Abstract
Background:
Neurogenic thoracic outlet syndrome (NTOS) is a dynamic compression of the brachial plexus. This study aimed to evaluate the correlation between the concave deformity of the posterior edge of the anterior scalene muscle (CDAS) on sagittal T1 with intraoperative findings of vascular compression. The second aim was to define the NTOS vascular subtypes and establish possible treatments.
Methods:
We retrospectively reviewed patients who met the Consortium for Research and Education on Thoracic Outlet Syndrome criteria for NTOS and were operated on after a failed rehabilitation program.
Results:
Forty-four patients were included; mean age was 29.51 years (range: 13–55 years), and 24 (54.5%) were women. CDAS on sagittal T1 magnetic resonance imaging (MRI) was identified in 20 of 44. Patients were divided into two categories: type A (pure NTOS) (20 of 44); and type B (mixed neurogenic-vascular variants) (24 of 44). Type B was divided into B1, B2, and B3, corresponding to subclavian artery (SCA) compression (seven of 44), subclavian vein compression (SCV) (five of 44), and both SCA and SCV compression (12 of 44), respectively. All patients with B1 had CDAS on MRI T1 sagittal, whereas CDAS was found on 5%, 60%, and 58.3% in types A, B2, and B3, respectively. Intraoperatively, all patients had at least one structural anomaly. Preoperative symptoms of lower or middle-lower brachial plexus trunk compressions were more prominent in patients with the vascular variant (B1: 85%, B2: 83%, and B3: 83%) than the pure NTOS (type A) (40%).
Conclusions:
NTOS presents as four subtypes: pure neurogenic (A) and vascular (B1, B2, and B3). Preoperative middle/lower trunk symptoms combined with positive upper limb duplex ultrasound of the SCA, SCV, and sagittal MRI show that a CDAS is correlated with the vascular form of NTOS and predicts failure of preoperative rehabilitation program.
Takeaways
Questions: Does magnetic resonance imaging (MRI) of the scalene triangle predict associated vascular compression in neurogenic thoracic outlet syndrome (NTOS)? How does the imaging finding of vascular compression impact surgical decision-making?
Findings: Concave deformity of the posterior edge of the anterior scalene muscle (CDAS) on MRI is strongly associated with the arterial NTOS variant. Surgical decompression is eventually warranted, as conservative therapy is ineffective.
Meaning: MRI is a useful tool for diagnosing vascular variants of NTOS. CDAS predicts failure of nonsurgical management in arterial NTOS.
INTRODUCTION
Neurogenic thoracic outlet syndrome (NTOS) is a dynamic compressive neuropathy of the brachial plexus. Compression can occur at the scalene triangle, underneath a cervical rib, in the subcoracoid space, or near fibrous bands (s bands) anywhere along the plexus.1 Usually, it presents as pain in the neck, shoulder, arm, and hand.2 Of the NTOS clinical presentations, symptoms of lower trunk brachial plexus compression are most frequently reported (68%), followed by symptoms of both upper and lower trunk compression (20%), with fewer symptoms of upper trunk compression alone (12%).3
Definitive diagnosis of NTOS is notoriously difficult. Differential diagnoses include motor neuron diseases, double crush syndrome, ulnar or median neuropathy, cervical radiculopathy, and other brachial plexus pathologies from inflammatory or neoplastic lesions. Electrophysiological studies are necessary to differentiate between these conditions. Imaging studies have yet to offer significant aid in diagnosing NTOS. Plain radiography is usually performed to diagnose a cervical rib,4 although most patients with NTOS lack cervical ribs.5 The complexity of treating challenging medical conditions encountered in both plastic and reconstructive peripheral nerve surgery mandates objective diagnostic and outcome measurement tools to facilitate an optimal surgical decision-making process. Therefore, there is a need for an accurate preoperative diagnostic modality for confirming the diagnosis of NTOS. Ultrasound (US) and high-functional US have a growing role in the diagnosis of NTOS, and they were able to confirm the presence of abnormal fibromuscular structures within the scalene triangle affecting mainly the lower trunk of the brachial plexus; however, they are operator-dependent, unlike other diagnostic imaging tools. Magnetic resonance imaging (MRI) is emerging as the preferred tool of imaging investigation for NTOS.6 However, some studies suggest a poor correlation between MRI and intraoperative findings.7
Associated vascular compression in NTOS has been scantily addressed. Previous reports in the literature quote an incidence of 2%–76.5%8; however, they all involved subjective evaluation of the radial pulse. Other methods, such as plethysmography or oscillometry, have been found to be unreliable as well,8 including the Adson test, which entails subjective interpretation.9 Only one report using Doppler ultrasonography reported arterial occlusion in 51% and venous compression in 8% of patients with a provocative maneuver.10 The correlation between different imaging modalities and associated trunk symptoms to allocate preoperative pathoanatomy and guide treatment has been poorly addressed.
