Abstract
Mitochondrial dysfunction has been implicated in numerous common diseases as well as aging and plays an important role in the pathogenesis of sensorineural hearing loss (SNHL). In the current study, we showed that supplementation with germanium dioxide (GeO2) in CBA/J mice resulted in SNHL due to the degeneration of the stria vascularis and spiral ganglion, which were associated with down-regulation of mitochondrial respiratory chain associated genes and up-regulation in apoptosis associated genes in the cochlea. Supplementation with taurine, coenzyme Q10, or hydrogen-rich water, attenuated the cochlear degeneration and associated SNHL induced by GeO2. These results suggest that daily supplements or consumption of antioxidants, such as taurine, coenzyme Q10, and hydrogen-rich water, may be a promising intervention to slow SNHL associated with mitochondrial dysfunction.
Keywords: Germanium dioxide, Hearing loss, Mitochondrial dysfunction, Taurine, Coenzyme Q10, Hydrogen water
1. Introduction
Mitochondria are the primary organ generating cellular adenosine triphosphate (ATP) and play a central role in a variety of cellular processes, including calcium signaling, reactive oxygen spices (ROS) generation, and apoptosis (Yamasoba et al., 2007). Based on these important roles, impairment of mitochondrial function has been implicated in numerous common diseases and conditions, such as cardiovascular disease, neurodegenerative diseases, metabolic disorders, and even in normal aging (Wang et al., 2016).
Mitochondrial dysfunction is typically associated with sensorineural hearing loss (SNHL). For example, in humans, several mutations and deletion in mitochondrial DNA (mtDNA) have been reported to cause both syndromic and non-syndromic forms of SNHL. Further, patients with age-related hearing loss (ARHL) have a significant load of acquired mtDNA mutations in their auditory tissues (Gopinath et al., 2009). In animal models, accumulation of mtDNA mutations by the mutator allele of the mitochondrial Polg DNA polymerase has shown ARHL acceleration (Kujoth et al., 2005). Even in acute SNHL, such as noise- and aminoglycoside-induced hearing loss, impairment of mitochondrial function has been shown to play an important role (Böttger and Schacht, 2013; Fujimoto and Yamasoba, 2019). Considering these findings, establishing a good animal model presenting mitochondrial dysfunction with hearing loss and discovering the methods for preventing the symptoms occurring in these animals seems to play an important role in overcoming many SNHL diseases as well as many other systemic diseases.
It has been demonstrated that chronic intake of germanium dioxide (GeO2) both in humans and animal models causes symptoms and pathological findings similar to those in patients with mitochondrial encephalomyopathy, which is known as mtDNA mutation disease (Higuchi et al., 1989; Takeuchi et al., 1992; Asaka et al., 1995; Kim et al., 1998; Higuchi et al., 1991; Sanai et al., 1990; Wu et al., 1992; Li et al., 2001; Lin et al., 2006). For example, the skeletal muscles from rats treated with GeO2 for 23 weeks contained numerous ragged-red fibers and cytochrome-c oxidase (COX)-deficient fibers and showed reduced enzyme activities in the mitochondrial respiratory chain, such as rotenone-sensitive NADH-cytochrome-c reductase and COX (Higuchi et al., 1991). These results suggest that GeO2 administration can reproduce several pathological conditions caused by mitochondrial dysfunction, which may be useful in elucidating diseases associated with mitochondrial dysfunction and their treatment.
Moreover, we have previously reported that diet supplemented with 0.5% GeO2 caused profound SNHL associated with degeneration of the stria vascularis and supporting cells in guinea pigs (Yamasoba et al., 2006). This result indicated that GeO2 application would be also used to create SNHL animal with mitochondrial dysfunction. Although we speculated that cochlear degeneration caused by GeO2 intake was associated with mitochondrial damage, mitochondrial function was not investigated in this previous study.
ROS are known to be closely related to mitochondrial dysfunction. ROS are continuously produced via normal metabolism by the electron transport chain in mitochondria. In normal status, cells effectively remove ROS by their innate ROS defense systems, such as superoxide dismutase (SOD), catalase, and glutathione (Finkel and Holbrook, 2000; Raha and Robinson, 2000; Dereköy et al., 2004). However, uncontrolled leakage of ROS by irregular respiratory chain and/or decrease in the defense systems can lead to cellular dysfunction. It has been shown that the lack of SOD1 or glutathione peroxidase resulted in severe hearing loss or higher susceptibility to noise exposure, which causes excessive ROS production and induces damage to the outer and inner hair cells (Ohlemiller et al., 2000). These reports suggest that daily dietary intake of ROS scavengers may augment the defense system against ROS and thereby prevent cellular damage caused by mitochondrial damage or dysfunction.
In the current study, we investigated whether chronic intake of GeO2 results in cochlear mitochondrial impairment and associated SNHL in CBA/J mice. Next, we investigated the effects of ROS scavengers, taurine, coenzyme Q10 (CoQ10), or hydrogen-rich water (Huxtable, 1992; Erdem et al., 2000; Qiao et al., 2015; Koh et al., 2014; Das et al., 2009; Manna et al., 2009; Alam and Hafiz, 2011; Roy and Sil, 2012; M Sikorska et al., 2014; M Sikorska et al., 2014; Sohet et al., 2009; Someya et al., 2009; Yamada et al., 2015; Ohsawa et al., 2008; Sato et al., 2008; Ohsawa et al., 2007; Hayashida et al., 2008; Yoshida et al., 2012; Fukuda et al., 2007; Nakashima-Kamimura et al., 2009; Lin et al., 2011; Fransson et al., 2021), on cochlear degeneration and SNHL induced by GeO2.
2. Material and methods
Female CBA/J mice were purchased from CLEA Japan (Tokyo, Japan). The experimental protocol was approved by the Committee for the Use and Care of Animals at the University of Tokyo and conformed to the NIH Guidelines for the Care and Use of Laboratory Animals.
2.1. Experimental protocols
2.1.1. Experiment 1: development and analysis of mouse model of progressive hearing loss by chronic oral intake of GeO2
Ten 2-month-old CBA/J mice were used. Five of them were given chow containing 0.15% GeO2 for 4 months. The amount of GeO2 was determined from a previous report using rats (Wu et al., 1992). The remaining five animals were given the normal chow serving as control. In the preliminary experiment, auditory brainstem response (ABR) thresholds were measured at 0, 2, 3 and 4 months at 2, 4, 8, and 16 kHz (Supplemental Figure 1). Animals given GeO2 showed increase of ABR thresholds and became profoundly deaf at 4 months. Therefore, histological changes and gene expression were evaluated 4 months after the start of germanium administration. The left cochlea, muscle, and kidney was fixed with 2% PFA and 2.5% glutaraldehyde, the cochlea was additionally decalcified, and embedded in epoxy resin. Ultrathin sections were examined under transmission electron microscope. The right cochleae were used for gene transcriptional analysis of the cochlea by DNA micro array.
Another 10 two-month-old CBA/J mice were used to confirm gene expression by quantitative reverse transcription-polymerase chain reaction (qRT-PCR). Five animals were given chow containing 0.15% GeO2 and the remaining Five animals were given the normal chow for 4 months. The cochleae were dissected and RNA was extracted.
2.1.2. Experiment 2: prevention of GeO2 –induced cochlear damage by oral intake of ROS scavengers
Two-month-old CBA/J mice that showed auditory brainstem response thresholds within the normal laboratory range were used. Forty animals were given chow containing 0.15% GeO2 for 3 months and assigned to one of the four groups (n = 10 each) according to the content of their drinking water: 1) water without antioxidant; 2) water containing 0.3% taurine (Wako Inc., Osaka, Japan); 3) water containing 150 μM water-soluble CoQ10 (Aqua Q10L10, Nisshin Pharma Inc., Tokyo, Japan); and 4) hydrogen water (Blue Mercury, Tokyo, Japan). The hydrogen water was placed in a closed glass vessel and changed every other day, which minimized the leakage of hydrogen from the water and maintained the concentration to be greater than 0.4 mM 1 day later (Ohsawa et al., 2007), and the remaining 10 animals were fed with normal chow and water as a control. Amounts of chow that each group ate were measured to confirm that there was no difference of the eating amounts among groups. Body weight of each animal was also measured before they were euthanized.
The 3-month ABR measurement took longer than usual to obtain the ABR threshold, and several animals died during the ABR measurement due to additional anesthesia. As a result, the final numbers analyzed were 10, 7, 9, 6, and 7 animals for the control, GeO2±normal water, GeO2±taurine, GeO2±hydrogen, and GeO2±CoQ10 groups, respectively. In this experiment, the significant protective effect of the ROS scavenger on GeO2 was confirmed by 3 months, so we decided to euthanize the animals at 3 months instead of 4 months to avoid further discomfort to the animals, to prevent further sample loss, and to reduce the expense of the agents.
2.2. Assessment of hearing function
Detailed protocols for ABR measurements have been described elsewhere (Kinoshita et al., 2013). Briefly, two examiners who were blinded to the experiment and measured ABRs with a tone burst stimulus (2, 4, 8, 16, and 32 kHz) using an ABR recording system (Neuropack Σ MEB5504, Nihon Kohden, Tokyo, Japan). Mice were anesthetized with a mixture of xylazine hydrochloride (10 mg/kg, i.m.) and ketamine hydrochloride (40 mg/kg, i.m.). Needle electrodes were placed subcutaneously at the vertex (active electrode), beneath the left pinna (reference electrode), and beneath the right ear (ground). The sound stimulus consisted of a 15-ms tone burst, with a rise-fall time of 1 ms at frequencies of 2, 4, 8, 16, and 32 kHz. The sound intensity varied in 5-dB intervals near threshold. To obtain a waveform, 1024 tone presentations given at the rate of 17/s were averaged with the Neuropack MEB-2208 evoked potential measuring system (Nihon Kohden, Tokyo, Japan). The threshold was defined as the lowest intensity level at which a clear reproducible wave V could be observed in the trace. When an ABR waveform could not be evoked, the threshold was determined to be 110 dB SPL (5 dB greater than the maximum intensity (105 dB SPL) produced by the system). ABR thresholds were measured at 2 and 6 months of age in experiment 1 and 2 and 5 months of age in experiment 2.
