Abstract
Microplastics have been found in the gastrointestinal (GI) fluid of bottlenose dolphins (Tursiops truncatus), inhabiting Sarasota Bay, FL, suggesting exposure by ingestion, possibly via contaminated fish. To better understand the potential for trophic transfer, muscle and GI tissues from 11 species of dolphin prey fish collected from Sarasota Bay were screened for microplastics (particles <5 mm diameter). Suspected microplastics were found in 82% of muscle samples (n=89), and 97% of GI samples (n=86). Particle abundance and shapes varied by species (p<0.05) and foraging habit (omnivore vs. carnivore, p<0.05). Pinfish (Lagodon rhomboides) had the highest particle abundance for both tissue types (muscle: 0.38 particles/g; GI: 15.20 particles/g), which has implications for dolphins as they are a common prey item. Findings from this study support research demonstrating the ubiquity of estuarine plastic contamination and underscore the risks of ingestion exposure for wildlife and potentially seafood consumers.
Keywords: plastic pollution, bottlenose dolphin, One Health, contaminant, trophic transfer
1. Introduction
Microplastics are plastic particles less than 5mm in diameter [1,2] and are found everywhere, including terrestrial [3], polar [4], freshwater [5–7], and marine environments [8]. Additionally,[8] estimated that our oceans contain roughly 171 trillion plastic particles. Microplastics enter the environment through various pathways, including degradation of macroplastic litter [9–11], direct contamination as primary microplastics (i.e., microbeads from personal care products; [12–14]), landfill and urban runoff [15–17], or via sewage and wastewater discharge [18,19].
Although widespread, the extent of microplastic contamination is not spatially uniform, and the variability in contamination can be attributed to particle properties (e.g., density and surface area, [20–22]), degrees of urbanization [23–25], and oceanographic currents [26–28]. For instance, particles with a higher density are more likely to sink and accumulate in sediment layers [20,22], and particles with larger surface areas may serve as substrates for biofouling, which can also contribute to their descent in water [21,22]. Geographically, the impact of urbanization on marine microplastic pollution is evident, with studies showing that waters surrounding urban centers are significantly more polluted than those near rural coastlines [24,29], likely due to urban runoff and wastewater discharge [15,17,25]. Additionally, research has demonstrated that microplastic abundance decreases as the distance from shoreline increases, in both rural and urban settings [23,25]. The influence of ocean currents is significant, with lower microplastic concentrations found in regions with strong currents and higher concentrations in areas with slower-moving currents, particularly noticeable in ocean gyres where both micro- and macro-plastics are trapped and circulate indefinitely [26–28].
Although the distribution of marine microplastics varies, their widespread prevalence makes all marine fauna vulnerable to exposure. Microplastics have been detected in multiple tissue types (e.g., muscle, liver, gills, gastrointestinal tracts, “GI”; [30–33]) across a wide range of taxa including jellyfish [34], bivalves [35–37], crustaceans [38], cephalopods [39], turtles [40], marine mammals[41–50], and numerous fish species [30,31,51,52]. Studies indicate that fish harbor the highest concentration of microplastics within their gastrointestinal tracts [53], with fibers being the most commonly observed shape [52,54]. Microplastic exposure in fish can occur via branchial intrusion in which particles enter via gills [32,53,55–57], as well as by incidental or direct ingestion [16,32,34,53,55,56,58–60]. For example, visually-oriented predators can mistake microplastics for prey due to their size and color resemblance [56,59,60], leading to higher gastrointestinal concentrations than chemosensory foragers [56]. However, not all fish actively pursue microplastics or confuse them for food; ingestion can also be an unintentional consequence of feeding in contaminated water [55] or through trophic transfer. An experimental study demonstrated that snowy sculpin (Myoxocephalus brandti) had higher concentrations of microplastics in their gastrointestinal tracts when placed in tanks with mysids (Neomysis spp) that had previously ingested microplastics, compared to tanks with only suspended microplastics [58]. Trophic transfer has also been observed in higher-order taxa such as grey seals (Halichoerus grypus), in which analyses of scat samples revealed microplastic characteristics similar to those found in their prey fish [48].
Recent studies of free-ranging bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida, demonstrated prevalent exposure to plasticizers [61,62] and microplastic ingestion [44]. Given previous evidence of microplastics in fish tissues and the potential for trophic transfer, we suspect that dolphin microplastic and plasticizer exposure is likely due to the consumption of contaminated prey. Building on initial findings from a study that compared microplastics in fish tissues and dolphin gastric fluid [51], we sought to quantify and characterize microplastics in a broader and more diverse sample of fish species, which are part of the Sarasota Bay bottlenose dolphin diet. Findings from this study will enhance our understanding of the types of microplastics that fish are exposed to, identify microplastic trophic exposure risks for bottlenose dolphins and local seafood consumers, and support ongoing efforts to monitor microplastic contamination in Sarasota Bay.
2. Materials and Methods
Study Location
The Sarasota Bay estuary (Figure 1) spans 50 miles along the central Gulf Coast of Florida and connects to the Gulf of Mexico through four inlets or passes [63]. Although tides are shallow (less than 2 feet), tidal exchange with the Gulf of Mexico is the dominant force for water circulation within Sarasota Bay. Several tidal creeks empty into Sarasota Bay along the eastern coast, with drainage areas varying in size (smallest: Palma Sola Creek, 900 acres; largest: Phillippi Creek, 36,417 acres [64]). Daily freshwater inflow averages 11.33 m3/s, and salinity throughout the Bay averages 30.00 ppt [65]. Sarasota Bay is an urbanized watershed and consists of agricultural, residential, commercial, and industrial land uses, so stormwater runoff due to the 45 inches of annual rainfall can be a significant contributor of pollutants to the Bay [64]. In fact, nitrogen deposition from wastewater and stormwater is the primary pollution concern for Sarasota Bay, but concentrations have been declining in recent years as a result of changes in stormwater management and efforts to improve wastewater treatment practices [64]. For example, septic systems have been transitioned to centralized sewer systems and regional wastewater treatment plants have been converted to pump stations for transport to larger, centralized facilities. Sarasota County now has three centralized wastewater treatment plants with capacities ranging between 3 and 12 million gallons per day, and reclaimed water is stored in tanks or ponds for residential, municipal and commercial irrigation practices throughout the county [66]. Each plant has plans to become an advanced wastewater treatment (AWT) facility [66], which can be over 90% effective in removing microplastics if employing both primary (i.e., physical process) and secondary (i.e., biological process) treatment practices [67]. In 1989, the United States Congress designated the bay as an estuary of national significance [68], leading to initiatives aimed at reducing pollution, including measures such as plastic straw bans and mandatory recycling protocols [69]. Despite these efforts, recent studies have found evidence of microplastics within the gastrointestinal tracts of fish and dolphins inhabiting the area [44,51].
Figure 1 - .
Fish collection locations in Sarasota Bay, Florida, USA (September 2022 - July 2023).
Fish Collection
Fish for this study were collected via purse-seining from Sarasota Bay, FL (Figure 1) by the Brookfield Zoo Chicago’s Sarasota Dolphin Research Program (SDRP) between September 2022 and July 2023 as part of efforts to monitor seasonal abundance [70, 71]. SDRP fish survey methods and procedures have been previously described [71,72]. Briefly, the study area for SDRP’s seasonal fish abundance surveys was chosen based on the spatial distribution of the resident dolphins, covering estuarine waters from Passage Key Inlet at the southwestern edge of Tampa Bay (27.5528° N / 82.7423° W) southward to Phillippi Creek, south of Sarasota Bay (27.27096° N / 82.53757° W). Five distinct habitat types were characterized within this study area including creek/mangrove edge, seagrass beds, open bay, sand flat, and nearshore gulf waters, based on location, water depth, and bottom type (vegetated vs. unvegetated) [71]. Using a 200 x 200-m resolution sampling grid originally created in ArcGIS 8.0 (Environmental Systems Research Institute, Redlands, CA, USA), sampling stations were located at the centroids of each grid cell and habitat type was identified at each centroid. Sampling stations were then chosen at random based on habitat type. For the present study, fish were collected during surveys focusing exclusively on seagrass habitat within Sarasota Bay, as the primary prey fish of resident dolphins in Sarasota Bay are associated with seagrass habitat [71]. Twelve species were targeted based on reports from stomach content analyses and observed feeding in the field [72,73]; these included hardhead catfish (Ariopsis felis), sheepshead (Archosargus probatocephalus), menhaden (Brevoortia tyrannus), spotted seatrout (Cynoscion nebulosus), ladyfish (Elops saurus), scaled sardine (Harengula jaguana), pinfish (Lagodon rhomboides), spot (Leiostomus xanthurus), striped mullet (Mugil cephalus), Gulf toadfish (Opsanus beta), pigfish (Orthopristis chrysoptera), and Atlantic thread herring (Opisthonema oglinum). Fish collection was approved by Mote Marine Laboratory’s Institutional Animal Care and Use Committee (IACUC, Permit nos. 22-09-RW2, 23-09-RW2) and Florida Fish and Wildlife Conservation Commission Special Activity License nos. 19-0809A-SR and 22-0809-SR.