The main purpose of our study was to establish the correlation between concave deformity of the anterior scalene (CDAS) on sagittal T1 MRI and the presence of a vascular variant of neurogenic TOS and suggest possible treatment implications. Our secondary goal was to define the NTOS subtypes using three criteria after ruling out the cervical rib on plain radiograph or double crush syndrome by electrodiagnostic studies: (1) standard protocol of US Doppler and photoplethysmography, (2) sagittal T1 MRI of the scalene triangle combined with (3) the preoperative presenting symptoms.
METHODS
After approval by the institutional review board, 46 patients with NTOS who met the predefined Consortium for Research and Education on Thoracic Outlet Syndrome clinical diagnostic criteria for NTOS11 and were operated on after a failed rehabilitation program trial, were evaluated at a single university hospital between January 2019 and January 2023. Data were collected from office notes, hospital records, imaging studies (MRI and cervical plain radiography), and electrodiagnostic studies. Two patients after revision scalenectomy were excluded. Brachial plexus MRI with coronal and axial STIR views, T1-weighted coronal, sagittal, and axial acquisitions were obtained by a musculoskeletal radiologist using a 3T scanner. Thicknesses of slices for coronal, sagittal, and axial views were 3, 3, and 4 mm, respectively. The sagittal T1 views were parallel to the scalene triangle. The TOS arterial photoplethysmography12 test was performed using the Viasonix system (Ra’anana, Israel): photoplethysmography sensors were placed on the right and left hands. After acquisition of the waveform in the resting position, patients were asked to actively perform the after provocative maneuvers: abduction to 90- and 180-degree angle, military, costoclavicular, hands up 180-degree angle, Allen, hands up straight 180-degree angle and hands up 90-degree angle front positions. The waveforms were recorded in each position. [See figure, Supplemental Digital Content 1, which displays (A) photoplethysmography in a patient with bilateral TOS type B1, resting and provocative maneuvers. Note the wave’s obliteration with provocation maneuvers. B, US duplex of the right subclavian vein with resting position. C, US duplex of the right subclavian vein with hands up demonstrating obstruction of the venous inflow. http://links.lww.com/PRSGO/D569.]
Subclavian vein duplex US examination involved visualizing the subclavian vein’s flow with the above-mentioned resting and TOS provocative maneuvers. In the presence of neurogenic and/or positive subclavian arterial compression, patients underwent supraclavicular thoracic outlet decompression, including complete first rib resection, anterior and middle scalenectomy, resection of any aberrant fibromuscular bands, mobilization and external neurolysis of the brachial plexus, and pectoralis minor tenotomy if the patient also had physical examination findings localized to the subcoracoid space.13
In patients with additional venous compression, a paraclavicular approach was performed, which allowed full resection of the entire first rib and complete removal of the anterior scalene (AS), middle scalene, and subclavius muscles, and full exposure of the axillary-subclavian vein by which we performed a thorough external venolysis.14 The intraoperative findings were photographed, and videos were recorded.
