Abstract
Ependymal cells are arranged along the inner surfaces of the ventricles and the central canal of the spinal cord, providing anatomical, physiological and immunological barriers that maintain cerebrospinal fluid (CSF) homeostasis. Based on this, studies have found that alterations in gene expression, cell junctions, cytokine secretion and metabolic disturbances can lead to dysfunction of ependymal cells, thereby participating in the onset and progression of central nervous system (CNS) infections. Additionally, ependymal cells can exhibit proliferative and regenerative potential as well as secretory functions during CNS injury, contributing to neuroprotection and post-injury recovery. Currently, studies on ependymal cell primarily focus on the basic investigations of their morphology, function and gene expression; however, there is a notable lack of clinical translational studies examining the molecular mechanisms by which ependymal cells are involved in disease onset and progression. This limits our understanding of ependymal cells in CNS infections and the development of therapeutic applications. Therefore, this review will discuss the molecular mechanism underlying the involvement of ependymal cells in CNS infections, and explore their potential for application in clinical treatment modalities.
Keywords: Ependymal cell, Central nervous system infections, Molecular mechanism, Clinical treatment
Key points
Ependymal cells play an important role in the maintenance of CSF homeostasis and CNS health by forming physical and immune barriers against pathogen invasion.
PPRs signaling pathways, cilia and intercellular junctions, cytokine secretion or senescence of ependymal cells can lead to dysfunction, which in turn is involved in the onset and progression of CNS infection.
We propose potential therapeutic applications including gene transfer and novel biomarkers.
Studies of ependymal cells have provided new ideas for pathophysiology and treatment, but further research is needed to fully understand their role in CNS infection and evaluate therapeutic effect.
Introduction
Ependymal cell
Ependymal cells are glial cells that form an epithelium lining the inner surfaces of the brain ventricles and the central canal of the spinal cord [1, 2]. They are located at the interface between cerebrospinal fluid (CSF) and brain parenchyma, playing a crucial role in the formation of the brain-CSF barrier [3]. They provide significant mechanical support and sensory functions, facilitating the transport of substances between CSF and parenchyma to maintain the composition of CSF while isolating damaging agents to protect the central nervous system (CNS). The apical ciliary clusters of ependymal cells beat in a coordinated manner to regulate the unidirectional flow of CSF. Moreover, ependymal cells can secret cytokines and other signaling molecules, providing nutritional support and contributing to immune and metabolic regulation. Reports indicate that ependymal cells can be activated under pathological conditions, such as spinal cord injury (SCI), exhibiting robust proliferative capacity and multipotent responses to injury, positioning them as potential endogenous stem cells [4] (See Fig 1). The heterogeneity of ependymal cells has been widely explored. Recent studies have identified different subgroups of ependymal cells according to their different localizations and morphologies, including multiciliated E1, biciliated E2 and uniciliated E3 [5, 6]. They possess distinct molecular markers and regeneration characteristics, playing varying roles in neurodevelopment, homeostasis maintenance, and damage response [7]. Among them, the E1 is the major subgroup, playing a key role in the brain homeostasis. In this review, we put emphasis on the multiciliated ependymal cells.
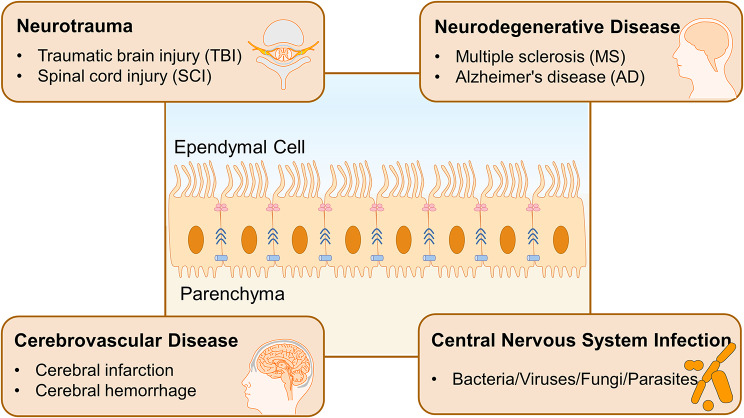
Fig. 1.
The structure, location and roles of ependymal cells Multiciliated ependymal cells are located at the interface between CSF and CNS parenchyma, participating in the brain-CSF barrier formation. Their cilia beat in a coordinated manner to regulate the unidirectional flow of CSF. There exist tight junctions, gap junctions, and adhesion junctions between cells, which maintain ependymal integrity and permeability and isolate harmful substances to protect the CNS. Ependymal cells are involved in the onset and progression of many CNS diseases, including neurotrauma, neurodegenerative diseases, cerebrovascular diseases, and CNS infections
Ependymal cell and CNS diseases
Due to the critical role of ependyma integrity and CSF homeostasis in regulating normal CNS activity, ependymal cells are implicated in the pathogenesis of various CNS disorders [6] (See Fig 1). Numerous studies have focused on the relationship between ependymal cells and hydrocephalus, identifying mechanisms such as ciliary dysfunction and loss of ependymal integrity caused by impaired cell junctions, both of which are considered to be the mechanisms of hydrocephalus [8, 9]. In addition, ependymal cell abnormality is found in the early pathological stages of neurotrauma. For example, after traumatic brain injury (TBI), there is a dramatic reduction in ependymal cilia, as well as DNA damage in cells, potentially affecting the barrier function and disrupting CSF composition, circulation, and waste clearance [10]. Dysfunction of ependymal cells may heighten the risk of further neuropathology and disease, particularly neurodegenerative disorders. Evidence of ependymal dysfunction has been noted in several neurodegenerative diseases, including multiple sclerosis (MS), Alzheimer’s disease (AD) and other neurodegenerative diseases. In autoimmune demyelinating diseases, after exposure to autoantibodies, such as IgG against Aquaporin-4 and GlialCAM, it can induce changes in the substance expression and morphology of ependymal cells and lead to lesions in the periventricular area [11, 12]. Though whether ependymal cells could serve as a potential origin of these diseases remains to be further explored [13]. However, ependymal cells also participate in the restrictive repair and prognosis. In the later stages of SCI, ependymal cells can actively promote neurological recovery by activating proliferation and differentiation, as well as providing neurotrophic support and limiting secondary injury [14]. The CNS can be infected by various pathogens, including viruses, bacteria, fungi, and parasites, which invade neural tissue, meninges, and vasculature, resulting in CNS infections with diverse pathological changes. Among them, ependymal cells are also susceptible to infections. It is worth mentioning that Stratton, J. A. and her team have carried out detailed and comprehensive work on ependymal cells. They also wrote a review to update the biology and pathology of ependymal cells in the adult brain, introducing the role of multiciliated ependymal cells in homeostasis and in response to CNS pathologies and aging [2].
While the significance of ependymal cells in CNS disorders is recognized, little is known about their molecular mechanisms in disease progression, particularly in infectious diseases. Recently, several reviews and articles have discussed and hypothesized about ependymal cells in the pathogenesis of SCI [15, 16], AD [13, 17], hydrocephalus [3, 18], autoimmunity [11, 12] and neurodegenerative diseases [13, 19]. Nevertheless, their involvement in CNS infection has received scant attention. In Stratton, J. A.‘s latest review, a summary table regarding recent studies on ependymal cells in infections was presented in Supplementary Table 4 [2]. This review describes the mechanism by which ependymal cells are involved in CNS infections and their potential application in clinical practice. These findings will help clarify the role of ependymal cells in CNS infections and may encourage targeted therapies for CNS diseases based on ependymal cell function (See Fig 1).
Ependymal dysfunction in infections
Ependymal cells can be invaded or affected by various pathogens. Receptors for coxsackie-adenovirus, measles virus [20] and the poliovirus [21] are present on ependymal cell surface. Nectin, expressed on the ependymal cells, functions not only as an adhesion molecule but also as an entry receptor for some viruses [22, 23]. For the newly emerging pathogen SARS-CoV-2, low-level expression of both angiotensin-converting enzyme-2 (ACE2) and transmembrane protease, serine 2 (TMPRSS2), necessary for viral entry, has been observed in human choroid plexus (CP) and ependymal cells [24, 25]. There are plaques that are immunopositive for the prion protein (PrP) in ependymal cells and around the base of adjacent vessels [26–28] following prion infection. Interactions between cells and prions lead to the formation and production of scrapie PrP amyloid filaments, along with the synthesis of PrP mRNA in ependymal cells [29], suggesting that ependymal cells may serve as one of the targets of prions [30]. HSV-1 infection shows a tropism for the ependyma, resulting in a loss of ciliated ependymal cells, lateral ventricular enlargement, and increased intracranial pressure during acute infection [31]. However, during latency, the expression of HSV-1 lytic genes is detected in the ependyma, correlating with a sustained dysfunctional response from resident T cells [32]. Ependymal cell dysfunction after infection is associated with various pathological changes in the CNS.
Ependymal dysfunction and post-infectious hydrocephalus
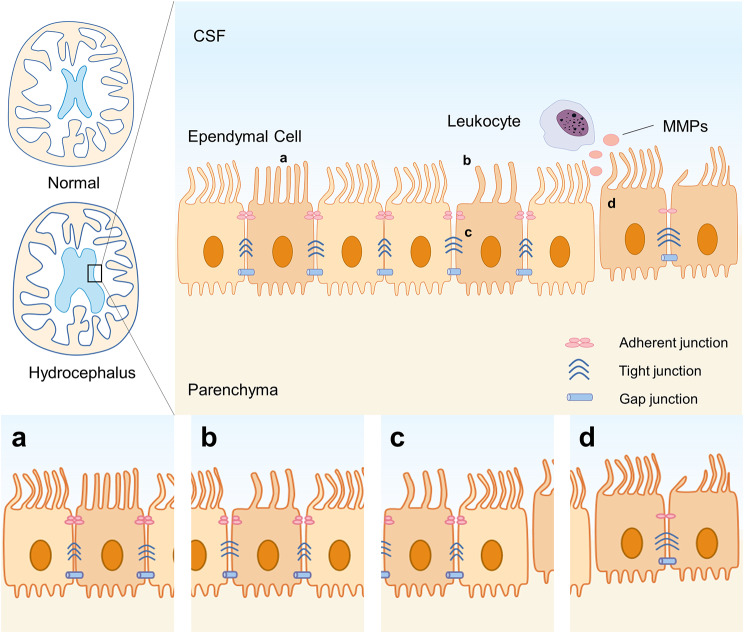
Ependymal ciliary dysfunction, inflammation, and increased intercellular space may contribute to hydrocephalus. In malaria, ependymal cells display varying degrees of damage, including ciliary thickening or loss, increased intercellular space, and dissociation of the ependymal layer. These alterations may enhance the permeability of the CSF-brain barrier [33]. In a model of Streptococcus pneumoniae meningitis, ependymal cells exhibit structural and functional ciliary abnormalities [34]. Zika virus’s NS5 protein causes severe ciliopathy through interacting with cilia in ependymal cells, which may be associated with microcephaly [35, 36]. Ciliary dysfunction may contribute to the neuropathological changes following CNS infections, especially hydrocephalus. In neurocysticercosis (NCC) caused by the larval form of Taenia Solium, ependymal and arachnoidal inflammation, along with the obstruction of the CSF pathway can lead to hydrocephalus, the most common cause of hydrocephalus in adults in endemic regions [37] (See Fig 2).