Sample Processing and Analysis
Dissections to remove muscle tissue and the gastrointestinal tract were conducted on metal trays using stainless steel scalpels and forceps, and tissues were stored at −20 °C glass jars until digestion [51]. To digest organic material, muscle and GI tissues were incubated in a potassium hydroxide (KOH, 10%) solution at 60 °C [74] for 24-72 hours. The resulting digestate was vacuum filtered onto a GF/A 1.6 μm glass fiber filter within a fume hood [51,75]. Samples containing large quantities of inorganic solids or durable organic remnants (i.e., sediment, crustacean exoskeletons, bone, and scales) were pre-filtered through 63 μm and 500 μm sieves prior to vacuum filtration. Filters were then placed in covered petri dishes and stored in a cabinet to dry.
Suspected microplastics were visually identified under a Leica EZ4 microscope at 16-35x magnification [74–76]. Characteristics of suspected microplastics included homogenous coloring, absence of organic or cellular structures, and uniform thickness of fibrous particles [74]. Suspected microplastics were categorized by color and shape. Fibers appeared significantly longer than they were wide [2,77], foams were round and porous, changing shape upon touch [2,77–79], films were flat with greater length and width than depth [2,77,79], and fragments had distinct corners [2,77]. Tire wear particles (TWP) were identified as black, rubbery fragments that retained their shape upon manipulation [76]. Plastic testing was conducted with a heated (250 °C) soldering needle [75], which causes plastic particles to bend or melt, as most polymers melt near a temperature of 250°C [74,75,80]. Fourier Transform Infrared (FTIR) spectroscopy (Nicolet iS20, Thermo Scientific, Waltham, MA, USA) was available for polymer determination; however, the particle sizes in this study were smaller than the instrument’s detection threshold (500 μm to 5 mm). Therefore, our findings report suspected microplastics identified via visual characteristics and hot needle responses [44,51].
QA/QC
Before each dissection, all tools were triple-rinsed with Milli-Q® purified water [2,74,75]. During the dissections, a petri dish containing a glass fiber filter was placed on the benchtop to capture ambient microplastics, serving as a “dissection blank” [2,74]. This blank was processed identically to the fish tissues to control for ambient contamination. Additionally, 100% cotton lab coats dyed orange (an uncommon microplastic color) and clean nitrile gloves were worn during dissection, digestion, filtration, and counting procedures to avoid contamination by personnel [2,74,76]. For quality assurance and control (QA/QC), blanks were collected at each step of the analysis. One lab/procedural blank without any tissue was processed with each digestion batch to account for contamination during sample processing [74]. To evaluate the efficiency of microplastic recovery, three positive controls containing polyethylene films, polystyrene foams, and polyester fibers were included [2]. These controls demonstrated mean recoveries of 60% for films, 83% for foams, and 85% for fibers. Finally, microplastic particles that matched the shape and color of those found in the corresponding blanks were excluded from the total counts in the sample data [2,51,74].
Statistical Analysis
The proportion of muscle and GI samples with suspected microplastics was determined for all fish combined and by species. Particle counts were categorized by shape and color and summarized for both tissue types across all species sampled. For each tissue type, particle load was quantified as the number of suspected microplastics per gram of tissue [51]. Mean particle load was compared across species using a Kruskal-Wallis test and between foraging habits (i.e., carnivore vs. omnivore) using a Mann-Whitney U test. All statistical analyses were conducted using Statistica software (version 13, Tibco, Inc., Palo Alto, CA, USA), with statistical significance set at ɑ=0.05.
3. Results
3.1. Sample Characteristics
From September 2022 through July 2023, 11 fish species were collected from 17 locations in Sarasota Bay (Figure 1). In total, 94 fish were screened for suspected microplastics, with 2 to 24 individuals per species (Table 1). Muscle tissue (n=89) mass varied between species, with the largest belonging to the ladyfish (Elops saurus; n=2; range: 100.20g - 117.30g), and the smallest belonging to the Atlantic thread herring (Opisthonema oglinum; n=4; range: 0.90g - 9.20g; Table 1). Among GI samples (n=86), the hardhead catfish (Ariopsis felis) had the largest tissue mass (n=6; range: 11.40g - 48.90), and the scaled sardine (Harengula jaguana) had the smallest (n=8; range: 0.50g - 5.10g; Table 1). For all fish, the muscle tissue mass was higher than their GI sample counterpart (Table 1).
Table 1.
Characteristics of fish screened for suspected microplastics. Characteristics include species, foraging type81, tissue sample counts, tissue sample mass (g), and mean particle load (# particles / g tissue) for muscle and gastrointestinal (GI) samples.
| Common Name (Genus species) | Foraging Type1 | Muscle Samples (n) | Muscle Mass (g) mean (sd) | Muscle Particle Load (# particles/g) mean (sd) | GI Samples (n) | GI Mass (g) mean (sd) | GI Particle Load (# particles/g) mean (sd) |
|---|---|---|---|---|---|---|---|
| Hard-head Catfish (Ariopsis felis) | Carnivore | 6 | 34.33 (14.45) | 0.08 (0.06) | 6 | 30.43 (16.69) | 6.04 (4.67) |
| Sheeps-head (Archosargus probatocephalus) | Omnivore | 2 | (16.40 - 47.40)* | (0.06 - 0.27)* | 2 | (3.10 - 19.90)* | (1.81 - 7.10)* |
| Menhaden (Brevoortia tyrannus) | Carnivore | 5 | 30.18 (11.90) | 0.11 (0.07) | 4 | 12.30 (2.99) | 2.70 (1.23) |
| Spotted Seatrout (Cynoscion nebulosus) | Carnivore | 5 | 68.66 (78.83) | 0.02 (0.05) | 5 | 15.68 (11.81) | 0.99 (1.29) |
| Ladyfish (Elops saurus) | Carnivore | 2 | (100.20 - 117.30)* | (0.04 - 0.15)* | 2 | (20.60 - 25.00)* | (0.68 - 1.16)* |
| Scaled Sardine (Harengula jaguana) | Carnivore | 8 | 6.46 (2.03) | 0.15 (0.12) | 8 | 2.39 (1.49) | 10.87 (5.51) |
| Pinfish (Lagodon rhomboides) | Omnivore | 24 | 15.01 (15.02) | 0.38 (0.64) | 25 | 4.50 (1.78) | 15.20 (22.79) |
| Spot (Leiostomus xanthurus) | Carnivore | 5 | 26.24 (2.83) | 0.07 (0.05) | 4 | 5.13 (0.86) | 0.91 (0.97) |
| Gulf Toadfish (Opsanus beta ) | Carnivore | 12 | 7.06 (3.75) | 0.38 (0.56) | 12 | 5.61 (2.86) | 4.66 (5.14) |
| Pigfish (Orthopristis chrysoptera) | Carnivore | 16 | 10.19 (7.19) | 0.23 (0.18) | 15 | 4.16 (3.76) | 4.45 (5.45) |
| Atlantic Thread Herring (Opisthonema oglinum) | Carnivore | 4 | 4.3 (3.55) | 1.08 (0.92) | 3 | 3.63 (1.06) | 11.61 (11.08) |
Minimum and maximum are presented
3.2. Microparticles in Muscle Samples
Suspected microplastic particles were found in 82.02% (n=73) of the muscle samples observed. Overall, particle counts in muscle tissue were relatively low; 75.28% of muscle samples contained <10 particles (Table 2). Among the particle shapes observed, single fibers were most common (71.91%), followed by films (26.97%), fragments (11.24%; both non-TWP and TWP), foams (5.62%), and fiber bundles (3.37%; Table 2). No mixed bundles were present. Among the colors observed, yellowed and transparent particles were found in the muscles of every species screened (Figure 2).
Table 2.