Data Analysis
One-way analysis of variance was used to compare the age and duration of symptoms across the TOS subtypes. Chi-square tests were used to examine the correlation between nominal variables with TOS subtypes. All statistical tests were performed using Statistical Package for the Social Sciences, version 27.0, with significance set at a P value of less than 0.05.
RESULTS
Forty-four patients operated on for NTOS were included. The mean duration of symptoms was 4.7 years (range: 6 months to 18 years), and the mean age was 29.4 years (range: 13–55 years). Photoplethysmography and Doppler duplex examination of the 44 patients demonstrated definite arterial pinching or obstruction with abduction in seven B1 patients (16%); venous pinching in five B2 patients (11%), both arterial and venous in 12 B3 (27%), and absence of vascular pinching in 20 type A patients (45%). [See Video 1 (online), which displays the ultrasound duplex of the right subclavian vein with hands up demonstrating narrowing of the vein lumen and decreased venous flow.] Eleven patients (20%) with pure NTOS type A were not included in the study as their symptoms improved with a rehabilitation trial. Type B3 patients were younger than the other group subtypes; B3 patients were diagnosed at an earlier age than the other subtype patients (22.8 ± 7.3) (P = 0.034) (Table 1).
Table 1.
Demographic Data, Mean Duration of Symptoms, and Type B3 [Venous-arterial Neurogenic TOS Patients Were the Youngest among the Other Subtypes (P = 0.0.034)]
| Type A | Type B1 | Type B2 | Type B3 | P | |
|---|---|---|---|---|---|
| No. patients | 20 (46%) | 7 (16%) | 5 (11%) | 12 (27%) | |
| Age, mean (range), y | 30.3 (15–42) | 31.42 (23–44) | 39.2 (20–55) | 22.88 (13–37) | 0.034* |
| Male:female ratio | 9:11 | 3:4 | 3:2 | 5:7 | |
| Affected dominant hand, n (%) | 9 (45) | 1 (14) | 3 (60) | 5 (42) | 0.36 |
| Duration of symptoms, mean (range), y | 4.61 (0.16–15) | 7.57 (2–18) | 7 (1.5–10) | 4.29 (0.5–14) | 0.339 |
Video 1. which displays the ultrasound duplex of the right subclavian vein with hands up demonstrating narrowing of the vein lumen and decreased venous flow.
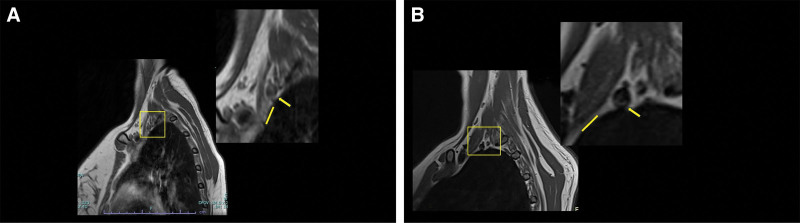
The dominant hand was the least affected in the B1 group; however, most patients in group B1 had a cervical rib (Table 2). Sagittal MRI was positive for posterior concave deformity of the AS muscle in 100% of patients with Type B1 compared with 5%, 60%, and 58.3% of patients with types A, B2, and B3, respectively (P = 0.001) (Table 3) (Fig. 1). None of the 20% patients with type A who were treated successfully with a trial of rehabilitation had posterior concave deformity on sagittal T1 MRI. Preoperatively, middle-lower trunk symptoms were associated with types B1 and B2 (P = 0.048), whereas type A was associated with middle trunk symptoms (P = 0.04) (Table 4). Among the neurogenic and compressive neuropathic-associated symptoms, only neck pain was significantly associated with type A (P = 0.02) (Table 5), whereas hand heaviness was only significantly associated with type B2 (the venous variant) (P = 0.03). (See figure, Supplemental Digital Content 2, which displays the distribution of preoperative compressive neurogenic-associated symptoms. http://links.lww.com/PRSGO/D570.) (See figure, Supplemental Digital Content 3, which displays the prevalence of venous-associated symptoms. http://links.lww.com/PRSGO/D571.) The arterial-associated symptoms did not differ significantly. (See figure, Supplemental Digital Content 4, which displays the prevalence of arterial-associated symptoms. http://links.lww.com/PRSGO/D572.)