Fig. 2.
Hydrocephalus and ependymal cell dysfunction Hydrocephalus often occurs after infection, with structural and functional abnormalities in ependymal cells: (a) a decreased ciliary beating frequency; (b) an increased intercellular space, with junctions changing or disappearing; (c) cilia thickening or loss; (d) ependymal denudation. Besides, leukocytes express active MMPs that allow them to cross the ependymal barrier and infiltrate the ventricles. MMPs: matrix metalloproteinases
In fact, post-infectious hydrocephalus is the leading cause of hydrocephalus in children worldwide, and hydrocephalus resulting from tuberculous meningitis (TBM) imposes a considerable burden in regions with a high tuberculosis prevalence [38]. Different reovirus serotypes mediate infectious tropism and pathogenesis: serotype 1 infects ependymal cells, leading to hydrocephalus, while serotype 3 targets neurons, resulting in encephalitis [39, 40]. This suggests that ependymal cell injury during infection may be a key pathophysiological mechanism underlying post-infectious hydrocephalus [41]. On the one hand, in the acute phase, ongoing damage to choroidal epithelial cells, ependymal cells, and brain tissue coupled with inflammation, may impair CSF resorption. On the other hand, in the chronic phase, ependymal scarring leads to intraventricular obstruction. In both instances, they account for the pathophysiologic mechanism of post-infectious hydrocephalus [38, 41]. In addition, microglia are also involved in the damage and death of ependymal cells. Intracerebroventricular (ICV) injection of recombinant HIV-1 tat protein can lead to ependymal damage and activate subependymal microglia. These microglia phagocytose ependymal fragments and migrate and infiltrate into the periventricular area and parenchyma, thereby causing inflammation [42]. VPS35 is highly expressed in ependymal cells and is involved in ependymal cell differentiation and ciliogenesis. In mice with VPS35 specifically knocked out in ependymal cells, hydrocephalus is observed, accompanied by damaged ependymal cells and local activation of microglia. After depletion, an alleviation in hydrocephalus and a restoration of ependymal cell homeostasis are observed [43].
Ependymal barrier dysfunction and pathogen invasion
Significant structural features of ependymal cells are the apical ciliary cluster and the tight junctions, gap junctions and adhesion junctions between them [44–46]. Together, they maintain ependymal integrity and permeability, regulate CSF circulation and ion transport, and form the brain-CSF barrier, which is crucial for maintaining CSF homeostasis. In CNS infection, ependymal cells present with ciliary loss, decreased beating frequency, and ultrastructural disruption, making neurons more likely susceptible to bacterial toxins [34], accompanied by hydrocephalus. Some pathogens, such as cryptococcus, enter the CNS through the blood-CSF-brain pathway. Following intracerebral inoculation, the virus initially spreads in the CSF and extensively infects ependymal cells before entering the brain parenchyma to infect neurons and glial cells [47, 48], thereby establishing a pathway for encephalitis development [49]. Cytomegalovirus (CMV) is the most common opportunistic viral pathogen in immunocompromised adults. It preferentially affects ependymal cells, and then expands to the parenchyma [50]. Additionally, SARS-CoV-2 can induce neurological symptoms and complications, and its staining is observed in CP and ependymal cells [51]. SARS-CoV-2 exhibits tropism for CP epithelial cells, accompanied by increased cell syncytia and increased cell death. And RNA-seq reveals heightened inflammatory cellular responses and downregulation of genes related to transport, cilia, and cell junctions, resulting in impaired barrier and secretory functions [52, 53]. And as for neurocysticercosis (NCC) infection, Alvarez proposes a fourth route from blood to the CSF through the disrupted ependymal layer via internal leptomeninges vessels [54]. Notable structural changes and loss of junction proteins occur in ependymal cells adjacent to the internal leptomeninges, correlating with active matrix metalloproteinases (MMPs) expressed by leukocytes, which facilitate their infiltration into the ventricles [54–56]. In malaria, the observed intercellular dissociation of ependymal cells appears to enhance the paracellular permeability of the CSF-brain barrier, making inflammatory mediators and toxins to enter the brain and cause injury [33]. In early bacteremia of tuberculosis, Mycobacterium tuberculosis (Mtb) deposits in the subpial or subependymal region of the brain and remains dormant for a long time. When the lesion expands and ruptures into the ventricle or subarachnoid space [57], it can lead to TBM. Mtb can also cross the ependymal layer or CP to enter the brain parenchyma [58]. Similarly, pathogens can invade the blood-CSF barrier and penetrate CP epithelium via the Trojan horse strategy, resulting in barrier breakdown and subsequent CNS infection [59].
In summary, ependymal cells may serve as a route for the entry of pathogens and inflammatory mediators into the brain. This occurs via the transcellular pathway through receptors, the paracellular pathway that disrupts with ependymal cell junctions, and leukocyte infiltration [53]. In addition to pathogens, tumors, immune cells, antibodies and cytokines can also access the brain parenchyma through the blood-CSF-brain pathway (See Fig 2).
The mechanism of ependymal cells in CNS infection
Pattern-recognition receptors are involved in inflammatory signaling
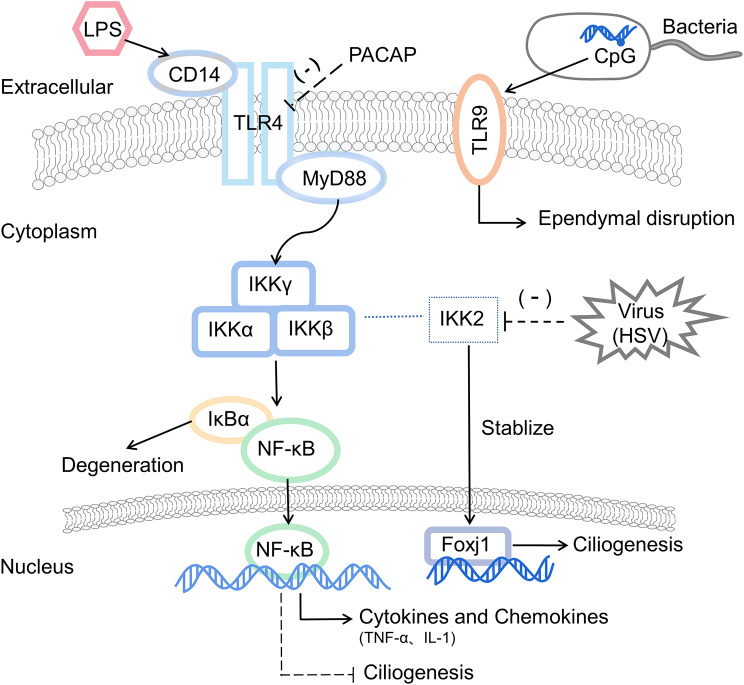
Intraventricular administration of lipopolysaccharide (LPS) induces a robust inflammatory response at the periventricular margin of the cerebral cortex. The inflammatory signaling in the brain may involve two pathways: local diffusion of LPS/inflammatory molecules across the meninges and ependyma, as well as signaling through cerebral blood circulation [60]. During CNS infection, ependymal cells at the blood-CSF barrier and CSF-brain barrier function as both a physical and immunological barrier. Pattern-recognition receptors (PPRs) on these cells, including Toll-like receptors (TLRs), CD14 and C-type lectin PRR, can recognize pathogens prior to their entry into the parenchyma and participate in inflammatory signaling transduction.
The expression of TLRs on ependymal cells increases upon infection, such as TLR4, TLR7, TLR8, and TLR13. TLR-mediated innate immunity plays a crucial role in recognizing pathogens such as bacteria, viruses, fungi and parasites, and facilitates interactions between immune cells and CNS cells [61–63]. The downstream of signaling of TLR4 involves the signaling molecules myeloid differentiation factor 88 (MyD88), nuclear factor kappa B (NF-κB) and inflammatory factors interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α). These factors not only induce the release of inflammatory factors, and apoptosis of phagocytic cells but also mediate persistent brain inflammation and neurological tissue damage during endotoxemia [60, 64]. Additionally, the ependyma also expresses the scavenger receptor CD14, which interacts with TLR4 to promote the phagocytosis of apoptotic neutrophils in CSF, thereby reducing the severity of inflammation [65].
In addition, unmethylated CpG motifs in bacteria are highly immunogenic and activate TLR9 signaling, leading to damage and subsequent activation of periventricular microglia, ependymal destruction and reactive astrocyte proliferation. This cascade may result in impaired neurological function, neuroinflammation, and even neurodegeneration [66], suggesting a potential association among infection, innate immunity and neurodegeneration. Pituitary adenylate cyclase-activating polypeptide (PACAP) can inhibit LPS-induced TLR4 signaling and its downstream responses, reducing the secondary inflammatory response and indicating a neuroprotective role [67]. Meanwhile, the regulation of TLRs can help maintain balance within the innate immune system, preventing excessive antimicrobial inflammatory response that could lead to secondary brain damage. TLRs may serve not only as targets for immunomodulation in CNS infection, but also for improving the prognosis of nerve injury and neurodegenerative conditions. Further research on TLRs is essential to elucidate how infections and innate immunity influence the onset and progression of neurodegenerative disorders.
