Abstract
Classical approaches to the study of pathological tissue have relied mainly on morphological techniques. In an attempt to quantitate the abnormalities and to investigate the pathogenesis of tissue disorders at a subcellular level we have combined analytical subcellular fractionation by sucrose density gradient centrifugation with microanalysis of tissue enzymic activities. The methodological problems of performing these studies on milligram quantities of tissue are discussed. Details of the appropriate equipment are provided, and its application to the study of human liver specimens is described. As an example of this approach, biochemical and subcellular fractionation experiments on tissue from patients with both primary and secondary hepatic lysosomal storage diseases are discussed. Examination of the lysosomal changes reveals that increased enzyme activity is a common finding in these disorders but tissue damage occurs only when there is evidence of enhances lysosomal fragility with intracellular release of degradative enzymes. Other tissues which have proved amenable to study in this manner and in which profitable results in the investigation of their disorders have been obtained are listed.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar-Costesec A., Wibo M., Thinès-Sempoux D., Beaufay H., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. IV. Biochemical, physical, and morphological modifications of microsomal components induced by digitonin, EDTA, and pyrophosphate. J Cell Biol. 1974 Sep;62(3):717–745. doi: 10.1083/jcb.62.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axline S. G., Cohn Z. A. In vitro induction of lysosomal enzymes by phagocytosis. J Exp Med. 1970 Jun 1;131(6):1239–1260. doi: 10.1084/jem.131.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BESSIS M., CAROLI J. A comparative study of hemochromatosis by electron microscopy. Gastroenterology. 1959 Nov;37:538–549. [PubMed] [Google Scholar]
- Barth R. F., Grimley P. M., Berk P. D., Bloomer J. R., Howe R. B. Excess lipofuscin accumulation in constitutional hepatic dysfunction (Gilbert's syndrome). Light and electron microscopic observations. Arch Pathol. 1971 Jan;91(1):41–47. [PubMed] [Google Scholar]
- Batt R. M., Bush B. M., Peters T. J. Biochemical changes in the jejunal mucosa of dogs with naturally occurring exocrine pancreatic insufficiency. Gut. 1979 Aug;20(8):709–715. doi: 10.1136/gut.20.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson G. D. Hepatic copper accumulation in primary biliary cirrhosis. Yale J Biol Med. 1979 Jan-Feb;52(1):83–88. [PMC free article] [PubMed] [Google Scholar]
- Bhuyan U. N., Welbourn C. R., Evans D. J., Peters T. J. Biochemical studies of the isolated rat glomerulus and the effects of puromycin aminonucleoside administration. Br J Exp Pathol. 1980 Feb;61(1):69–75. [PMC free article] [PubMed] [Google Scholar]
- Björntorp P., Björkerud S., Scherstén T. Subcellular fractionation of human liver. Biochim Biophys Acta. 1965 Dec 16;111(2):375–383. doi: 10.1016/0304-4165(65)90047-4. [DOI] [PubMed] [Google Scholar]
- Bojar H., Basler M., Fuchs F., Dreyfürst R., Staib W., Broelsch C. Preparation of parenchymal and non-parenchymal cells from adult human liver--morphological and biochemical characteristics. J Clin Chem Clin Biochem. 1976 Nov;14(11):527–532. doi: 10.1515/cclm.1976.14.1-12.527. [DOI] [PubMed] [Google Scholar]
- Bors W., Saran M., Lengfelder E., Spöttl R., Michel C. The relevance of the superoxide anion radical in biological systems. Curr Top Radiat Res Q. 1974 May;9(3):247–309. [PubMed] [Google Scholar]
