Abstract
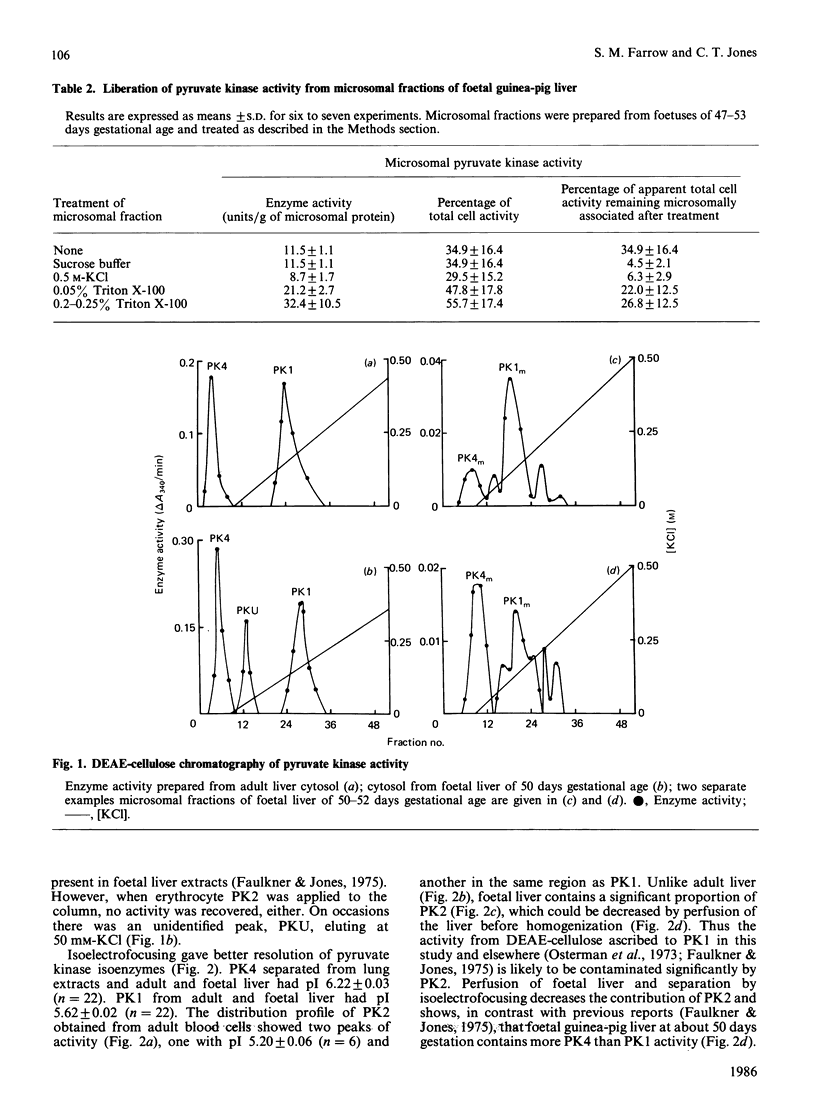
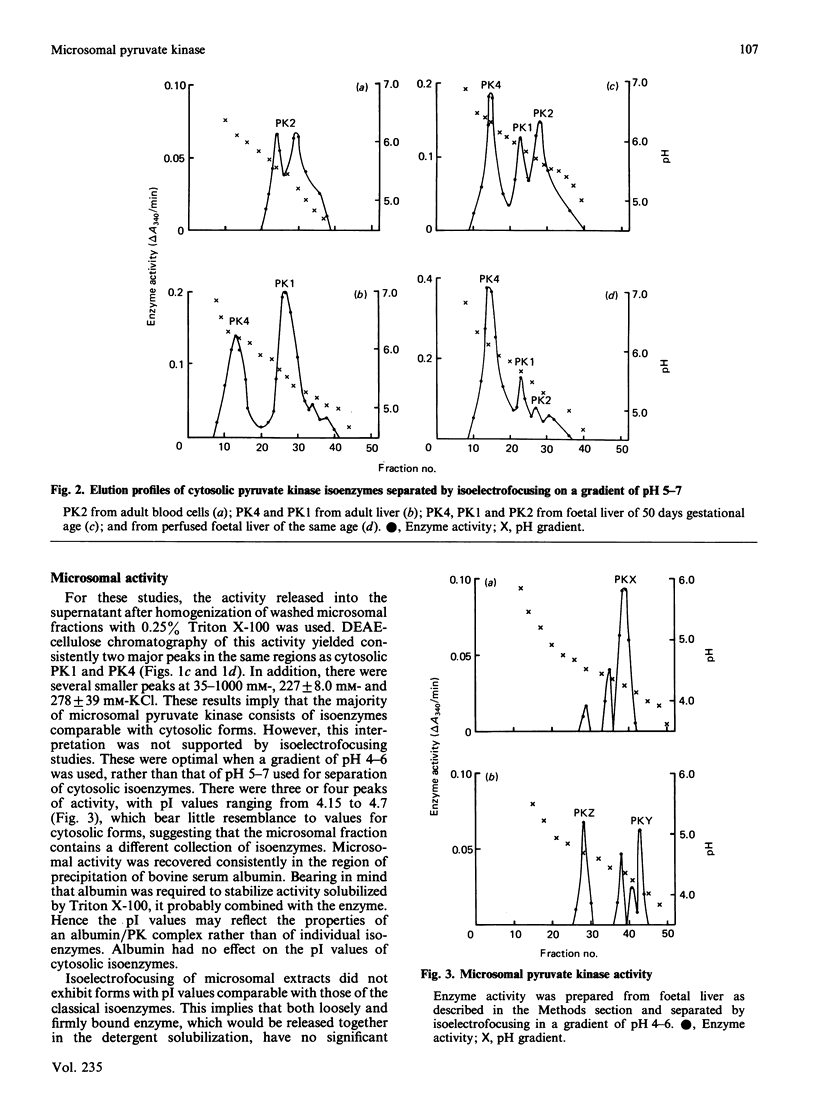
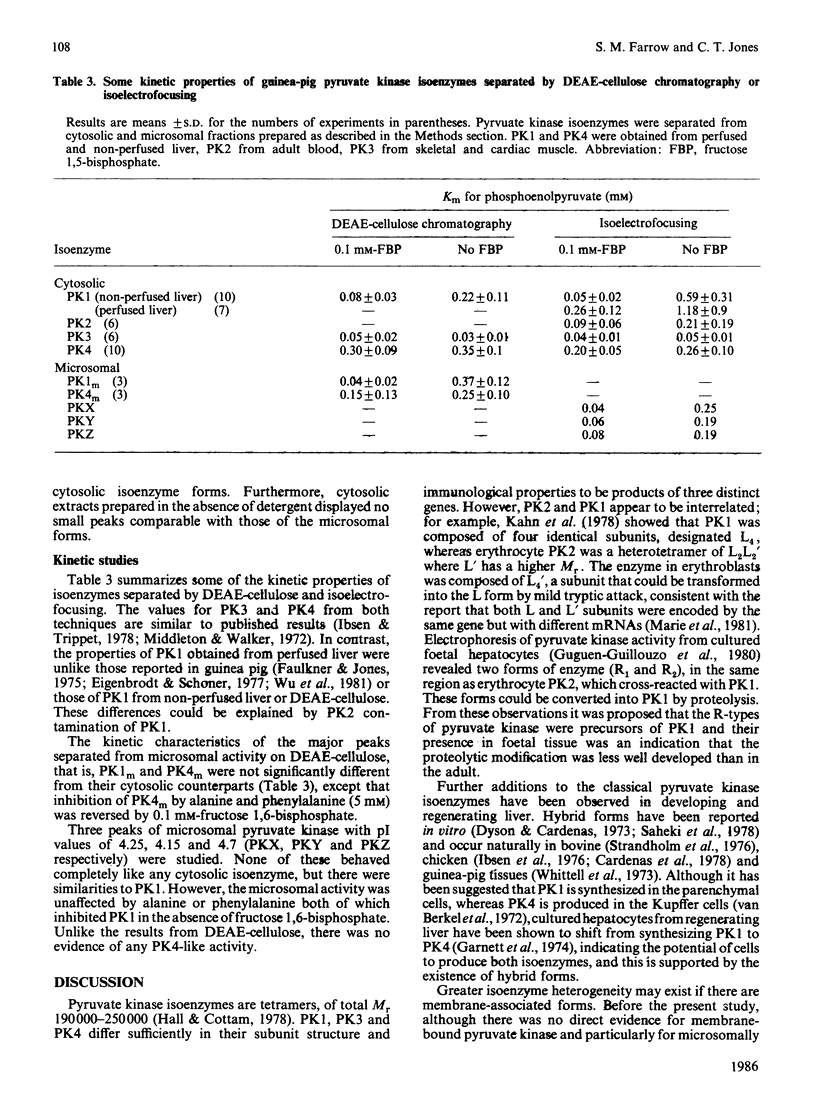
During analysis of pyruvate kinase distribution in developing guinea-pig liver it was observed that a substantial proportion of the activity remained associated with the microsomal membrane fraction ('microsomes'). Although some of this could be removed by washing with sucrose, the majority required detergent treatment for liberation, and even then at least one-half remained attached to the microsomes. Estimates of the contribution of this fraction to total cell pyruvate kinase activity indicated that it was more than 50% of the total, and this is likely to be an underestimate because of the continued latency of the enzyme even in the presence of detergent. The susceptibility of the microsomal enzyme, whether released by detergent or sucrose washing, to inactivation by Triton X-100 suggested it to be different from the cytosolic enzyme, which was stable under such conditions. (The microsomal enzyme required the presence of additional protein, such as bovine serum albumin, to maintain stability.) This view was confirmed by DEAE-cellulose chromatography and particularly isoelectric focusing, where the microsomal enzyme was shown to consist of at least four forms, which