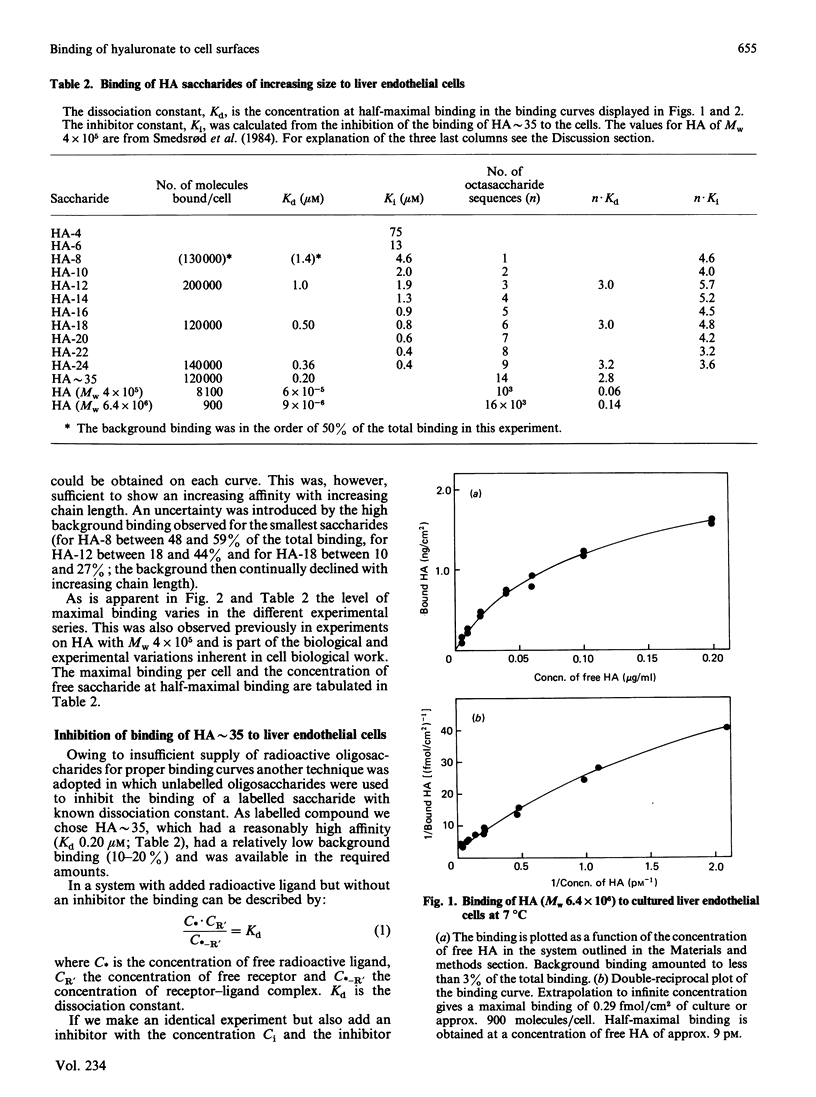
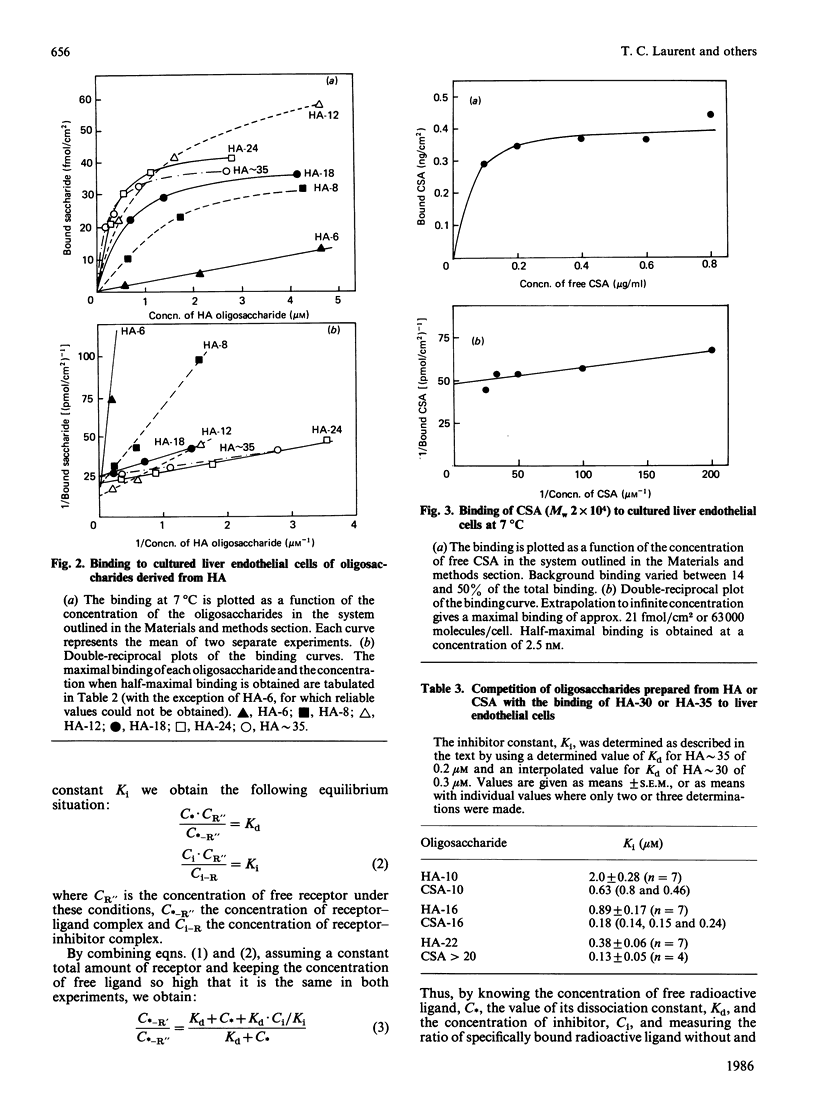
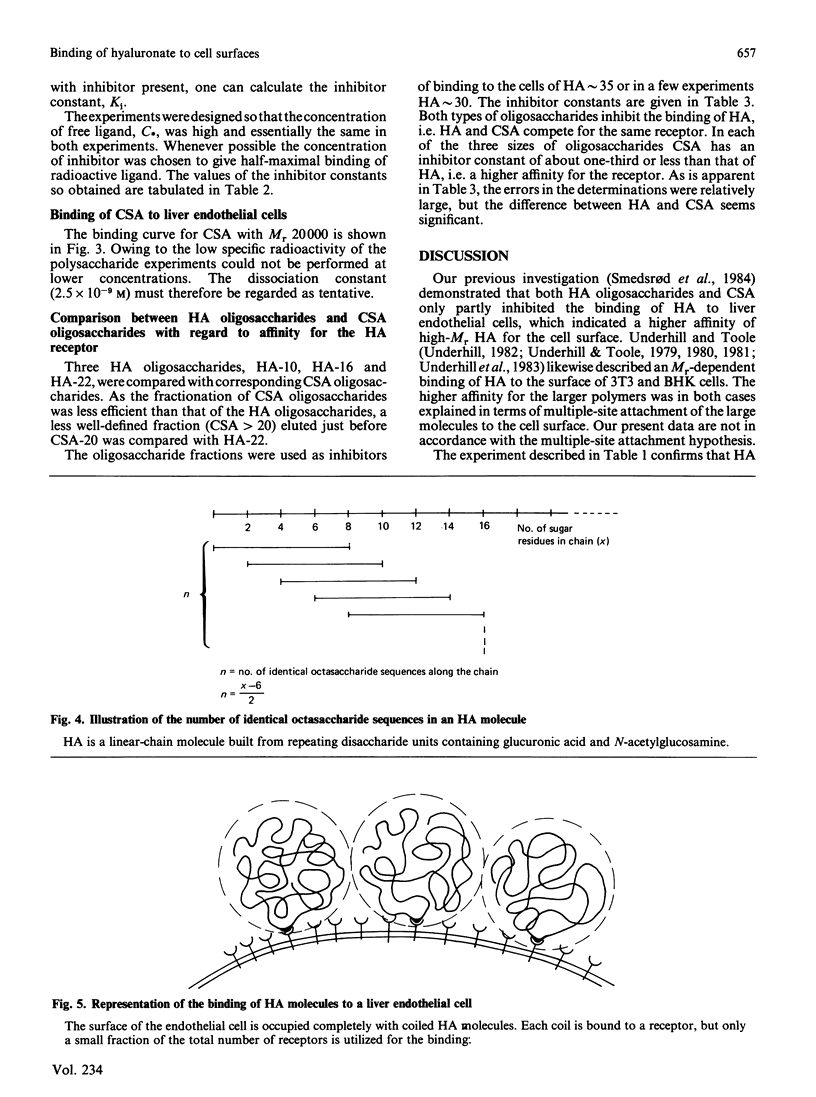
Abstract
Hyaluronate is taken up and metabolized in liver endothelial cells by means of a receptor. To characterize the interaction with the receptor, two preparations of 3H-labelled hyaluronate, of Mr 4 X 10(5) and 6.4 X 10(6), and a series of hyaluronate oligosaccharides were bound to cultured liver endothelial cells at 7 degrees C. The dissociation constant varied between 4.6 X 10(-6) M for an octasaccharide and 9 X 10(-12) M for the largest polymer. The Mr-dependence for the series of oligosaccharides was explained by the increased probability of binding due to the repetitive sequence along the chain. The high affinity of high-Mr hyaluronate for the receptor could also be mainly ascribed to this effect, which rules out any major contribution of co-operative multiple-site attachment to the cell surface. Each liver endothelial cell can bind 10(5) oligosaccharides, about 10(4) molecules with Mr 4 X 10(5) and about 10(3) molecules with Mr 6.4 X 10(6). This is explained by mutual exclusion of large molecules from the cell surface. Chondroitin sulphate is also bound to liver endothelial cells. Inhibition studies showed that it binds to the same receptor as hyaluronate and with an affinity that is about 3-fold higher than that of hyaluronate of the same degree of polymerization.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Fraser J. R., Laurent T. C., Pertoft H., Smedsrød B. Endothelial cells are a site of uptake and degradation of hyaluronic acid in the liver. Exp Cell Res. 1983 Mar;144(1):223–228. doi: 10.1016/0014-4827(83)90458-5. [DOI] [PubMed] [Google Scholar]
