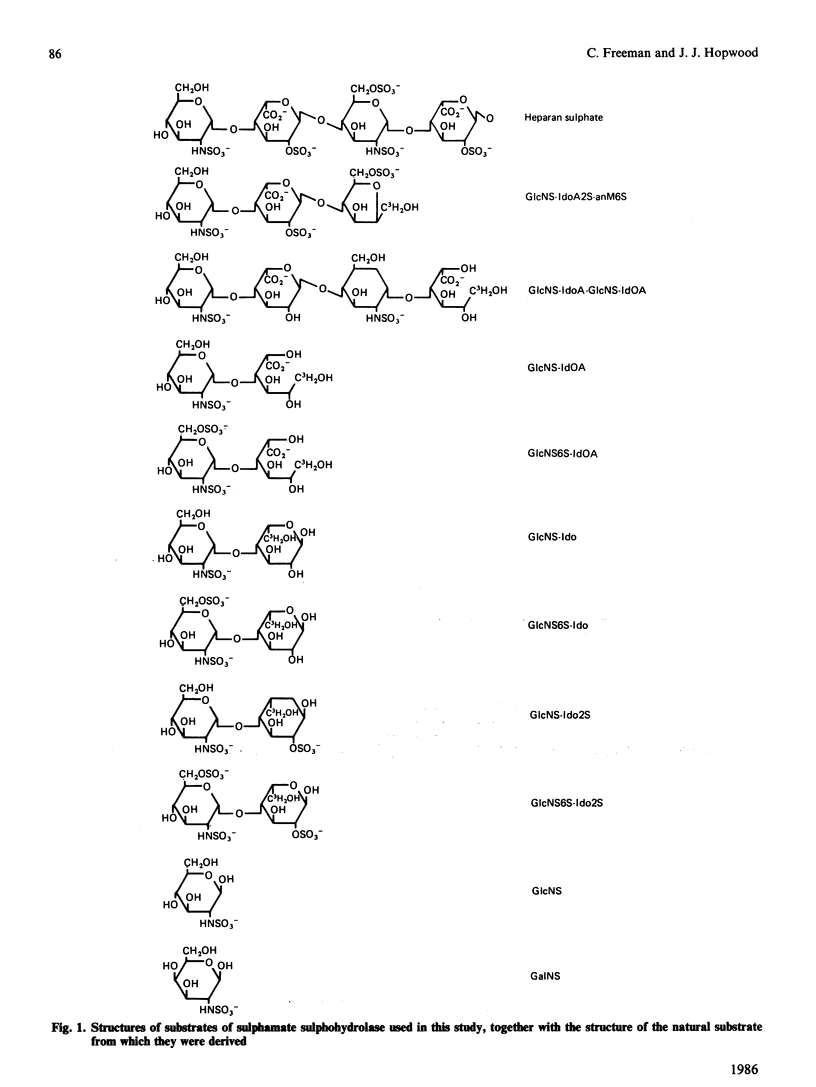
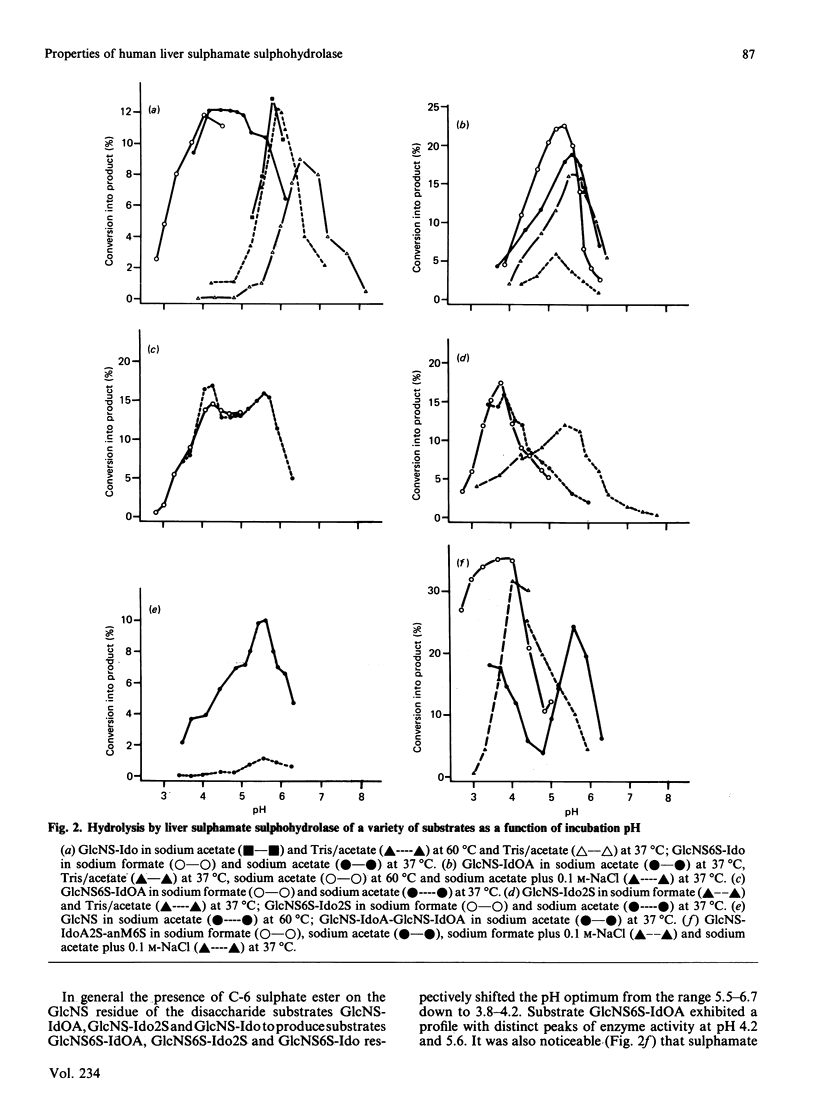
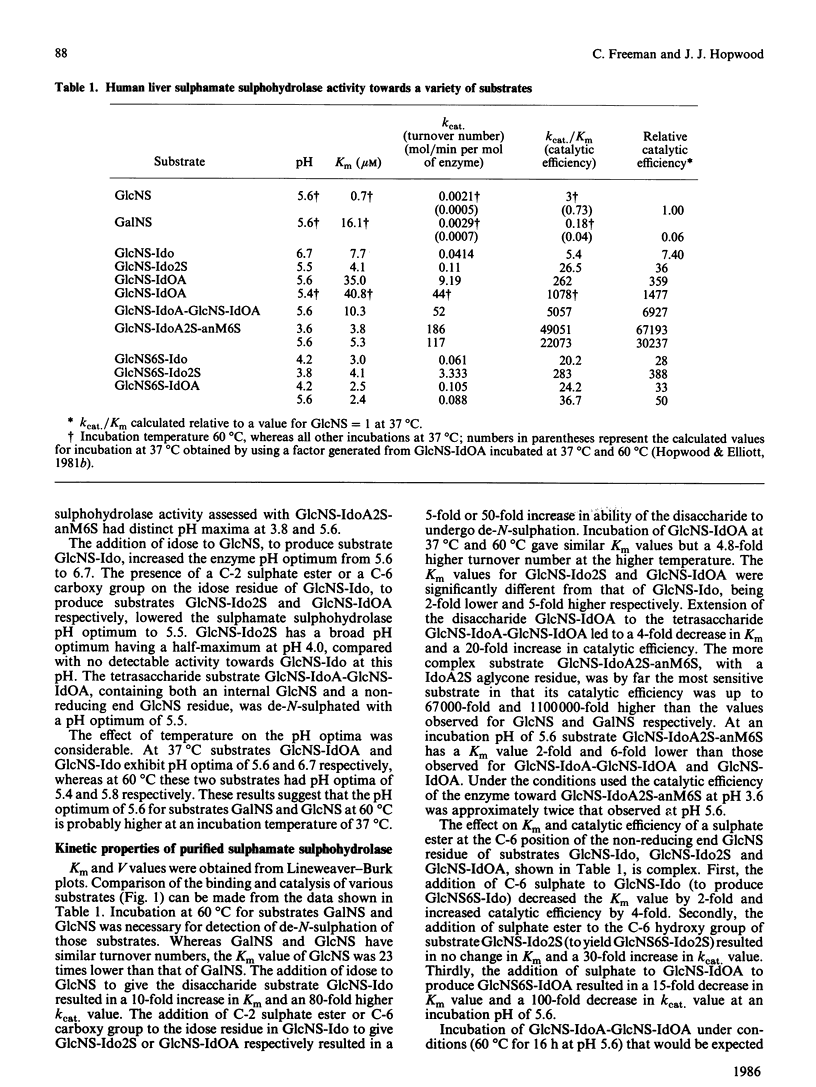
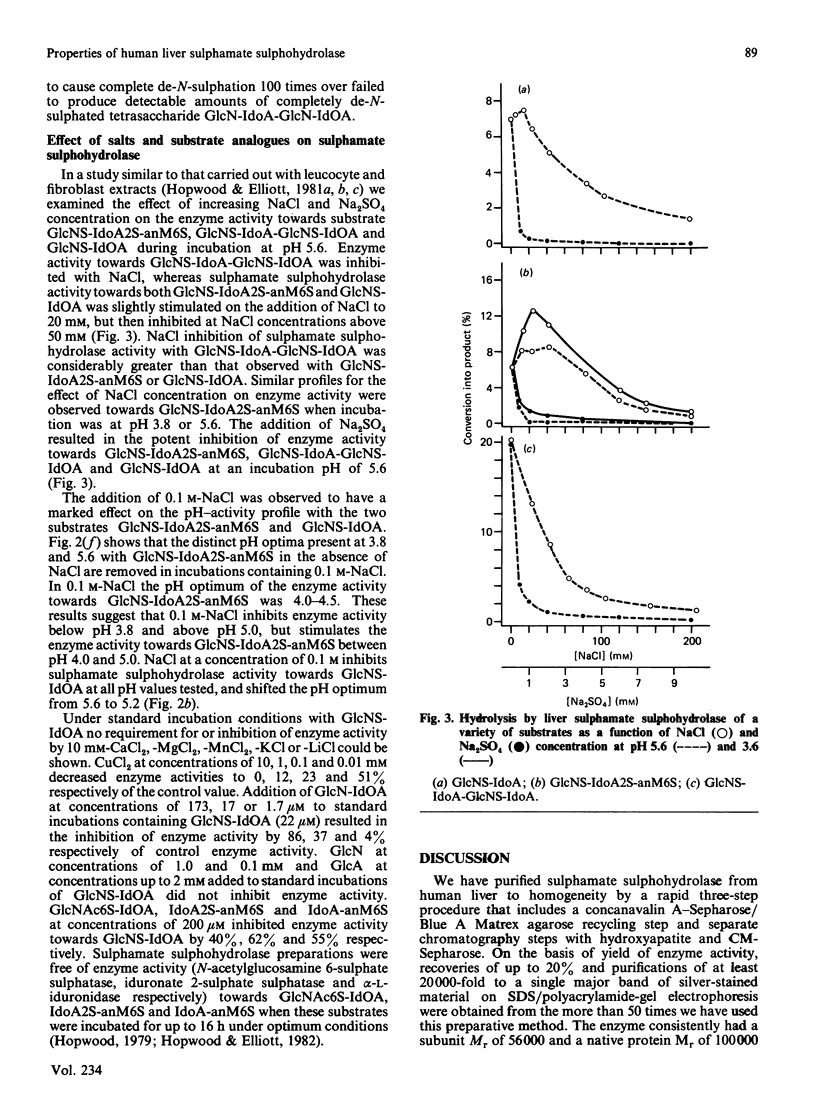
Abstract
Human sulphamate sulphohydrolase was purified at least 20,000-fold to homogeneity from liver with a three-step four-column procedure, which consisted of a concanavalin A-Sepharose/Blue A agarose coupled step, and Bio-Gel HT step and then a CM-Sepharose step. The procedure was also used to purify enzyme from kidney and placenta. The subunit Mr of liver, kidney and placenta sulphamate sulphohydrolase was assessed to be 56,000 by using SDS/polacrylamide-gel electrophoresis. The native protein Mr of enzyme from all three tissue sources was assessed by gel-permeation chromatography to be approx. 120,000 on Sephacryl S-300 and 100,000 on Fractogel TSK. It is probable that the native enzyme results from dimerization of subunits. Kinetic parameters (km and kcat.) of human liver sulphamate sulphohydrolase were determined with a variety of substrates matching structural aspects of the physiological substrates in vivo, namely heparin and heparan sulphate. More structurally complex substrates, in which several aspects of the aglycone structure of the natural substrate were maintained, are turned over up to 372000 times faster than the monosaccharide substrate 2-sulphaminoglucosamine. Aglycone structures that influence substrate binding and/or enzyme activity were penultimate-residue C-6 carboxy and C-2 sulphate ester groups and a post-penultimate 2-sulphaminoglucosamine residue. The C-4 hydroxy group of the 2-sulphaminoglucosamine under enzymic attack is involved in binding of substrate to enzyme. The presence of C-6 sulphate ester on the non-reducing end 