Indeed, CNS infection can disrupt ependymal ciliary status and motility through the TLR/MyD88/NF-κB signaling pathway, contributing to acquired hydrocephalus [68]. In the presence of inflammatory factors and chemokines such as TNF-α and IL-1, NF-κB activation and pro-inflammatory mediators lead to macrophage recruitment and inhibition of ciliogenesis [69]. Furthermore, the NF-κB-independent inhibitor of kappa B kinase 2 (IKK2) stabilizes Foxj1, whereas some viruses such as HSV-1 and growth factors can inhibit IKK, resulting in Foxj1 degradation, dedifferentiation of ependymal cells, and progression to hydrocephalus [70]. On the other hand, cilia length and function may regulate TLR4-mediated NF-κB signaling activation and pro-inflammatory cytokine expression [71], suggesting that the interactions between the NF-κB signaling pathway and cilia in neuroinflammation and innate immune responses warrant further investigation. As mentioned earlier, ependymal cilia are crucial for maintaining CSF homeostasis and undergo pathological changes during infection. Ciliogenetic genes are highly expressed in ependymal cells and are tightly regulated by a complex network of transcription factors, with Foxj1 serving as the central transcription factor of ciliogenesis in the network [72] (See Fig 3). Additionally, there is a strong connection between ciliogenesis and cell cycle regulation. After SCI, the expression of cilia-related genes in ependymal cells may be suppressed due to increased cell proliferation and downregulation of Foxj1 expression [72]. The axoneme, the fundamental structure of cilia, comprises microtubules and ATP-driven dynein motors [73], enabling cilia to beat synchronously in a rhythmic, wave-like manner through gap junctions or innervation. However, structural and functional abnormalities within the cilia may impair intraciliary transport, ciliary maintenance, material transport, and CSF regulation [9], leading to ventricular enlargement and hydrocephalus. Mutations or defects in cilia-related genes and proteins can result in compromised ciliary motility and failure of mucosal clearance [68], resulting in primary ciliary dyskinesia (PCD) [74]. PCD is a hereditary and clinically heterogeneous syndrome characterized by recurrent respiratory infections, infertility and early postnatal hydrocephalus [75]. At least 40 genes have been found to be associated with PCD [76–80]. Consequently, hydrocephalus can arise from the production of structurally abnormal cilia that cause dyskinesia and disrupt normal CSF flow.
Fig. 3.
Pattern recognition receptors on ependymal cells are involved in inflammatory signaling The TLR4/MyD88/NF-κB signaling pathway induces the release of inflammatory cytokines like IL-1β and TNF-α, while promoting phagocytic apoptosis. This mediates persistent brain inflammation and neurological tissue damage. Activation of NF-κB and pro-inflammatory mediators facilitates macrophage recruitment and inhibits ependymal ciliogenesis. NF-κB-independent IKK2 can stabilize Foxj1, while some viruses such as HSV-1 and growth factors can inhibit IKK, which in turn strongly induces Foxj1 degradation and subsequently leads to hydrocephalus. Besides, unmethylated CpG in bacteria relies on TLR9 signaling to mediate damage and induce ependymal destruction. LPS: lipopolysaccharide; TLR: toll-like receptor; PACAP: Pituitary adenylate cyclase-activating polypeptide; NF-κB: nuclear factor kappa B; IKK: inhibitor of kappa B kinase
Inflammatory cytokines and neurotrophic support
Ependymal cells are also involved in immune regulation and inflammatory responses through cytokines. Interferon (IFN) is a cytokine involved in antiviral, antitumor and immunomodulation activities, particularly in viral encephalitis and autoimmune diseases within the CNS. Following viral infection, ependymal cells produce IFN-α/β, which strongly inhibits viral transmission [81, 82]. Additionally, IFN-γ induces ependymal cells to express chemokines that recruit T cells into the CNS synergizing with peripheral infection stimulation [83]. Loss of myelin is a prominent pathological hallmark of MS and viral infection in humans. Defects in IFN-γ signaling are linked to enhanced oligodendrocyte tropism and delayed virus clearance but do not significantly impact the extent or distribution of demyelination [84, 85]. Interferon regulatory factor 3 (IRF3) is selectively expressed in brain cells, with particularly strong expression in ependymal cells [86]. Upon activation, IRF3 induces IFN-β production and antiviral immunity, effectively inhibiting viral replication [86]. For example, during CNS infection with coronaviruses, the interplay between IFN I-related innate immunity and the cleavage of the viral spike glycoprotein by host protease contribute to reduced neurovirulence and control of neural invasion [87], highlighting two potential antiviral targets. In the absence of functional IFN I, the Semliki Forest virus exhibits rapid and widespread infection with tropism, affecting ependymal cells, meningeal cells, and oligodendrocytes, resulting in viral encephalitis. JC polyomavirus (JCPyV) infection can also cause encephalitis and induce progressive multifocal leukoencephalopathy [82]. In a CNS infection model with Mouse polyomavirus (MuPyV) to mimic JCPyV infection, STAT1 is involved in IFN receptor signaling and collaborates with CD8 T cells to alleviate MuPyV encephalopathy and control viral replication [88]. Additionally, by inducing acute neuroinflammation through the application of LPS and IFN-γ both in vivo and in vitro, ependymal cells can be triggered to exhibit reactive characteristics, manifested as the increased expression of GFAP and STAT1 and closely related to some genes associated with cellular reorganization and immune regulation. The same applies to chronic neuroinflammation [89]. Macrophage migration inhibitory factor (MIF), present in ependymal cells and choroidal epithelial cells [90, 91], serves as a key upstream mediator of host innate and adaptive immunity, functioning as immunomodulators and pro-inflammatory cytokines that facilitate pathogen clearance and enhance host defenses [92].
The TLR pathway has been established as a crucial mediator of inflammatory signal recognition, with TNF-α and IL-1-mediated acute inflammatory response playing important roles in the initiation of inflammatory response to CNS infection. Following both HSV viral encephalitis and bacterial meningitis, induction of TNF-α was observed in the ependyma, alongside elevated levels of pro-inflammatory factors and chemokines in the CSF, such as IL-1β, IL-6, CXCL1, and CXCL10 [31, 93, 94]. In infant rats infected with Streptococcus pneumoniae, ependymal cells and CP express cathelin-related antimicrobial peptide, which are induced by IL-1β and TNF. Furthermore, the expression of the antimicrobial peptide LL-37 has also been detected in the CSF of patients with bacterial meningitis [95].
Moreover, ependymal cells likely consistently express receptors for IL-1 and TNF-α. In acute aseptic neuroinflammation induced by neuraminidase (NA) injection, activated microglia are contribute to ependymal injury in the ventricles, with IL-1β likely serving as the mediator [96]. This mechanism may explain how neurological infections, such as those caused by Streptococcus pneumoniae, lead to ependymal cell death and hydrocephalus. However, it is also evident that NA can partially induce ependymal damage in vitro, indicating that its role should not be discounted. Additionally, IL-1 signaling is directly or indirectly involved in the changes of the blood-CSF barrier. High levels of IL-1β in CP enhance the activity of MMPs, which degrade substrates and junction proteins, increasing barrier permeability and inducing edema. This process also facilitates the release of chemokines and leukocyte trafficking [97]. Activating transcription factor 3 (ATF3) acts as a negative regulatory transcription factor in TLR pathways [98], thereby reducing the expression of genes encoding inflammatory cytokines such as IL-1β, IFN-γ and TNF-α during infection and injury [99, 100]. Quiescent ependymal cells express ATF3; while activated in vitro or after SCI, ATF3 expression is upregulated, accompanied by the migration of ependymal cells [101]. Inhibiting ATF3 suppresses the migration of ependymal cells and upregulates the expression of inflammatory factors. These data suggest that ATF3 may contribute to the survival of motor neurons and the maintenance of axonal connections in the zebrafish SCI model by regulating the inflammatory response. ATF3 is proposed as a novel dynamic marker of ependymal derived stem/progenitor cell (epSPC) [101], and the upregulation of ATF3 after SCI acts as a negative regulator of proinflammatory cytokines, promoting motor recovery and axonal regeneration [102].
Interestingly, ependymal cells can also express immunomodulatory proteins, including GPI-anchored molecules and immunoglobulin superfamily molecules, to regulate excessive immune responses in both health and disease, thereby modulating innate immune responses in the CNS [103]. In meningitis, there is upregulation of complement activators in ependymal cells and choroidal epithelial cells, such as strong CD46 and CD35 staining, which helps balance anti-inflammatory and protective responses. This expression renders ependymal cells resistant to complement-mediated attacks during strong activation of the complement pathway in the infected CNS, thereby mitigating innate complement-mediated inflammatory damage. Notably, some viruses can exploit this mechanism to gain immune privilege [104].
It is worth noting that ependymal cells can also secrete neurotrophic factors that promote neurogenesis and angiogenesis, regulate local inflammation, and protect neurons from death. During the recovery from TBI and cerebrovascular disease, the CP/ependyma upregulate the expression of growth factors and neurotrophic factors, including brain-derived neurotrophic factor (BDNF), nerve growth factor, vascular endothelial growth factor (VEGF), transforming growth factor and so on. These factors are released into CSF through endocrine-like mechanisms and then transmitted to injured areas [105], potentially activating similar signaling pathways that protect neurons and improve the neurogenesis niche [106]. This process is modulated by inflammatory cytokines.
Ependymal cells and changes in innate immunity-related genes
Changes in the expression of innate immunity-related genes have been observed in ependymal cells. Exposure to LPS significantly increases the expression of inflammation-related genes [65]. In ependymal cells from NCC-infected mice, genes associated with the innate immune response, antigen presentation, and leukocyte infiltration are upregulated, including MHC-II and various chemokines [56]. Meanwhile, a recent study has reported significant transcriptional alterations of ependymal cells in a Bacillus Calmette-Guerin-induced model of TBM, revealing a significant enrichment of genes related to metal ion and protein transport, as well as antigen presentation and processing, as detected by single-cell RNA sequencing. Notably, a reduced expression of the FERM structural domain 4 A (Frmd4a) may correlate with clinical symptoms of hydrocephalus and neurodegeneration [107]. Additionally, another study has identified miRNAs that are associated with changes in gene expression, particularly targeted mRNAs focusing on ion and protein transport. MiR-21a-3p is one of miRNAs involved in the miRNA-mRNA network of ependymal cells and neurons [108], which targets several components in the network that work in the innate immune response. Furthermore, it has been shown that miR-21a-3p targets IFN-γ mRNA, negatively regulating anti-mycobacterial immune responses [109]. These evidences suggest that ependymal cells play a role in innate immunity and material transport in TBM.
