Abstract
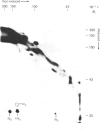
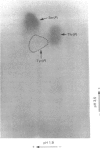
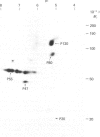
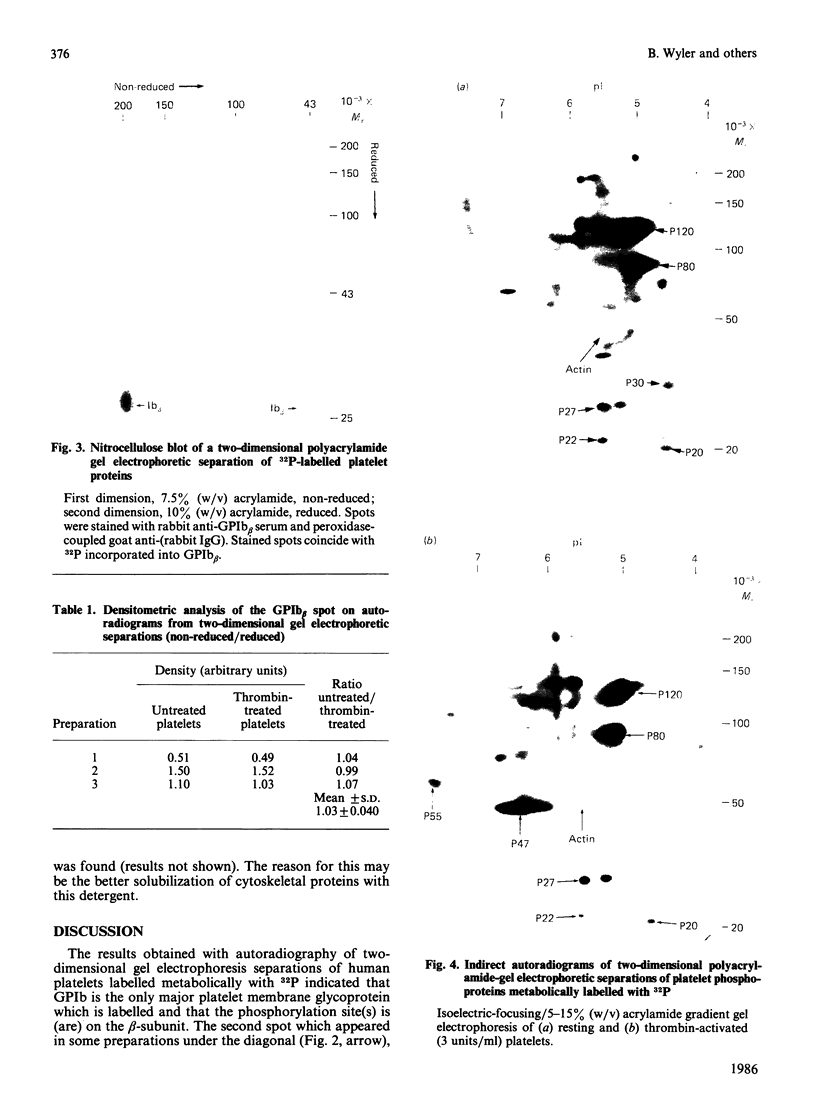
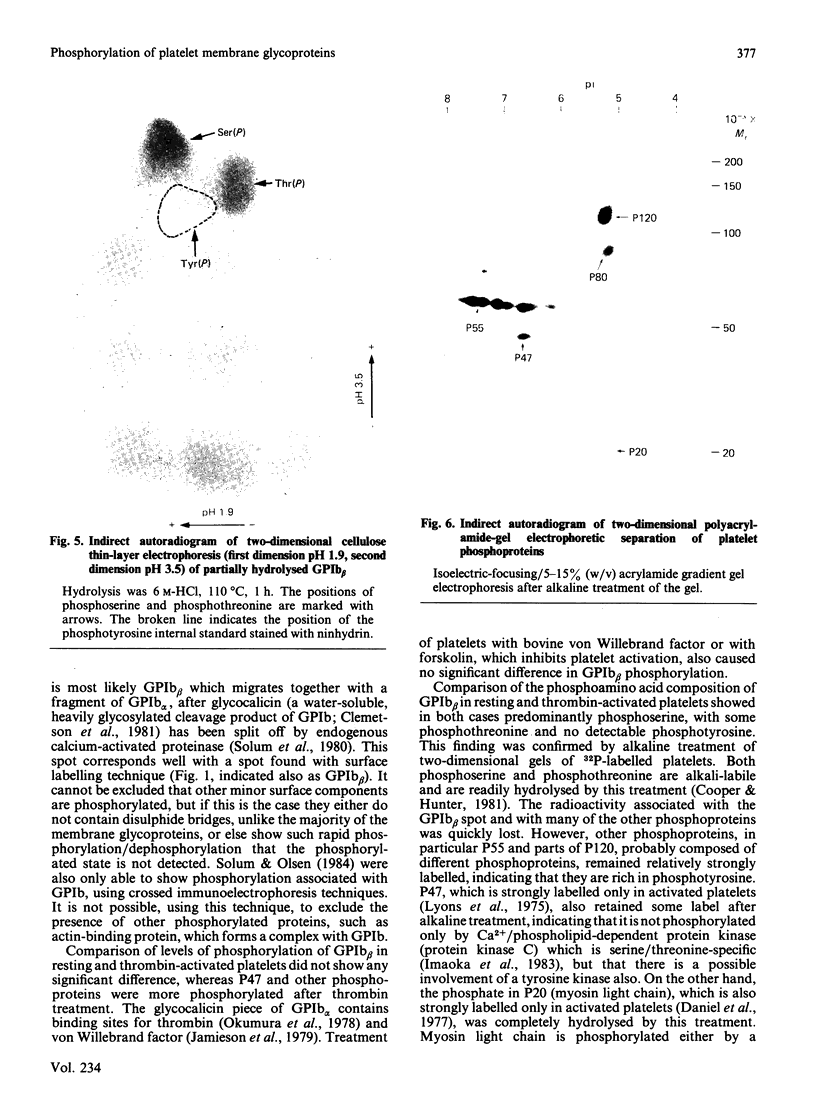
Platelets were metabolically labelled with 32P and the phosphoproteins examined by two-dimensional non-reduced/reduced gel electrophoresis and isoelectric-focusing/gel electrophoresis. Comparison with similar separations of surface-labelled platelets showed that the only major glycoprotein which is phosphorylated is the beta-subunit of glycoprotein Ib, indicating that this subunit contains a cytoplasmic segment. The identification was confirmed using immunoblotting with an antibody to the beta-subunit. Phosphoserine was the principal phosphorylation site, with some phosphothreonine, but phosphotyrosine was absent. No quantitative or qualitative differences could be detected in the phosphorylation of glycoprotein Ib beta from resting or activated platelets. These results exclude changes in phosphorylation of the major platelet membrane glycoproteins as a method of signal transmission by these receptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S. Regulation of contractile proteins by phosphorylation. J Clin Invest. 1983 Dec;72(6):1863–1866. doi: 10.1172/JCI111148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch J., Nemenoff R. A., Blackshear P. J., Pierce M. W., Osathanondh R. Insulin-stimulated tyrosine phosphorylation of the insulin receptor in detergent extracts of human placental membranes. Comparison to epidermal growth factor-stimulated phosphorylation. J Biol Chem. 1982 Dec 25;257(24):15162–15166. [PubMed] [Google Scholar]
- BETTEX-GALLAND M., LUSCHER E. F. Studies on the metabolism of human blood platelets in relation to clot retraction. Thromb Diath Haemorrh. 1960 Mar 1;4:178–195. [PubMed] [Google Scholar]
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhout T. A., Schiphorst M. E., Wirtz K. W., Sixma J. J. Identification of membrane proteins of human blood platelets with a hydrophobic photolabel. Biochim Biophys Acta. 1984 Dec 5;778(2):298–304. doi: 10.1016/0005-2736(84)90372-9. [DOI] [PubMed] [Google Scholar]
- Berndt M. C., Caen J. P. Platelet glycoproteins. Prog Hemost Thromb. 1984;7:111–150. [PubMed] [Google Scholar]
