Abstract
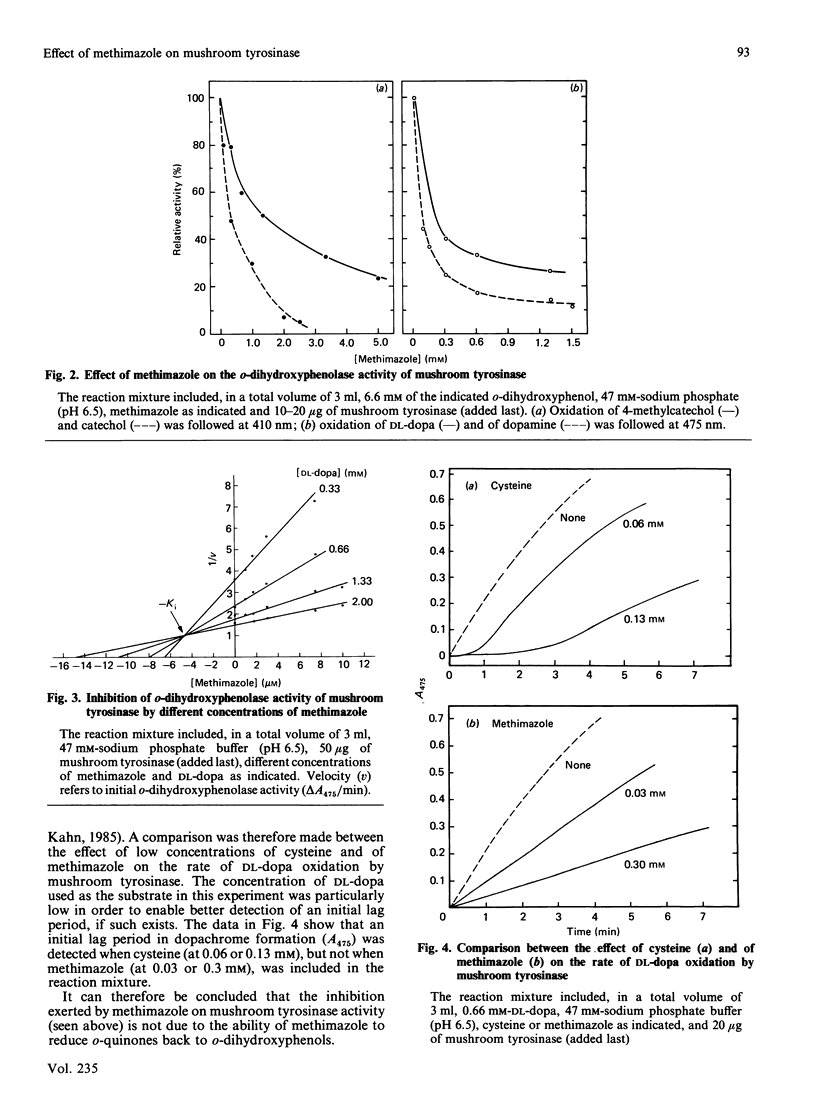
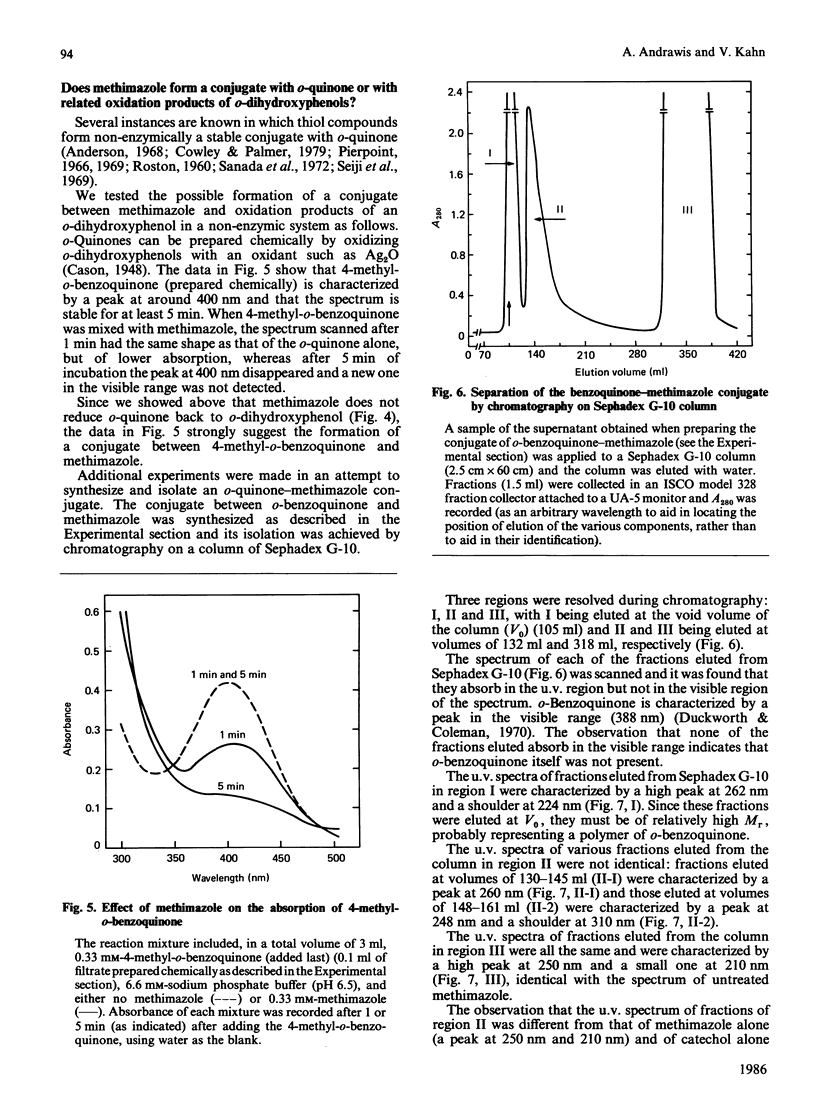
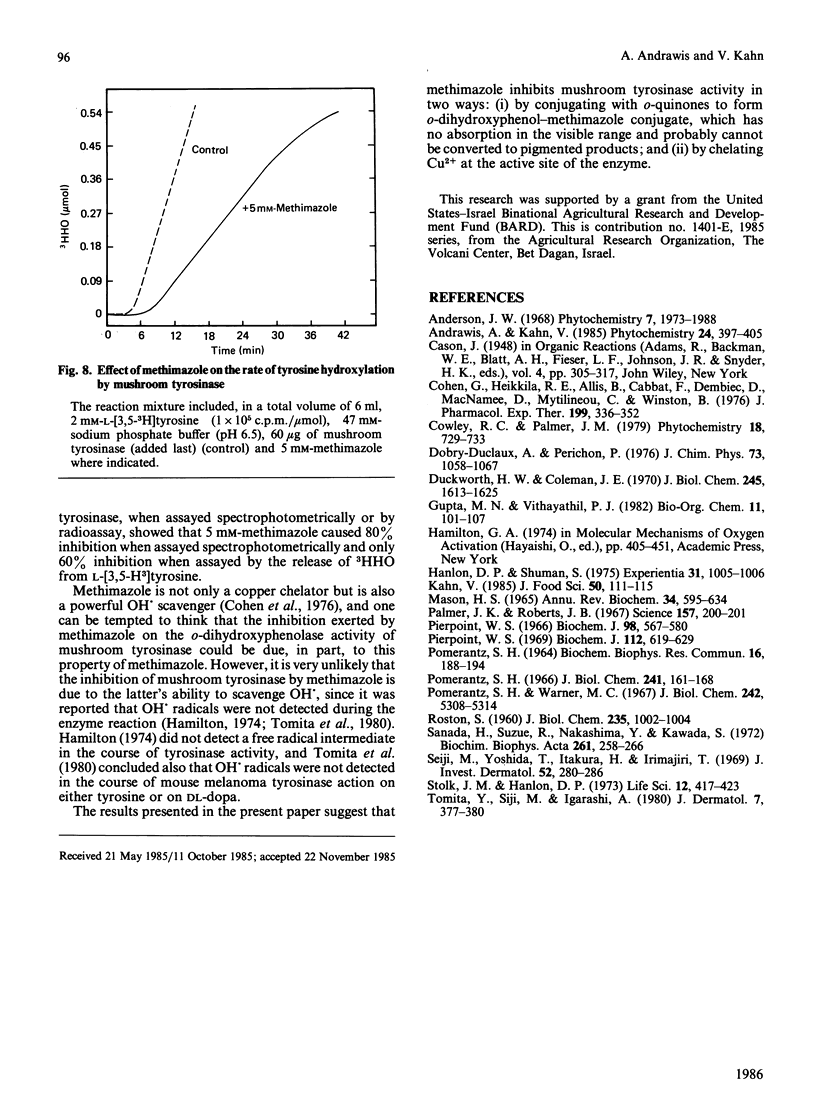
Methimazole (1-methyl-2-mercaptoimidazole) inhibits both the mono- and the o-dihydroxyphenolase activities of mushroom tyrosinase when assayed spectrophotometrically. With DL-3,4-dihydroxyphenylalanine as substrate, the inhibition was found to be a mixed-type one with Ki 4.6 X 10(-6) M. We found that methimazole can interact with the oxidation products of o-dihydroxyphenols, probably with o-quinones, to form a conjugate. The conjugate formed between methimazole and o-benzoquinone was separated by chromatography on Sephadex G-10 and was characterized by an absorption maximum at 248-260 nm. Our data suggest that methimazole inhibits mushroom tyrosinase activity in two ways: by conjugating with o-quinones, thereby causing an apparent inhibition in pigmented product formation as judged by the spectrophotometric assay; and by chelating copper at the active site of the enzyme, as judged by assaying the release of 3HHO from L-[3,5-3H]tyrosine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen G., Heikkila R. E., Allis B., Cabbat F., Dembiec D., MacNamee D., Mytilineou C., Winston B. Destruction of sympathetic nerve terminals by 6-hydroxydopamine: protection by 1-phenyl-3-(2-thiazolyl)-2-thiourea, diethyldithiocarbamate, methimazole, cysteamine, ethanol and n-butanol. J Pharmacol Exp Ther. 1976 Nov;199(2):336–352. [PubMed] [Google Scholar]
