Abstract
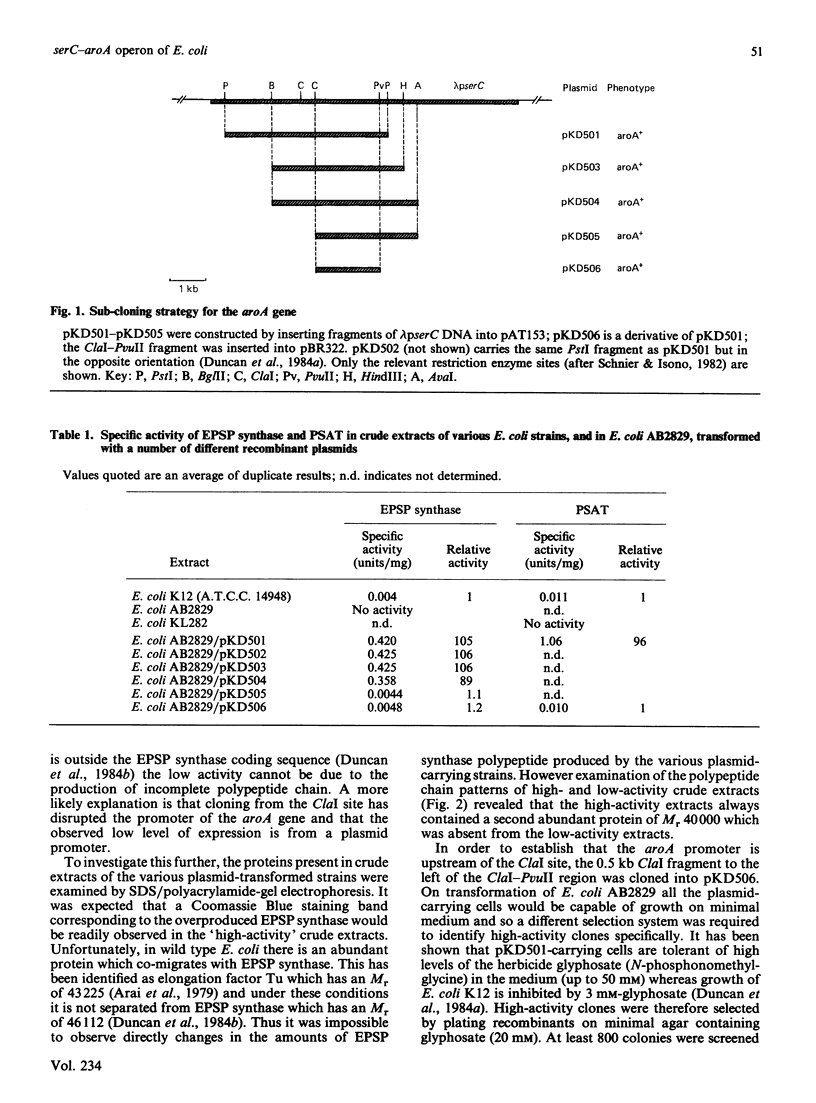
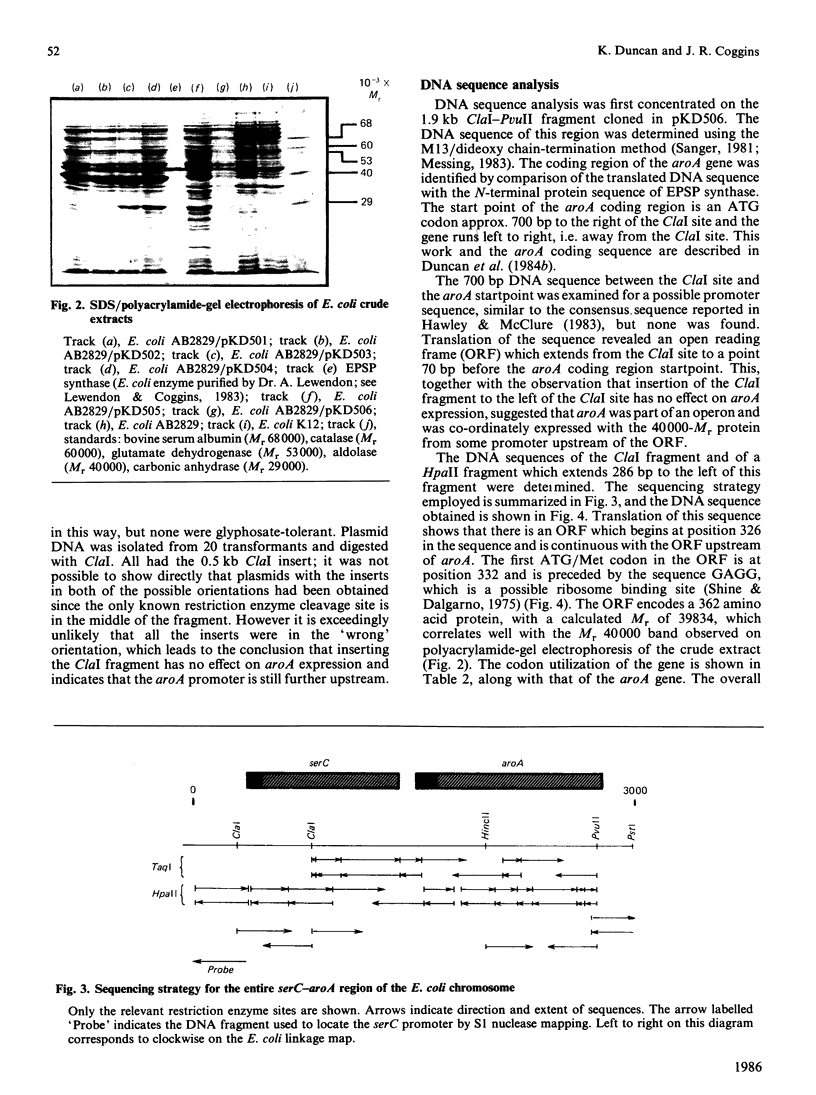
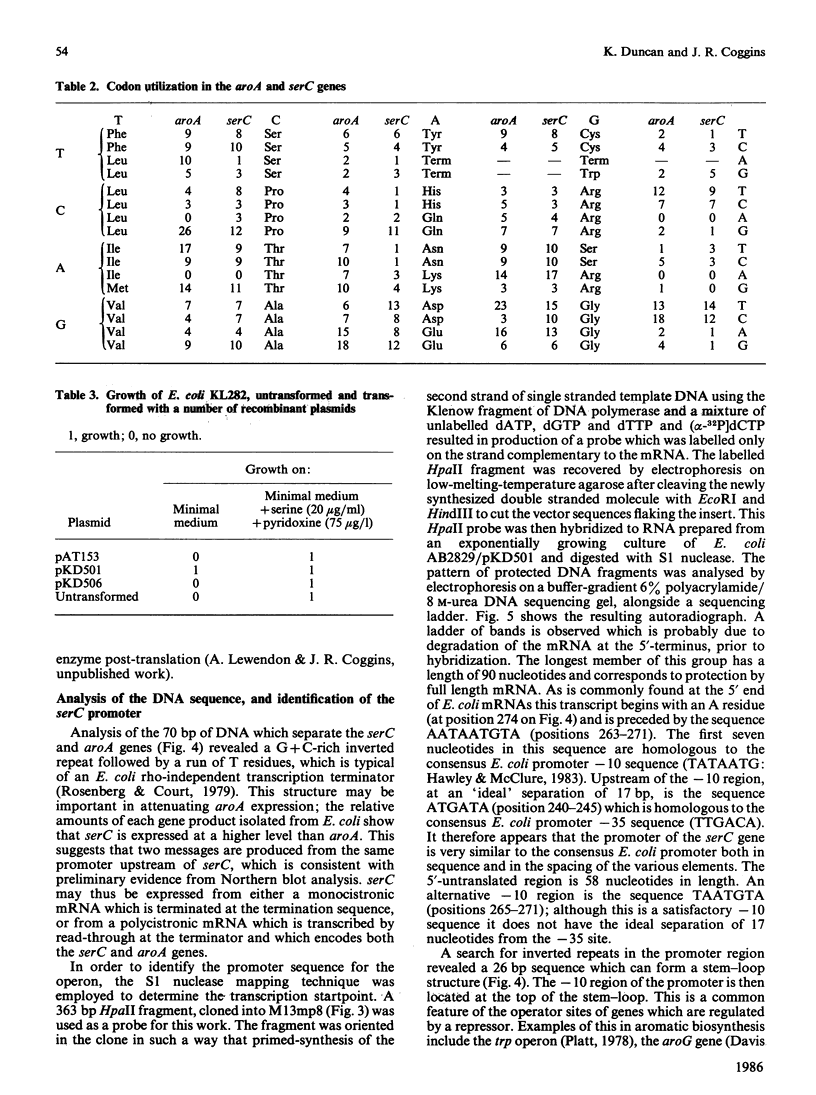
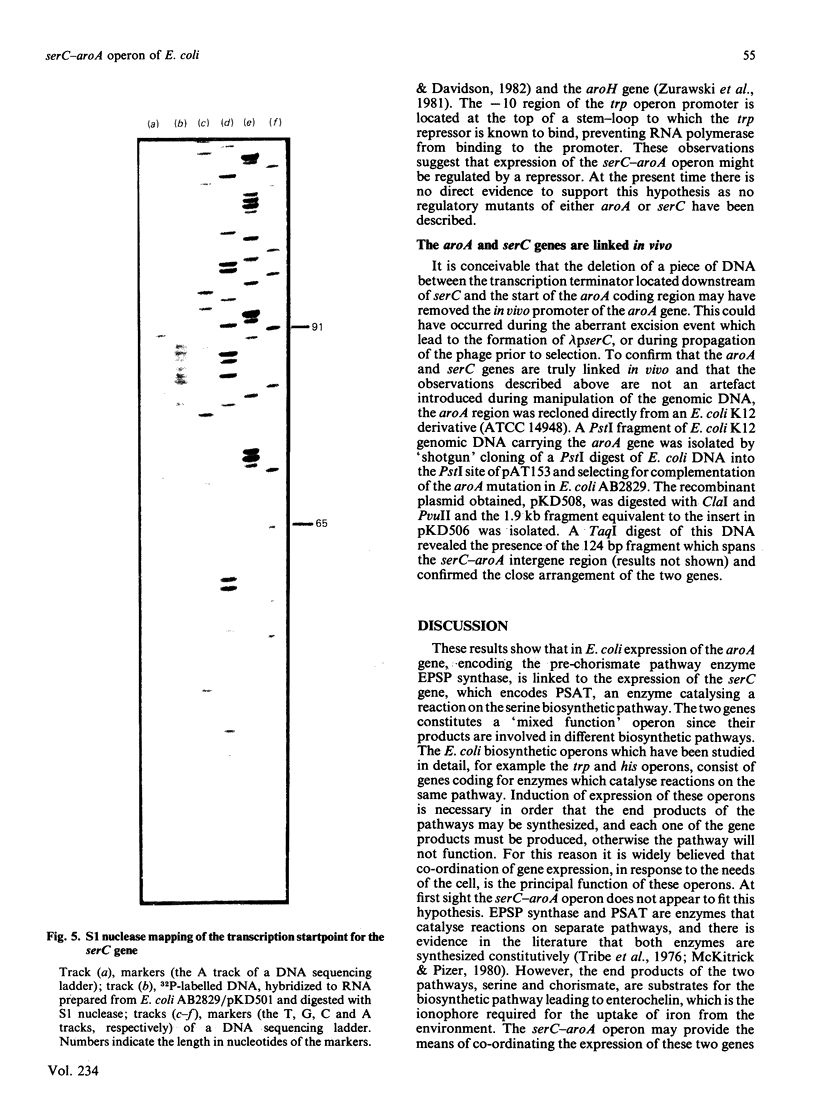
Sub-cloning experiments aimed at precisely locating the E. coli aroA gene, which encodes the shikimate pathway enzyme 5-enolpyruvylshikimate 3-phosphate synthase, showed that in certain constructions, which remain capable of complementing an auxotrophic aroA mutation, expression of aroA is reduced. DNA sequence analysis revealed that a sequence approx. 1200 base pairs (bp) upstream of aroA is necessary for its expression. An open reading frame was identified in this region which encodes a protein of 362 amino acids with a calculated Mr of 39,834 and which ends 70 bp before the start of the aroA coding sequence. This gene has been identified as serC, the structural gene for 3-phosphoserine aminotransferase, an enzyme of the serine biosynthetic pathway. Both genes are expressed as a polycistronic message which is transcribed from a promotor located 58 bp upstream of serC. Evidence is presented which confirms that the aroA and serC genes constitute an operon which has the novel feature of encoding enzymes from two different amino acid biosynthetic pathways.