Suspected microplastic abundance in muscle tissue of fish collected from Sarasota Bay, FL (n=89).
| Particle Shape | Total Muscle Samples with Particle Shape n (%) | Particle Shapes in Muscle Samples with <10 Particles n (%) | Particle Shapes in Muscle Samples with 10-50 Particles n (%) |
|---|---|---|---|
| Fiber Bundles | 3 (3.37) | 3 (3.37) | 0 |
| Single Fibers | 64 (71.91) | 63 (70.79) | 1 (1.12) |
| Films | 24 (26.97) | 24 (26.97) | 0 |
| Foams | 5 (5.62) | 5 (5.62) | 0 |
| Non-TWP Fragments | 10 (11.24) | 9 (10.11) | 1 (1.12) |
| TWP Fragments* | 10 (11.24) | 10 (11.24) | 0 |
TWP (tire-wear particle)
Figure 2.
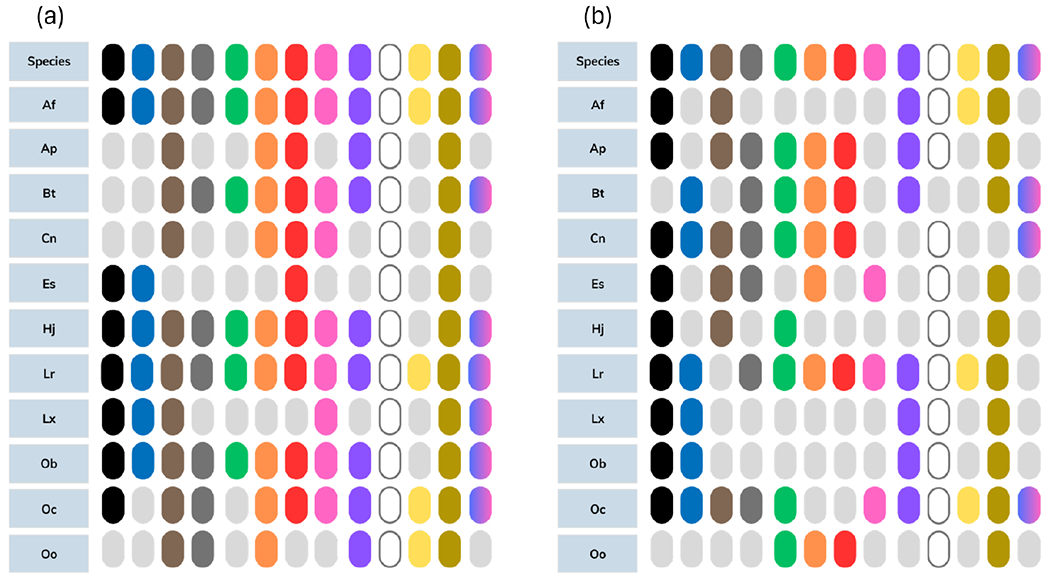
Suspected microplastic colors by species for GI samples (a) and muscle samples (b). From left to right the colors are black, blue, brown, gray, green, orange, red, pink, purple, transparent/white, yellow, yellowed, and multi-colored. From top to bottom the species are Af: hardhead catfish (Ariopsis felis); Ap: sheepshead (Archosargus probatocephalus); Bt: menhaden (Brevoortia tyrannus); Cn: spotted seatrout (Cynoscion nebulosus); Es: ladyfish (Elops saurus); Hj: scaled sardine (Harengula jaguana); Lr: pinfish (Lagodon rhomboides); Lx: spot (Leiostomus xanthurus); Ob: Gulf toadfish (Opsanus beta); Oc: pigfish (Orthopristis chrysoptera); and Oo: Atlantic thread herring (Opisthonema oglinum).
3.3. Microparticles in Gastrointestinal Samples
Among the 86 GI samples screened, 96.51% (n=83) contained at least one suspected microplastic particle. Microparticles were more abundant in GI samples; 60.05% of samples contained 10 particles or more (Table 3). In fact, nearly 300 suspected microplastics were observed in the GI tissue of a single hardhead catfish (Table 3). Particle shapes observed in GI samples varied, but similarly to muscle samples, single fibers were the most common (82.56% of samples screened; Table 3). Films and fiber bundles were also commonly observed (62.79% and 48.84%, respectively), while fewer samples contained fragments (non-TWP: 32.56% and TWP: 16.28%), mixed bundles (12.79%), and foams (4.65%; Table 3). GI particle colors were also variable, but similarly to muscle samples, transparent and yellowed were commonly observed across all species (Figure 2).
Table 3.
Suspected microplastic abundance in GI tissue of fish collected from Sarasota Bay, FL (n=86).
| Particle Shapes Observed In GI Tissue | Total GI Samples with Particle Shape n (%) | Particle Shapes in GI Samples with <10 Particles n (%) | Particle Shapes in GI Samples with 10-50 Particles n (%) | Particle Shapes in GI Samples with 51-100 Particles n (%) | Particle Shapes in GI Samples with 101-300 Particles n (%) |
|---|---|---|---|---|---|
| Fiber Bundles | 42 (48.84) | 26 (30.23) | 15 (17.44) | 0 | 1 (1.16) |
| Single Fibers | 71 (82.56) | 40 (46.51) | 25 (29.07) | 2 (2.33) | 4 (4.65) |
| Film | 54 (62.79) | 40 (46.51) | 11 (12.79) | 0 | 3 (3.49) |
| Foam | 4 (4.65) | 4 (4.65) | 0 | 0 | 0 |
| Non-TWP Fragment | 28 (32.56) | 25 (29.07) | 2 (2.33) | 1 (1.16) | 0 |
| TWP Fragment* | 14 (16.28) | 14 (16.28) | 0 | 0 | 0 |
| Mixed Bundle | 11 (12.79) | 6 (6.98) | 5 (5.81) | 0 | 0 |
TWP (tire-wear particle)
3.4. Comparisons Across Species
For both muscle and GI samples, the mean particle load (# particles per gram of tissue) was compared across species to account for differences in sample mass. Samples from sheepshead and ladyfish were excluded from species comparisons due to their limited sample size (n=2; Table 1). Significant differences in mean particle load were observed across species for both muscle (Kruskal-Wallis, p=0.006) and GI tissues (Kruskal-Wallis, p=0.003). Additionally, mean particle load was consistently higher for GI samples, compared to muscle tissue (Table 1). Particle abundance was highest in pinfish for both tissue types (n=24; muscle: 0.38 particles/g; GI: 15.20 particles/g), and high particle loads were also observed in the Atlantic thread herring (n=4; muscle: 1.08 particles/g; GI: 11.61 particles/g).
Among the three species with the highest mean particle load for muscle samples (pinfish, Atlantic threadfin herring, gulf toadfish), fibers were most abundant (Figure 3). Other common particle shapes in muscle samples from these fish included films (pinfish and Atlantic threadfin herring), non-tire wear fragments (pinfish), and tire wear fragments (gulf toadfish; Figure 3). For the fish with the highest particle loads in GI samples (pinfish, Atlantic threadfin herring, scaled sardine), fibers and films were most abundant (Figure 3).
Figure 3.
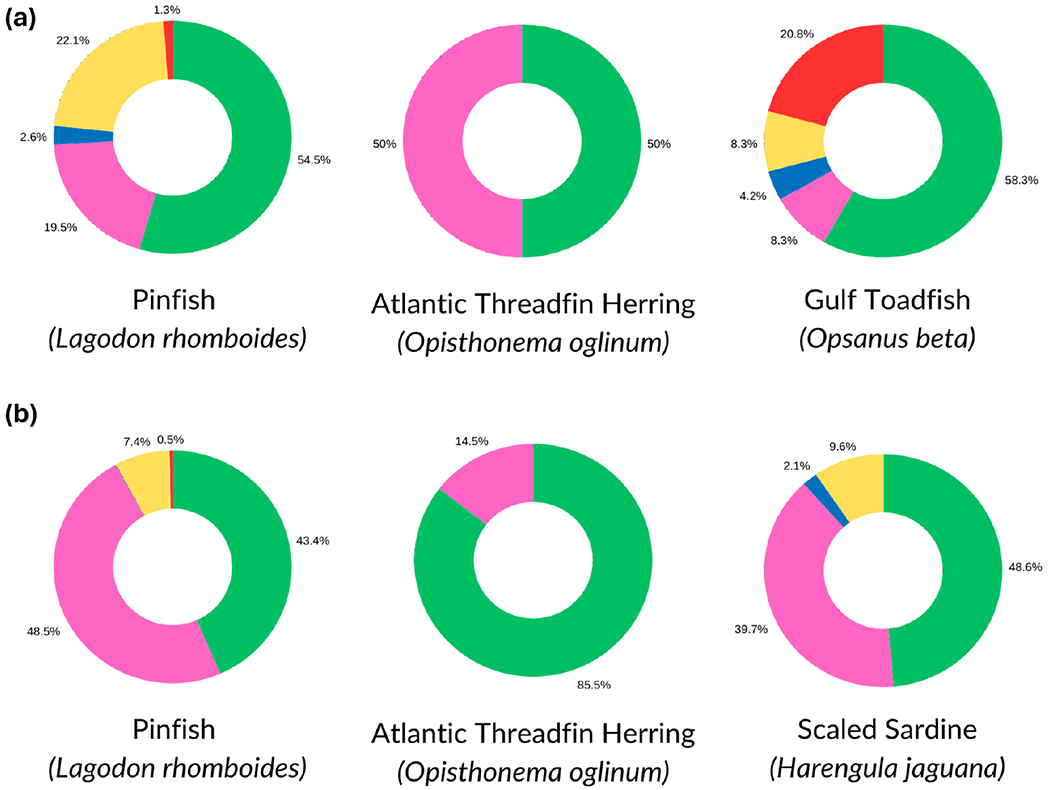
Particle shapes (green = fibers, pink = films, blue = foams, yellow = non-TWP fragments, and red = TWP fragments) among fish species with the highest particle loads for muscle (a) and GI (b) samples.