Table 2.
Etiology of TOS, Including Idiopathic, Trauma-related (Work-related Injury, Whiplash, Other Traumatic) and Cervical Rib
| Type A, N = 20 | Type B1, N = 7 | Type B2, N = 5 | Type B3, N = 12 | P | |
|---|---|---|---|---|---|
| Idiopathic, n (%) | 12 (60) | 1 (14) | 3 (60) | 8 (66) | 0.134 |
| Cervical rib, n (%) | 2 (10) | 4 (57) | 1 (20) | 2 (17) | 0.065 |
| Trauma, n (%) | 6 (30) | 2 (29) | 1 (20) | 2 (17) | 0.843 |
Table 3.
The Prevalence of Preoperative CDAS on Sagittal T1 MRI and Compressing Structural Anomalies
| Type A, N = 20 | Type B1, N = 7 | Type B2, N = 5 | Type B3, N = 12 | P | |
|---|---|---|---|---|---|
| SM, n (%) | 10 (50) | 3 (43) | 4 (80) | 8 (67) | 0.516 |
| AS posterior fibrous edge, n (%) | 10 (50) | 5 (71) | 2 (40) | 9 (75) | 0.5 |
| Roos, n (%) | 12 (60) | 5 (71) | 2 (40) | 6 (50) | 0.39 |
| MRI CDAS, n (%) | 1 (5) | 7 (100) | 3 (60) | 7 (58.3) | 0.001 |
More than one structure could be found intraoperatively. The prevalence of each anomaly did not differ significantly between the subgroups.
Fig. 1.
A, Sagittal T1 MRI of the scalene triangle with posterior concave deformity of AS in a patient with type B3. Yellow rectangle: magnification of the scalene triangle (arrow: SCA, line: AS insertion). B, Sagittal T1 MRI of the scalene triangle without posterior concave deformity of AS in a patient with type A. Yellow rectangle: magnification of the scalene triangle (arrow: SCA, line: AS insertion).
Table 4.
Preoperative Patient-reported Pain and Numbness
| Type A, n = 20 | Type B1, n = 7 | Type B2, n = 5 | Type B3, n = 12 | P | |
|---|---|---|---|---|---|
| Upper trunk, n (%) | 2 (10) | 0 (0) | 1 (20) | 1 (8) | 0.08 |
| Middle trunk, n (%) | 7 (35) | 0 (0) | 0 (0) | 1 (8) | 0.04* |
| Lower trunk, n (%) | 6 (30) | 3 (43) | 0 (0) | 5 (42) | 0.12 |
| Upper middle trunks, n (%) | 3 (15) | 1 (14) | 0 (0) | 0 (0) | 0.42 |
| Middle-lower trunks, n (%) | 2 (10) | 3 (43) | 4 (80) | 3 (25) | 0.048* |
The brachial plexus trunk distribution is based on patients’ pain drawings and physical examination (response to Tinel over the brachial plexus.
Table 5.