- Bryant M. G., Dawson J., Bloom S. R., Peters T. J. Separation of the gut hormone endocrine-cell storage granules of human jejunum using analytical subcellular fractionation. Gut. 1980 Mar;21(3):177–180. doi: 10.1136/gut.21.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton R., Lloyd J. B. Latency of some glycosidases of rat liver lysosomes. Biochem J. 1976 Dec 15;160(3):631–638. doi: 10.1042/bj1600631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper B. T., Candy D. C., Harries J. T., Peters T. J. Subcellular fractionation studies of the intestinal mucosa in congenital sucrase--isomaltase deficiency. Clin Sci (Lond) 1979 Aug;57(2):181–185. doi: 10.1042/cs0570181. [DOI] [PubMed] [Google Scholar]
- Dawson J., Bryant M. G., Bloom S. R., Peters T. J. Subcellular fractionation studies of human gastric antrum: localization of the mucosal peptide hormones. Clin Sci (Lond) 1980 Jul;59(1):1–6. doi: 10.1042/cs0590001. [DOI] [PubMed] [Google Scholar]
- Dawson J., Seymour C. A., Peters T. J. Gilbert's syndrome: analytical subcellular fractionation of liver biopsy specimens. Enzyme activities, organelle pathology and evidence for subpopulations of the syndrome. Clin Sci (Lond) 1979 Dec;57(6):491–497. doi: 10.1042/cs0570491. [DOI] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Evans W. H. A biochemical dissection of the functional polarity of the plasma membrane of the hepatocyte. Biochim Biophys Acta. 1980 May 27;604(1):27–64. doi: 10.1016/0005-2736(80)90584-2. [DOI] [PubMed] [Google Scholar]
- Fitchett D. H., Wells G., Peters T. J. Analytical subcellular fractionation of human heart: a comparison of left and right ventricle with hypertrophic obstructive myopathic tissue. Cardiovasc Res. 1979 Sep;13(9):532–540. doi: 10.1093/cvr/13.9.532. [DOI] [PubMed] [Google Scholar]
- Fong K. L., McCay P. B., Poyer J. L., Keele B. B., Misra H. Evidence that peroxidation of lysosomal membranes is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem. 1973 Nov 25;248(22):7792–7797. [PubMed] [Google Scholar]
- GOLBERG L., MARTIN L. E., BATCHELOR A. Biochemical changes in the tissues of animals injected with iron: acid phosphatase and other enzymes. Biochem J. 1960 Nov;77:252–262. doi: 10.1042/bj0770252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfischer S., Sternlieb I. Changes in the distribution of hepatic copper in relation to the progression of Wilson's disease (hepatolenticular degeneration). Am J Pathol. 1968 Dec;53(6):883–901. [PMC free article] [PubMed] [Google Scholar]
- Gregor H. D. A new method for the rapid separation of cell organelles. Anal Biochem. 1977 Sep;82(1):255–257. doi: 10.1016/0003-2697(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: is it a mechanism for hydroxyl radical production in biochemical systems? FEBS Lett. 1978 Aug 15;92(2):321–326. doi: 10.1016/0014-5793(78)80779-0. [DOI] [PubMed] [Google Scholar]
- Iancu T. C., Neustein H. B. Ferritin in human liver cells of homozygous beta-thalassaemia: ultrastructural observations. Br J Haematol. 1977 Dec;37(4):527–535. doi: 10.1111/j.1365-2141.1977.tb01026.x. [DOI] [PubMed] [Google Scholar]
- Jenkins W. J., Peters T. J. Mitochondrial enzyme activities in liver biopsies from patients with alcoholic liver disease. Gut. 1978 May;19(5):341–344. doi: 10.1136/gut.19.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins W. J., Peters T. J. Selectively reduced hepatic acetaldehyde dehydrogenase in alcoholics. Lancet. 1980 Mar 22;1(8169):628–629. doi: 10.1016/s0140-6736(80)91121-6. [DOI] [PubMed] [Google Scholar]