2-sulphaminoglucosamine stimulates sulphamate bond hydrolysis and substrate affinity if the adjacent monosaccharide residue is idose or 2-sulphoidose, but strongly inhibits hydrolysis if the adjacent monosaccharide residue is iduronic acid. Sulphamate sulphohydrolase is an exoenzyme, since activity toward internal sulphamate bonds was not detected. The effect of incubation pH on enzyme activity towards the variety of substrates evaluated was complex and dependent on substrate aglycone structure. The presence of aglycone C-2 sulphate ester and aglycone C-6 carboxy groups and C-6 sulphate ester groups on the 2-sulphaminoglucosamine residue under attack considerably affect the pH response. Structurally complex substrates had two pH optima. Incubation temperature and buffer ionic strength markedly influenced pH optima and enzyme activity. Cu2+ and SO4(2-)ions are potent inhibitors of enzyme activity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- Brown W. E., Seamon K. B. Quantitation and characterization of the trifluoroacetonyl derivative of cysteine: a useful NMR probe. Anal Biochem. 1978 Jun 15;87(1):211–222. doi: 10.1016/0003-2697(78)90587-0. [DOI] [PubMed] [Google Scholar]
- Clements P. R., Brooks D. A., Saccone G. T., Hopwood J. J. Human alpha-L-iduronidase. 1. Purification, monoclonal antibody production, native and subunit molecular mass. Eur J Biochem. 1985 Oct 1;152(1):21–28. doi: 10.1111/j.1432-1033.1985.tb09158.x. [DOI] [PubMed] [Google Scholar]
- Clements P. R., Mahuran D. J., Hopwood J. J. Improved concanavalin A-Sepharose elution by specific readsorption of glycoproteins. J Chromatogr. 1983 May 20;261(1):77–82. doi: 10.1016/s0021-9673(01)87920-6. [DOI] [PubMed] [Google Scholar]