2.3. Transmission electron microscopic observation of the cochlea, kidneys, and soleus muscles in animals given GeO2
In experiment 1, animals were euthanized at the age of 6 months after the last ABR measurements. The left cochlea, muscle, and kidney were fixed with 2% paraformaldehyde and 2.5% glutaraldehyde, cochlea was additionally decalcified, and embedded in epoxy resin. Ultrathin sections were examined under transmission electron microscope.
2.4. Histological analysis of the cochlea under light microscope
In experiment 2, the cochlear pathology was examined under light microscope. Detailed preparation and examination protocols for determining cochlear pathology have been described previously (Lin et al., 2006; Kinoshita et al., 2013). Briefly, all animals were euthanized under deep anesthesia with xylazine hydrochloride and ketamine hydrochloride at the age of 5 months. The left cochlea was immersed in 4% paraformaldehyde in 0.1 M phosphate-buffered saline overnight at 4 °C and decalcified in 10% ethylenediaminetetraacetic acid solution. The specimens were then dehydrated through a graded alcohol series and embedded in paraffin. The embedded tissues were cut into 5-μm thick sections parallel to the modiolus, and two sequential sections were mounted on glass slides and deparaffinized. Five sections at an interval of three slides (i.e., at an interval of approximately 30-μm) were stained with hematoxylin and eosin and observed under a light microscope (Nikon Eclipse E800M, Tokyo, Japan, 40× objective) to evaluate spiral ganglion cell (SGC) densities and stria vascularis degeneration in the lower-basal turn.
The number of SGCs and the area of Rosenthal’s canal of the lower-basal turn were measured using Photoshop CS4 software, and SGC density (SGC number/mm2) was calculated, as previously reported (S Someya et al., 2007). In brief, the number of SGCs in each profile were counted with computer monitors. The area of the Rosenthal’s canal profile was determined in each photomicrograph by outlining the margin of bony canal using ‘Select’ tool. The number of the pixel of the Rosenthal’s canal was measured using ‘Histogram’ tool. The pixels were then converted to the area by calculating the number of pixels per unit area. The density of SGC was calculated for each profile of the ganglion as the number of SGCs divided by the area of Rosenthal’s canal (mm2).
The area of the stria vascularis of the lower-basal turns was measured in digital photomicrographs using Photoshop CS4 software. The proportions of affected areas were also measured in digital photomicrographs. From these data, degeneration rate was calculated by the vacuolar degenerated area divided by the total area of stria vascularis.
2.5. Gene transcriptional analysis of the cochlea by DNA micro array
Detailed protocols for gene expression profiling analysis using Affymetrix microarray analysis have been described (Affymetrix 2004; Lee et al., 1999). Briefly, the right cochleae of the animals were used in this study. The cochleae were placed in a micro centrifuge tube, flash frozen in liquid nitrogen, and stored at −80 °C. Total RNA was extracted from the frozen cochleae by using the TRIzol reagent (Life Technologies, Grand Island, NY). We hybridized each sample to a single Affymetrix MOE 430A Gene Chip (Affymetrix, Santa Clara, CA). Signals in each image were normalized to minimize an overall variability in hybridization intensities by a global scaling method using the Affymetrix software as described in the previous report (S Someya et al., 2007). A gene was considered “expressed” if it displayed a “present” call in at least one GeneChip based on the Affymetrix “present/absent call” algorithms. All genes considered “not expressed” were eliminated from our analysis. To identify genes whose expression was significantly altered by GeO2, each control sample (n = 5) was compared to each GeO2 sample (n = 5), generating a total of 25 pairwise comparisons. Gene expression change was considered significant when the P value was <0.05 and the fold change was >1.2. We then used Database for Annotation, Visualization, and Integrated Discovery (DAVID) (Dennis et al., 2003) and Expression Analysis Systematic Explorer (EASE) (Hosack et al., 2003) to assign identified genes to “GO (Gene Ontology): Biological Process” categories of Gene Ontology Consortium (www.geneontology.org). We also used EASE to determine the total number of identified genes that were assigned to each Biological Process category and the total number of genes on the array in each Biological Process category and to identify “GO: Biological Process” categories statistically associated with AHL-correlated genes by performing Fisher exact tests. The Fisher exact score represents the probability that an overrepresentation of germanium-induced hearing loss-correlated genes in a certain GO: Biological Process category occurs by chance (Hosack et al., 2003). When the Fisher Exact score is < 0.05 for a given GO: Biological Process category, this gene list is considered to be specifically associated (enriched) in the Biological Process category. Gene probe sets were considered “genes” if they had been assigned a “gene symbol” annotation by DAVID.
2.6. Quantitative RT-PCR
We used the same mRNA pools for both microarray and quantitative RT-PCR analyses. Detection of mRNA was performed with an Applied Biosystems Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). Duplicate reactions for each primer set were run simultaneously in a 96-well plate using the TaqMan EZ RT-PCR kit. β-Actin was used as an internal standard. Oligonucleotide primers and MGB fluorescent probes (TaqMan Gene Expression Assays) were purchased from Applied Biosystems. Detailed protocols for analysis by qRT-PCR have been described (Someya et al., 2008). All data were reported as mean ± SEM.
2.7. Statistical analysis
Sigma Stat statistical software was used and all data were expressed as mean ± SD. ABR thresholds, HC survival rates, SGC densities, and SV thicknesses were compared among groups by one-way analysis of variance, and then pairwise comparisons were performed by using Bonferroni’s test.
3. Results
3.1. Auditory and histopathological findings of animals given GeO2
In experiment 1, CBA/J mice were orally given GeO2-containing chows for 4 months from the age of 2 months. The ABRs examined at 2 months of age before experiments were within the normal laboratory range in all 10 animals and did not differ between animals given chows with and without GeO2. At the age of 6 months, ABRs showed that animals given GeO2-containing chows developed profound hearing loss at all frequencies examined, while those given normal chows maintained normal ABR thresholds (Fig. 1).
Fig. 1.
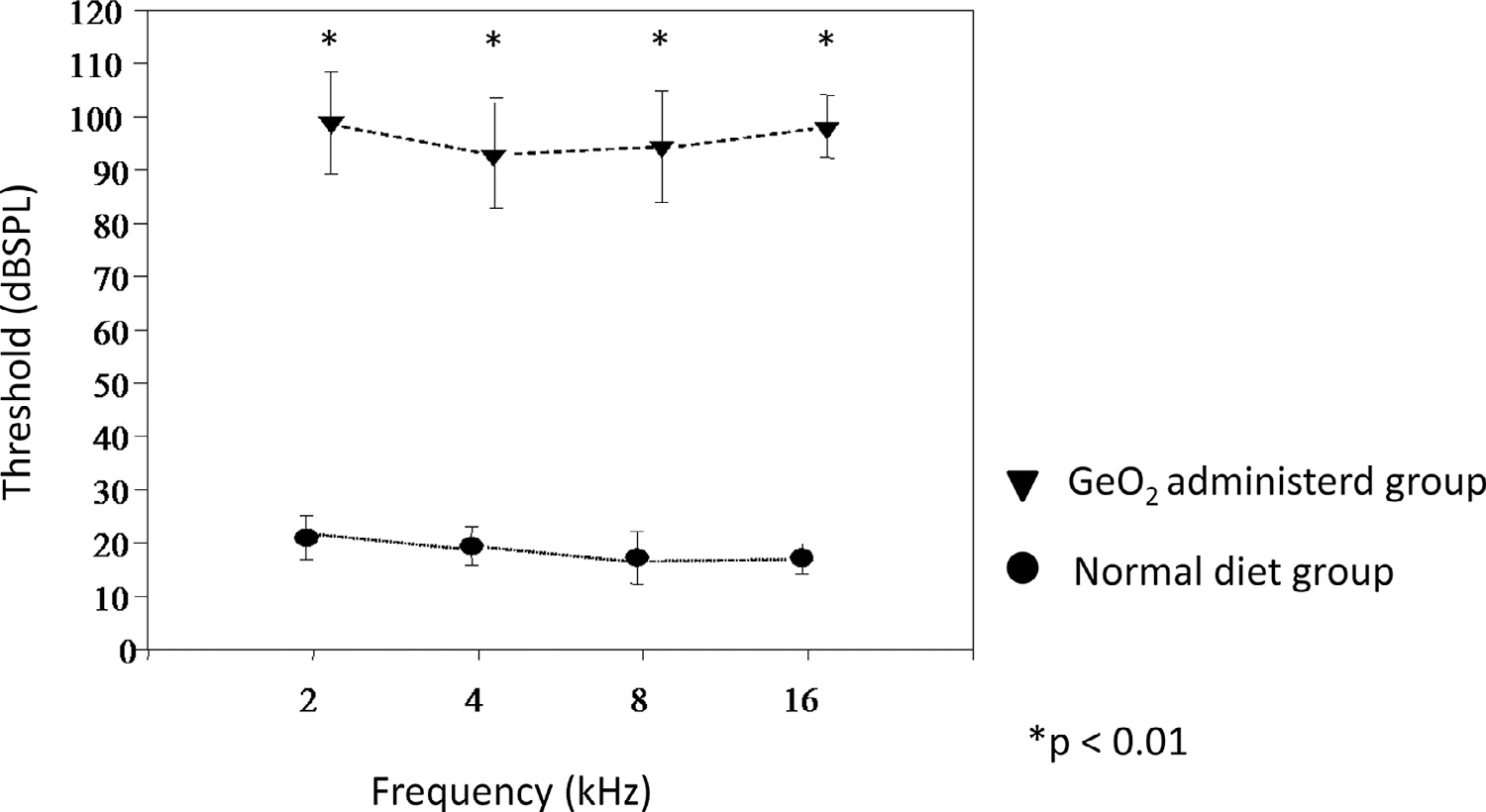
Threshold after 4 months of GeO2 treatment in CBA mice
CBA/J mice treated GeO2-containing chows for 4 months showed profound hearing loss in all frequency (triangle), while CBA mice with normal diet showed normal hearing (circle).