The Prevalence of Various Neurogenic Pain Symptoms Preoperatively
| Type A, n = 20 | Type B1, n = 7 | Type B2, n = 5 | Type B3, n = 12 | P | |
|---|---|---|---|---|---|
| Headache, n (%) | 14 (70) | 6 (86) | 4 (80) | 10 (83) | 0.42 |
| Neck pain, n (%) | 18 (90) | 4 (57) | 3 (60) | 7 (58) | 0.02* |
| Shoulder pain, n (%) | 15 (75) | 4 (57) | 3 (60) | 7 (58) | 0.99 |
| Arm pain, n (%) | 15 (75) | 6 (86) | 3 (60) | 10 (83) | 0.25 |
| Hand pain, n (%) | 15 (75) | 7 (100) | 3 (60) | 10 (83) | 0.25 |
| Neck stiffness, n (%) | 13 (65) | 3 (43) | 2 (40) | 6 (50) | 0.46 |
| Pain in excertion, n (%) | 15 (75) | 7 (100) | 3 (60) | 10 (83) | 0.39 |
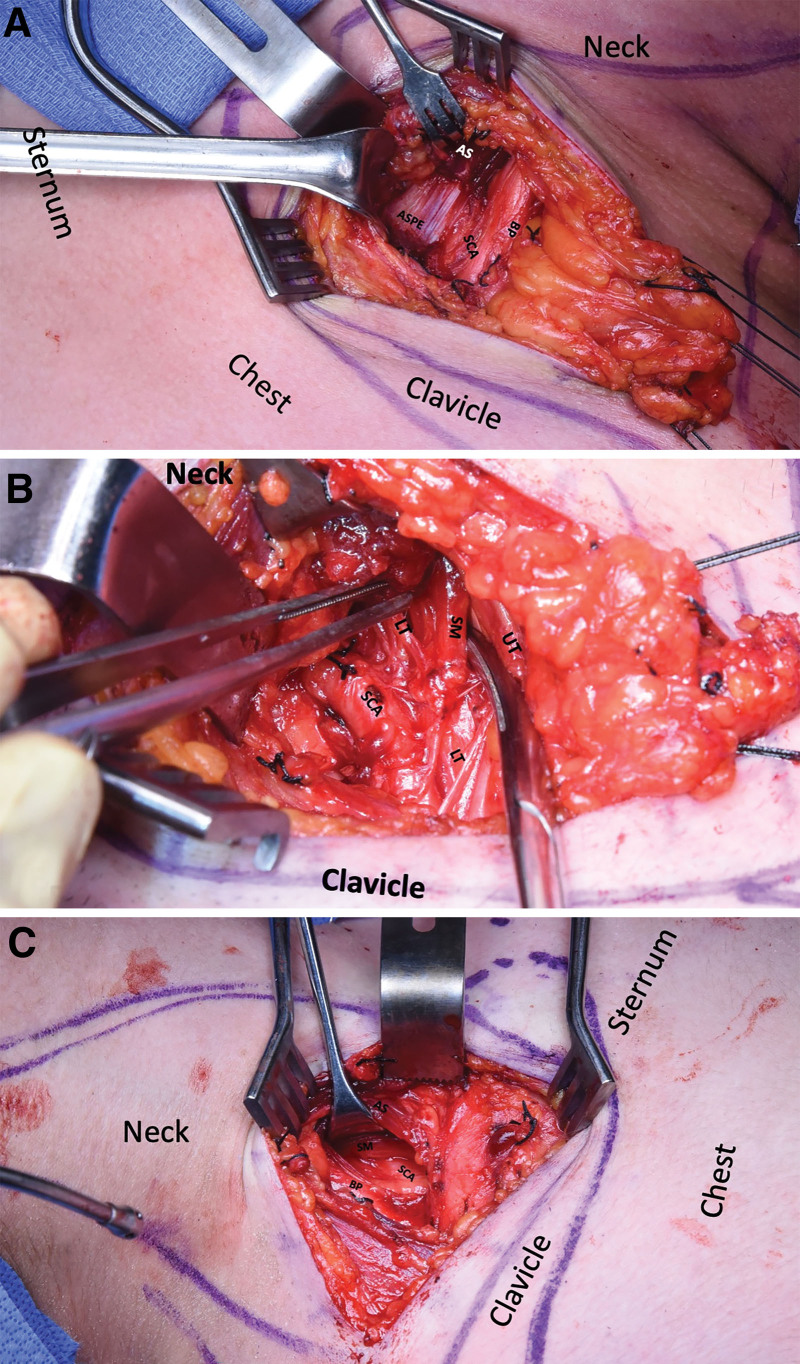
Intraoperatively, all patients had at least one structural abnormality: fibrous band at the posterior edge of the AS muscle, scalenus minimus (SM), and Roo bands (Fig. 2).15 [See Video 2 (online), which displays the intraoperative video of the posterior edge of the AS directly compressing the subclavian artery (SCA).] [See Video 3 (online), which displays the intraoperative video of the SM compressing the lower trunk of the brachial plexus.] There was no significant difference in the presence of fibrous bands at the posterior edge of the AS between the vascular-arterial subtypes and pure neurogenic (type A). Intraoperatively, only type B1 was found to compress the SCA. In two patients (B1 arterial variant) who had SM, the posterior edge of the AS directly compressed the SCA and the SM compressed the lower trunk (Fig. 2) (Videos 2, 3). However, when the posterior edge was found to compress the middle and/or lower trunks, it also compressed the SCA. This finding was predominantly noticed in the B1 and B3 patients.