- Duckworth H. W., Coleman J. E. Physicochemical and kinetic properties of mushroom tyrosinase. J Biol Chem. 1970 Apr 10;245(7):1613–1625. [PubMed] [Google Scholar]
- Hanlon D. P., Shuman S. Copper ion binding and enzyme inhibitory properties of the antithyroid drug methimazole. Experientia. 1975 Sep 15;31(9):1005–1006. doi: 10.1007/BF02326924. [DOI] [PubMed] [Google Scholar]
- MASON H. S. OXIDASES. Annu Rev Biochem. 1965;34:595–634. doi: 10.1146/annurev.bi.34.070165.003115. [DOI] [PubMed] [Google Scholar]
- Palmer J. K., Roberts J. B. Inhibition of banana polyphenoloxidase by 2-mercaptobenzothiazole. Science. 1967 Jul 14;157(3785):200–201. doi: 10.1126/science.157.3785.200. [DOI] [PubMed] [Google Scholar]
- Pierpoint W. S. The enzymic oxidation of chlorogenic acid and some reactions of the quinone produced. Biochem J. 1966 Feb;98(2):567–580. doi: 10.1042/bj0980567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpoint W. S. o-Quinones formed in plant extracts. Their reaction with bovine serum albumin. Biochem J. 1969 May;112(5):619–629. doi: 10.1042/bj1120619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz S. H. The tyrosine hydroxylase activity of mammalian tyrosinase. J Biol Chem. 1966 Jan 10;241(1):161–168. [PubMed] [Google Scholar]