The histopathological examination showed that animals given GeO2-containing chows exhibited marked degeneration of the stria vascularis in almost all cochlear turns, more markedly in the lower turn (Fig. 2). Animals given normal chows did not develop any of such pathologies (data not shown).
Fig. 2.
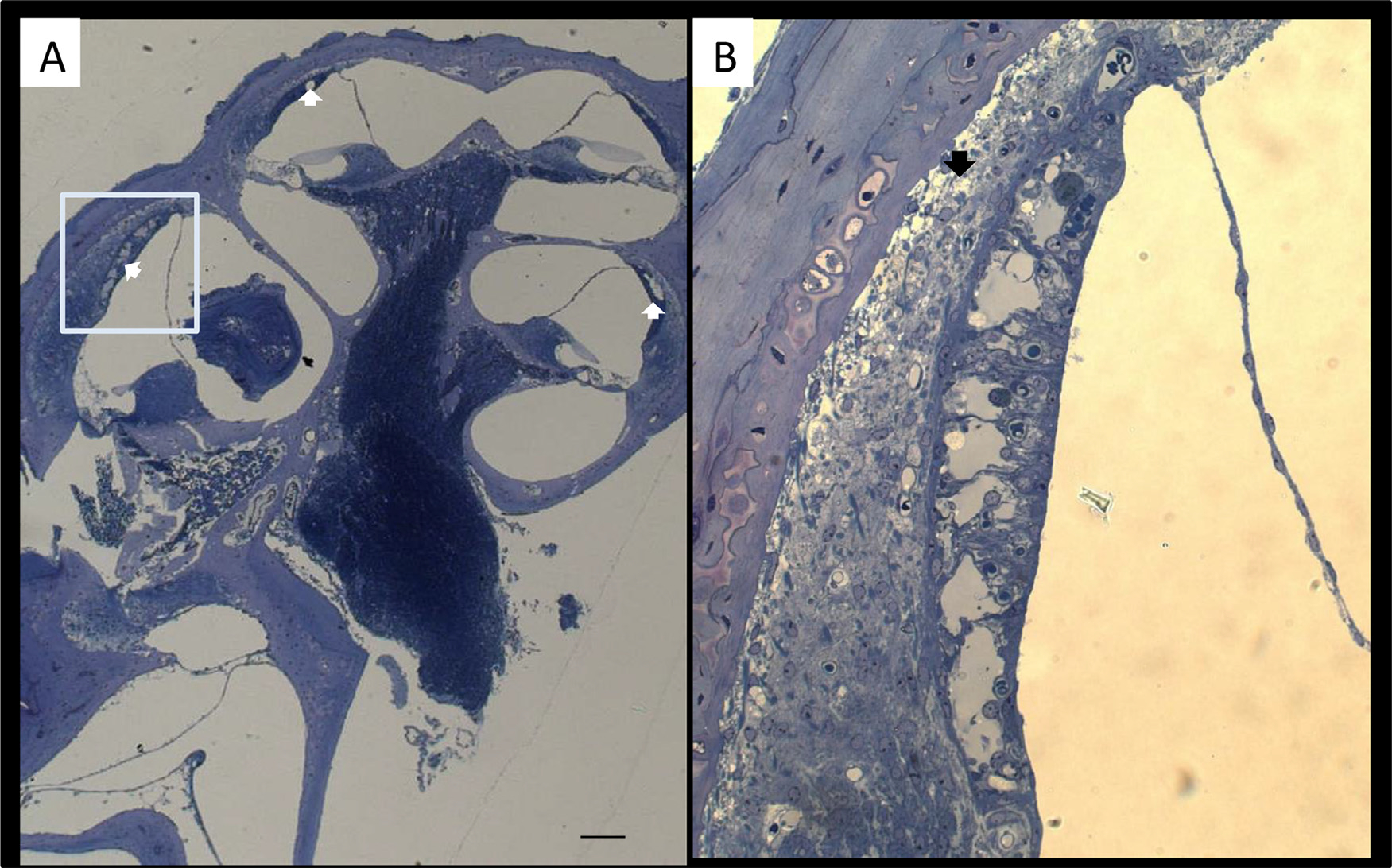
Representative light micrographs of the cochlea after administration of GeO2 for 4 months. Enlarged image of stria vascularis for captured area (B). Degeneration of the stria vascularis indicated with white arrow is seen in almost all cochlear turns, more markedly in the lower turn (A). Severe vacuolar degeneration of the stria vascularis is found in the lower basal turn (B). Bar = 100 μm in (A), 50 μm in (B).
Transmission electron microscope examination revealed marked vacuolar degeneration in the stria vascularis, where almost all mitochondria contained electron-dense inclusions. Similarly, the distal tubular epithelium of the kidney and the sole muscles showed many electron-dense deposits inside the degenerated mitochondria (Fig. 3).
Fig. 3.
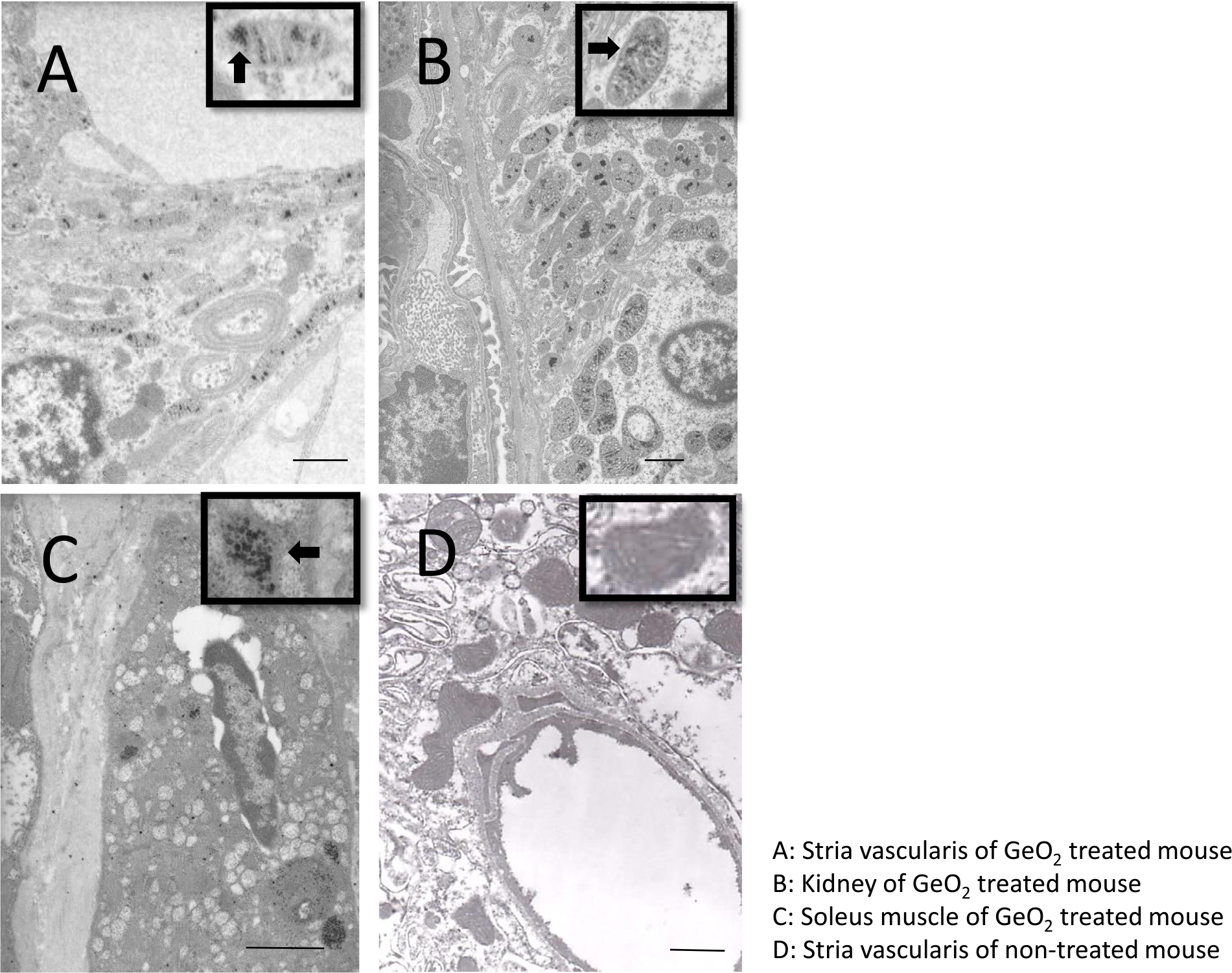
Ultrastructural findings of the intermediate cells of stria vascularis (A), kidney (B), soleus muscle(C) of GeO2 treated mice. Transmission electron microscope showed vacuolar degeneration of the stria vascularis (A). The arrow head indicates degenerated mitochondria containing electron-dense inclusion. The distal tubular epithelium of the kidney (B) and soleus muscles (C) showed many electron-dense deposits inside the degenerated mitochondria. Ultrastructural findings from a stria vascularis of non-treated mice (D).Inset: high-power view of mitochondria. andBar = 1μm.
3.2. Overview of microarray analysis
To identify genes and Biological Process categories associated with GeO2-induced hearing loss, we conducted genome-wide gene expression analysis using RNA samples isolated from the cochlear tissues of 6-month-old CBA mice (n = 5). Using Affymetrix Gene Chip, we found that 3827 gene probe sets were significantly down-regulated, and 3327 gene probe sets were significantly up-regulated in the cochlear tissues of 6-month-old mice treated with GeO2 compared to 6-month-old controls given normal chow. These significantly altered gene probe sets were further assigned to “GO: Biological Process” categories using Database for Annotation, Visualization, and Integrated Discovery (Huang da et al., 2009), which assigned a classification to 3827 of the downregulated and 3327 of the upregulated genes. A summary of the “Gene Ontology (GO): Biological Process” categories associated with germanium-induced hearing loss is shown in Table 1. The complete set of microarray data has been submitted to the GEO (Gene Expression Omnibus) repository (http://www.ncbi.nlm.nih.gov/geo/) with GEO Accession number GSE84735. The EASE analysis revealed that 16 Go: Biological Process categories, including “mitochondrion,” “mitochondrial inner membrane,” “mitochondrial electron transport chain,” “oxidative phosphorylation,” and tricarboxylic acid cycle, were significantly associated with germanium-induced mitochondrial dysfunction genes (Fisher exact score p < 0.05), and 818 out of 1863 genes in these categories on the Gene Chip were significantly downregulated in the cochleae of germanium-applied animals (Table 1).