Potential applications implicating ependymal cells in clinical treatment
Gene transfer in ependymal cells
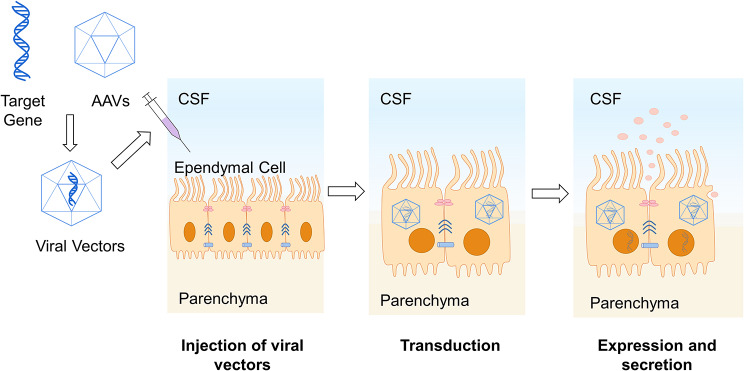
Due to the blood-brain barrier, the efficacy of some drugs targeting the CNS is limited. As brain-CSF barrier, ependymal cells are considered as promising targets for enzyme replacement therapies to improve neurological metabolic disorders and for delivering cytokines or drugs through ventricular, subarachnoid, and perivascular spaces. Therefore, non-replicating viral vectors carrying therapeutic genes have been engineered to effectively infect ependymal cells, facilitating the synthesis and secretion of transgenic therapeutic products into CSF to target neurons and achieve sustained transgene expression without significant toxicity. This approach holds potential for treating various CNS diseases [110]. Among these, adeno-associated virus (AAV) vectors enable ependymal-specific transduction and transgene expression in the CNS, making them suitable for local intracerebral gene therapy [111–113] (See Fig 4). Comparative studies have shown that AAV2/5 and AAV2/8 display remarkable infections in the CP, while AAV2/1 infects both ependymal cells and cells in the CP. In contrast, lentivirus vectors demonstrate low infection intensity in the CP. Therefore, serotype-specific AAVs 5 and 8 are promising tools for ICV gene delivery [112].
Fig. 4.
Gene transfer application in ependymal cell Design non-replicating viral vectors containing therapeutic genes. These vectors are injected into CSF through lumbar puncture. They effectively infect ependymal cells for long-term therapeutic gene expression. The resulting products are secreted into CSF, providing potential treatments for neurodegenerative diseases and metabolic disorders. AAVs: adeno-associated virus
Batten disease is caused by a deficiency of the soluble lysosomal enzyme tripeptidyl peptidase 1 (TPP1) due to mutations in the TPP1 gene. Transduction of recombinant AVVs expressing the TPP1 gene into ependymal cells, which have high TPP1 expression and secrete the enzyme into the CSF, has been shown to halt disease progression and rectify neuropathological features in TPP1-deficient dogs [114]. Additionally, the feasibility of ICV injection of self-complementary (sc) AAV to treat metachromatic leukodystrophy (MLD), caused by functional deficiency in human arylsulfatase A (hASA), has been reported. However, controlling the immune response to prevent antibody production remains a challenge for long-term therapeutic application [115, 116]. Beyond enzyme replacement therapy, AAV-mediated gene therapy can be applied for the delivery of cytokines and neurotrophic factors. For example, AAV4-mediated expression of insulin-like growth factor-1 (IGF-1)/VEGF in the ependymal lining, CP and central canal of the spinal cord, significantly delays exercise decline and extends survival in amyotrophic lateral sclerosis (ALS) mice [106]. Enhancer-based HB-EGF delivery by AAV improves axon densities in neonatal crush SCI, suggesting a potential strategy to improve spinal cord repair in mammals [117].
Several studies have demonstrated that intrathecal or intraventricular injection of AAV vectors can achieve extensive and prolonged, though not permanent, expression of exogenous genes in ependymal cells [118]. In addition to the effective and stable sustained secretion [115, 119], this approach facilitates high-level and wide delivery in the CNS, showing noteworthy benefits for aging and several diseases. Furthermore, the transduction of cytokine genes can achieve high levels in the CNS without affecting the peripheral immune system [120]. Recently, Carrell and his colleagues have identified a promoter for ependyma-derived transgene expression, derived from the VWA3A gene, demonstrating its functional utility in diseases and aging, and its cross-species function in both mice and rhesus macaques. This strategy enhances safety and continuity while leading to higher protein secretion for better therapeutic applications [121].
Given the accessibility of the ventricles, gene transduction therapy through the ependymal pathway using viral infection presents a promising alternative for treating neurodegenerative and neuropathic metabolic diseases. Notably, ependymal cells are susceptible to viral infections. Currently, lysosomal viruses that selectively target cancer cells require thorough evaluation for CNS safety and their potential to infect and damage ependymal cells [122, 123]. The sigma 1 sequence of echoviruses determines the tropism for infecting specific cells, suggesting a direction for rational design and improvement [39, 40]. However, current research still focuses on in vitro experiments and animal experiments, and has not yet obtained sufficient data to support for clinical application. Thus, its safety and feasibility need to be carefully evaluated by more studies.
Ependymal cells and novel biomarkers
Ependymal cells play crucial barrier and secretory roles in maintaining CSF composition and homeostasis, while abnormalities in CSF often reflect CNS lesions or diseases. Evaluation of CSF can identify immune cells, tumor cells, or microorganisms indicative of disease spread in CSF, as well as biomarkers therein that reflect parenchymal changes [124]. However, due to the temporal and spatial variation of CSF parameters, definitive diagnosis of CNS infection using routine clinical chemistry or cytology parameters remains challenging. It still relies on identifying causative pathogens by culture, antigen detection or molecular methods such as polymerase chain reaction (PCR) and next-generation sequencing (NGS) [125]. Nevertheless, recent advances in novel biomarkers and molecular methods could open new ways for future definitive diagnosis of CNS infections and disease evaluation.
Surfactant proteins (SP) are crucial for regulating CSF flow and CNS innate immunity, similar to their function in the lung. Surfactant proteins A, B, C and D exhibit immunoreactivity in the CP and in the ependymal cell layer of the CNS, playing a role in host defense, regulating inflammation and maintaining CSF flow. In patients with CNS infections, the level of both SP-A and SP-D in CSF is statistically significantly decreased compared to healthy individuals. Considering their known functions, they are assumed to participate in the clearance of pathogens and apoptotic polymorphnuclear neutrophils [126]. Additionally, SP-G, a recently identified surfactant protein, is produced by ependymal cells and CP and secreted into CSF. Its concentration significantly increases in children with intraventricular hemorrhage or CNS infection, indicating its potential as a novel CSF biomarker for reflecting the CNS innate immune response and dynamic parameter changes of CSF components [127].
Levels of non-LTA4H-dependent cytokines in TBM affect the therapeutic efficacy of dexamethasone [128, 129]. Ependymal cells secrete cytokines such as IL-1, TNF, and IFN into CSF following infection, which affects cytokine levels. A deeper understanding of the mechanisms governing cytokine secretion by ependymal cells and how cytokines affect infection treatment, utilizing gene transduction in ependymal cells for expressing and secreting cytokines may provide new insights to guide clinical treatment and prognosis. Additionally, a meta-study of CSF cytokines in TBM provides a reference set of cytokines as a potential adjunct to the diagnosis of TBM and to differentiate it from other etiologies of meningitis [130].
In addition, correlation of imaging findings with biomarkers can be used for clinical management. Ventricular tuberculosis shows ependymal enhancement, swelling, and enhancement of CP and intraventricular tuberculomas on magnetic resonance imaging (MRI) [131]; some patients with TBM shows enplaque-like ependymal granulomas associated with the ventricular ependymal lining on computed tomography (CT) [132]. Guerini proposed an empirical review of periventricular ependymal enhancement characteristics on MRI to reflect etiological types, such as neurological infection or tumor, based on the patient’s immune status, type of enhancement, and treatment response [133]. The release of brain-derived proteins such as GFAP and neurofilament light chain (NFL) into the CSF indicates a disruption of the brain-CSF barrier [124]. In HIV patients with cryptococcal meningoencephalitis, correlating MRI imaging findings with NFL, which reflects axonal damage, and sCD27, a predictor of intrathecal T-cell-mediated inflammation, can serve as a measure of severity and an individualized guide to treatment. Results indicate that brain MRI ependymitis is the best predictor of higher sCD27 levels, while choroidal plexitis is the best predictor of higher NFL levels [134]. Additionally, intraventricular debris and stranding, and an irregular and echogenic ependyma in cranial sonography are highly indicative of ventriculitis. Sonography is also capable of detecting post-infectious hydrocephalus and parenchymal involvement from cerebritis or early abscess [135]. Therefore, imaging changes in the ependyma provide an effective, noninvasive method for reflecting inflammatory biomarkers, assessing inflammatory immune activation, and facilitating subpopulation management, follow-up, and prognostic evaluation.
Summary and prospects
In CNS infections, relevant molecules and receptors, expressed by glial cells and neurons, are involved in immune recognition, defense and clearance of pathogens and toxic cellular components, which serve as innate immunity [136]. Ependymal cells constitute a vital physical and immune barrier essential for maintaining CNS health, with dysfunction often evident in the early stages of the disease. Current research on ependymal cells has been progressively advanced from cellular studies to molecular mechanisms, exploring interactions among signaling pathways such as TLR/MyD88/NF-κB, various cytokines, cilia and intercellular junctions, through in vivo/vitro experiments, single-cell sequencing and other techniques. Notably, single-cell sequencing technology facilitates the precise identification of specific gene expression profiles and allows for a detailed analysis of underlying molecular mechanisms, paving the way for future investigations into the roles and mechanisms of ependymal cells in CNS infections.