- Pomerantz S. H. Tyrosine hydroxylation catalyzed by mammalian tyrosinase: an improved method of assay. Biochem Biophys Res Commun. 1964 Jun 1;16(2):188–194. doi: 10.1016/0006-291x(64)90359-6. [DOI] [PubMed] [Google Scholar]
- Pomerantz S. H., Warner M. C. 3,4-dihydroxy-L-phenylalanine as the tyrosinase cofactor. Occurrence in melanoma and binding constant. J Biol Chem. 1967 Nov 25;242(22):5308–5314. [PubMed] [Google Scholar]
- ROSTON S. Reaction of the sulfhydryl group with an oxidation product of beta-3,4-dihydroxyphenyl-L-alanine. J Biol Chem. 1960 Apr;235:1002–1004. [PubMed] [Google Scholar]
- Sanada H., Suzue R., Nakashima Y., Kawada S. Effect of thiol compounds on melanin formation by tyrosinase. Biochim Biophys Acta. 1972 Jan 28;261(1):258–266. doi: 10.1016/0304-4165(72)90336-4. [DOI] [PubMed] [Google Scholar]
- Seiji M., Yoshida T., Itakura H., Irimajiri T. Inhibition of melanin formation by sulfhydryl compounds. J Invest Dermatol. 1969 Mar;52(3):280–286. [PubMed] [Google Scholar]
- Stolk J. M., Hanlon D. P. Inhibition of brain dopamine- -hydroxylase activity by methimazole. Life Sci II. 1973 May 8;12(9):417–423. doi: 10.1016/0024-3205(73)90324-x. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Seiji M., Igarashi A. The hydroxyl radical is not involved with tyrosinase inactivation. J Dermatol. 1980 Oct;7(5):377–380. doi: 10.1111/j.1346-8138.1980.tb01986.x. [DOI] [PubMed] [Google Scholar]