- Fraser J. R., Alcorn D., Laurent T. C., Robinson A. D., Ryan G. B. Uptake of circulating hyaluronic acid by the rat liver. Cellular localization in situ. Cell Tissue Res. 1985;242(3):505–510. doi: 10.1007/BF00225415. [DOI] [PubMed] [Google Scholar]
- Fraser J. R., Laurent T. C., Pertoft H., Baxter E. Plasma clearance, tissue distribution and metabolism of hyaluronic acid injected intravenously in the rabbit. Biochem J. 1981 Nov 15;200(2):415–424. doi: 10.1042/bj2000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURENT T. C., GERGELY J. Light scattering studies on hyaluronic acid. J Biol Chem. 1955 Jan;212(1):325–333. [PubMed] [Google Scholar]
- LAURENT T. C. Studies on hyaluronic acid in the vitreous body. J Biol Chem. 1955 Sep;216(1):263–271. [PubMed] [Google Scholar]
- Laurent U. B., Granath K. A. The molecular weight of hyaluronate in the aqueous humour and vitreous body of rabbit and cattle eyes. Exp Eye Res. 1983 Apr;36(4):481–492. doi: 10.1016/0014-4835(83)90042-8. [DOI] [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- Smedsrød B., Kjellén L., Pertoft H. Endocytosis and degradation of chondroitin sulphate by liver endothelial cells. Biochem J. 1985 Jul 1;229(1):63–71. doi: 10.1042/bj2290063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedsrød B., Pertoft H., Eriksson S., Fraser J. R., Laurent T. C. Studies in vitro on the uptake and degradation of sodium hyaluronate in rat liver endothelial cells. Biochem J. 1984 Nov 1;223(3):617–626. doi: 10.1042/bj2230617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedsrød B., Pertoft H. Preparation of pure hepatocytes and reticuloendothelial cells in high yield from a single rat liver by means of Percoll centrifugation and selective adherence. J Leukoc Biol. 1985 Aug;38(2):213–230. doi: 10.1002/jlb.38.2.213. [DOI] [PubMed] [Google Scholar]
- Underhill C. B., Chi-Rosso G., Toole B. P. Effects of detergent solubilization on the hyaluronate-binding protein from membranes of simian virus 40-transformed 3T3 cells. J Biol Chem. 1983 Jul 10;258(13):8086–8091. [PubMed] [Google Scholar]
- Underhill C. B. Interaction of hyaluronate with the surface of simian virus 40-transformed 3T3 cells: aggregation and binding studies. J Cell Sci. 1982 Aug;56:177–189. doi: 10.1242/jcs.56.1.177. [DOI] [PubMed] [Google Scholar]
- Underhill C. B., Toole B. P. Binding of hyaluronate to the surface of cultured cells. J Cell Biol. 1979 Aug;82(2):475–484. doi: 10.1083/jcb.82.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill C. B., Toole B. P. Physical characteristics of hyaluronate binding to the surface of simian virus 40-transformed 3T3 cells. J Biol Chem. 1980 May 25;255(10):4544–4549. [PubMed] [Google Scholar]
- Underhill C. B., Toole B. P. Receptors for hyaluronate on the surface of parent and virus-transformed cell lines: binding and aggregation studies. Exp Cell Res. 1981 Feb;131(2):419–423. doi: 10.1016/0014-4827(81)90248-2. [DOI] [PubMed] [Google Scholar]
- Wasteson A. Properties of fractionated chondroitin sulphate from ox nasal septa. Biochem J. 1971 May;122(4):477–485. doi: 10.1042/bj1220477. [DOI] [PMC free article] [PubMed] [Google Scholar]