Due to the special location of ependymal cells between CSF and parenchyma, they broaden our perspective for detecting, diagnosing and managing CNS infections, and provide an important manipulable target for the treatment of CNS diseases. The application of viral vectors containing therapeutic genes for long-term expression in ependymal cells is promising, extending beyond CNS infections to encompass injuries [117], neurodegeneration [106] and cerebrovascular diseases [137]. However, their potential application in infectious diseases, such as antibiotic delivery, requires further exploration. Additionally, the specificity and sensitivity of the proposed novel biomarkers above are currently low, and the imaging findings are often based on empirical observations, necessitating further research and validation. Further studies on ependymal cells may lead to develop innovative therapeutic strategies aimed at enhancing neurological recovery and functional improvement in CNS infections, particularly by leveraging the neural stem cell characteristic of ependymal cells. However, it is important to note that ethical considerations and accessibility issues have limited most studies on ependymal cells to animal models, predominantly adult specimens, leaving a gap in our understanding of age-related responses in ependymal cells. Therefore, the therapeutic potential observed in animal models should be approached with caution [138].
In conclusion, ependymal cells play a significant role in CNS infections by resisting pathogen invasion, activating innate immunity, participating in inflammatory signaling and secreting cytokines and chemokines. As infection progresses, pathogens penetrate the parenchyma, leading to structural alterations, dysfunction, and necrosis of ependymal cells, often resulting in hydrocephalus as a clinical manifestation. These processes offer insights for clinical diagnosis and treatment, particularly through the detection of biomarkers in CSF and producing therapeutic proteins into CSF.
Acknowledgements
Not applicable.
Abbreviations
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- AVV
Adeno-associated virus
- BDNF
Brain-derived neurotrophic factor
- CNS
Central nervous system
- CP
Choroid plexus
- CSF
Cerebrospinal fluid
- CT
Computed tomography
- epSPC
Ependymal derived stem/progenitor cells
- HSV
Herpes simplex virus
- IFN
Interferon
- IKK
NF-κB-independent inhibitor of kappa B kinase
- IL
Interleukin
- IRF3
Interferon regulatory factor 3
- JCPyV
JC polyomavirus
- LPS
Lipopolysaccharide
- MARCKS
Myristoylated alanine-rich C kinase substrate
- MIP
Migration inhibitory factor
- MMP
Matrix metalloproteinase
- MRI
Magnetic resonance imaging
- MS
Multiple sclerosis
- Mtb
Mycobacterium tuberculosis
- MuPyV
Mouse polyomavirus
- MyD88
Myeloid differentiation factor 88
- NA
Neuraminidase
- NCC
Neurocysticercosis
- NF-κB
Nuclear factor kappa B
- NFL
Neurofilament light chain
- NGS
Next Generation Sequencing
- PACAP
Pituitary adenylate cyclase-activating polypeptide
- PCD
Primary ciliary dyskinesia
- PCR
Polymerase chain reaction
- PPR
Pattern-recognition receptor
- PrP
Prion protein
- SCI
Spinal cord injury
- SP
Surfactant proteins
- TBI
Traumatic brain injury
- TBM
Tuberculous meningitis
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor α
- TPP-1
Tripeptidyl peptidase 1
- VEGF
Vascular endothelial growth factor
Author contributions
SX: Conceptualization, Writing—original draft. FL: Conceptualization, Supervision, Writing — review & editing.
Funding
Supported by Science and Technology Commission of Shanghai Municipality (21Y11901700, 20Z11901002). Supported by Shanghai Municipal Science and Technology Major Project (ZD2021CY001).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Del Bigio MR. Ependymal cells: biology and pathology. Acta Neuropathol. 2010;119:55–73. [DOI] [PubMed] [Google Scholar]
- 2.Groh AMR, Song YL, Tea F, Lu B, Huynh S, Afanasiev E, Bigotte M, Del Bigio MR, Stratton JJA. Multiciliated ependymal cells: an update on biology and pathology in the adult brain. Acta Neuropathol. 2024;148:39. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez AJ, Dominguez-Pinos M-D, Guerra MM, Fernandez-Llebrez P, Perez-Figares J-M. Structure and function of the ependymal barrier and diseases associated with ependyma disruption. Tissue Barriers. 2014;2:e28426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, Frisen J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:1494–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng S, Gan L, Liu C, Xu T, Zhou S, Guo Y, Zhang Z, Yang GY, Tian H, Tang Y. Roles of Ependymal Cells in the Physiology and Pathology of the Central Nervous System. Aging Dis. 2023;14:468–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirzadeh Z, Kusne Y, Duran-Moreno M, Cabrales E, Gil-Perotin S, Ortiz C, Chen B, Garcia-Verdugo JM, Sanai N, Alvarez-Buylla A. Bi- and uniciliated ependymal cells define continuous floor-plate-derived tanycytic territories. Nat Commun. 2017;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saugier-Veber P, Marguet F, Lecoquierre F, Adle-Biassette H, Guimiot F, Cipriani S, Patrier S, Brasseur-Daudruy M, Goldenberg A, Layet V, et al. Hydrocephalus due to multiple ependymal malformations is caused by mutations in the MPDZ gene. Acta Neuropathol Commun. 2017;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swiderski RE, Agassandian K, Ross JL, Bugge K, Cassell MD, Yeaman C. Structural defects in cilia of the choroid plexus, subfornical organ and ventricular ependyma are associated with ventriculomegaly. Fluids Barriers CNS. 2012;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelles DG, Hazrati LN. The pathological potential of ependymal cells in mild traumatic brain injury. Front Cell Neurosci. 2023;17:1216420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bigotte M, Gimenez M, Gavoille A, Deligiannopoulou A, El Hajj A, Croze S, Goumaidi A, Malleret G, Salin P, Giraudon P, et al. Ependyma: a new target for autoantibodies in neuromyelitis optica? Brain Commun. 2022;4:fcac307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bigotte M, Groh AMR, Marignier R, Stratton JA. Pathogenic role of autoantibodies at the ependyma in autoimmune disorders of the central nervous system. Front Cell Neurosci. 2023;17:1257000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelles DG, Hazrati LN. Ependymal cells and neurodegenerative disease: outcomes of compromised ependymal barrier function. Brain Commun. 2022;4:fcac288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore SA. The spinal Ependymal Layer in Health and Disease. Vet Pathol. 2016;53:746–53. [DOI] [PubMed] [Google Scholar]
- 15.Fabbiani G, Reali C, Valentin-Kahan A, Rehermann MI, Fagetti J, Falco MV, Russo RE. Connexin Signaling is involved in the reactivation of a latent stem cell niche after spinal cord Injury. J Neurosci. 2020;40:2246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Jimenez FJ, Jendelova P, Erceg S. The activation of dormant ependymal cells following spinal cord injury. Stem Cell Res Ther. 2023;14:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton LK, Dufresne M, Joppé SE, Petryszyn S, Aumont A, Calon F, Barnabé-Heider F, Furtos A, Parent M, Chaurand P, Fernandes KJ. Aberrant lipid metabolism in the Forebrain Niche suppresses adult neural stem cell proliferation in an animal model of Alzheimer’s Disease. Cell Stem Cell. 2015;17:397–411. [DOI] [PubMed] [Google Scholar]
- 18.Huh MS, Todd MAM, Picketts DJ. SCO-ping out the mechanisms underlying the etiology of Hydrocephalus. Physiology. 2009;24:117–26. [DOI] [PubMed] [Google Scholar]
- 19.Hatrock D, Caporicci-Dinucci N, Stratton JA. Ependymal cells and multiple sclerosis: proposing a relationship. Neural Regeneration Res. 2020;15:263–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McQuaid S, Cosby SL. An immunohistochemical study of the distribution of the measles virus receptors, CD46 and SLAM, in normal human tissues and subacute sclerosing panencephalitis. Lab Invest. 2002;82:403–9. [DOI] [PubMed] [Google Scholar]
- 21.Ida-Hosonuma M, Iwasaki T, Taya C, Sato Y, Li J, Nagata N, Yonekawa H, Koike S. Comparison of neuropathogenicity of poliovirus in two transgenic mouse strains expressing human poliovirus receptor with different distribution patterns. J Gen Virol. 2002;83:1095–105. [DOI] [PubMed] [Google Scholar]
- 22.Pratakpiriya W, Teh APP, Radtanakatikanon A, Pirarat N, Lan NT, Takeda M, et al. Expression of canine distemper virus receptor nectin-4 in the central nervous system of dogs. Sci Rep. 2017;7:349. [DOI] [PMC free article] [PubMed]
- 23.Shukla D, Scanlan PM, Titvari V, Sheth V, Clement C, Guzman-Hartman G, Dermody TS, Valyi-Nagy T. Expression of nectin-1 in normal and herpes simplex virus type 1-infected murine brain. Appl Immunohistochem Mol Morphol. 2006;14:341–7. [DOI] [PubMed] [Google Scholar]
- 24.Haverty R, McCormack J, Evans C, Purves K, O’Reilly S, Gautier V, et al. SARS-CoV-2 infects neurons, astrocytes, choroid plexus epithelial cells and pericytes of the human central nervous system in vitro. J Gen Virol. 2024;105:002009. [DOI] [PMC free article] [PubMed]
- 25.Lindskog C, Mear L, Virhammar J, Fallmar D, Kumlien E, Hesselager G, Casar-Borota O, Rostami E. Protein expression Profile of ACE2 in the normal and COVID-19-Affected human brain. J Proteome Res. 2022;21:2137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sikorska B, Liberski PP, Brown P. Subependymal plaques in scrapie-affected hamster brains–why are they so different from compact kuru plaques? Folia Neuropathol. 2008;46:32–42. [PubMed] [Google Scholar]
- 27.Liberski PP, Bratosiewicz J, Waliś A, Kordek R, Jeffrey M, Brown P. A special report I. prion protein (PrP)--amyloid plaques in the transmissible spongiform encephalopathies, or prion diseases revisited. Folia Neuropathol. 2001;39:217–35. [PubMed] [Google Scholar]
- 28.Karmysheva V, Pogodina VV. [PRP(CJD) reproduction in the cerebral vascular plexus epitheliocytes in a new type of Creutzfeldt-Jakob disease]. Arkh Patol. 2007;69:28–31. [PubMed] [Google Scholar]
- 29.Johanson CE, Duncan JA 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. [DOI] [PMC free article] [PubMed]
- 30.Fournier JG, Adjou K, Grigoriev V, Deslys JP. Ultrastructural evidence that ependymal cells are infected in experimental scrapie. Acta Neuropathol. 2008;115:643–50. [DOI] [PubMed] [Google Scholar]
- 31.Conrady CD, Zheng M, van Rooijen N, Drevets DA, Royer D, Alleman A, Carr DJ. Microglia and a functional type I IFN pathway are required to counter HSV-1-driven brain lateral ventricle enlargement and encephalitis. J Immunol. 2013;190:2807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menendez CM, Jinkins JK, Carr DJ. Resident T cells are unable to control herpes simplex Virus-1 activity in the Brain Ependymal Region during latency. J Immunol. 2016;197:1262–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández NR, Barenque LC, Silva SER, Garrido GS, Rosey SGJ, Rodríguez AZ, Romero LPR, Gómez AM, Gutiérrez FM. Ependymal damage in a Plasmodium Yoelii Yoelii lethal murine malaria model. Histol Histopathol. 2015;30:245–53. [DOI] [PubMed] [Google Scholar]
- 34.McLennan NF, Rennison KA, Bell JE, Ironside JW. In situ hybridization analysis of PrP mRNA in human CNS tissues. Neuropathol Appl Neurobiol. 2001;27:373–83. [DOI] [PubMed] [Google Scholar]
- 35.Ganapathiraju MK, Karunakaran KB, Correa-Menéndez J. Predicted protein interactions of IFITMs may shed light on mechanisms of Zika virus-induced microcephaly and host invasion. F1000Res 2016, 5:1919. [DOI] [PMC free article] [PubMed]
- 36.Saade M, Ferrero DS, Blanco-Ameijeiras J, Gonzalez-Gobartt E, Flores-Mendez M, Ruiz-Arroyo VM, Martinez-Saez E, Ramon YCS, Akizu N, Verdaguer N, Marti E. Multimerization of Zika Virus-NS5 causes ciliopathy and forces premature neurogenesis. Cell Stem Cell. 2020;27:920–e936928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torrescorzo J. Intraventricular and subarachnoid basal cisterns Neurocysticercosis treated with flexible cerebral endoscopy. In 13th World Congress of Neurological Surgery; Jun 19–24; Marrakech, MOROCCO. 2005: 227–230.