Full text
PDF




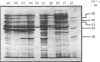
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Clark B. F., Duffy L., Jones M. D., Kaziro Y., Laursen R. A., L'Italien J., Miller D. L., Nagarkatti S., Nakamura S. Primary structure of elongation factor Tu from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1326–1330. doi: 10.1073/pnas.77.3.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlyn M. B., Giles N. H. Organization of enzymes in the polyaromatic synthetic pathway: separability in bacteria. J Bacteriol. 1969 Jul;99(1):222–230. doi: 10.1128/jb.99.1.222-230.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boocock M. R., Coggins J. R. Kinetics of 5-enolpyruvylshikimate-3-phosphate synthase inhibition by glyphosate. FEBS Lett. 1983 Apr 5;154(1):127–133. doi: 10.1016/0014-5793(83)80888-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buck M., Griffiths E. Iron mediated methylthiolation of tRNA as a regulator of operon expression in Escherichia coli. Nucleic Acids Res. 1982 Apr 24;10(8):2609–2624. doi: 10.1093/nar/10.8.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., Griffiths E. Regulation of aromatic amino acid transport by tRNA: role of 2-methylthio-N6-(delta2-isopentenyl)-adenosine. Nucleic Acids Res. 1981 Jan 24;9(2):401–414. doi: 10.1093/nar/9.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. J., Low B., Konigsberg W. H. Close linkage of the genes serC (for phosphohydroxy pyruvate transaminase) and serS (for seryl-transfer ribonucleic acid synthetase) in Escherichia coli K-12. J Bacteriol. 1973 Mar;113(3):1091–1095. doi: 10.1128/jb.113.3.1091-1095.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins J. R., Boocock M. R., Campbell M. S., Chaudhuri S., Lambert J. M., Lewendon A., Mousdale D. M., Smith D. D. Functional domains involved in aromatic amino acid biosynthesis. Biochem Soc Trans. 1985 Apr;13(2):299–303. doi: 10.1042/bst0130299. [DOI] [PubMed] [Google Scholar]
- Comai L., Sen L. C., Stalker D. M. An Altered aroA Gene Product Confers Resistance to the Herbicide Glyphosate. Science. 1983 Jul 22;221(4608):370–371. doi: 10.1126/science.221.4608.370. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Davies W. D., Davidson B. E. The nucleotide sequence of aroG, the gene for 3-deoxy-D-arabinoheptulosonate-7-phosphate synthetase (phe) in Escherichia coli K12. Nucleic Acids Res. 1982 Jul 10;10(13):4045–4058. doi: 10.1093/nar/10.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B. 3-Phosphoserine transaminase mutants of Escherichia coli B. J Bacteriol. 1969 Nov;100(2):1114–1115. doi: 10.1128/jb.100.2.1114-1115.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K., Lewendon A., Coggins J. R. The purification of 5-enolpyruvylshikimate 3-phosphate synthase from an overproducing strain of Escherichia coli. FEBS Lett. 1984 Jan 2;165(1):121–127. doi: 10.1016/0014-5793(84)80027-7. [DOI] [PubMed] [Google Scholar]
- Fleming T. P., Nahlik M. S., McIntosh M. A. Regulation of enterobactin iron transport in Escherichia coli: characterization of ent::Mu d(Apr lac) operon fusions. J Bacteriol. 1983 Dec;156(3):1171–1177. doi: 10.1128/jb.156.3.1171-1177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertner F. H., Cole K. W. A cluster-gene: evidence for one gene, one polypeptide, five enzymes. Biochem Biophys Res Commun. 1977 Mar 21;75(2):259–264. doi: 10.1016/0006-291x(77)91037-3. [DOI] [PubMed] [Google Scholar]
- Garner C. C., Herrmann K. M. Operator mutations of the Escherichia coli aroF gene. J Biol Chem. 1985 Mar 25;260(6):3820–3825. [PubMed] [Google Scholar]
- Giles N. H., Case M. E., Partridge C. W., Ahmed S. I. A gene cluster in Nuerospora crassa coding for an aggregate of five aromatic synthetic enzymes. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1453–1460. doi: 10.1073/pnas.58.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw C. E., Sogo S. G., Knowles J. R. The fate of the hydrogens of phosphoenolpyruvate in the reaction catalyzed by 5-enolpyruvylshikimate-3-phosphate synthase. Isotope effects and isotope exchange. J Biol Chem. 1982 Jan 25;257(2):596–598. [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Genes aroA and serC of Salmonella typhimurium constitute an operon. J Bacteriol. 1985 Jul;163(1):355–361. doi: 10.1128/jb.163.1.355-361.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitakawa M., Blumenthal L., Isono K. Isolation and characterization of specialized transducing lambda phages carrying ribosomal protein genes of Escherichia coli. Mol Gen Genet. 1980;180(2):343–349. doi: 10.1007/BF00425846. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laird A. J., Ribbons D. W., Woodrow G. C., Young I. G. Bacteriophage Mu-mediated gene transposition and in vitro cloning of the enterochelin gene cluster of Escherichia coli. Gene. 1980 Nov;11(3-4):347–357. doi: 10.1016/0378-1119(80)90074-8. [DOI] [PubMed] [Google Scholar]