- Clements P. R., Muller V., Hopwood J. J. Human alpha-L-iduronidase. 2. Catalytic properties. Eur J Biochem. 1985 Oct 1;152(1):29–34. doi: 10.1111/j.1432-1033.1985.tb09159.x. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Sjöberg I., Havsmark B. Structural studies on heparan sulphates. Characterization of oligosaccharides; obtained by periodate oxidation and alkaline elimination. Eur J Biochem. 1980 May;106(1):59–69. [PubMed] [Google Scholar]
- Freeman C., Clements P. R., Hopwood J. J. Acetyl CoA:alpha-glucosaminide N-acetyl transferase: partial purification from human liver. Biochem Int. 1983 May;6(5):663–671. [PubMed] [Google Scholar]
- Gacesa P., Savitsky M. J., Dodgson K. S., Olavesen A. H. Modification of functional arginine residues in purified bovine testicular hyaluronidase with butane-2, 3-dione. Biochim Biophys Acta. 1981 Oct 13;661(2):205–212. doi: 10.1016/0005-2744(81)90005-x. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Diagnosis of Sanfilippo type A syndrome by estimation of sulfamidase activity using a radiolabelled tetrasaccharide substrate. Clin Chim Acta. 1982 Aug 18;123(3):241–250. doi: 10.1016/0009-8981(82)90168-1. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Radiolabelled oligosaccharides as substrates for the estimation of sulfamidase and the detection of the Sanfilippo type A syndrome. Clin Chim Acta. 1981 Apr 27;112(1):55–66. doi: 10.1016/0009-8981(81)90268-0. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Selective depolymerisation of heparin to produce radio-labelled substrates for sulfamidase, 2-acetamido-2-deoxy-alpha-D-glucosidase, acetyl-CoA:2-amino-2-deoxy-alpha-D-glucoside N-acetyltransferase, and 2-acetamido-2-deoxy-D-glucose 6-sulfate sulfatase. Carbohydr Res. 1981 May 1;91(2):165–190. doi: 10.1016/s0008-6215(00)86029-2. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Sulphamidase activity in leucocytes, cultured skin fibroblasts and amniotic cells: diagnosis of the Sanfilippo A syndrome with the use of radiolabelled disaccharide substrate. Clin Sci (Lond) 1981 Dec;61(6):729–735. doi: 10.1042/cs0610729. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Freeman C., Clements P. R., Stein R., Miller A. L. Cellular location of N-acetyltransfer activities toward glucosamine and glucosamine-6-phosphate in cultured human skin fibroblasts. Biochem Int. 1983 Jun;6(6):823–830. [PubMed] [Google Scholar]
- Hopwood J. J. alpha-L-iduronidase, beta-D-glucuronidase, and 2-sulfo-L-iduronate 2-sulfatase: preparation and characterization of radioactive substrates from heparin. Carbohydr Res. 1979 Mar;69:203–216. doi: 10.1016/s0008-6215(00)85765-1. [DOI] [PubMed] [Google Scholar]
- Jacobsson I., Lindahl U. Biosynthesis of heparin. Concerted action of late polymer-modification reactions. J Biol Chem. 1980 Jun 10;255(11):5094–5100. [PubMed] [Google Scholar]
- Jacobsson I., Lindahl U., Jensen J. W., Rodén L., Prihar H., Feingold D. S. Biosynthesis of heparin. Substrate specificity of heparosan N-sulfate D-glucuronosyl 5-epimerase. J Biol Chem. 1984 Jan 25;259(2):1056–1063. [PubMed] [Google Scholar]
- Kresse H. Mucopolysaccharidosis 3 A (Sanfilippo A disease): deficiency of a heparin sulfamidase in skin fibroblasts and leucocytes. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1111–1118. doi: 10.1016/0006-291x(73)90807-3. [DOI] [PubMed] [Google Scholar]
- Mahuran D., Clements P., Carrella M., Strasberg P. M. A high recovery method for concentrating microgram quantities of protein from large volumes of solution. Anal Biochem. 1983 Mar;129(2):513–516. doi: 10.1016/0003-2697(83)90585-7. [DOI] [PubMed] [Google Scholar]