- 38.Karimy JK, Reeves BC, Damisah E, Duy PQ, Antwi P, David W, Wang K, Schiff SJ, Limbrick DD Jr., Alper SL, et al. Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets. Nat Rev Neurol. 2020;16:285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutherland DM, Aravamudhan P, Dietrich MH, Stehle T, Dermody TS. Reovirus neurotropism and virulence are dictated by sequences in the head domain of the viral attachment protein. J Virol. 2018;92:e00974–18. [DOI] [PMC free article] [PubMed]
- 40.Stencel-Baerenwald J, Reiss K, Blaum BS, Colvin D, Li XN, Abel T, et al. Glycan engagement dictates hydrocephalus induction by serotype 1 reovirus. Mbio. 2015;6:e02356. [DOI] [PMC free article] [PubMed]
- 41.MacRae C, Varma H. Chronic Hydrocephalus following Mumps Encephalitis: Neuropathological Correlates and review. J Neuropathol Exp Neurol. 2020;79:113–7. [DOI] [PubMed] [Google Scholar]
- 42.Carbonell WS, Murase SI, Horwitz AF, Mandell JW. Infiltrative microgliosis: activation and long-distance migration of subependymal microglia following periventricular insults. J Neuroinflammation. 2005;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu KY, Tang FL, Lee D, Zhao Y, Song H, Zhu XJ, Mei L, Xiong WC. Ependymal Vps35 promotes Ependymal Cell differentiation and survival, suppresses Microglial activation, and prevents neonatal Hydrocephalus. J Neurosci. 2020;40:3862–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliver C, Gonzalez CA, Alvial G, Flores CA, Rodriguez EM, Batiz LF. Disruption of CDH2/N-Cadherin-based Adherens junctions leads to apoptosis of Ependymal Cells and denudation of brain ventricular walls. J Neuropathol Exp Neurol. 2013;72:846–60. [DOI] [PubMed] [Google Scholar]
- 45.Roales-Bujan R, Paez P, Guerra M, Rodriguez S, Vio K, Ho-Plagaro A, Garcia-Bonilla M, Rodriguez-Perez LM, Dominguez-Pinos MD, Rodriguez EM, et al. Astrocytes acquire morphological and functional characteristics of ependymal cells following disruption of ependyma in hydrocephalus. Acta Neuropathol. 2012;124:531–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldner A, Adam MG, Tetzlaff F, Moll I, Komljenovic D, Sahm F, Bauerle T, Ishikawa H, Schroten H, Korff T, et al. Loss of Mpdz impairs ependymal cell integrity leading to perinatal-onset hydrocephalus in mice. EMBO Mol Med. 2017;9:890–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terry LA, Stewart JP, Nash AA, Fazakerley JK. Murine gammaherpesvirus-68 infection of and persistence in the central nervous system. J Gen Virol. 2000;81:2635–43. [DOI] [PubMed] [Google Scholar]
- 48.Reuter JD, Gomez DL, Wilson JH, van den Pol AN. Systemic immune deficiency necessary for cytomegalovirus invasion of the mature brain. J Virol. 2004;78:1473–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duprex WP, McQuaid S, Roscic-Mrkic B, Cattaneo R, McCallister C, Rima BK. In vitro and in vivo infection of neural cells by a recombinant measles virus expressing enhanced green fluorescent protein. J Virol. 2000;74:7972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon JY, Danielson B, Mathis D, Karamchandani J, Munoz DG. Cytomegalovirus in the human dentate gyrus and its impact on neural progenitor cells: report of two cases. Clin Neuropathol. 2017;36:240–5. [DOI] [PubMed] [Google Scholar]
- 51.Gomes I, Karmirian K, Oliveira JT, Pedrosa CDG, Mendes MA, Rosman FC, et al. SARS-CoV-2 infection of the central nervous system in a 14-month-old child: a case report of a complete autopsy. Lancet Reg Health-Americas. 2021;2:100046. [DOI] [PMC free article] [PubMed]
- 52.Jacob F, Pather SR, Huang WK, Zhang F, Wong SZH, Zhou H, Cubitt B, Fan W, Chen CZ, Xu M, et al. Human pluripotent stem cell-derived neural cells and Brain Organoids reveal SARS-CoV-2 Neurotropism predominates in Choroid Plexus Epithelium. Cell Stem Cell. 2020;27:937–e950939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mihaljevic S, Michalicova A, Bhide M, Kovac A. Pathophysiology of the choroid plexus in brain diseases. Gen Physiol Biophys. 2021;40:443–62. [DOI] [PubMed] [Google Scholar]
- 54.Alvarez JI, Teale JM. Differential changes in junctional complex proteins suggest the ependymal lining as the main source of leukocyte infiltration into ventricles in murine neurocysticercosis. J Neuroimmunol. 2007;187:102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alvarez JI, Teale JM. Multiple expression of matrix metalloproteinases in murine neurocysticercosis: implications for leukocyte migration through multiple central nervous system barriers. Brain Res. 2008;1214:145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mishra PK, Teale JM. Transcriptome analysis of the ependymal barrier during murine neurocysticercosis. J Neuroinflammation. 2012;9:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma Q, Chen J, Kong X, Zeng Y, Chen Z, Liu H, Liu L, Lu S, Wang X. Interactions between CNS and immune cells in tuberculous meningitis. Front Immunol. 2024;15:1326859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barnacle JR, Davis AG, Wilkinson RJ. Recent advances in understanding the human host immune response in tuberculous meningitis. Front Immunol. 2023;14:1326651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Solar P, Zamani A, Kubickova L, Dubovy P, Joukal M. Choroid plexus and the blood-cerebrospinal fluid barrier in disease. Fluids Barriers Cns. 2020;17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xia Y, Yamagata K, Krukoff TL. Differential expression of the CD14/TLR4 complex and inflammatory signaling molecules following i.c.v. administration of LPS. Brain Res. 2006;1095:85–95. [DOI] [PubMed] [Google Scholar]
- 61.Wojtkowiak-Giera A, Derda M, Kolasa-Wołosiuk A, Hadaś E, Kosik-Bogacka D, Solarczyk P, Jagodziński PP, Wandurska-Nowak E. Toll-like receptors in the brain of mice following infection with Acanthamoeba spp. Parasitol Res. 2016;115:4335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mishra BB, Mishra PK, Teale JM. Expression and distribution of toll-like receptors in the brain during murine neurocysticercosis. J Neuroimmunol. 2006;181:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mishra BB, Gundra UM, Teale JM. Expression and distribution of toll-like receptors 11–13 in the brain during murine neurocysticercosis. J Neuroinflammation. 2008;5:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maślińska D, Laure-Kamionowska M, Maśliński S. Toll-like receptors in rat brains injured by hypoxic-ischaemia or exposed to staphylococcal alpha-toxin. Folia Neuropathol. 2004;42:125–32. [PubMed] [Google Scholar]
- 65.Neal JW, Gasque P. How does the brain limit the severity of inflammation and tissue injury during bacterial meningitis? J Neuropathol Exp Neurol. 2013;72:370–85. [DOI] [PubMed] [Google Scholar]
- 66.Tauber SC, Ebert S, Weishaupt JH, Reich A, Nau R, Gerber J. Stimulation of toll-like receptor 9 by chronic intraventricular unmethylated cytosine-guanine DNA infusion causes neuroinflammation and impaired spatial memory. J Neuropathol Exp Neurol. 2009;68:1116–24. [DOI] [PubMed] [Google Scholar]
- 67.Mao SS, Hua R, Zhao XP, Qin X, Sun ZQ, Zhang Y, Wu YQ, Jia MX, Cao JL, Zhang YM. Exogenous administration of PACAP Alleviates Traumatic Brain Injury in rats through a mechanism involving the TLR4/MyD88/NF-kappa B pathway. J Neurotrauma. 2012;29:1941–59. [DOI] [PubMed] [Google Scholar]
- 68.Bramall AN, Anton ES, Kahle KT, Fecci PE. Navigating the ventricles: novel insights into the pathogenesis of hydrocephalus. EBioMedicine. 2022;78:103931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lattke M, Magnutzki A, Walther P, Wirth T, Baumann B. Nuclear factor κB activation impairs Ependymal Ciliogenesis and Links Neuroinflammation to Hydrocephalus formation. J Neurosci. 2012;32:11511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abdi K, Lai CH, Paez-Gonzalez P, Lay M, Pyun J, Kuo CT. Uncovering inherent cellular plasticity of multiciliated ependyma leading to ventricular wall transformation and hydrocephalus. Nat Commun. 2018;9:1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baek H, Shin HJ, Kim JJ, Shin N, Kim S, Yi MH, Zhang E, Hong J, Kang JW, Kim Y, et al. Primary cilia modulate TLR4-mediated inflammatory responses in hippocampal neurons. J Neuroinflammation. 2017;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chevreau R, Ghazale H, Ripoll C, Chalfouh C, Delarue Q, Hemonnot-Girard AL, et al. RNA profiling of Mouse Ependymal Cells after spinal cord Injury identifies the oncostatin pathway as a potential key regulator of spinal cord stem cell fate. Cells. 2021;10:3332. [DOI] [PMC free article] [PubMed]