Each fish species was grouped by feeding habit (i.e., carnivore, omnivore, herbivore; [81]; Table 1), for additional comparisons of particle load. Herbivores were not included in the analysis, as none of the species screened were herbivorous. Despite the larger sample size and sample masses for carnivorous fish species (n = 63), mean particle load was significantly higher among omnivorous fish (n = 26) for GI samples (14.40 vs. 5.12 particles/g; Mann–-Whitney U test, p = 0.03). No significant differences in mean particle load were observed for comparisons of muscle tissue be-tween omnivorous (0.36 particles/g) and carnivorous (0.25 particles/g) fish (Mann–-Whitney U test, p = 0.11). It should be noted, however, that the majority of omnivorous fish (92%) were pinfish.
4. Discussion
Microplastic contamination of fish commonly consumed by Sarasota Bay dolphins was substantial. Suspected microplastics were observed in 82% of muscle samples (n = 73) and 97% (n = 83) of GI samples (Tables 2,3), which is higher than some previous studies at other sites. For example, [9] found suspected microplastics in only 35% of GI samples from Nile tilapia (Oreochromis niloticus), African sharptooth catfish (Clarias gariepinus), common Carp (Cyprinus carpio) and Crucian carp (Carassius carassius; n=125). Similarly, [82] observed microplastics in approximately 25% of muscle samples and 40% of GI samples from red mullet (Mullus barbatus; n= 82) and pontic shad (Alosa immaculata; n= 82). Other studies have provided results in a similar range to ours; [53] observed microplastics in 100% of sin croaker (Johnius dussumieri) GI samples (n = 188) from Mumbai, India, and, [83] found microplastics in 100% of both GI and muscle samples screened from painted combers (Serranus scriba) sampled near the Tunisian coast. The high proportion of fish with suspected microplastics in our study could be attributed to Sarasota Bay’s location. To our knowledge, systematic studies of microplastic pollution in Sarasota Bay have not been performed; however, research by [84] suggested that the neighboring Tampa Bay could contain up to 4 billion microplastic particles. Also, Sarasota Bay is an urban estuary that receives freshwater input from multiple sources. Since freshwater tributaries can carry agricultural and urban runoff, [85] suggest that they may serve as substantial conduits for estuarine microplastics. Additionally, these freshwater creeks can create mixing zones with saltwater from the ocean, potentially trapping debris and acting as a sink for microplastics [86].
Consistent with previous studies [57,82,87], particle counts were higher in GI samples than in muscle tissue, as ingestion is a primary exposure route for microplastics [16,32,34,53,55,56,58–60]. Studies of fish from Turkey, Iran, and Tunisia have shown similar results, with lower concentrations in muscle tissues compared to GI and stomach samples [82,87,88]. The mechanism by which particles enter muscle tissue remains unclear, but it is theorized that they escape the GI tract through cellular gaps in the stomach lining [89]. This translocation hypothesis was demonstrated in European sea bass (Dicentrarchus labrax) fed fluorescently-labeled particles (1-5μm; [89]).
Despite all fish being collected within Sarasota Bay (Figure 1), particle abundance varied across species. These findings are consistent with research by the authors of [9], which demonstrated variable contamination in fish sampled from different sites within the same body of water. Differences in foraging habits could help explain this variation by species. For example, studies have shown that benthic fish (such as catfish) ingest more plastic than surface feeders [9,90], likely due to higher concentrations of microplastics in sediment compared to surface waters [20–22]. Additionally, as microplastics undergo weathering and biofouling, they can sink [20–22], increasing the likelihood of consumption by fish that feed lower in the water column.
Our findings suggest that diet may influence contamination, as particle load was higher among omnivorous fish. However, caution is warranted in interpreting this result because the majority of omnivorous fish in our study were pinfish, which had the highest particle abundance. Although pinfish are considered omnivorous, seagrasses, a significant component of their diet [91], could be a substantial sink for microplastics. For example, [92] observed microplastic particles on 75% of examined seagrass blades. Similar trends of higher particle counts in omnivorous fish have been reported in other studies [59,90], where the authors hypothesize that the diverse diet of omnivorous fish increases their chances of ingesting particles. Microplastics have been detected in plants, various fish species, and lower trophic organisms [30,31,92,93], all of which could be food sources for omnivorous fish. Lastly, while some studies suggest that fish may inadvertently consume microplastics that resemble their typical food in color or shape [56,59,60], our results do not support this theory, as we did not observe color preferences among different species.
Significance of Findings
Our previous studies observed ingested microplastics in Sarasota Bay dolphins [44], and the results of this study provide insight into possible sources of their exposure. We detected suspected microplastics in every fish species examined, all of which are commonly consumed by bottlenose dolphins in Sarasota Bay, Florida, with pinfish being the species most frequently found in Sarasota dolphin stomach contents [72,73,94]. Considering the evidence of trophic transfer in marine mammal studies [48,95], it is possible that contaminated fish could be a substantial source of microplastic exposure for Sarasota Bay dolphins. Although the impacts of microplastic exposure are not yet understood for dolphins and other marine mammals, in vitro laboratory studies suggest that adverse health effects such as inflammation [96,97], reproductive impairment [98,99], neurological impairment [100,101], and metabolic issues [102,103] are possible.
Additionally, our findings of microplastics in these fish are concerning for seafood safety. In 2021, Florida was ranked 11th in the United States for the highest production of fresh seafood, accounting for 4.2% of the national total value [104]. The species examined in our study hold commercial value or are sought after for sport fishing [105]. The most contaminated species in our study, the pinfish, is commonly used as bait fish in both commercial and recreational fishing [106,107]. Through trophic transfer, these larger commercial species, such as spotted seatrout, could become contaminated, thereby increasing exposure risk for seafood consumers [108].
Strengths and Limitations
One challenge for microplastic research is the potential contamination from ambient particles [109]. To mitigate and monitor ambient contamination, several precautions were implemented, such as wearing 100% cotton laboratory coats, rinsing instruments with filtered water, and collecting laboratory and procedural blanks. Additionally, a conservative approach to blank-correction was adopted in which sample particles resembling the shape and color of suspected microplastics in blanks were excluded from abundance counts and particle characterization. This method enhanced the reliability of findings by reducing the likelihood of reporting ambient or procedural contaminants as suspected tissue particles. Particle size was also a limitation of this study, as suspected microplastics were too small to confirm their polymer composition using FTIR, which requires particles to be between 500 μm and 5 mm in diameter. Due to this constraint, particles suspected to be microplastics were identified using microscopy and the hot needle method. The hot needle test is less reliable than FTIR analysis because it depends on specific reactions in plastic that can vary (e.g., burning, melting, curling; [110]). Although the hot needle test is not as precise as FTIR, it can still effectively identify microplastics when used by individuals familiar with plastic reactions to heat [110].
5. Conclusion
Plastic pollution is a persistent and widespread issue, leading to ubiquitous microplastic contamination. In this study, we examined the muscle and GI tissues of 11 fish species and found suspected microplastics in each one. These fish are commonly consumed by bottlenose dolphins in Sarasota Bay, Florida, suggesting a trophic exposure route for dolphins and other apex predators. Some species examined are also commonly used as bait fish for commercial fishers, suggesting a risk to seafood safety. However, we detected the fewest particles in fillet tissue, indicating a lower exposure risk compared to apex predators that consume whole fish. Additionally, particle loads were higher in omnivorous fish compared to carnivorous fish, possibly due to their varied diet. Therefore, microplastic exposure through trophic transfer could be higher for apex predators and seafood consumers that eat omnivorous fish. While suspected microplastics are abundant in many of these fish, their small sizes may limit plastic confirmation by standard methodologies (e.g., FTIR). Future fish studies should employ methods that use smaller size thresholds (e.g., micro-Raman spectroscopy).