were distinctly different from those in the cytosol. Those data and the kinetic properties of the four forms in the membrane fraction indicate that the microsomal pyruvate kinase could consist of four counterparts to the cytosolic isoenzyme forms. These results are discussed in relation to the two possible explanations for the phenomenon (not mutually exclusive): that the more hydrophobic membrane forms are precursors of the cytosolic enzyme and that they may be part of functional glycolytic pathway in the microsomes of developing liver.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELS J., BOUMA W., NIEWEG H. O. Assay of intrinsic factor with anti-intrinsic factor serum in vitro. Biochim Biophys Acta. 1963 Apr 2;71:227–229. doi: 10.1016/0006-3002(63)91016-3. [DOI] [PubMed] [Google Scholar]
- ALVAREZ E. F. The kinetics and mechanism of the hydrolysis of phosphoric acid esters by potato acid phosphatase. Biochim Biophys Acta. 1962 Jun 4;59:663–672. doi: 10.1016/0006-3002(62)90646-7. [DOI] [PubMed] [Google Scholar]
- Agostoni A., Vergani C., Villa L. Intracellular distribution of the different forms of lactic dehydrogenase. Nature. 1966 Mar 5;209(5027):1024–1025. doi: 10.1038/2091024a0. [DOI] [PubMed] [Google Scholar]