Fig. 2.
A, Intraoperative fibrous posterior edge of AS compressing the SCA after detachment of the AS muscular insertion on the first rib; in a patient with TOS type B3. B, The same patient as in (A) after AS-scalenectomy and removal of the ASPE, note the SM compressing the LT, the brachial plexus UT, is retracted laterally. C, Intraoperative posterior edge of AS without compressing the SA in a patient with TOS type A. Note the presence of SM traversing the SCA underneath the AS; the BP is retracted laterally. ASPE, posterior edge of AS; LT, lower trunk; UT, upper trunk.
Video 2. which displays the intraoperative video of the posterior edge of the AS directly compressing the subclavian artery (SCA).
Video 3. which displays the intraoperative video of the SM compressing the lower trunk of the brachial plexus.
DISCUSSION
In this retrospective study, we characterized patients with NTOS whose rehabilitation failed, resulting in surgical treatment. Vascular compression was identified in 55% of patients with NTOS. The combined venous-arterial variant (B3) was the most prominent neurogenic-vascular subtype, followed by the isolated arterial variant (B1) and the isolated venous variant (B2). All patients with the vascular variant (B 1, 2, 3) underwent a TOS rehabilitation trial that was discontinued due to exacerbation of their neurogenic symptoms; however, about 20% of the patients with pure NTOS type A improved with a rehabilitation trial and were not included in this study. Hence, the presence of preoperative middle and lower trunk symptoms combined with a concave deformity of the AS muscle on sagittal T1 MRI suggest the presence of at least one structural anomaly. When combined with positive findings of vascular compression on plethysmography and US duplex of the subclavian vein compression, the rehabilitation trial is likely to fail, and surgical decompression is warranted.
The thoracic outlet includes three spaces: the scalene triangle, the costoclavicular space and the subcoracoid space.14 Narrowing of the scalene triangle is associated with either neurogenic and/or arterial symptoms. Additional venous compression usually occurs at the costoclavicular space. The subcoracoid space is another pinching point where compression by the pectoralis minor is associated with neurogenic-vascular symptoms. Delineating the potential site of compression relies on physical examination including directed pressure over the scalene triangle or subcoracoid space together with the provocation maneuvers.14 US duplex, photoplethysmography, and US neurography aid in the localization of the primary pinching site; however, all three spaces might be involved, and thus, in the presence of positive provocative test over the subcoracoid space, pectoralis minor tenotomy is advocated in NTOS decompression first rib resection.
Vascular components of NTOS are poorly addressed in the literature.10,16 Our findings are in line with Molina and D’Cunha10 who demonstrated the presence of arterial impingement in 51% and venous impingement in 11% of cases. However, we identified another subgroup in which both arterial and venous impingement (B3) occurred. Interestingly, in the arterial subtype (B1), a concave deformity of the AS on preoperative sagittal T1 sequence was found in all patients, suggesting the scalene triangle as the location of the primary pathology. This is compared with patients with subtypes B3 (58%) and B2 (60%), where the pathology might also be localized to the costoclavicular space, either because of abnormal insertion of the AS,17 or narrowing of the costoclavicular space, as evidenced by decreased venous flow on duplex subclavian vein compression US with the costoclavicular maneuver.
