
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.