- Laird A. J., Young I. G. Tn5 mutagenesis of the enterochelin gene cluster of Escherichia coli. Gene. 1980 Nov;11(3-4):359–366. doi: 10.1016/0378-1119(80)90075-x. [DOI] [PubMed] [Google Scholar]
- Lambert J. M., Boocock M. R., Coggins J. R. The 3-dehydroquinate synthase activity of the pentafunctional arom enzyme complex of Neurospora crassa is Zn2+-dependent. Biochem J. 1985 Mar 15;226(3):817–829. doi: 10.1042/bj2260817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer F. W., Morse C. C., Beck A. K., Cole K. W., Gaertner F. H. Isolation of the ARO1 cluster gene of Saccharomyces cerevisiae. Mol Cell Biol. 1983 Sep;3(9):1609–1614. doi: 10.1128/mcb.3.9.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewendon A., Coggins J. R. Purification of 5-enolpyruvylshikimate 3-phosphate synthase from Escherichia coli. Biochem J. 1983 Jul 1;213(1):187–191. doi: 10.1042/bj2130187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden J., Coggins J. R. The subunit structure of the arom multienzyme complex of Neurospora crassa. A possible pentafunctional polypeptide chain. Biochem J. 1977 Mar 1;161(3):599–607. doi: 10.1042/bj1610599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray J. W., Jr, Herrmann K. M. Derepression of certain aromatic amino acid biosynthetic enzymes of Escherichia coli K-12 by growth in Fe3+-deficient medium. J Bacteriol. 1976 Feb;125(2):608–615. doi: 10.1128/jb.125.2.608-615.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKitrick J. C., Pizer L. I. Regulation of phosphoglycerate dehydrogenase levels and effect on serine synthesis in Escherichia coli K-12. J Bacteriol. 1980 Jan;141(1):235–245. doi: 10.1128/jb.141.1.235-245.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. G., Brand L. A., Holder S. B., Sharps E. S., Brackin M. J. Amplification of the aroA gene from Escherichia coli results in tolerance to the herbicide glyphosate. Appl Environ Microbiol. 1983 Jul;46(1):37–43. doi: 10.1128/aem.46.1.37-43.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F. Determination of nucleotide sequences in DNA. Science. 1981 Dec 11;214(4526):1205–1210. doi: 10.1126/science.7302589. [DOI] [PubMed] [Google Scholar]
- Schnier J., Isono K. The DNA sequence of the gene rpsA of Escherichia coli coding for ribosomal protein S1. Nucleic Acids Res. 1982 Mar 25;10(6):1857–1865. doi: 10.1093/nar/10.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Dempsey W. B. 3-hydroxypyruvate substitutes for pyridoxine in serC mutants of Escherichia coli K-12. J Bacteriol. 1978 Jun;134(3):944–949. doi: 10.1128/jb.134.3.944-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Steinrücken H. C., Amrhein N. 5-Enolpyruvylshikimate-3-phosphate synthase of Klebsiella pneumoniae. 1. Purification and properties. Eur J Biochem. 1984 Sep 3;143(2):341–349. doi: 10.1111/j.1432-1033.1984.tb08378.x. [DOI] [PubMed] [Google Scholar]
- Steinrücken H. C., Amrhein N. The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1207–1212. doi: 10.1016/0006-291x(80)90547-1. [DOI] [PubMed] [Google Scholar]
- Tribe D. E., Camakaris H., Pittard J. Constitutive and repressivle enzymes of the common pathway of aromatic biosynthesis in Escherichia coli K-12: regulation of enzyme synthesis at different growth rates. J Bacteriol. 1976 Sep;127(3):1085–1097. doi: 10.1128/jb.127.3.1085-1097.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Gunsalus R. P., Brown K. D., Yanofsky C. Structure and regulation of aroH, the structural gene for the tryptophan-repressible 3-deoxy-D-arabino-heptulosonic acid-7-phosphate synthetase of Escherichia coli. J Mol Biol. 1981 Jan 5;145(1):47–73. doi: 10.1016/0022-2836(81)90334-x. [DOI] [PubMed] [Google Scholar]