Acknowledgments:
The authors would like to thank Bonnie Ertel and Dana Norton for providing training in microplastic isolation and quantification. They would also like to thank Maggie Knight for providing assistance with manuscript editing and positive controls for QA/QC methods. The authors would also like to thank Christina Toms, Jonathan Crossman, and Kylee DiMaggio for their assistance with sample collection. We would also like to thank SDRP interns and volunteers who provided crew support for fishing operations. Finally, the authors would like to thank The Citadel, the College of Charleston’s Department of Health and Human Performance, and the Center for Coastal Environmental and Human Health for providing laboratory and logistical support.
Funding:
Research reported in this publication was supported by the National Institute Of Environmental Health Sciences of the National Institutes of Health under Award Number R15ES034169. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Primary support for fish sampling operations in Sarasota Bay was provided to the SDRP by the Charles and Margery Barancik Foundation.
Footnotes
Conflicts of Interest: The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Data Availability Statement:
Data supporting the reported results can be found in the DRYAD data repository (datadryad.org) using this DOI: 10.5061/dryad.nvx0k6
References
- 1.Andrady AL Microplastics in the Marine Environment. Marine Pollution Bulletin 2011, 62, 1596–1605, doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 2.Rochman CM; Brookson C; Bikker J; Djuric N; Earn A; Bucci K; Athey S; Huntington A; McIlwraith H; Munno K; et al. Rethinking Microplastics as a Diverse Contaminant Suite. Environmental Toxicology and Chemistry 2019, 38, 703–711, doi: 10.1002/etc.4371. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y; Liu Q; Jia W; Yan C; Wang J Agricultural Plastic Mulching as a Source of Microplastics in the Terrestrial Environment. Environmental Pollution 2020, 260, 114096. [DOI] [PubMed] [Google Scholar]
- 4.Lusher AL; Tirelli V; O’Connor I; Officer R Microplastics in Arctic Polar Waters: The First Reported Values of Particles in Surface and Sub-Surface Samples. Scientific Reports 2015, 5, 14947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castañeda RA; Avlijas S; Simard MA; Ricciardi A Microplastic Pollution in St. Lawrence River Sediments. Canadian Journal of Fisheries and Aquatic Sciences 2014, 71, 1767–1771. [Google Scholar]
- 6.Vermaire JC; Pomeroy C; Herczegh SM; Haggart O; Murphy M Microplastic Abundance and Distribution in the Open Water and Sediment of the Ottawa River, Canada, and Its Tributaries. Facets 2017, 2, 301–314. [Google Scholar]
- 7.Mani T; Primpke S; Lorenz C; Gerdts G; Burkhardt-Holm P Microplastic Pollution in Benthic Midstream Sediments of the Rhine River. Environmental Science & Technology 2019, 53, 6053–6062. [DOI] [PubMed] [Google Scholar]
- 8.Eriksen M; Cowger W; Erdle LM; Coffin S; Villarrubia-Gómez P; Moore CJ A Growing Plastic Smog, Now Estimated to Be over 170 Trillion Plastic Particles Afloat in the World’s Oceans—Urgent Solutions Required. PLoS ONE 2023, 18, 0281596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merga LB; Redondo-Hasselerharm PE; Brink PJ; Koelmans AA Distribution of Microplastic and Small Macroplastic Particles across Four Fish Species and Sediment in an African Lake. Science of the Total Environment 2020, 741, 140527. [DOI] [PubMed] [Google Scholar]
- 10.Song YK; Hong SH; Eo S; Shim WJ The Fragmentation of Nano-and Microplastic Particles from Thermo-plastics Accelerated by Simulated-Sunlight-Mediated Photooxidation. Environmental Pollution 2022, 311, 119847. [DOI] [PubMed] [Google Scholar]
- 11.Wardlaw CM; Corcoran PL; Neff BD Factors Influencing the Variation of Microplastic Uptake in Demersal Fishes from the Upper Thames River Ontario. Environmental Pollution 2022, 313, 120095. [DOI] [PubMed] [Google Scholar]
- 12.Huang L; Li QP; Li H; Lin L; Xu X; Yuan X; Li H Microplastic Contamination in Coral Reef Fishes and Its Potential Risks in the Remote Xisha Areas of the South China Sea. Marine Pollution Bulletin 2023, 186, 114399. [DOI] [PubMed] [Google Scholar]
- 13.Isobe A. Percentage of Microbeads in Pelagic Microplastics within Japanese Coastal Waters. Marine Pollution Bulletin 2016, 110, 432–437. [DOI] [PubMed] [Google Scholar]
- 14.Velmurugan PM; Vijayaprabhakaran K; Devika PT Baseline Study on Identification, Characterization, Distribution and Abundance of Microplastics in Surface Water from Ennore to Kovalam along the East Coast of India. Physics and Chemistry of the Earth, Parts A/B/C 2023, 130, 103391. [Google Scholar]
- 15.Hajiouni S; Mohammadi A; Ramavandi B; Arfaeinia H; De-la-Torre GE; Tekle-Röttering A; Dobaradaran S Occurrence of Microplastics and Phthalate Esters in Urban Runoff: A Focus on the Persian Gulf Coastline. Science of the Total Environment 2022, 806, 150559. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q; Li J; Zhu X; Sun C; Teng J; Chen L; Zhao J Microplastics in Fish Meals: An Exposure Route for Aquaculture Animals. Science of the Total Environment 2022, 807, 151049. [DOI] [PubMed] [Google Scholar]
- 17.Xue W; Maung GYT; Otiti J; Tabucanon AS Land Use-Based Characterization and Source Apportionment of Microplastics in Urban Storm Runoffs in a Tropical Region. Environmental Pollution 2023, 329, 121698. [DOI] [PubMed] [Google Scholar]
- 18.Napper IE; Thompson RC Release of Synthetic Microplastic Plastic Fibres from Domestic Washing Machines: Effects of Fabric Type and Washing Conditions. Marine Pollution Bulletin 2016, 112, 39–45. [DOI] [PubMed] [Google Scholar]
- 19.Sun J; Dai X; Wang Q; Loosdrecht MC; Ni BJ Microplastics in Wastewater Treatment Plants: Detection, Occurrence and Removal. Water Research 2019, 152, 21–37. [DOI] [PubMed] [Google Scholar]
- 20.Choy CA; Robison BH; Gagne TO; Erwin B; Firl E; Halden RU; Houtan SK; The Vertical Distribution and Biological Transport of Marine Microplastics across the Epipelagic and Mesopelagic Water Column. Scientific Reports 2019, 9, 7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai Z; Zhang H; Zhou Q; Tian Y; Chen T; Tu C; Luo Y Occurrence of Microplastics in the Water Column and Sediment in an Inland Sea Affected by Intensive Anthropogenic Activities. Environmental Pollution 2018, 242, 1557–1565. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D; Cui Y; Zhou H; Jin C; Yu X; Xu Y; Zhang C Microplastic Pollution in Water, Sediment, and Fish from Artificial Reefs around the Ma’an Archipelago, Shengsi, China. Science of the Total Environment 2020, 703, 134768. [DOI] [PubMed] [Google Scholar]
- 23.Desforges JPW; Galbraith M; Dangerfield N; Ross PS Widespread Distribution of Microplastics in Subsurface Seawater in the NE Pacific Ocean. Marine Pollution Bulletin 2014, 79, 94–99. [DOI] [PubMed] [Google Scholar]
- 24.Kwon OY; Kang JH; Hong SH; Shim WJ Spatial Distribution of Microplastic in the Surface Waters along the Coast of Korea. Marine Pollution Bulletin 2020, 155, 110729. [DOI] [PubMed] [Google Scholar]
- 25.Wan Y; Chen X; Liu Q; Hu H; Wu C; Xue Q Informal Landfill Contributes to the Pollution of Microplastics in the Surrounding Environment. Environmental Pollution 2022, 293, 118586. [DOI] [PubMed] [Google Scholar]