- Kane S. P., Peters T. J. Analytical subcellular fractionation of human granulocytes with reference to the localization of vitamin B12-binding proteins. Clin Sci Mol Med. 1975 Aug;49(2):171–182. doi: 10.1042/cs0490171. [DOI] [PubMed] [Google Scholar]
- Kane S. P., Vincenti A. C. Mucosal enzymes in human inflammatory bowel disease with reference to neutrophil granulocytes as mediators of tissue injury. Clin Sci (Lond) 1979 Oct;57(4):295–303. doi: 10.1042/cs0570295. [DOI] [PubMed] [Google Scholar]
- Knook D. L., Blansjaar N., Sleyster E. C. Isolation and characterization of Kupffer and endothelial cells from the rat liver. Exp Cell Res. 1977 Oct 15;109(2):317–329. doi: 10.1016/0014-4827(77)90011-8. [DOI] [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEIJER A. E., WILLIGHAGEN R. G. THE ACTIVITY OF GLUCOSE-6-PHOSPHATASE, ADENOSINE TRIPHOSPHATASE, SUCCINIC DEHYDROGENASE, AND ACID PHOSPHATASE AFTER DEXTRAN OF POLYVINYLPYRROLIDONE UPTAKE BY LIVER IN VIVO. Biochem Pharmacol. 1963 Sep;12:973–980. doi: 10.1016/0006-2952(63)90020-0. [DOI] [PubMed] [Google Scholar]
- Mellors A., Tappel A. L., Sawant P. L., Desai I. D. Mitochondrial swelling and uncoupling of oxidative phosphorylation by lysosomes. Biochim Biophys Acta. 1967 Sep 6;143(2):299–309. doi: 10.1016/0005-2728(67)90084-9. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Venkatesan S., Balasubramaniam S., Peters T. J. The submicrosomal localization of 3-hydroxy-3-methylglutaryl-coenzyme-A reductase, cholesterol 7alpha-hydroxylase and cholesterol in rat liver. Eur J Biochem. 1978 Jan 16;82(2):419–429. doi: 10.1111/j.1432-1033.1978.tb12036.x. [DOI] [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Berg T., Seljelid R. Distribution of lysosomal enzymes in different types of rat liver cells. Exp Cell Res. 1976 Apr;99(1):146–154. doi: 10.1016/0014-4827(76)90689-3. [DOI] [PubMed] [Google Scholar]
- Neerunjun J. S., Dubowitz V., Peters T. J. Analytical subcellular fractionation and enzymic analysis of dystrophic mouse skeletal muscle [proceedings]. Biochem Soc Trans. 1978;6(6):1266–1268. doi: 10.1042/bst0061266. [DOI] [PubMed] [Google Scholar]
- Nicholson J. A., Peters T. J. The subcellular localization of peptidase activity in the human jejunum. Eur J Clin Invest. 1979 Oct;9(5):349–354. doi: 10.1111/j.1365-2362.1979.tb00895.x. [DOI] [PubMed] [Google Scholar]
- Owen C. A., Jr, Dickson E. R., Goldstein N. P., Baggenstoss A. H., McCall J. T. Hepatic subcellular distribution of copper in primary biliary cirrhosis. Comparison with other hyperhepatocupric states and review of the literature. Mayo Clin Proc. 1977 Feb;52(2):73–80. [PubMed] [Google Scholar]
- PORTER H. TISSUE COPPER PROTEINS IN WILSON'S DISEASE. INTRACELLULAR DISTRIBUTION AND CHROMATOGRAPHIC FRACTIONATION. Arch Neurol. 1964 Oct;11:341–349. doi: 10.1001/archneur.1964.00460220003001. [DOI] [PubMed] [Google Scholar]
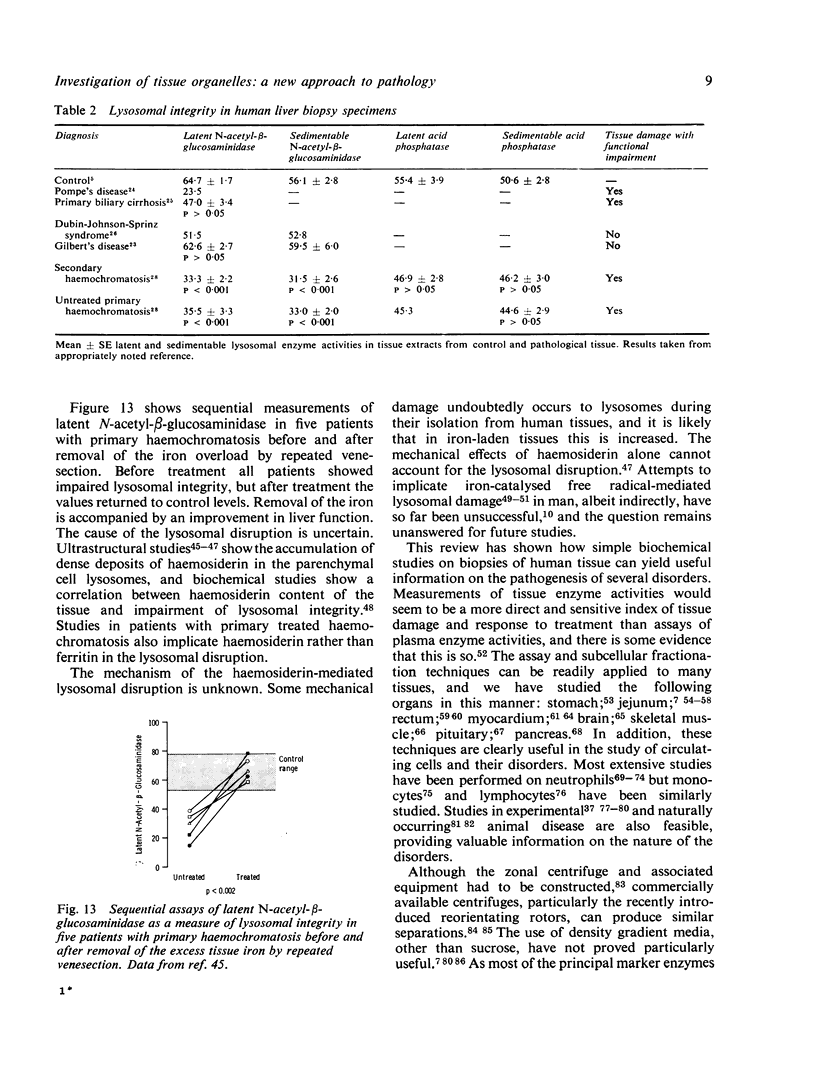
- Peters T. J. Analytical subcellular fractionation of jejunal biopsy specimens: methodology and characterization of the organelles in normal tissue. Clin Sci Mol Med. 1976 Dec;51(6):557–574. doi: 10.1042/cs0510557. [DOI] [PubMed] [Google Scholar]
- Peters T. J. Application of analytical subcellular fractionation techniques and tissue enzymic analysis to the study of human pathology. Clin Sci Mol Med. 1977 Dec;53(6):505–511. doi: 10.1042/cs0530505. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Brooksby I. A., Webb-Peploe M. M., Wells G., Jenkins B. S., Coltart D. J. Enzymic analysis of cardiac biopsy material from patients with valvular heart-disease. Lancet. 1976 Feb 7;1(7954):269–270. doi: 10.1016/s0140-6736(76)91401-x. [DOI] [PubMed] [Google Scholar]