Table 1.
Summary of the “GO: Biological Process” categories associated with germanium-induced hearing loss.
| Biological Process Categories | N | TN | EASE |
|---|---|---|---|
|
| |||
| Down-regulated (3827 classified genes) | |||
| Mitochondrion | 225 | 679 | 0.000 |
| Mitochondrial membrane | 81 | 226 | 0.000 |
| Mitochondrial envelope | 85 | 242 | 0.000 |
| Mitochondrial inner membrane | 76 | 209 | 0.000 |
| Mitochondrial electron transport chain | 14 | 27 | 0.000 |
| Mitochondrial ribosome | 17 | 39 | 0.000 |
| Mitochondrial matrix | 21 | 57 | 0.001 |
| Mitochondrial lumen | 21 | 57 | 0.001 |
| NADH dehydrogenase activity | 15 | 35 | 0.001 |
| NADH dehydrogenase (Quinone) activity | 15 | 35 | 0.001 |
| NADH dehydrogenase (Ubiquinone) activity | 15 | 35 | 0.001 |
| Tricarboxylic acid cycle | 10 | 20 | 0.004 |
| Acetyl-CoA catabolism | 10 | 21 | 0.005 |
| Oxidative phosphorylation | 17 | 51 | 0.010 |
| Acetyl-CoA metabolism | 12 | 31 | 0.012 |
| ATP binding | 184 | 99 | 0.049 |
| Up-regulated (3327 classified genes) | |||
| Transcription, DNA-dependent | 289 | 1418 | 0.000 |
| Regulation of transcription, DNA-dependent | 283 | 1397 | 0.000 |
| Ligase activity | 71 | 293 | 0.001 |
| Endocytosis | 35 | 1540 | 0.002 |
| Apoptosis | 88 | 433 | 0.018 |
| Programmed cell death | 88 | 439 | 0.025 |
Column titles: N, the number of identified genes in the category; FC, fold change; TN, the total number of genes in the category on the Gene Chip; EASE, EASE test score.
3.3. Downregulation of genes associated with germanium-induced mitochondrial dysfunction
Table 2 shows a list of down-regulated genes encoding components of the mitochondrial respiratory chain in the cochlea. Twenty-eight genes encoding components of the mitochondrial respiratory chain were found to be significantly down-regulated (P value < 0.05) (Table 2). Of these, three genes encode for components of the “respiratory chain complex I” (NADH dehydrogenase complex), including Ndufs2, Ndufs7, and Ndufv2; two genes encode for components of the “respiratory chain complex II” (succinate dehydrogenase complex), including Sdhb and Sdhc genes; two genes encode for components of the “respiratory chain complex III”, including Cyc1 and Cycs; one gene encode for components of the “respiratory chain complex IV” (cytochrome c oxidase subunits), including Cox5a; and 11 genes encode for components of the “respiratory chain complex V” (ATP synthase subunits), including Atp5k, Atp5e, and Atp5a1. The analyses of qRT-PCR were conducted for Atp5a1, Atp5e, Cox5a, Sdhb, and Sdhc, to validate the microarray results. The qRT-PCR results were in good agreement with the microarray findings that expression of these mitochondrial function-associated genes were significantly decreased in the cochleae of GeO2-treated mice (Fig. 4). These results provide the evidence that GeO2-induced hearing loss is associated with the down-regulation of genes involved in the mitochondrial respiratory chain complexes in the cochlea of CBA/J mice.
Table 2.
List of down-regulated genes encoding components of the mitochondrial respiratory chain in the cochlea.
| Gene | Gene ID | Affy ID | P Value | FC |
|---|---|---|---|---|
|
| ||||
| Oxidative Phosphorylation | ||||
| Atp5a1 | C78762 | 1,420,037_at | 0.011 | −2.028 |
| Atp5d | BC008273 | 1,423,716_s_at | 0.033 | −1.571 |
| Atp5e | NM_025983 | 1,416,567_s_at | 0.000 | −1.835 |
| Atp5g1 | NM_007506 | 1,416,020_a_at | 0.000 | −1.567 |
| Atp5g2 | NM_026468 | 1,415,980_at | 0.002 | −1.727 |
| Atp5j | NM_016755 | 1,416,143_at | 0.008 | −1.191 |
| Atp5k | AV216686 | 1,434,053_x_at | 0.004 | −1.727 |
| Atp5l | NM_013795 | 1,448,203_at | 0.009 | −1.593 |
| Atp5o | NM_138,597 | 1,416,278_a_at | 0.000 | −1.563 |
| Atp5o /// LOC432676 | AV066932 | 1,437,164_x_at | 0.005 | −1.558 |
| Atp6ap1 | AI316502 | 1,449,622_s_at | 0.000 | −1.795 |
| Cox5a | NM_007747 | 1,448,153_at | 0.025 | −1.345 |
| Cyc1 | NM_025567 | 1,416,604_at | 0.005 | −1.266 |
| Cycs | NM_007808 | 1,422,483_a_at | 0.007 | −1.235 |
| Ndufc2 | NM_024220 | 1,416,366_at | 0.000 | −2.120 |
| Ndufs7 | BC013503 | 1,451,312_at | 0.001 | −1.458 |
| Ndufv2 | AV046532 | 1,438,159_x_at | 0.003 | −1.259 |
| Tricarboxylic acid cycle | ||||
| Aco1 | BB504570 | 1,456,728_x_at | 0.003 | −1.360 |
| Aco2 | AU019938 | 1,436,934_s_at | 0.047 | −1.345 |
| Cs | AB056479 | 1,450,667_a_at | 0.022 | −1.248 |
| Dlst | BC006702 | 1,423,710_at | 0.005 | −1.616 |
| Idh3b | NM_130,884 | 1,418,886_s_at | 0.001 | −1.316 |
| Idh3g | NM_008323 | 1,416,789_at | 0.000 | −1.736 |
| Mdh2 | NM_008617 | 1,416,478_a_at | 0.000 | −1.342 |
| Polr3h | AK019868 | 1,424,227_at | 0.000 | −1.181 |
| Sdhb | BC013509 | 1,418,005_at | 0.000 | −1.387 |
| Sdhc | NM_025321 | 1,448,630_a_at | 0.012 | −1.537 |
Column titles: Gene, gene symbol; Gene ID, representative public gene ID.
Affy ID, Affymetrix probe set ID; FC, fold change.
Fig. 4.
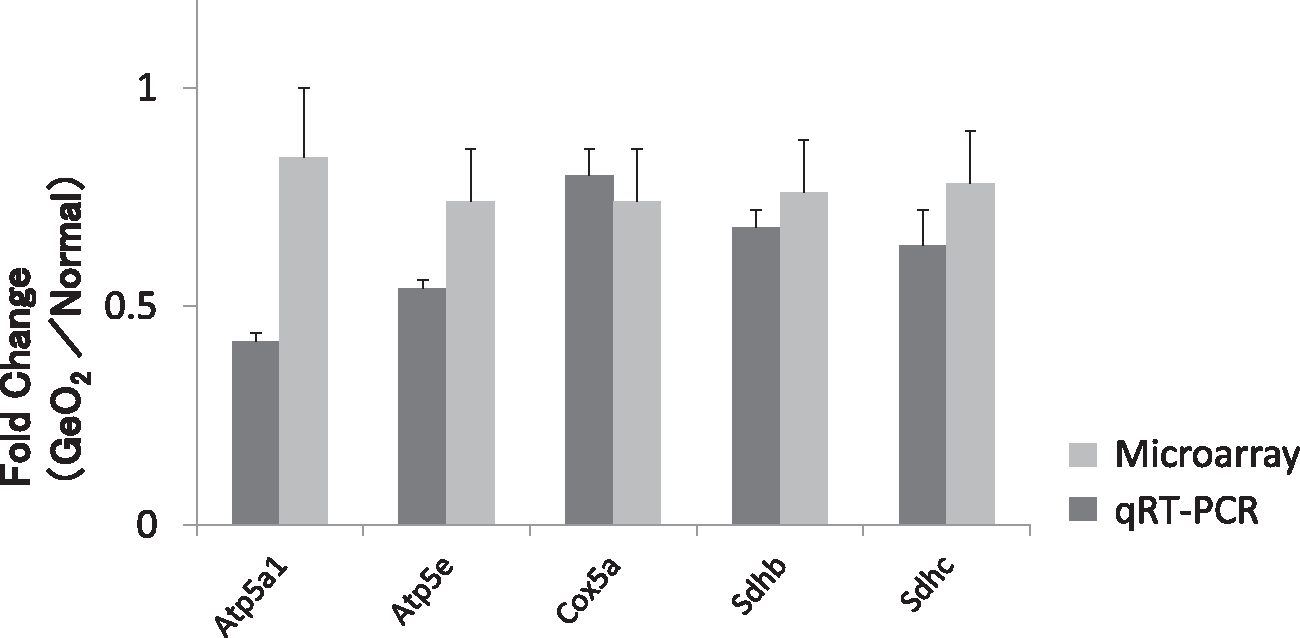
The qRT-PCR validation of microarray data. The data represent the fold change in gene expression of 6-month-old mice treated with GeO2 compared to 6-month-old controls given normal chow. The qRT-PCR analyses for Atp5a1, Atp5e, Cox5a, Sdhb, and Sdhc. The qRT-PCR results were in agreement with the microarray findings (light gray bar) that the expression of these mitochondrial function-associated genes was significantly decreased in the cochleae of germanium-treated mice (dark gray bar).
3.4. Effect of antioxidants on ABR threshold shifts induced by GeO2
The total amount of weekly dietary intake and final body weights for each group is shown in supplementary Table 1.
Animals given normal chow and water almost maintained ABR thresholds until 5 months of age, whereas animals given GeO2-containing chow and normal water from 2 months of age for 3 months showed approximately 30 to 50 dB threshold shifts. The difference of the threshold shifts was significantly different between controls and animals given GeO2+normal water at all frequencies (Fig. 5).