- 26.Galli M; Baini M; Panti C; Giani D; Caliani I; Campani T; Fossi MC Oceanographic and Anthropogenic Variables Driving Marine Litter Distribution in Mediterranean Protected Areas: Extensive Field Data Supported by Forecasting Modelling. Science of the Total Environment 2023, 903, 166266. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Y; Yang F; Zhao Y; Wang J Greenland Sea Gyre Increases Microplastic Pollution in the Surface Waters of the Nordic Seas. Science of the Total Environment 2020, 712, 136484. [DOI] [PubMed] [Google Scholar]
- 28.Jorquera A; Castillo C; Murillo V; Araya J; Pinochet J; Narváez D; Urbina MA Physical and Anthropogenic Drivers Shaping the Spatial Distribution of Microplastics in the Marine Sediments of Chilean Fjords. Science of the Total Environment 2022, 814, 152506. [DOI] [PubMed] [Google Scholar]
- 29.Ding R; Ouyang F; Peng D; You J; Ding L; Ouyang Z; Guo X A Case Study of Distribution and Characteristics of Microplastics in Surface Water and Sediments of the Seas around Shenzhen, Southern Coastal Area of China. Science of The Total Environment 2022, 838, 156063. [DOI] [PubMed] [Google Scholar]
- 30.Daniel DB; Ashraf PM; Thomas SN Microplastics in the Edible and Inedible Tissues of Pelagic Fishes Sold for Human Consumption in Kerala, India. Environmental Pollution 2020, 266, 115365. [DOI] [PubMed] [Google Scholar]
- 31.Giacinto F; Renzo L; Mascilongo G; Notarstefano V; Gioacchini G; Giorgini E; Berti M Detection of Microplastics, Polymers and Additives in Edible Muscle of Swordfish (Xiphias Gladius) and Bluefin Tuna (Thunnus Thynnus) Caught in the Mediterranean Sea. Journal of Sea Research 2023, 192, 102359. [Google Scholar]
- 32.Lu Y; Zhang Y; Deng Y; Jiang W; Zhao Y; Geng J; Ren H Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio Rerio) and Toxic Effects in Liver. Environmental Science & Technology 2016, 50, 4054–4060. [DOI] [PubMed] [Google Scholar]
- 33.McIlwraith HK; Kim J; Helm P; Bhavsar SP; Metzger JS; Rochman CM Evidence of Microplastic Translocation in Wild-Caught Fish and Implications for Microplastic Accumulation Dynamics in Food Webs. Environmental Science & Technology 2021, 55, 12372–12382. [DOI] [PubMed] [Google Scholar]
- 34.Sucharitakul P; Pitt KA; Welsh DT Trophic Transfer of Microbeads to Jellyfish and the Importance of Aging Microbeads for Microplastic Experiments. Marine Pollution Bulletin 2021, 172, 112867. [DOI] [PubMed] [Google Scholar]
- 35.Joshy A; Sharma SK; Mini KG Microplastic Contamination in Commercially Important Bivalves from the Southwest Coast of India. Environmental Pollution 2022, 305, 119250. [DOI] [PubMed] [Google Scholar]
- 36.Rios-Fuster B; Alomar C; González GP; Martínez RMG; Rojas DLS; Hernando PF; Deudero S Assessing Microplastic Ingestion and Occurrence of Bisphenols and Phthalates in Bivalves, Fish and Holothurians from a Mediterranean Marine Protected Area. Environmental Research 2022, 214, 114034. [DOI] [PubMed] [Google Scholar]
- 37.Rojas-Jimenez K; Villalobos-Rojas F; Gatgens-García J; Rodríguez-Arias M; Hernández-Montero N; Wehrtmann IS Presence of Microplastics in Six Bivalve Species (Mollusca, Bivalvia) Commercially Exploited at the Pacific Coast of Costa Rica, Central America. Marine Pollution Bulletin 2022, 183, 114040. [DOI] [PubMed] [Google Scholar]
- 38.Zhang F; Wang X; Xu J; Zhu L; Peng G; Xu P; Li D Food-Web Transfer of Microplastics between Wild Caught Fish and Crustaceans in East China Sea. Marine Pollution Bulletin 2019, 146, 173–182. [DOI] [PubMed] [Google Scholar]
- 39.Justino AK; Ferreira GV; Fauvelle V; Schmidt N; Lenoble V; Pelage L; Lucena-Frédou F From Prey to Predators: Evidence of Microplastic Trophic Transfer in Tuna and Large Pelagic Species in the Southwestern Tropical Atlantic. Environmental Pollution 2023, 327, 121532. [DOI] [PubMed] [Google Scholar]
- 40.Ugwu K; Herrera A; Gómez M Microplastics in Marine Biota: A Review. Marine Pollution Bulletin 2021, 169, 112540, doi: 10.1016/j.marpolbul.2021.112540. [DOI] [PubMed] [Google Scholar]
- 41.Besseling E; Foekema EM; Van Franeker JA; Leopold MF; Kuhn S; Bravo Rebolledo EL; HeBe E; Mielke L; IJzer J; Kamminga P; et al. Microplastic in a Macro Filter Feeder: Humpback Whale Megaptera Novaeangliae. Marine Pollution Bulletin 2015, 95, 248–252, doi: 10.1016/j.marpolbul.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Donohue MJ; Masura J; Gelatt T; Ream R; Baker JD; Faulhaber K; Lerner DT Evaluating Exposure of Northern Fur Seals, Callorhinus Ursinus, to Microplastic Pollution through Fecal Analysis. Marine Pollution Bulletin 2019, 138, 213–221. [DOI] [PubMed] [Google Scholar]
- 43.Fossi MC; Panti C; Guerranti C; Coppola D; Giannetti M; Marsili L; Minutoli R Are Baleen Whales Exposed to the Threat of Microplastics? A Case Study of the Mediterranean Fin Whale (Balaenoptera Physalus). Marine Pollution Bulletin 2012, 64, 2374–2379, doi: 10.1016/j.marpolbul.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 44.Hart LB; Dziobak M; Wells RS; Ertel B; Weinstein J Microplastics in Gastric Samples from Common Bottlenose Dolphins (Tursiops Truncatus) Residing in Sarasota Bay FL (USA). Front. Mar. Sci 2022, 9, 947124, doi: 10.3389/fmars.2022.947124. [DOI] [Google Scholar]
- 45.Hernandez-Gonzalez A; Saavedra C; Gago J; Covelo P; Santos MB; Pierce GJ Microplastics in the Stomach Contents of Common Dolphin (Delphinus Delphis) Stranded on the Galician Coasts (NW Spain, 2005–2010. Marine Pollution Bulletin 2018, 137, 526–532. [DOI] [PubMed] [Google Scholar]
- 46.Mclvor A; Pires R; Raimundo J; Campos P; Pais M; Lopes C; Canning-Clode J; Dinis A Assessing Microplastic Exposure of the Critically Endangered Mediterranean Monk Seal (Monachus Monachus) on a Remote Oceanic Island. Science of The Total Environment 2023, 856. [DOI] [PubMed] [Google Scholar]
- 47.Moore RC; Loseto L; Noel M; Etemadifar A; Brewster JD; MacPhee S; Ross PS Microplastics in Beluga Whales (Delphinapterus Leucas) from the Eastern Beaufort Sea. Marine Pollution Bulletin 2020, 150, 110723. [DOI] [PubMed] [Google Scholar]
- 48.Nelms SE; Galloway TS; Godley BJ; Jarvis DS; Lindeque PK Investigating Microplastic Trophic Transfer in Marine Top Predators. Environmental Pollution 2018, 238, 999–1007, doi: 10.1016/j.envpol.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 49.Zantis LJ; Bosker T; Lawler F; Nelms SE; O’Rorke R; Constantine R; Carroll EL Assessing Microplastic Exposure of Large Marine Filter-Feeders. Science of The Total Environment 2022, 818, 151815. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X; Luo D; Yu RQ; Xie Z; He L; Wu Y Microplastics in the Endangered Indo-Pacific Humpback Dolphins (Sousa Chinensis) from the Pearl River Estuary, China. Environmental Pollution 2021, 270, 116057. [DOI] [PubMed] [Google Scholar]
- 51.Hart LB; Dziobak M; Wells RS; McCabe EB; Conger E; Curtin T; Knight M; Weinstein J Plastic, It’s What’s for Dinner. A Preliminary Comparison of Ingested Particles in Bottlenose Dolphins and Their Prey. Oceans 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matupang DM; Zulkifli HI; Arnold J; Lazim AM; Ghaffar MA; Musa SM Tropical Sharks Feasting on and Swimming through Microplastics: First Evidence from Malaysia. Marine Pollution Bulletin 2023, 189, 114762. [DOI] [PubMed] [Google Scholar]