- Peters T. J., De Duve C. Lysosomes of the arterial wall. II. Subcellular fractionation of aortic cells from rabbits with experimantal atheroma. Exp Mol Pathol. 1974 Apr;20(2):228–256. doi: 10.1016/0014-4800(74)90057-4. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Heath J. R., Wansbrough-Jones M. H., Foe W. F. Enzyme activities and properties of lysosomes and brush borders in jejunal biopsies from control subjects and patients with coeliac disease. Clin Sci Mol Med. 1975 Apr;48(4):259–267. doi: 10.1042/cs0480259. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Jenkins W., Dubowitz V. Subcellular fractionation studies on hepatic tissue from a patient with Pompe's disease (type II glycogen-storage disease). Clin Sci (Lond) 1980 Jul;59(1):7–12. doi: 10.1042/cs0590007. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Jones P. E., Wells G. Analytical subcellular fractionation of jejunal biopsy specimens: enzyme activities, organelle pathology and response to gluten withdrawal in patients with coeliac disease. Clin Sci Mol Med. 1978 Sep;55(3):285–292. doi: 10.1042/cs0550285. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Jones P. E., Wells G., Cook G. G. Sequential enzyme and subcellular fractionation studies on jejunal biopsy specimens from patients with post-infective tropical malabsorption. Clin Sci (Lond) 1979 May;56(5):479–486. doi: 10.1042/cs0560479. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T. J., Neale G., Heath J. R. Effect of bile-duct ligation on organelle marker enzymes in the liver and serum of rats. Clin Sci Mol Med. 1975 Apr;48(4):307–313. doi: 10.1042/cs0480307. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Seymour C. A. Analytical subcellular fractionation of needle-biopsy specimens from human liver. Biochem J. 1978 Aug 15;174(2):435–446. doi: 10.1042/bj1740435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T. J., Seymour C. A. The organelle pathology and demonstration of mitochondrial superoxide dismutase deficiency in two patients with Dubin--Johnson--Sprinz syndrome. Clin Sci Mol Med. 1978 May;54(5):549–553. doi: 10.1042/cs0540549. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Shio H. Analytical subcellular fractionation studies on rat liver and on isolated jejunal enterocytes with special reference to the separation of lysosomes, peroxisomes and mitochondria. Clin Sci Mol Med. 1976 May;50(5):355–366. doi: 10.1042/cs0500355. [DOI] [PubMed] [Google Scholar]
- Rustin G. J., Peters T. J. Studies on the subcellular organelles of neutrophils in chronic granulocytic leukaemia with special reference to alkaline phosphatase. Br J Haematol. 1979 Apr;41(4):533–543. doi: 10.1111/j.1365-2141.1979.tb05891.x. [DOI] [PubMed] [Google Scholar]
- Rustin G. J., Wilson P. D., Peters T. J. Studies on the subcellular localization of human neutrophil alkaline phosphatase. J Cell Sci. 1979 Apr;36:401–412. doi: 10.1242/jcs.36.1.401. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Peters T. J. Analytical subcellular fractionation of human granulocytes with special reference to the localization of enzymes involved in microbicidal mechanisms. Clin Sci Mol Med. 1977 Apr;52(4):429–442. doi: 10.1042/cs0520429. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Peters T. J. Analytical subcellular fractionation of neutrophils from patients with chronic granulomatous disease. Demonstration of the enzyme defect in four cases. Q J Med. 1978 Apr;47(186):213–220. [PubMed] [Google Scholar]