Fig. 5.
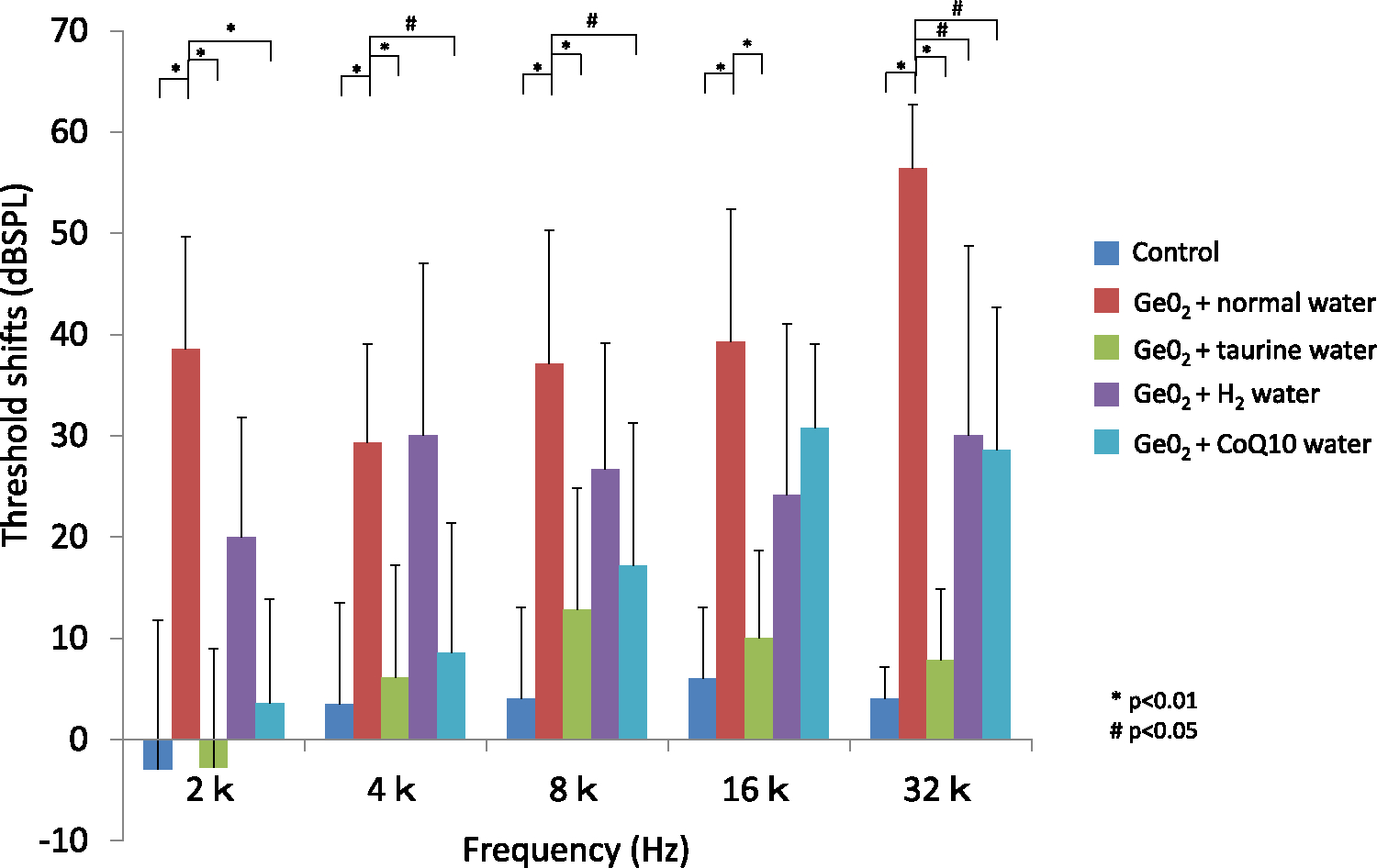
ABR threshold shifts after 3 months of GeO2 treatment
The control group showed almost no threshold shifts in all frequency. Animals given GeO2 and water without antioxidant showed significant threshold shift in all frequency from 2 to 32 kHz. The taurine supplemented group prevented the threshold shift in all frequency in the level that was nearly the same as the control group. CoQ10 group prevented a threshold shift in most of the frequency but there was a substantial threshold shift in higher frequencies compared to control animals. Hydrogen water group had a substantial threshold shift in all frequency compared to the control group but showed some protective effect in limited frequencies compared to animals given GeO2 +normal water.
ROS scavengers all provided preventive effect against GeO2-induced hearing loss, with taurine showing the strongest effect. Animal given GeO2+taurine developed only slight threshold shifts at all frequencies. The threshold shifts in animals given GeO2+taurine were significantly different (p<0.01) at all frequencies compared with those given GeO2+normal water and were not significantly different at any frequencies compared with controls. CoQ10 prevented GeO2-induced threshold shifts predominantly at lower frequencies, with substantial threshold shifts at higher frequencies. Threshold shifts in animals given GeO2+CoQ10 were significantly smaller at 2, 4, and 8 kHz (p<0.01 in 2 kHz, p<0.05 in 4 and 8 kHz) compared to animals given GeO2+normal water. Hydrogen water also provided some preventive effect, but the effect was smallest among the three ROS scavengers. The threshold shifts in animals given GeO2 and hydrogen water showed approximately 20–30 threshold shifts at all frequencies, which were significantly different from those in animals given GeO2+normal water only at 32 kHz (p<0.05). When compared among animals given GeO2 and ROS scavengers, threshold shifts in animals given taurine were significantly smaller at 2, 4, and 32 kHz (p<0.01) compared to those given hydrogen water and significantly smaller at 16 and 32 kHz (p<0.01) compared with animals given CoQ10. Threshold shifts in animals given CoQ10 were significantly smaller only at 4 kHz (p<0.05) compared to animals given hydrogen water. These data indicated that taurine has the strongest preventive effect, followed by CoQ10 and then hydrogen water.
3.5. Effect of antioxidants on degeneration of the spiral ganglion and stria vascularis induced by GeO2
The average of total area of stria vascularis at the lower basal turn for all groups are shown in Supplemental Figure 2. There were no significant differences in the average of total areas of stria vascularis among groups. Mice that did not take GeO2 showed nearly normal appearance of the stria vascularis and SGCs at the age of 5 months (data not shown), whereas mice administered GeO2+normal water for 3 months showed vacuolar degeneration of stria vascularis and severe degeneration of the SGCs mainly in the lower-basal and upper-basal turns in the cochlea.
The extent of degeneration in the stria vascularis and SGCs were significantly ameliorated (p<0.01) in animals given GeO2 and one of the antioxidants when compared to those given GeO2 + normal water (Figs. 6 and 7), indicative that all the antioxidants protected from GeO2-induced cochlear degeneration. The protective effect was most significant in taurine supplementation; there was no significant difference in the extent of degeneration either in the stria vascularis or SGCs between animals given GeO2+taurine and controls without GeO2 intake. The extent of degeneration in the stria vascularis and SGCs in animals given GeO2+hydrogen water or CoQ10 was significantly smaller (p<0.01) compared to those given GeO2+normal water, but significantly greater (p<0.01) compared to controls without GeO2 intake. When compared among animals given GeO2 and one of three antioxidants, animals given taurine showed significant protective effect against the degeneration of the stria vascularis and the SGCs when compared to those given hydrogen water or CoQ10 (p<0.001). There was no significant difference in the degeneration of either stria vascularis or the SGCs between animals given GeO2+CoQ10 and those given GeO2+hydrogen water.
Fig. 6.
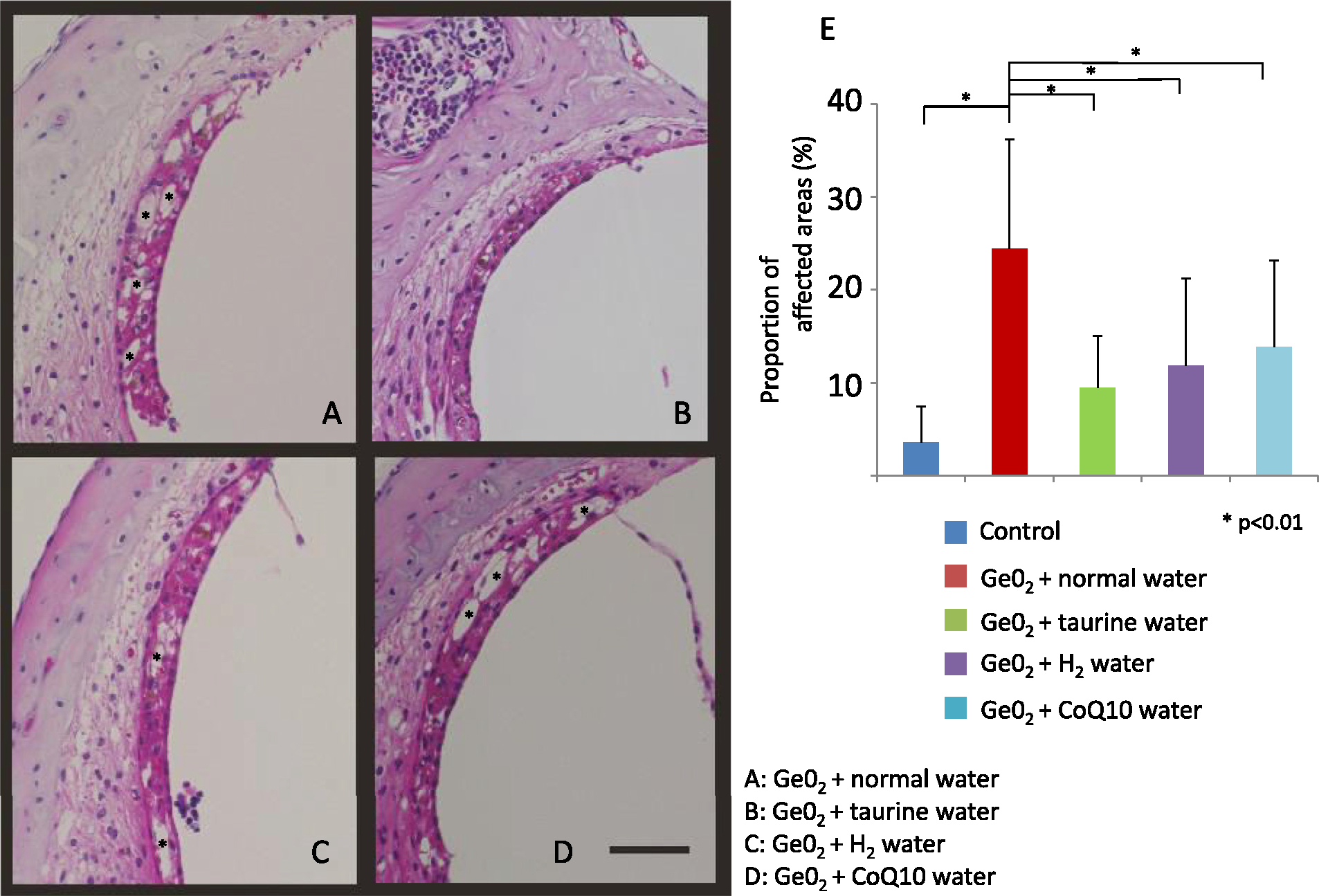
Representative light micrographs of the stria vascularis in animals given germanium and water (A), taurine (B), hydrogen water (C), and CoQ10 (D). The extent of the degeneration is significantly attenuated in animals given one of the antioxidants compared to those given water without antioxidant. Taurine provides the strongest effect, with the extent of degeneration not being different compared to the controls without germanium treatment (E). Representative vascuolar degeneration area are maked with “*”. Bar = 50 μm.