- Carroll R. C., Gerrard J. M. Phosphorylation of platelet actin-binding protein during platelet activation. Blood. 1982 Mar;59(3):466–471. [PubMed] [Google Scholar]
- Clemetson K. J., Bienz D., Zahno M. L., Lüscher E. F. Distribution of platelet glycoproteins and phosphoproteins in hydrophobic and hydrophilic phases in Triton X-114 phase partition. Biochim Biophys Acta. 1984 Dec 19;778(3):463–469. doi: 10.1016/0005-2736(84)90395-x. [DOI] [PubMed] [Google Scholar]
- Clemetson K. J., Capitanio A., Pareti F. I., McGregor J. L., Lüscher E. F. Additional platelet membrane glycoprotein abnormalities in Glanzmann's thrombasthenia: A comparison with normals by high resolution two-dimensional polyacrylamide gel electrophoresis. Thromb Res. 1980 Jun 15;18(6):797–806. doi: 10.1016/0049-3848(80)90202-9. [DOI] [PubMed] [Google Scholar]
- Clemetson K. J., McGregor J. L., James E., Dechavanne M., Lüscher E. F. Characterization of the platelet membrane glycoprotein abnormalities in Bernard-Soulier syndrome and comparison with normal by surface-labeling techniques and high-resolution two-dimensional gel electrophoresis. J Clin Invest. 1982 Aug;70(2):304–311. doi: 10.1172/JCI110618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemetson K. J., Naim H. Y., Lüscher E. F. Relationship between glycocalicin and glycoprotein Ib of human platelets. Proc Natl Acad Sci U S A. 1981 May;78(5):2712–2716. doi: 10.1073/pnas.78.5.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. Studies with a murine monoclonal antibody that abolishes ristocetin-induced binding of von Willebrand factor to platelets: additional evidence in support of GPIb as a platelet receptor for von Willebrand factor. Blood. 1983 Jan;61(1):99–110. [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J. L., Holmsen H., Adelstein R. S. Thrombin-stimulated myosin phosphorylation in intact platelets and its possible involvement secretion. Thromb Haemost. 1977 Dec 15;38(4):984–989. [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Furlan M., Perret B. A., Beck E. A. Studies on factor VIII-related protein. III. Size distribution and carbohydrate content of human and bovine factor VIII. Biochim Biophys Acta. 1979 Aug 28;579(2):325–333. doi: 10.1016/0005-2795(79)90060-6. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977 Aug 25;252(16):5888–5894. [PubMed] [Google Scholar]