- Selden C., Owen M., Hopkins J. M., Peters T. J. Studies on the concentration and intracellular localization of iron proteins in liver biopsy specimens from patients with iron overload with special reference to their role in lysosomal disruption. Br J Haematol. 1980 Apr;44(4):593–603. doi: 10.1111/j.1365-2141.1980.tb08714.x. [DOI] [PubMed] [Google Scholar]
- Selden C., Wootton A. M., Moss D. W., Peters T. J. Analytical subcellular fractionation studies on different cell types isolated from normal rat liver. Clin Sci Mol Med. 1978 Nov;55(5):423–427. doi: 10.1042/cs0550423. [DOI] [PubMed] [Google Scholar]
- Seymour C. A., Neale G., Peters T. J. Lysosomal changes in liver tissue from patients with the Dubin-Johnson-Sprinz syndrome. Clin Sci Mol Med. 1977 Mar;52(3):241–248. doi: 10.1042/cs0520241. [DOI] [PubMed] [Google Scholar]
- Seymour C. A., Peters T. J. Changes in hepatic enzymes and organelles in alcoholic liver disease. Clin Sci Mol Med. 1978 Oct;55(4):383–389. doi: 10.1042/cs0550383. [DOI] [PubMed] [Google Scholar]
- Seymour C. A., Peters T. J. Enzyme activities in human liver biopsies: assay methods and activities of some lysosomal and membrane-bound enzymes in control tissue and serum. Clin Sci Mol Med. 1977 Mar;52(3):229–239. doi: 10.1042/cs0520229. [DOI] [PubMed] [Google Scholar]
- Seymour C. A., Peters T. J. Organelle pathology in primary and secondary haemochromatosis with special reference to lysosomal changes. Br J Haematol. 1978 Oct;40(2):239–253. doi: 10.1111/j.1365-2141.1978.tb03661.x. [DOI] [PubMed] [Google Scholar]
- Smith G. D., Peters T. J. Analytical subcellular fractionation of rat liver with special reference to the localisation of putative plasma membrane marker enzymes. Eur J Biochem. 1980 Feb;104(1):305–311. doi: 10.1111/j.1432-1033.1980.tb04429.x. [DOI] [PubMed] [Google Scholar]
- Smith G. P., Peters T. J. Studies on the activities, kinetic properties and subcellular localisation of cyclic AMP phosphodiesterase in human neutrophil leukocytes. Clin Chim Acta. 1980 Apr 25;103(2):193–201. doi: 10.1016/0009-8981(80)90213-2. [DOI] [PubMed] [Google Scholar]
- Solyom A., Trams E. G. Enzyme markers in characterization of isolated plasma membranes. Enzyme. 1972;13(5-6):329–372. doi: 10.1159/000459682. [DOI] [PubMed] [Google Scholar]
- Soulé J. C., Neale G., Peters T. J. Functional and biochemical evidence of damage to enterocytes induced by triparanol: role of lysosomes and the effect of gluten-free diet. Clin Sci Mol Med. 1976 Jul;51(1):19–25. doi: 10.1042/cs0510019. [DOI] [PubMed] [Google Scholar]
- Tilleray J., Peters T. J. Analytical subfractionation of microsomal fractions from the livers of control and Gunn-strain rats. Biochem Soc Trans. 1976;4(2):248–250. doi: 10.1042/bst0040248. [DOI] [PubMed] [Google Scholar]
- Tomiyama O., Shiigai T., Ideura T., Tomita K., Mito Y., Shinohara S., Takeuchi J. Baroreflex sensitivity in renal failure. Clin Sci (Lond) 1980 Jan;58(1):21–27. doi: 10.1042/cs0580021. [DOI] [PubMed] [Google Scholar]
- Van Hoof F., Hers H. G. The abnormalities of lysosomal enzymes in mucopolysacc- haridoses. Eur J Biochem. 1968 Dec;7(1):34–44. doi: 10.1111/j.1432-1033.1968.tb19570.x. [DOI] [PubMed] [Google Scholar]
- Wibo M., Amar-Costesec A., Berthet J., Beaufay H. Electron microscope examination of subcellular fractions. 3. Quantitative analysis of the microsomal fraction isolated from rat liver. J Cell Biol. 1971 Oct;51(1):52–71. doi: 10.1083/jcb.51.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton A. M., Neale G., Moss D. W. Some properties of alkaline-phosphatases in parenchymal and biliary tract cells separated from rat liver. Clin Chim Acta. 1975 Jun 2;61(2):183–190. doi: 10.1016/0009-8981(75)90313-7. [DOI] [PubMed] [Google Scholar]