- 73.Walton T, Gui M, Velkova S, Fassad MR, Hirst RA, Haarman E, O’Callaghan C, Bottier M, Burgoyne T, Mitchison HM, Brown A. Axonemal structures reveal mechanoregulatory and disease mechanisms. Nature. 2023;618:625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zariwala MA, Knowles MR, Omran H. Genetic defects in ciliary structure and function. Annu Rev Physiol. 2007;69:423–50. [DOI] [PubMed] [Google Scholar]
- 75.Lucas JS, Davis SD, Omran H, Shoemark A. Primary ciliary dyskinesia in the genomics age. Lancet Respiratory Med. 2020;8:202–16. [DOI] [PubMed] [Google Scholar]
- 76.Ibañez-Tallon I, Pagenstecher A, Fliegauf M, Olbrich H, Kispert A, Ketelsen UP, North A, Heintz N, Omran H. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum Mol Genet. 2004;13:2133–41. [DOI] [PubMed] [Google Scholar]
- 77.Ibañez-Tallon I, Gorokhova S, Heintz N. Loss of function of axonemal dynein Mdnah5 causes primary ciliary dysk inesia and hydrocephalus. Hum Mol Genet. 2002;11:715–21. [DOI] [PubMed] [Google Scholar]
- 78.Merveille A-C, Davis EE, Becker-Heck A, Legendre M, Amirav I, Bataille G, Belmont J, Beydon N, Billen F, Clément A, et al. CCDC39 is required for assembly of inner dynein arms and the dynein re gulatory complex and for normal ciliary motility in humans and dogs. Nat Genet. 2011;43:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davy BE, Robinson ML. Congenital hydrocephalus in hy3 mice is caused by a frameshift mutation in Hydin, a large novel gene. Hum Mol Genet. 2003;12:1163–70. [DOI] [PubMed] [Google Scholar]
- 80.Chiani F, Orsini T, Gambadoro A, Pasquini M, Putti S, Cirilli M, et al. Functional loss of Ccdc151 leads to hydrocephalus in a mouse model of primary ciliary dyskinesia. Dis Model Mech. 2019;12:dmm038489. [DOI] [PMC free article] [PubMed]
- 81.Delhaye S, Paul S, Blakqori G, Minet M, Weber F, Staeheli P, et al. Neurons produce type I interferon during viral encephalitis. Proc Natl Acad Sci USA. 2006;103:7835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fragkoudis R, Breakwell L, McKimmie C, Boyd A, Barry G, Kohl A, et al. The type I interferon system protects mice from Semliki Forest virus by preventing widespread virus dissemination in extraneural tissues, but does not mediate the restricted replication of avirulent virus in central nervous system neurons. J Gen Virol. 2007;88:3373–84. [DOI] [PubMed] [Google Scholar]
- 83.Millward JM, Caruso M, Campbell IL, Gauldie J, Owens T. IFN-γ-induced chemokines synergize with pertussis toxin to promote T cell entry to the central nervous system. J Immunol. 2007;178:8175–82. [DOI] [PubMed] [Google Scholar]
- 84.González JM, Bergmann CC, Ramakrishna C, Hinton DR, Atkinson R, Hoskin J, Macklin WB, Stohlman SA. Inhibition of interferon-gamma signaling in oligodendroglia delays cor onavirus clearance without altering demyelination. Am J Pathol. 2006;168:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parra B, Hinton DR, Marten NW, Bergmann CC, Lin MT, Yang CS, Stohlman SA. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J Immunol. 1999;162:1641–7. [PubMed] [Google Scholar]
- 86.Tarassishin L, Bauman A, Suh HS, Lee SC. Anti-viral and anti-inflammatory mechanisms of the Innate Immune Transcription Factor Interferon Regulatory Factor 3: relevance to human CNS diseases. J Neuroimmune Pharmacol. 2013;8:132–44. [DOI] [PubMed] [Google Scholar]
- 87.Le Coupanec A, Desforges M, Kaufer B, Dubeau P, Côté M, Talbot PJ. Potential differences in cleavage of the S protein and type-1 interferon together control human coronavirus infection, propagation, and neuropathology within the central nervous system. J Virol. 2021;95:e00140–21. [DOI] [PMC free article] [PubMed]
- 88.Mockus TE, Netherby-Winslow CS, Atkins HM, Lauver MD, Jin G, Ren HM, et al. CD8 T cells and STAT1 signaling are essential codeterminants in Protection from Polyomavirus Encephalopathy. J Virol. 2020;94:e02038–19. [DOI] [PMC free article] [PubMed]
- 89.Groh AMR, Caporicci-Dinucci N, Afanasiev E, Bigotte M, Lu B, Gertsvolf J, et al. Ependymal cells undergo astrocyte-like reactivity in response to neuroinflammation. J Neurochem. 2024. [DOI] [PubMed]
- 90.Ogata A, Nishihira J, Suzuki T, Nagashima K, Tashiro K. Identification of macrophage migration inhibitory factor mRNA expression in neural cells of the rat brain by in situ hybridization. Neurosci Lett. 1998;246:173–7. [DOI] [PubMed] [Google Scholar]
- 91.Nishibori M, Nakaya N, Tahara A, Kawabata M, Mori S, Saeki K. Presence of macrophage migration inhibitory factor (MIF) in ependyma, astrocytes and neurons in the bovine brain. Neurosci Lett. 1996;213:193–6. [DOI] [PubMed] [Google Scholar]
- 92.Sumaiya K, Langford D, Natarajaseenivasan K, Shanmughapriya S. Macrophage migration inhibitory factor (MIF): a multifaceted cytokine regulated by genetic and physiological strategies. Pharmacol Ther. 2022;233:108024. [DOI] [PubMed] [Google Scholar]
- 93.Kim YS, Honkaniemi J, Sharp FR, Täuber MG. Expression of proinflammatory cytokines tumor necrosis factor-alpha and interleukin-1beta in the brain during experimental group B streptococcal meningitis. Brain Res Mol Brain Res. 2004;128:95–102. [DOI] [PubMed] [Google Scholar]
- 94.Elwakil BH, Bakr BA, Aljeldah MM, Shehata NS, Shahin YH, Olama ZA, et al. Memory impairment, pro-inflammatory host response and brain histopathologic severity in rats infected with K. pneumoniae or P. Aeruginosa Meningitis. Pathogens. 2022;11:933. [DOI] [PMC free article] [PubMed]
- 95.Brandenburg LO, Varoga D, Nicolaeva N, Leib SL, Wilms H, Podschun R, Wruck CJ, Schröder JM, Pufe T, Lucius R. Role of glial cells in the functional expression of LL-37/rat cathelin-related antimicrobial peptide in meningitis. J Neuropathol Exp Neurol. 2008;67:1041–54. [DOI] [PubMed] [Google Scholar]
- 96.Fernandez-Arjona MDM, Leon-Rodriguez A, Lopez-Avalos MD, Grondona JM. Microglia activated by microbial neuraminidase contributes to ependymal cell death. Fluids Barriers CNS. 2021;18:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bodnar CN, Watson JB, Higgins EK, Quan N, Bachstetter AD. Inflammatory regulation of CNS barriers after traumatic Brain Injury: a Tale Directed by Interleukin-1. Front Immunol. 2021;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Whitmore MM, Iparraguirre A, Kubelka L, Weninger W, Hai T, Williams BR. Negative regulation of TLR-signaling pathways by activating transcription factor-3. J Immunol. 2007;179:3622–30. [DOI] [PubMed] [Google Scholar]
- 99.Cartagena CM, Phillips KL, Williams GL, Konopko M, Tortella FC, Dave JR, Schmid KE. Mechanism of action for NNZ-2566 anti-inflammatory effects following PBBI involves upregulation of immunomodulator ATF3. Neuromolecular Med. 2013;15:504–14. [DOI] [PubMed] [Google Scholar]
- 100.Rosenberger CM, Clark AE, Treuting PM, Johnson CD, Aderem A. ATF3 regulates MCMV infection in mice by modulating IFN-gamma expression in natural killer cells. Proc Natl Acad Sci U S A. 2008;105:2544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mladinic M, Bianchetti E, Dekanic A, Mazzone GL, Nistri A. ATF3 is a novel nuclear marker for migrating ependymal stem cells in the rat spinal cord. Stem Cell Res. 2014;12:815–27. [DOI] [PubMed] [Google Scholar]
- 102.Wang LF, Huang SB, Zhao HD, Liu CJ, Yao L, Shen YQ. Activating transcription factor 3 promotes spinal cord regeneration of adult zebrafish. Biochem Biophys Res Commun. 2017;488:522–7. [DOI] [PubMed] [Google Scholar]
- 103.Hoarau JJ, Krejbich-Trotot P, Jaffar-Bandjee MC, Das T, Thon-Hon GV, Kumar S, Neal JW, Gasque P. Activation and Control of CNS Innate Immune Responses in Health and Diseases: A Balancing Act Finely Tuned by Neuroimmune Regulators (NIReg). Cns & Neurological Disorders-Drug Targets 2011, 10:25–43. [DOI] [PubMed]
- 104.Canova C, Neal JW, Gasque P. Expression of innate immune complement regulators on brain epithelial cells during human bacterial meningitis. J Neuroinflammation. 2006;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johanson C, Stopa E, Baird A, Sharma H. Traumatic brain injury and recovery mechanisms: peptide modulation of periventricular neurogenic regions by the choroid plexus-CSF nexus. J Neural Transm. 2011;118:115–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dodge JC, Treleaven CM, Fidler JA, Hester M, Haidet A, Handy C, Rao M, Eagle A, Matthews JC, Taksir TV, et al. AAV4-mediated expression of IGF-1 and VEGF within cellular components of the ventricular system improves survival outcome in familial ALS mice. Mol Ther. 2010;18:2075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang X, Zhao Z, Wu Q, Wang L, Li L, Wang M, Ren Y, Pan L, Tang H, Li F. Single-cell analysis reveals changes in BCG vaccine-injected mice modeling tuberculous meningitis brain infection. Cell Rep. 2023;42:112177. [DOI] [PubMed] [Google Scholar]