- Haslam R. J., Lynham J. A., Fox J. E. Effects of collagen, ionophore A23187 and prostaglandin E1 on the phosphorylation of specific proteins in blood platelets. Biochem J. 1979 Feb 15;178(2):397–406. doi: 10.1042/bj1780397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway D. R., Adelstein R. S. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1653–1657. doi: 10.1073/pnas.76.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981 Jun;24(3):741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaoka T., Lynham J. A., Haslam R. J. Purification and characterization of the 47,000-dalton protein phosphorylated during degranulation of human platelets. J Biol Chem. 1983 Sep 25;258(18):11404–11414. [PubMed] [Google Scholar]
- Jamieson G. A., Okumura T., Hasitz M. Structure and function of platelet glycocalicin. Thromb Haemost. 1980 Feb 29;42(5):1673–1678. [PubMed] [Google Scholar]
- Kawahara Y., Takai Y., Minakuchi R., Sano K., Nishizuka Y. Phospholipid turnover as a possible transmembrane signal for protein phosphorylation during human platelet activation by thrombin. Biochem Biophys Res Commun. 1980 Nov 17;97(1):309–317. doi: 10.1016/s0006-291x(80)80169-0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lyons R. M., Stanford N., Majerus P. W. Thrombin-induced protein phosphorylation in human platelets. J Clin Invest. 1975 Oct;56(4):924–936. doi: 10.1172/JCI108172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi S. L., Chasis J. A. Isolation of human platelet glycoproteins. Biochim Biophys Acta. 1979 Aug 23;555(3):442–459. doi: 10.1016/0005-2736(79)90398-5. [DOI] [PubMed] [Google Scholar]
- Massini P., Lüscher E. F. Some effects of ionophores for divalent cations on blood platelets. Comparison with the effects of thrombin. Biochim Biophys Acta. 1974 Nov 4;372(1):109–121. doi: 10.1016/0304-4165(74)90077-4. [DOI] [PubMed] [Google Scholar]
- Nachman R. L., Leung L. L. Complex formation of platelet membrane glycoproteins IIb and IIIa with fibrinogen. J Clin Invest. 1982 Feb;69(2):263–269. doi: 10.1172/JCI110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka M., Nishikawa M., Adelstein R. S., Hidaka H. Phorbol ester-induced activation of human platelets is associated with protein kinase C phosphorylation of myosin light chains. Nature. 1983 Dec 1;306(5942):490–492. doi: 10.1038/306490a0. [DOI] [PubMed] [Google Scholar]