Conservative management, such as physical therapy and scalene blocks, is contraindicated in arterial thoracic outlet patients, as chronic arterial compression increases endothelial wall damage and risks SCA occlusion injury. Our NTOS patients, particularly the vascular (B) subtypes, experienced worsening of their symptoms with ongoing preoperative rehabilitation, suggesting a similar mechanism of injury to the brachial plexus, as opposed to some of type A (pure neurogenic group). The long-standing compression of the brachial plexus by SM, Roo bands, or AS anomaly induces chronic inflammation from repetitive microtrauma with subsequent fibrosis and scarring of the extravascular space and brachial plexus, thereby creating a vicious cycle of repeated microtrauma, fibrosis, compression, and pain.
It is still the choice of some surgeons to perform primary operations with scalenectomy and brachial plexus neurolysis alone.18,19 In a case series of 90 patients undergoing reoperation for NTOS recurrence, Gadiwalla et al20 reported complete retained first rib in 48% of patients versus 12% who did not have any residual bony or muscle structures. Our findings support their conclusion regarding the need for thoracic outlet decompression, combined with first rib resection, to decrease the failure rates in patients with NTOS. Our study suggests that first rib resection may be highly recommended when vascular compression is preoperatively evident, particularly in the venous (B2) and venous-arterial (B3) subtypes. Moreover, in all group types, more than one structural anomaly was noticed intraoperatively (Table 3). Therefore, performing scalenectomy entails increased risk for postoperative symptom recurrence. The presence of concave deformity on sagittal T1 MRI preoperatively suggests compression of the SCA and the plexus by one or more of the above-mentioned structures due mainly to their abnormal insertion in the first rib. This fact should not be confused with the intraoperative findings of AS fibrous edge in the pure neurogenic group (type A) as AS abnormal insertion is another factor that must be present to exert compression of the SCA.
As a rule, a trial of preoperative rehabilitation should be attempted in patients with NTOS; however, it is unlikely to succeed in the presence of a vascular pinching component. The presence of CDAS on sagittal T1 MRI predicts symptoms worsening with ongoing rehabilitation trial and makes surgical decompression inevitable.
CONCLUSIONS
NTOS presents as four subtypes: pure neurogenic (A), arterial (B1), venous (B2), and venous-arterial (B3). The presence of preoperative middle/lower trunk symptoms combined with (1) positive US Doppler compression of the SVA, SCV, and (2) sagittal MRI showing a concave deformity of the AS posterior edge is correlated with the presence of the vascular form of NTOS secondary to structural anomalies. Preoperative rehabilitation may be attempted; however, it is unlikely to alleviate symptoms without surgical decompression and first rib resection.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
PATIENT CONSENT
Written informed consent was obtained from all patients for their anonymized information to be published in the study.
ETHICAL APPROVAL
Hadassah University Medical Center does not require ethical approval for reporting individual cases or case series.
Supplementary Material
Footnotes
Published online 10 October 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
Drs. El-Haj and Beyth contributed equally to this work.