Fig. 7.
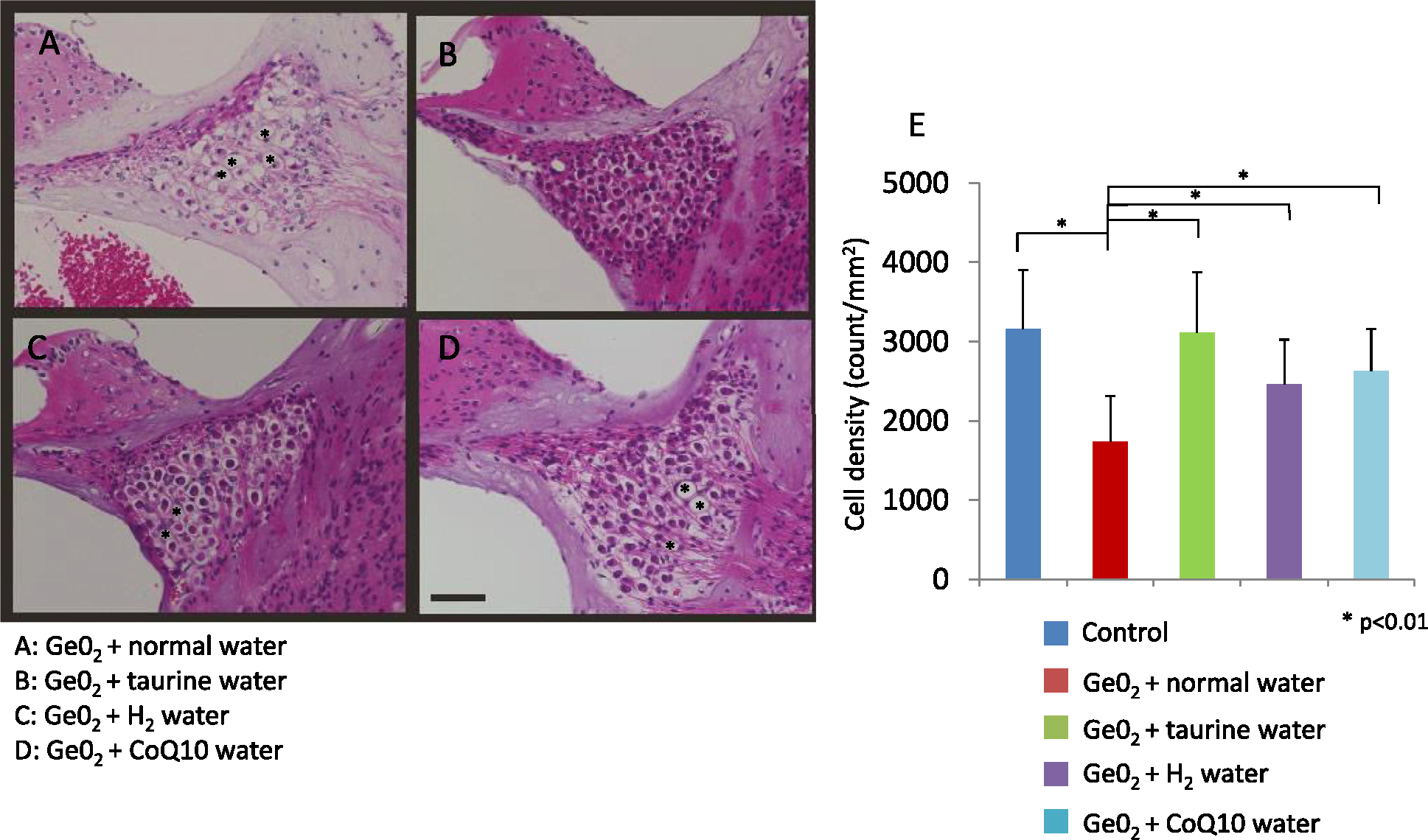
Representative light micrographs of the spiral ganglion cells in animals given germanium and water (A), taurine (B), hydrogen water (C), and CoQ10 (D). The extent of the degeneration is significantly attenuated in animals given one of the antioxidants compared to those given water without an antioxidant. Taurine provides the strongest effect, with the extent of degeneration not being different compared to the controls without germanium treatment (E). Representative degenerated area are marked with “*” Bar = 50 μm.
4. Discussion
The current study demonstrated that oral intake of 0.15% GeO2 for 4 months caused profound hearing loss associated with severe degeneration of the stria vascularis and SGCs in CBA mice. Transmission electron microscopic examination revealed electron-dense inclusions in degenerated mitochondria not only in the cochlea but in the kidney and muscle. Microarray gene expression analysis of the cochlea revealed down-regulation of 16 categories, including the “mitochondrion,” “mitochondrial inner membrane,” “mitochondrial electron transport chain,” and “oxidative phosphorylation.” qRT-PCR confirmed the down-regulation of five representative genes associated with mitochondrial respiratory chain in the cochlea. These findings indicate that dietary oral administration of 0.15% GeO2 in CBA mice is a promising animal model to investigate SNHL associated with mitochondrial dysfunction. We also observed that GeO2-induced SNHL and cochlear degeneration could be ameliorated by dietary intake of water containing taurine, CoQ10, or hydrogen, with taurine providing the strongest protection. These findings suggest that dietary intake of these antioxidants could be used to slow or treat SNHL and other phenotypes associated with mitochondrial dysfunction such as mitochondrial encephalomyopathy.
As mentioned above, we have shown that dietary intake of GeO2 induces SNHL in CBA mice, which could be a good model to study SNHL associated with impairment of mitochondrial function. It has been reported that topical application of mitochondrial toxin, 3-nitropropionic acid (3-NP) on the round window of the cochlea can cause acute SNHL; both permanent and temporary threshold shifts were observed in this model depending on the amount of 3-NP used (Hoya et al., 2004; Okamoto et al., 2005). In the permanent threshold shift model, marked degeneration was observed in type 2 fibrocytes in the spiral prominence, type 4 fibrocytes in the spiral ligament, marginal cells, and intermediate cells in the stria vascularis 3 h after 3-NP administration, indicative that SNHL caused by topical application of 3-NP is primarily mediated by cellular degeneration in the lateral wall of the cochlea. Compared to this animal model, in our mouse model, the degeneration was observed not only in the stria vascularis but the SGCs. The difference of the affected sites may be due to the different methods of drug application. 3-NP was applied acutely and topically, whereas GeO2 was applied chronically and systemically.
The current study revealed previously unrecognized pathways associated with GeO2-induced SNHL, such as the down-regulation of genes involved in the mitochondrial respiratory chain. The DNA microarray analysis revealed that chronic application of GeO2 down-regulated 27 genes in the respiratory chain complexes I, II, III, IV and V. Someya et al. (S Someya et al., 2007) reported changes of gene expression in the cochlea of DBA/2 J mice, which show severe progressive age-related hearing loss. In their study, gene analysis revealed that the aged DBA/2 J mice showed significant down-regulation of genes encoding components of the mitochondrial respiratory chain complexes I, II, III, IV, and V. Deficiency of complex IV is reported to be associated with SNHL (Horváth et al., 2005; Lamperti et al., 2012) in other reports. Gutiérrez Cortés et al. (Gutiérrez Cortés et al., 2012) also suggested that mutations of genes in complex I, III, and IV could be the cause of maternally inherited non-syndromic hearing loss. These results are in line with our findings that the dysfunction of mitochondrial respiratory chain complexes was closely related to SNHL. Moreover, the deficiencies of mitochondrial respiratory chain complexes have been reported to be closely related to neural degeneration. For example, Atp5a1 deficiency has been reported to cause severe neonatal encephalopathy, and Atp5e causes early onset lactic acidosis, 3-methylglutaconic aciduria, mild mental retardation, and severe peripheral neuropathy development. Both genes were confirmed to be down-regulated in the current study. Complex V deficiency mediated by other genes also has been observed in neurodegenerative diseases (Kantrow et al., 1997). Complex II deficiency also has been reported to cause neurodegenerative disorders (EA. Shoubridge, 2001; EA. Shoubridge, 2001). Although there has been no report examining the degeneration of SGCs, one of the peripheral neurons, in these deficiencies, it is speculated that mitochondrial dysfunction caused by the down-regulation of genes in complex I, III, and IV may be related to the degeneration of the SGCs.