- Okita J. R., Pidard D., Newman P. J., Montgomery R. R., Kunicki T. J. On the association of glycoprotein Ib and actin-binding protein in human platelets. J Cell Biol. 1985 Jan;100(1):317–321. doi: 10.1083/jcb.100.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura T., Hasitz M., Jamieson G. A. Platelet glycocalicin. Interaction with thrombin and role as thrombin receptor of the platelet surface. J Biol Chem. 1978 May 25;253(10):3435–3443. [PubMed] [Google Scholar]
- Solum N. O., Hagen I., Filion-Myklebust C., Stabaek T. Platelet glycocalicin. Its membrane association and solubilization in aqueous media. Biochim Biophys Acta. 1980 Apr 10;597(2):235–246. doi: 10.1016/0005-2736(80)90102-9. [DOI] [PubMed] [Google Scholar]
- Solum N. O., Olsen T. M. Effects of diamide and dibucaine on platelet glycoprotein Ib, actin-binding protein and cytoskeleton. Biochim Biophys Acta. 1985 Jul 25;817(2):249–260. doi: 10.1016/0005-2736(85)90026-4. [DOI] [PubMed] [Google Scholar]
- Solum N. O., Olsen T. M. Glycoprotein Ib in the Triton-insoluble (cytoskeletal) fraction of blood platelets. Biochim Biophys Acta. 1984 Jun 29;799(3):209–220. doi: 10.1016/0304-4165(84)90263-0. [DOI] [PubMed] [Google Scholar]
- Spiker S., Isenberg I. Preparative polyacrylamide gel electrophoresis. Methods Enzymol. 1983;91:214–226. doi: 10.1016/s0076-6879(83)91018-2. [DOI] [PubMed] [Google Scholar]
- Steiner B., Clemetson K. J., Lüscher E. F. Improvement of the periodate-borohydride surface-labeling method for human blood platelets. Thromb Res. 1983 Jan 1;29(1):43–52. doi: 10.1016/0049-3848(83)90124-x. [DOI] [PubMed] [Google Scholar]
- TRAVERSO-CORI A., CHAIMOVICH H., CORI O. KINETIC STUDIES AND PROPERTIES OF POTATO APYRASE. Arch Biochem Biophys. 1965 Jan;109:173–184. doi: 10.1016/0003-9861(65)90303-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Richards F. M. An approach to nearest neighbor analysis of membrane proteins. Application to the human erythrocyte membrane of a method employing cleavable cross-linkages. J Biol Chem. 1974 Dec 25;249(24):8005–8018. [PubMed] [Google Scholar]