- 53.Debbarma N; Gurjar UR; Ramteke KK; Shenoy L; Nayak BB; Bhushan S; Xavier M Abundance and Characteristics of Microplastics in Gastrointestinal Tracts and Gills of Croaker Fish (Johnius Dussumieri) from off Mumbai Coastal Waters of India. Marine Pollution Bulletin 2022, 176, 113473. [DOI] [PubMed] [Google Scholar]
- 54.Neves D; Sobral P; Ferreira Joana Lia; Pereira T. Ingestion of Microplastics by Commercial Fish off the Portuguese Coast. Marine Pollution Bulletin 2015, 101, 119–126. [DOI] [PubMed] [Google Scholar]
- 55.Li B; Liang W; Liu QX; Fu S; Ma C; Chen Q; Shi H Fish Ingest Microplastics Unintentionally. Environmental Science & Technology 2021, 55, 10471–10479. [DOI] [PubMed] [Google Scholar]
- 56.Roch S; Friedrich C; Brinker A Uptake Routes of Microplastics in Fishes: Practical and Theoretical Approaches to Test Existing Theories. Scientific Reports 2020, 10, 3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su L; Deng H; Li B; Chen Q; Pettigrove V; Wu C; Shi H The Occurrence of Microplastic in Specific Organs in Commercially Caught Fishes from Coast and Estuary Area of East China. Journal of Hazardous Materials 2019, 365, 716–724. [DOI] [PubMed] [Google Scholar]
- 58.Hasegawa T; Nakaoka M Trophic Transfer of Microplastics from Mysids to Fish Greatly Exceeds Direct Ingestion from the Water Column. Environmental Pollution 2021, 273, 116468. [DOI] [PubMed] [Google Scholar]
- 59.Mizraji R; Ahrendt C; Perez D; Vargas J; Pulgar J; Aldana M; Galban C Is the Feeding Type Related with the Content of Microplastics in Intertidal Fish Gut? Marine Pollution Bulletin 2017, 116, 498–500. [DOI] [PubMed] [Google Scholar]
- 60.Ory NC; Sobral P; Ferreira JL; Thiel M Amberstripe Scad Decapterus Muroadsi (Carangidae) Fish Ingest Blue Microplastics Resembling Their Copepod Prey along the Coast of Rapa Nui (Easter Island) in the South Pacific Subtropical Gyre. Science of the Total Environment 2017, 586, 430–437. [DOI] [PubMed] [Google Scholar]
- 61.Dziobak MK; Wells RS; Pisarski EC; Wirth EF; Hart LB Demographic Assessment of Mono(2-ethylhexyl) Phthalate (MEHP) and Monoethyl Phthalate (MEP) Concentrations in Common Bottlenose Dolphins ( Tursiops Truncatus ) From Sarasota Bay, FL, USA. GeoHealth 2021, 5, doi: 10.1029/2020GH000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hart LB; Beckingham B; Wells RS; Alten Flagg M; Wischusen K; Moors A; Kucklick J; Pisarski E; Wirth E Urinary Phthalate Metabolites in Common Bottlenose Dolphins (Tursiops Truncatus) From Sarasota Bay, FL, USA. GeoHealth 2018, 2, 313–326, doi: 10.1029/2018GH000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.University of South Florida, W.A. Sarasota Bay Watershed: Geography and Land Use - Sarasota.WaterAtlas.Org Available online: https://www.sarasota.wateratlas.usf.edu/watershed/geography.asp?wshedid=5&wbodyat-las=watershed (accessed on 2 February 2023).
- 64.Sarasota Bay Estuary Program Sarasota Bay Estuary Program Comprehensive Conservation and Management Plan Available online: https://drive.google.com/file/d/1PwMtAe4YZyKG-TZmnv59OkVswD3Rfyy_/view (accessed on 4 August 2024).
- 65.Gulfbase.Org Available online: https://www.gulfbase.org/ (accessed on 4 August 2024).
- 66.Wastewater Division | Sarasota County, FL: Available online: https://www.scgov.net/government/public-utilities/wastewater-division (accessed on 4 August 2024). [Google Scholar]
- 67.Cristaldi A; Fiore M; Zuccarello P; Oliveri Conti G; Grasso A; Nicolosi I; Copat C; Ferrante M Efficiency of Wastewater Treatment Plants (WWTPs) for Microplastic Removal: A Systematic Review. International Journal of Environmental Research and Public Health 2020, 17, 8014, doi: 10.3390/ijerph17218014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.33 USC 1330: National Estuary Program. In 33-NAVIGATION AND NAVIGABLE WATERS; 2024.
- 69.Sarasota, Florida Code of Ordinances ARTICLE IV. - REGULATION OF EXPANDED POLYSTYRENE AND SINGLE-USE DRINKING STRAWS; 2019; Vol. 19–5287;. [Google Scholar]
- 70.Berens McCabe EJ; Wells RS; Toms CN; Barleycorn AA; Wilkinson KA; Palubok VI Effects of Multiple Karenia Brevis Red Tide Blooms on a Common Bottlenose Dolphin (Tursiops Truncatus) Prey Fish Assemblage: Patterns of Resistance and Resilience in Sarasota Bay, Florida. Frontiers in Marine Science 2021, 8, 711114, doi: 10.3389/fmars.2021.711114. [DOI] [Google Scholar]
- 71.Gannon DP; McCabe EJB; Camilleri SA; Gannon JG; Brueggen MK; Barleycorn AA; Wells RS Effects of Karenia Brevis Harmful Algal Blooms on Nearshore Fish Communities in Southwest Florida. Marine Ecology Progress Series 2009, 378, 171–186. [Google Scholar]
- 72.Berens McCabe EJ; Gannon DP; Barros NB; Wells RS Prey Selection by Resident Common Bottlenose Dolphins (Tursiops Truncatus) in Sarasota Bay, Florida. Mar Biol 2010, 157, 931–942, doi: 10.1007/s00227-009-1371-2. [DOI] [Google Scholar]
- 73.Wells RS; McHugh KA; Douglas DC; Shippee S; McCabe EB; Barros NB; Phillips GT Evaluation of Potential Protective Factors against Metabolic Syndrome in Bottlenose Dolphins: Feeding and Activity Patterns of Dolphins in Sarasota Bay, Florida. Frontiers in Endocrinology 2013, 4, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lusher AL; Welden NA; Sobral P; Cole M Sampling, Isolating and Identifying Microplastics Ingested by Fish and Invertebrates. Analytical Methods 2017, 9, 1346–1360, doi: 10.1039/C6AY02415G. [DOI] [Google Scholar]
- 75.De Witte B; Devriese L; Bekaert K; Hoffman S; Vandermeersch G; Cooreman K; Robbens J Quality Assessment of the Blue Mussel (Mytilus Edulis): Comparison between Commercial and Wild Types. Marine Pollution Bulletin 2014, 85, 146–155. [DOI] [PubMed] [Google Scholar]
- 76.Leads RR; Weinstein JE Occurrence of Tire Wear Particles and Other Microplastics within the Tributaries of the Charleston Harbor Estuary, South Carolina, USA. Marine Pollution Bulletin 2019, 145, 569–582. [DOI] [PubMed] [Google Scholar]
- 77.Hartmann NB; Huffer T; Thompson RC; Hassellov M; Verschoor A; Daugaard AE; Wagner M Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environmental Science & Technology 2019, 53, 1039–1047. [DOI] [PubMed] [Google Scholar]
- 78.Bharath KM; Muthulakshmi AL; Natesan U Microplastic Contamination around the Landfills: Distribution, Characterization and Threats: A Review. Current Opinion in Environmental Science & Health 2023, 31, 100422. [Google Scholar]
- 79.Lusher AL; Bråte ILN; Munno K; Hurley RR; Welden NA Is It or Isn’t It: The Importance of Visual Classification in Microplastic Characterization. Applied Spectroscopy 2020, 74, 1139–1153. [DOI] [PubMed] [Google Scholar]
- 80.Devriese LI; van der Meulen MD; Maes T; Bekaert K; Paul-Pont I; Frère L; Robbens J; Vethaak AD Microplastic Contamination in Brown Shrimp (Crangon Crangon, Linnaeus 1758) from Coastal Waters of the Southern North Sea and Channel Area. Marine Pollution Bulletin 2015, 98, 179–187, doi: 10.1016/j.marpolbul.2015.06.051. [DOI] [PubMed] [Google Scholar]
- 81.Robertson DR; Tassell JV Shorefishes of the Greater Caribbean: Online Information System. Version 3.0 Available online: https://biogeodb.stri.si.edu/caribbean/en/pages (accessed on 7 April 2024).