In the current study, the up-regulation of genes involved in apoptosis was also observed. It has been reported that the defect of the respiratory chain system is associated with the induction of apoptosis (Kantrow et al., 1997). An in vivo study using Neuro-2A cells showed that treatment of GeO2 to the neuron A2 cell induced the release of cytochrome c from mitochondria, loss of mitochondrial membrane potential, and translocation of the Bax, resulting in apoptosis by the mitochondrial-dependent pathway (Lin et al., 2006). Interestingly, such phenomena have also been reported by applying similar semiconductor elements, such as arsenic, indium, and gallium (Bustamante et al., 1997; Chang et al., 2003; Hu et al., 2003; Milton et al., 2004). Studies investigating the mechanism for arsenic-induced apoptosis have revealed that the apoptosis is triggered by inhibition of mitochondrial respiratory function, resulting in the induction of ROS. ROS inactivate enzymes and damage DNA molecules by the direct chemical attack on their structure (Pelicano et al., 2003; Shen et al., 2003). Considering these, it can be assumed that the accumulation of germanium in the mitochondria would affect mitochondrial respiratory function, thereby resulting in ROS generation and induced mitochondria-mediated apoptosis of the stria vascularis and SGCs.
Taken together, these reports suggest that GeO2 accumulation causes mitochondrial dysfunction, leading to the degeneration of the cochlea and subsequent progressive hearing loss via apoptotic pathway. However, the current study does not directly demonstrate a causal relationship between mitochondrial dysfunction and cochlear degeneration, which should be evaluated by future studies.
In the current study, we observed that antioxidants, such as taurine, CoQ10, and hydrogen water, attenuated GeO2-induced SNHL and degeneration of the stria vascularis and SGCs, which implies that ROS play a key role in GeO2-mediated damage in the cochlea. Those antioxidants have been proven to have powerful antioxidant effects in various fields. For example, taurine has been exhibited to protect various organs from oxidative stress caused by alminium (Qiao et al., 2015), diabetes (Koh et al., 2014), and various drugs (Das et al., 2009; Manna et al., 2009; Alam and Hafiz, 2011; Roy and Sil, 2012). It has also been reported that adminisitration of taurine induces a significant reduction of intracelular ROS level and recovery of mitochondria membrane potential caused by arsenic in mouse neuroblastoma N2a cells. (Chou et al., Apr). CoQ10 has been shown to protect neuronal cells from UVB- and ROS-induced damage (M Sikorska et al., 2014), brain ischemia/reperfusion, gentamicin-induced cochlear damage and hearing loss, and hepatic oxidative stress and inflammation (M Sikorska et al., 2014). In addition, supplementation of CoQ10 has been reported to show a therapeutic effect in patients with mitrochonrial respiratory chain disorders (Hargreaves, 2014). Hydrogen gas has shown protective effects from ischemia/reperfusion injuries in cerebral (Sato et al., 2008) and myocardial infarction (Hayashida et al., 2008; Yoshida et al., 2012), hepatic injury (Fukuda et al., 2007), cisplatin-induced nephrotoxicity (Nakashima-Kamimura et al., 2009), and noise-induced hearing loss (Lin et al., 2011; Fransson et al., 2021). Although these antioxidants showed protective effect in the current study, hydrogen showed weakest effect compared to other supplements. This may be explained by the limited concentration of hydrogen when given in water. The solubility of the hydrogen is limited and easily leaked from water. Although the glass bottle used minimized the leakage of hydrogen from water and the water was changed every other day to keep the hydrogen concentration above 0.4 mM, the dose may be not sufficient to achieve satisfactory effects. Another reason may be the unique mechanism of scavenging system of hydrogen, which selectively scavenges free hydroxyl radicals (•OH) (Sato et al., 2008; Ohsawa et al., 2007). Other types of free radicals may be more relevant to GeO2-induced damage, and as a result, the effects of hydrogen may be limited.
5. Conclusion
Chronic dietary intake of GeO2 in CBA mice could induce SNHL due to the degeneration of stria vascularis and the SGCs, which was associated with down-regulation of mitochondrial respiratory chain associated genes and up-regulation of apoptosis-associated genes. Antioxidant supplements, such as taurine, CoQ10, and hydrogen water, could attenuate cochlear damage and SNHL induced by GeO2 intake. SNHL induced by oral intake of GeO2 can be a promising animal model to investigate SNHL associated with mitochondrial dysfunction. Daily supplements of antioxidants may be one of the solutions to prevent or slow SNHL associated with mitochondrial dysfunction.
Supplementary Material
Acknowledgment
We thank Prof. Mitsuya Suzuki, Mr. Yoshiro Mori, Ms. Atsuko Tsuyuzaki, and Ms. Yukari Kurasawa for their technical assistance in tissue preparation including transmission electron microscopy and immunostaining.
Funding source
This study was supported by Grant Number JP20H00546, JP20K21646JP from Government of Japan Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), and Grant Number 20FC1019 from Government of Japan Ministry of Labour and Welfare.
Footnotes
This article is part of the Special Issue Mitochondrial Function and Dysfunction in the Inner Ear Edited by Shinichi Someya, Anna Lysakowski, Katie Kindt, and David Raible. So, Dr. Lysakowski serve as a Guest Editor of this Special Issue.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.heares.2022.108678.
Data availability
Data will be made available on request.
References
- Affymetrix, 2004. Expression Analysis Technical Manual. Affymetrix, Santa Clara, CA: Version 5. [Google Scholar]
- Alam SS, Hafiz NA, 2011. Abd El-Rahim AH. Protective role of taurine against genotoxic damage in mice treated with methotrexate and tamoxfine. Environ Toxicol Pharmacol 31, 143–152. [DOI] [PubMed] [Google Scholar]
- Asaka T, Nitta E, Makifuchi T, Shibazaki Y, Kitamura Y, Ohara H, et al. , 1995. Germanium intoxication with sensory ataxia. J Neurol Sci 130, 220–223. [DOI] [PubMed] [Google Scholar]
- Böttger EC, Schacht J, 2013. The mitochondrion: a perpetrator of acquired hearing loss. Hear Res 303, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante J, Dock L, Vahter M, Fowler B, 1997. Orrenius S. The semiconductor elements arsenic and indium induce apoptosis in rat thymocytes. Toxicology 118, 129–136. [DOI] [PubMed] [Google Scholar]
- Chang KL, Liao WT, Yu CL, Lan CC, Chang LW, Yu HS, 2003. Effects of gallium on immune stimulation and apoptosis induction in human peripheral blood mononuclear cells. Toxicol Appl Pharmacol 193, 209–217. [DOI] [PubMed] [Google Scholar]
- Chou CT, Lin HT, Hwang PA, Wang ST, Hsieh CH, Hwang DF, 2015. Apr. Taurine resumed neuronal differentiation in arsenite-treated N2a cells through reducing oxidative stress, endoplasmic reticulum stress, and mitochondrial dysfunction. Amino Acids 47 (4), 735–744. [DOI] [PubMed] [Google Scholar]
- Das J, Ghosh J, Manna P, Sinha M, Sil PC, 2009. Arsenic-induced oxidative cerebral disorders: protection by taurine. Drug Chem Toxicol 32, 93–102. [DOI] [PubMed] [Google Scholar]
- Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. , 2003. DAVID: database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 4 (5), P3. [PubMed] [Google Scholar]
- Dereköy FS, Köken T, Yilmaz D, Kahraman A, Altuntaş A, 2004. Effects of ascorbic acid on oxidative system and transient evoked otoacoustic emissions in rabbits exposed to noise. Laryngoscope 114, 1775–1779. [DOI] [PubMed] [Google Scholar]
- Erdem A, Gündoğan NU, Usubütün A, Kilinç K, Erdem SR, Kara A, et al. , 2000. The protective effect of taurine against gentamicin-induced acute tubular necrosis in rats. Nephrol Dial Transplant 15, 1175–1182. [DOI] [PubMed] [Google Scholar]
- Finkel T1, Holbrook NJ, 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247. [DOI] [PubMed] [Google Scholar]
- Fransson AE, Videhult Pierre P, Risling M, Laurell GFE, 2021. Inhalation of molecular hydrogen, a rescue treatment for noise-induced hearing loss. Front Cell Neurosci 15, 658662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto C, Yamasoba T, 2019. Mitochondria-targeted antioxidants for treatment of hearing loss: a systematic review. Antioxidants (Basel) 8 (4), 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S, 2007. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun 361, 670–674. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Schneider J, Rochtchina E, Leeder SR, Mitchell P, 2009. Association between age-related hearing loss and stroke in an older population. Stroke 40, 1496–1498. [DOI] [PubMed] [Google Scholar]
- Gutiérrez Cortés N, Pertuiset C, Dumon E, Börlin M, Hebert-Chatelain E, Pierron D, Feldmann D, Jonard L, Marlin S, Letellier T, Rocher C, 2012. Novel mitochondrial DNA mutations responsible for maternally inherited nonsyndromic hearing loss. Hum Mutat 33, 681–689. [DOI] [PubMed] [Google Scholar]
- Hargreaves IP, 2014. Coenzyme Q10 as a therapy for mitochondrial disease. Int J Biochem Cell Biol 49, 105–111. [DOI] [PubMed] [Google Scholar]
- Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, et al. , 2008. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun 373, 30–35. [DOI] [PubMed] [Google Scholar]
- Higuchi I, Izumo S, Kuriyama M, Suehara M, Nakagawa M, Fukunaga H, et al. , 1989. Germanium myopathy: clinical and experimental pathological studies. Acta Neuropathol 79, 300–304. [DOI] [PubMed] [Google Scholar]
- Higuchi I, Takahashi K, Nakahara K, Izumo S, Nakagawa M, Osame M, 1991. Experimental germanium myopathy. Acta Neuropathol 82, 55–59. [DOI] [PubMed] [Google Scholar]