- Mahuran D., Clements P., Hopwood J. A rapid four column purification of 2-deoxy-D-glucoside-2-sulphamate sulphohydrolase from human liver. Biochim Biophys Acta. 1983 Jun 9;757(3):359–365. doi: 10.1016/0304-4165(83)90062-4. [DOI] [PubMed] [Google Scholar]
- Matalon R., Dorfman A. Sanfilippo A syndrome: sulfamidase deficiency in cultured skin fibroblasts and liver. J Clin Invest. 1974 Oct;54(4):907–912. doi: 10.1172/JCI107830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Paschke E., Kresse H. Multiple forms of 2-deoxy-D-glucoside-2-sulphamate sulphohydrolase from human placenta. Biochem J. 1979 Sep 1;181(3):677–684. doi: 10.1042/bj1810677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann R., Klein U., Fromme H. G., von Figura K. Localisation of acetyl-CoA: alpha-glucosaminide N-acetyltransferase in microsomes and lysosomes of rat liver. Hoppe Seylers Z Physiol Chem. 1981 Sep;362(9):1199–1207. doi: 10.1515/bchm2.1981.362.2.1199. [DOI] [PubMed] [Google Scholar]
- Riordan J. F., McElvany K. D., Borders C. L., Jr Arginyl residues: anion recognition sites in enzymes. Science. 1977 Mar 4;195(4281):884–886. doi: 10.1126/science.190679. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Neufeld E. F. Human kidney alpha-L-iduronidase: purification and characterization. Arch Biochem Biophys. 1978 Aug;189(2):344–353. doi: 10.1016/0003-9861(78)90221-7. [DOI] [PubMed] [Google Scholar]
- Wombacher H. Molecular compartmentation by enzyme cluster formation. A view over current investigations. Mol Cell Biochem. 1983;56(2):155–164. doi: 10.1007/BF00227216. [DOI] [PubMed] [Google Scholar]