- 82.Atamanalp M; Köktürk M; Uçar A; Duyar HA; Özdemir S; Parlak V; Esenbuğa N; Alak G Microplastics in Tissues (Brain, Gill, Muscle and Gastrointestinal) of Mullus Barbatus and Alosa Immaculata. Archives of Environmental Contamination and Toxicology 2021, 81, 460–469, doi: 10.1007/s00244-021-00885-5. [DOI] [PubMed] [Google Scholar]
- 83.Zitouni N; Cappello T; Missawi O; Boughattas I; De Marco G; Belbekhouche S; Mokni M; Alphonse V; Guerbej H; Bousserrhine N Metabolomic Disorders Unveil Hepatotoxidty of Environmental Microplastics in Wild Fish Serranus Scriba (Linnaeus 1758). Science of the Total Environment 2022, 838, 155872. [DOI] [PubMed] [Google Scholar]
- 84.McEachern K; Alegria H; Kalagher AL; Hansen C; Morrison S; Hastings D Microplastics in Tampa Bay, Florida: Abundance and Variability in Estuarine Waters and Sediments. Marine pollution bulletin 2019, 148, 97–106. [DOI] [PubMed] [Google Scholar]
- 85.Luo W; Su L; Craig NJ; Du F;Wu C; Shi H Comparison of Microplastic Pollution in Different Water Bodies from Urban Creeks to Coastal Waters. Environmental Pollution 2019, 246, 174–182, doi: 10.1016/j.envpol.2018.11.081. [DOI] [PubMed] [Google Scholar]
- 86.Acha EM; Mianzan HW; Iribarne O; Gagliardini DA; Lasta C; Daleo P The Role of the Rıo de La Plata Bottom Salinity Front in Accumulating Debris. Marine Pollution Bulletin 2003, 46, 197–202. [DOI] [PubMed] [Google Scholar]
- 87.Zitouni N; Bousserrhine N; Belbekhouche S; Missawi O; Alphonse V; Boughatass I; Banni M First Report on the Presence of Small Microplastics (≤ 3 Mm) in Tissue of the Commercial Fish Serranus Scriba (Linnaeus. 1758) from Tunisian Coasts and Associated Cellular Alterations. Environmental Pollution 2020, 263, 114576. [DOI] [PubMed] [Google Scholar]
- 88.Makhdoumi P; Hossini H; Nazmara Z; Mansouri K; Pirsaheb M Occurrence and Exposure Analysis of Microplastic in the Gut and Muscle Tissue of Riverine Fish in Kermanshah Province of Iran. Marine Pollution Bulletin 2021, 173, 112915. [DOI] [PubMed] [Google Scholar]
- 89.Zeytin S; Wagner G; Mackay-Roberts N; Gerdts G; Schuirmann E; Klockmann S; Slater M Quantifying Microplastic Translocation from Feed to the Fillet in European Sea Bass Dicentrarchus Labrax. Marine Pollution Bulletin 2020, 156, 111210, doi: 10.1016/j.marpolbul.2020.111210. [DOI] [PubMed] [Google Scholar]
- 90.Zhang S; Wang N; Gong S; Gao S The Patterns of Trophic Transfer of Microplastic Ingestion by Fish in the Artificial Reef Area and Adjacent Waters of Haizhou Bay. Marine Pollution Bulletin 2022, 177, 113565, doi: 10.1016/j.marpolbul.2022.113565. [DOI] [PubMed] [Google Scholar]
- 91.Montgomery JLM; Targett TE The Nutritional Role of Seagrass in the Diet of the Omnivorous Pinfish Lagodon Rhomboides (L.). Journal of Experimental Marine Biology and Ecology 1992, 158, 37–57, doi: 10.1016/0022-0981(92)90307-V. [DOI] [Google Scholar]
- 92.Goss H; Jaskiel J; Rotjan R Thalassia Testudinum as a Potential Vector for Incorporating Microplastics into Benthic Marine Food Webs. Marine pollution bulletin 2018, 135, 1085–1089. [DOI] [PubMed] [Google Scholar]
- 93.Aytan U; Esensoy FB; Senturk Y Microplastic Ingestion and Egestion by Copepods in the Black Sea. Science of The Total Environment 2022, 806, 150921. [DOI] [PubMed] [Google Scholar]
- 94.Barros NB; Wells RS Prey and Feeding Patterns of Resident Bottlenose Dolphins (Tursiops Truncatus) in Sarasota Bay, Florida. Journal of Mammalogy 1998, 79, 1045–1059, doi: 10.2307/1383114. [DOI] [Google Scholar]
- 95.Moore R; Noel M; Etemadifar A; Loseto L; Posacka A; Bendell L; Ross P Microplastics in Beluga Whale (Delphinapterus Leucas) Prey: An Exploratory Assessment of Trophic Transfer in the Beaufort Sea. Science of the Total Environment 2022, 806, 150201. [DOI] [PubMed] [Google Scholar]
- 96.Jin Y; Xia J; Pan Z; Yang J; Wang W; Fu Z Polystyrene Microplastics Induce Microbiota Dysbiosis and Inflammation in the Gut of Adult Zebrafish. Environmental Pollution 2018, 235, 322–329. [DOI] [PubMed] [Google Scholar]
- 97.Solomando A; Capó X; Alomar C; Álvarez E; Compa M; Valencia JM; Pinya S; Deudero S; Sureda A Long-Term Exposure to Microplastics Induces Oxidative Stress and a pro-Inflammatory Response in the Gut of Sparus Aurata Linnaeus, 1758. Environmental Pollution 2020, 266, 115295. [DOI] [PubMed] [Google Scholar]
- 98.Gupta P; Mahapatra A; Suman A; Ray SS; Malafaia G; Singh RK Polystyrene Microplastics Disrupt Female Reproductive Health and Fertility via Sirt1 Modulation in Zebrafish (Danio Rerio). Journal of Hazardous Materials 2023, 460, 132359. [DOI] [PubMed] [Google Scholar]
- 99.Sayed E-DH; Hamed M; Ismail RF Natural Antioxidants Can Improve Microplastics-Induced Male Reproductive Impairment in the African Catfish (Clarias Gariepinus). Frontiers in Environmental Science 2022, 9, 811466. [Google Scholar]
- 100.Jin H; Yang C; Jiang C; Li L; Pan M; Li D; Han X; Ding J Evaluation of Neurotoxicity in BALB/c Mice Following Chronic Exposure to Polystyrene Microplastics. Environmental health perspectives 2022, 130, 107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liao X-L; Chen Z-F; Ou S-P; Liu Q-Y; Lin S-H; Zhou J-M; Wang Y; Cai Z Neurological Impairment Is Crucial for Tire Rubber-Derived Contaminant 6PPDQ-Induced Acute Toxicity to Rainbow Trout. Science Bulletin 2024, 69, 621–635. [DOI] [PubMed] [Google Scholar]
- 102.Jin Y; Lu L; Tu W; Luo T; Fu Z Impacts of Polystyrene Microplastic on the Gut Barrier, Microbiota and Metabolism of Mice. Science of the total environment 2019, 649, 308–317. [DOI] [PubMed] [Google Scholar]
- 103.Wan Z; Wang C; Zhou J; Shen M; Wang X; Fu Z; Jin Y Effects of Polystyrene Microplastics on the Composition of the Microbiome and Metabolism in Larval Zebrafish. Chemosphere 2019, 217, 646–658. [DOI] [PubMed] [Google Scholar]
- 104.Florida Seafood and Aquaculture Overview and Statistics / Agriculture Industry / Home - Florida Department of Agriculture & Consumer Services Available online: https://www.fdacs.gov/Agriculture-Industry/Florida-Sea-food-and-Aquaculture-Overview-and-Statistics (accessed on 7 April 2024).
- 105.Search FishBase Available online: https://www.fishbase.se/search.php (accessed on 25 August 2023) [Google Scholar]
- 106.Faletti ME; Chacin DH; Peake JA; MacDonald TC; Stallings CD Population Dynamics of Pinfish in the Eastern Gulf of Mexico (1998-2016). PLoS One 2019, 14, e0221131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ohs CL; Creswell RL; Dimaggio MA Growing Marine Baitfish: A Guide to Florida’s Common Marine Baitfish and Their Potential for Aquaculture 2013. [Google Scholar]
- 108.Akoueson F; Sheldon Lisa M.; Danopoulos E; Morris S; Hotten J; Chapman E; Li J; Jeanette M. Rotchell A Preliminary Analysis of Microplastics in Edible versus Non-Edible Tissues from Seafood Samples. Environmental Pollution 2020, 263 A. [DOI] [PubMed] [Google Scholar]
- 109.Foekema EM; De Gruijter C; Mergia MT; van Franeker JA; Murk AJ; Koelmans AA Plastic in North Sea Fish. Environmental science & technology 2013, 47, 8818–8824. [DOI] [PubMed] [Google Scholar]
- 110.Beckingham B; Apintiloaiei A; Moore C; Brandes J Hot or Not: Systematic Review and Laboratory Evaluation of the Hot Needle Test for Microplastic Identification. Microplastics and Nanoplastics 2023, 3, 8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the reported results can be found in the DRYAD data repository (datadryad.org) using this DOI: 10.5061/dryad.nvx0k6