- Horváth R, Schoser BG, Müller-Höcker J, Völpel M, Jaksch M, Lochmüller H, 2005. Mutations in mtDNA-encoded cytochrome c oxidase subunit genes causing isolated myopathy or severe encephalomyopathy. Neuromuscul Disord 15, 851–857. [DOI] [PubMed] [Google Scholar]
- Hosack DA, Dennis G Jr, Sherman BT, Lane HC, Lempicki RA, 2003. Identifying biological themes within lists of genes with EASE. Genome Biol 4 (10), R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoya N, Okamoto Y, Kamiya K, Fujii M, Matsunaga T, 2004. A novel animal model of acute cochlear mitochondrial dysfunction. Neuroreport 15, 1597–1600. [DOI] [PubMed] [Google Scholar]
- Hu XM, Hirano T, Oka K, 2003. Arsenic trioxide induces apoptosis in cells of MOLT-4 and its daunorubicin-resistant cell line via depletion of intracellular glutathione, disruption of mitochondrial membrane potential and activation of caspase-3. Cancer Chemother Pharmacol 52, 47–58. [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA, 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4, 44–57. [DOI] [PubMed] [Google Scholar]
- Huxtable RJ, 1992. Physiological actions of taurine. Physiol Rev 72, 101–163. [DOI] [PubMed] [Google Scholar]
- Kantrow SP, DE Taylor, Carraway MS, Piantadosi CA, 1997. Oxidative metabolism in rat hepatocytes and mitochondria during sepsis. Arch Biochem Biophys 345, 278–288. [DOI] [PubMed] [Google Scholar]
- Kim KM, Lim CS, Kim S, Kim SH, Park JH, Ahn C, et al. , 1998. Nephropathy and neuropathy induced by a germanium-containing compound. Nephrol Dial Transplant 13, 3218–3219. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Sakamoto T, Kashio A, Shimizu T, Yamasoba T, 2013. Age-related hearing loss in Mn-SOD heterozygous knockout mice. Oxid Med Cell Longev. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JH, Lee ES, Hyun M, Kim HM, Choi YJ, Lee EY, et al. , 2014. Taurine alleviates the progression of diabetic nephropathy in type 2 diabetic rat model. Int J Endocrinol, 397307 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, et al. , 2005. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484. [DOI] [PubMed] [Google Scholar]
- Lamperti C, Diodato D, Lamantea E, Carrara F, Ghezzi D, Mereghetti P, et al. , 2012. MELAS-like encephalomyopathy caused by a new pathogenic mutation in the mitochondrial DNA encoded cytochrome c oxidase subunit I. Neuromuscul Disord 22, 990–994. [DOI] [PubMed] [Google Scholar]
- Lee CK, Klopp RG, Weindruch R, Prolla TA, 1999. Gene expression profile of aging and its retardation by caloric restriction. Science 285, 1390–1393. [DOI] [PubMed] [Google Scholar]
- Li X, Gao F, Chen Q, 2001. The pathogenesis of experimental model of mitochondrial myopathy induced by germanium dioxide. Chin Med Sci J 16, 157–160. [PubMed] [Google Scholar]
- Lin CH, Chen SS, Lin YC, Lee YS, Chen TJ, 2006. Germanium dioxide induces mitochondria-mediated apoptosis in Neuro-2A cells. Neurotoxicology 27, 1052–1063. [DOI] [PubMed] [Google Scholar]
- Lin Y, Kashio A, Sakamoto T, Suzukawa K, Kakigi A, Yamasoba T, 2011. Hydrogen in drinking water attenuates noise-induced hearing loss in guinea pigs. Neurosci Lett 487, 12–16. [DOI] [PubMed] [Google Scholar]
- Manna P, Sinha M, Sil PC, 2009. Taurine plays a beneficial role against cadmium-induced oxidative renal dysfunction. Amino Acids 36, 417–428. [DOI] [PubMed] [Google Scholar]
- Milton AG1, Zalewski PD, Ratnaike RN, 2004. Zinc protects against arsenic-induced apoptosis in a neuronal cell line, measured by DEVD-caspase activity. Biometals 17, 707–713. [DOI] [PubMed] [Google Scholar]
- Nakashima-Kamimura N, Mori T, Ohsawa I, Asoh S, Ohta S, 2009. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother Pharmacol 64, 753–761. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding DL, Lear PM, Ho YS, 2000. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J Assoc Res Otolaryngol 1, 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, et al. , 2007. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 13, 688–694. [DOI] [PubMed] [Google Scholar]
- Ohsawa I, Nishimaki K, Yamagata K, Ishikawa M, Ohta S, 2008. Consumption of hydrogen water prevents atherosclerosis in apolipoprotein E knockout mice. Biochem Biophys Res Commun 377, 1195–1198. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Hoya N, Kamiya K, Fujii M, Ogawa K, Matsunaga T, 2005. Permanent threshold shift caused by acute cochlear mitochondrial dysfunction is primarily mediated by degeneration of the lateral wall of the cochlea. Audiol Neurootol 10, 220–233. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Feng L, Zhou Y, Carew JS, Hileman EO, Plunkett W, et al. , 2003. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J Biol Chem 278, 37832–37839. [DOI] [PubMed] [Google Scholar]
- Qiao M, Liu P, Ren X, Feng T, Zhang Z, 2015. Potential protection of taurine on antioxidant system and ATPase in brain and blood of rats exposed to aluminum. Biotechnol Lett 37, 1579–1584. [DOI] [PubMed] [Google Scholar]
- Raha S1, Robinson BH, 2000. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci 25, 502–508. [DOI] [PubMed] [Google Scholar]
- Roy A, Sil PC Pathophysiology. Tertiary butyl hydroperoxide induced oxidative damage in mice erythrocytes: protection by taurine. 19, 137–48 (2012). [DOI] [PubMed] [Google Scholar]
- Sanai T, Oochi N, Okuda S, Osato S, Kiyama S, Komota T, et al. , 1990. Subacute nephrotoxicity of germanium dioxide in the experimental animal. Toxicol Appl Pharmacol 103, 345–353. [DOI] [PubMed] [Google Scholar]
- Sato Y, Kajiyama S, Amano A, Kondo Y, Sasaki T, Handa S, et al. , 2008. Hydrogen-rich pure water prevents superoxide formation in brain slices of vitamin C-depleted SMP30/GNL knockout mice. Biochem Biophys Res Commun 375, 346–350. [DOI] [PubMed] [Google Scholar]
- Shen ZY, Shen WY, Chen MH, Shen J, Zeng Y, 2003. Reactive oxygen species and antioxidants in apoptosis of esophageal cancer cells induced by As2O3. Int J Mol Med 11, 479–484. [PubMed] [Google Scholar]
- Shoubridge EA, 2001a. Nuclear genetic defects of oxidative phosphorylation. Hum Mol Genet 10, 2277–2284. [DOI] [PubMed] [Google Scholar]
- Shoubridge EA, 2001b. Nuclear gene defects in respiratory chain disorders. Semin Neurol 21, 261–267. [DOI] [PubMed] [Google Scholar]
- Sikorska M, Lanthier P, Miller H, Beyers M, Sodja C, Zurakowski B, et al. , 2014a. Nanomicellar formulation of coenzyme Q10 (Ubisol-Q10) effectively blocks ongoing neurodegeneration in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model: potential use as an adjuvant treatment in Parkinson’s disease. Neurobiol Aging 35, 2329–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorska M, Lanthier P, Miller H, Beyers M, Sodja C, Zurakowski B, et al. , 2014b. Nanomicellar formulation of coenzyme Q10 (Ubisol-Q10) effectively blocks ongoing neurodegeneration in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model: potential use as an adjuvant treatment in Parkinson’s disease. Neurobiol Aging 35, 2329–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohet FM, Neyrinck AM, Pachikian BD, de Backer FC, Bindels LB, Niklowitz P, et al. , 2009. Coenzyme Q10 supplementation lowers hepatic oxidative stress and inflammation associated with diet-induced obesity in mice. Biochem Pharmacol 78, 1391–1400. [DOI] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, Weindruch R, Prolla TA, Tanokura M, 2007a. Caloric restriction suppresses apoptotic cell death in the mammalian cochlea and leads to prevention of presbycusis. Neurobiol Aging 28, 1613–1622. [DOI] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, Prolla TA, Tanokura M, 2007b. Genes encoding mitochondrial respiratory chain components are profoundly down-regulated with aging in the cochlea of DBA/2J mice. Brain Res 1182, 26–33. [DOI] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, Kujoth GC, Pugh TD, Weindruch R, Tanokura M, et al. , 2008. The role of mtDNA mutations in the pathogenesis of age-related hearing loss in mice carrying a mutator DNA polymerase gamma. Neurobiol Aging 29, 1080–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Xu J, Kondo K, Ding D, Salvi RJ, Yamasoba T, et al. , 2009. Age-related hearing loss in C57BL/6 J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci USA. 106, 19432–19437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A1, Yoshizawa N, Oshima S, Kubota T, Oshikawa Y, Akashi Y, et al. , 1992. Nephrotoxicity of germanium compounds: report of a case and review of the literature. Nephron 60, 436–442. [DOI] [PubMed] [Google Scholar]
- Wang W, Karamanlidis G, Tian R, 2016. Novel targets for mitochondrial medicine. Sci Transl Med 8, 326rv3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CM, Matsuoka T, Takemitsu M, Goto Y, Nonaka I, 1992. An experimental model of mitochondrial myopathy: germanium-induced myopathy and coenzyme Q10 administration. Muscle Nerve 15, 1258–1264. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Nakamura K, Abe J, Hyodo M, Haga S, Ozaki M, et al. , 2015. Mitochondrial delivery of Coenzyme Q10 via systemic administration using a MITO–Porter prevents ischemia/reperfusion injury in the mouse liver. J Control Release 213, 86–95. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Goto Y, Komaki H, Mimaki M, Sudo A, Suzuki M, 2006. Cochlear damage due to germanium-induced mitochondrial dysfunction in guinea pigs. Neurosci Lett 395, 18–22. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Someya S, Yamada C, Weindruch R, Prolla TA, Tanokura M, 2007. Role of mitochondrial dysfunction and mitochondrial DNA mutations in age-related hearing loss. Hear Res 226, 185–193. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Asanuma H, Sasaki H, Sanada S, Yamazaki S, Asano Y, et al. , 2012. H(2) mediates cardioprotection via involvements of K(ATP) channels and permeability transition pores of mitochondria in dogs. Cardiovasc Drugs Ther 26, 217–226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