REFERENCES
- 1.Klaassen Z, Sorenson E, Tubbs RS, et al. Thoracic outlet syndrome: a neurological and vascular disorder. Clin Anat. 2014;27:724–732. [DOI] [PubMed] [Google Scholar]
- 2.Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev. 2014;2014:CD007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urschel HC, Jr., Razzuk MA. Neurovascular compression in the thoracic outlet: changing management over 50 years. Ann Surg. 1998;228:609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilbey JH, Muller NL, Connell DG, et al. Thoracic outlet syndrome: evaluation with CT. Radiology. 1989;171:381–384. [DOI] [PubMed] [Google Scholar]
- 5.Henry BM, Vikse J, Sanna B, et al. Cervical rib prevalence and its association with thoracic outlet syndrome: a meta-analysis of 141 studies with surgical considerations. World Neurosurg. 2018;110:e965–e978. [DOI] [PubMed] [Google Scholar]
- 6.Expert Panels on Vascular Imaging Thoracic Imaging, Neurological Imaging, Zurkiya O, Ganguli S, et al. ACR appropriateness criteria(R) thoracic outlet syndrome. J Am Coll Radiol. 2020;17:S323–S334. [DOI] [PubMed] [Google Scholar]
- 7.Singh VK, Jeyaseelan L, Kyriacou S, et al. Diagnostic value of magnetic resonance imaging in thoracic outlet syndrome. J Orthop Surg (Hong Kong). 2014;22:228–231. [DOI] [PubMed] [Google Scholar]
- 8.Colon E, Westdorp R. Vascular compression in the thoracic outlet. Age dependent normative values in noninvasive testing. J Cardiovasc Surg (Torino). 1988;29:166–171. [PubMed] [Google Scholar]
- 9.Warrens AN, Heaton JM. Thoracic outlet compression syndrome: the lack of reliability of its clinical assessment. Ann R Coll Surg Engl. 1987;69:203–204. [PMC free article] [PubMed] [Google Scholar]
- 10.Molina JE, D’Cunha J. The vascular component in neurogenic-arterial thoracic outlet syndrome. Int J Angiol. 2008;17:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balderman J, Holzem K, Field BJ, et al. Associations between clinical diagnostic criteria and pretreatment patient-reported outcomes measures in a prospective observational cohort of patients with neurogenic thoracic outlet syndrome. J Vasc Surg. 2017;66:533–544.e2. [DOI] [PubMed] [Google Scholar]
- 12.Illig KA, Donahue D, Duncan A, et al. Reporting standards of the Society for Vascular Surgery for thoracic outlet syndrome. J Vasc Surg. 2016;64:e23–e35. [DOI] [PubMed] [Google Scholar]
- 13.Caputo FJ, Wittenberg AM, Vemuri C, et al. Supraclavicular decompression for neurogenic thoracic outlet syndrome in adolescent and adult populations. J Vasc Surg. 2013;57:149–157. [DOI] [PubMed] [Google Scholar]
- 14.Ohman JW, Thompson RW. Thoracic outlet syndrome in the overhead athlete: diagnosis and treatment recommendations. Curr Rev Musculoskelet Med. 2020;13:457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harry WG, Bennett JD, Guha SC. Scalene muscles and the brachial plexus: anatomical variations and their clinical significance. Clin Anat. 1997;10:250–252. [DOI] [PubMed] [Google Scholar]
- 16.Likes K, Rochlin DH, Call D, et al. Coexistence of arterial compression in patients with neurogenic thoracic outlet syndrome. JAMA Surg. 2014;149:1240–1243. [DOI] [PubMed] [Google Scholar]
- 17.Tucker DL, Freischlag JA. Anterior scalene anomaly in a patient With arterial and neurogenic thoracic outlet syndrome. Vasc Endovascular Surg. 2022;57:295–298. [DOI] [PubMed] [Google Scholar]
- 18.Dellon AL. The results of supraclavicular brachial plexus neurolysis (without first rib resection) in management of post-traumatic “thoracic outlet syndrome.” J Reconstr Microsurg. 1993;9:11–17. [DOI] [PubMed] [Google Scholar]
- 19.Sessions RT. Reoperation for thoracic outlet syndrome. J Cardiovasc Surg (Torino). 1989;30:434–444. [PubMed] [Google Scholar]
- 20.Gadiwalla Q, Dong S, Recarey M, et al. Recurrent arterial and new-onset neurogenic thoracic outlet syndrome as a complication after previously inadequately excised first and cervical ribs. J Vasc Surg Cases Innov Tech. 2022;8:328–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.