- 108.Zhang X, Pan L, Zhang P, Wang L, Shen Y, Xu P, Ren Y, Huang W, Liu P, Wu Q, Li F. Single-cell analysis of the miRNA activities in tuberculous meningitis (TBM) model mice injected with the BCG vaccine. Int Immunopharmacol. 2023;124:110871. [DOI] [PubMed] [Google Scholar]
- 109.Yang F, Yang Y, Chen Y, Li G, Zhang G, Chen L, Zhang Z, Mai Q, Zeng G. MiR-21 is remotely governed by the commensal Bacteria and impairs Anti-TB immunity by Down-regulating IFN-γ. Front Microbiol. 2020;11:512581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martino G, Furlan R, Comi G, Adorini L. The ependymal route to the CNS: an emerging gene-therapy approach for MS. Trends Immunol. 2001;22:483–90. [DOI] [PubMed] [Google Scholar]
- 111.Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, Zabner J, Ghodsi A, Chiorini JA. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci U S A. 2000;97:3428–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen X, He Y, Tian Y, Wang Y, Wu Z, Lan T, Wang H, Cheng K, Xie P. Different serotypes of Adeno-Associated Virus Vector- and lentivirus-mediated tropism in Choroid Plexus by Intracerebroventricular Delivery. Hum Gene Ther. 2020;31:440–7. [DOI] [PubMed] [Google Scholar]
- 113.Hinderer C, Miller R, Dyer C, Johansson J, Bell P, Buza E, Wilson JM. Adeno-associated virus serotype 1-based gene therapy for FTD caused by GRN mutations. Ann Clin Transl Neurol. 2020;7:1843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Katz ML, Tecedor L, Chen Y, Williamson BG, Lysenko E, Wininger FA, Young WM, Johnson GC, Whiting RE, Coates JR, Davidson BL. AAV gene transfer delays disease onset in a TPP1-deficient canine model of the late infantile form of Batten disease. Sci Transl Med. 2015;7:313ra180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yamazaki Y, Hirai Y, Miyake K, Shimada T. Targeted gene transfer into ependymal cells through intraventricular injection of AAV1 vector and long-term enzyme replacement via the CSF. Sci Rep. 2014;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hironaka K, Yamazaki Y, Hirai Y, Yamamoto M, Miyake N, Miyake K, Okada T, Morita A, Shimada T. Enzyme replacement in the CSF to treat metachromatic leukodystrophy in mouse model using single intracerebroventricular injection of self-complementary AAV1 vector. Sci Rep. 2015;5:13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cigliola V, Shoffner A, Lee N, Ou J, Gonzalez TJ, Hoque J, Becker CJ, Han Y, Shen G, Faw TD, et al. Spinal cord repair is modulated by the neurogenic factor Hb-egf under direction of a regeneration-associated enhancer. Nat Commun. 2023;14:4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suzuki J-i, Dezawa M, Kitada M. Prolonged but non-permanent expression of a transgene in ependymal cells of adult rats using an adenovirus-mediated transposon gene transfer system. Brain Res. 2017;1675:20–7. [DOI] [PubMed] [Google Scholar]
- 119.Dindot S, Piccolo P, Grove N, Palmer D, Brunetti-Pierri N. Intrathecal injection of helper-dependent adenoviral vectors results in long-term transgene expression in Neuroependymal Cells and neurons. Hum Gene Ther. 2011;22:745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Furlan R, Poliani PL, Galbiati F, Bergami A, Grimaldi LME, Comi G, Adorini L, Martino G. Central nervous system delivery of interleukin 4 by a nonreplicative herpes simplex type 1 viral vector ameliorates autoimmune demyelination. Hum Gene Ther. 1998;9:2605–17. [DOI] [PubMed] [Google Scholar]
- 121.Carrell EM, Chen YH, Ranum PT, Coffin SL, Singh LN, Tecedor L, Keiser MS, Hudry E, Hyman BT, Davidson BL. VWA3A-derived ependyma promoter drives increased therapeutic protein secretion into the CSF. Mol Ther Nucleic Acids. 2023;33:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang BT, Guo ZS, Bartlett DL, Liu J, McFadden G, Shisler JL, Roy EJ. A cautionary note on the selectivity of oncolytic poxviruses. Oncolytic Virotherapy. 2019;8:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mehta H, Muller J, Markovitz NS. Ultrastructural analysis of ICP34.5 herpes Simplex Virus 1 replication in mouse brain cells in vivo. J Virol. 2010;84:10982–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cousins O, Hodges A, Schubert J, Veronese M, Turkheimer F, Miyan J, et al. The blood-CSF-brain route of neurological disease: the indirect pathway into the brain. Neuropathol Appl Neurobiol. 2022;48:e12789. [DOI] [PubMed]
- 125.Djukic M, Lange P, Erbguth F, Nau R. Spatial and temporal variation of routine parameters: pitfalls in the cerebrospinal fluid analysis in central nervous system infections. J Neuroinflammation. 2022;19:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schob S, Schicht M, Sel S, Stiller D, Kekule AS, Paulsen F, Maronde E, Brauer L. The detection of surfactant proteins A, B, C and D in the human brain and their regulation in cerebral infarction, autoimmune conditions and infections of the CNS. PLoS ONE. 2013;8:e74412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Enos N, Takenaka H, Scott S, Salfity HVN, Kirk M, Egar MW, Sarria DA, Slayback-Barry D, Belecky-Adams T, Chernoff EAG. Meningeal foam cells and Ependymal Cells in Axolotl spinal cord regeneration. Front Immunol. 2019;10:2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Whitworth LJ, Troll R, Pagan AJ, Roca FJ, Edelstein PH, Troll M, et al. Elevated cerebrospinal fluid cytokine levels in tuberculous meningitis predict survival in response to dexamethasone. Proc Natl Acad Sci U S A. 2021;118:e2024852118. [DOI] [PMC free article] [PubMed]
- 129.Thuong NTT, Heemskerk D, Tram TTB, Thao LTP, Ramakrishnan L, Ha VTN, Bang ND, Chau TTH, Lan NH, Caws M, et al. Leukotriene A4 hydrolase genotype and HIV infection influence intracerebral inflammation and survival from tuberculous meningitis. J Infect Dis. 2017;215:1020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saghazadeh A, Rezaei N. Central Inflammatory cytokines in Tuberculous meningitis: a systematic review and Meta-analysis. J Interferon Cytokine Res. 2022;42:95–107. [DOI] [PubMed] [Google Scholar]
- 131.Li D, Lv P, Lv Y, Ma D, Yang J. Magnetic resonance imaging characteristics and treatment aspects of ventricular tuberculosis in adult patients. Acta Radiol. 2017;58:91–7. [DOI] [PubMed]
- 132.Ravenscroft A, Schoeman JF, Donald PR. Tuberculous granulomas in childhood tuberculous meningitis: radiological features and course. J Trop Pediatr. 2001;47:5–12. [DOI] [PubMed] [Google Scholar]
- 133.Guerini H, Helie O, Leveque C, Adem C, Hauret L, Cordoliani YS. Diagnosis of periventricular ependymal enhancement in MRI in adults. J Neuroradiol. 2003;30:46–56. [PubMed] [Google Scholar]
- 134.Hammoud DA, Mahdi E, Panackal AA, Wakim P, Sheikh V, Sereti I, Bielakova B, Bennett JE, Williamson PR. Choroid Plexitis and Ependymitis by Magnetic Resonance Imaging are biomarkers of neuronal damage and inflammation in HIV-negative cryptococcal meningoencephalitis. Sci Rep. 2017;7:9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yikilmaz A, Taylor GA. Sonographic findings in bacterial meningitis in neonates and young infants. Pediatr Radiol. 2008;38:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hauwel M, Furon E, Canova C, Griffiths M, Neal J, Gasque P. Innate (inherent) control of brain infection, brain inflammation and brain repair: the role of microglia, astrocytes, protective glial stem cells and stromal ependymal cells. Brain Res Rev. 2005;48:220–33. [DOI] [PubMed] [Google Scholar]
- 137.Kumai Y, Ooboshi H, Kitazono T, Takada J, Ibayashi S, Fujishima M, Iida M. Brain ischemia augments exo-focal transgene expression of adenovirus-mediated gene transfer to ependyma in hypertensive rats. Exp Neurol. 2003;184:904–11. [DOI] [PubMed] [Google Scholar]
- 138.Garcia-Ovejero D, Arevalo-Martin A, Paniagua-Torija B, Florensa-Vila J, Ferrer I, Grassner L, Molina-Holgado E. The ependymal region of the adult human spinal cord differs from other species and shows ependymoma-like features. Brain. 2015;138:1583–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.