- Arnold H., Pette D. Binding of aldolase and triosephosphate dehydrogenase to F-actin and modification of catalytic properties of aldolase. Eur J Biochem. 1970 Aug;15(2):360–366. doi: 10.1111/j.1432-1033.1970.tb01016.x. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Brattin W. J., Jr, Waller R. L., Recknagel R. O. Analysis of microsomal calcium sequestration by steady state isotope exchange. Enzyme kinetics and role of membrane permeability. J Biol Chem. 1982 Sep 10;257(17):10044–10051. [PubMed] [Google Scholar]
- Cardenas J. M., Bandman E., Strohman R. C. Hybrid isozymes of pyruvate kinase appear during avian cardiac development. Biochem Biophys Res Commun. 1978 Feb 14;80(3):593–599. doi: 10.1016/0006-291x(78)91610-8. [DOI] [PubMed] [Google Scholar]
- Cardenas J. M., Dyson R. D. Bovine pyruvate kinases. II. Purification of the liver isozyme and its hybridization with skeletal muscle pyruvate kinase. J Biol Chem. 1973 Oct 25;248(20):6938–6944. [PubMed] [Google Scholar]
- Cardenas J. M., Hubbard D. R., Anderson S. Subunit structure and hybrid formation of bovine pyruvate kinases. Biochemistry. 1977 Jan 25;16(2):191–197. doi: 10.1021/bi00621a005. [DOI] [PubMed] [Google Scholar]
- Cartier P., Najman A., Leroux J. P., Temkine H. Les anomalies de la glycolyse au cours de l'anémie hémolytique par déficit du globule rouge en pyruvate kinase. Clin Chim Acta. 1968 Oct;22(2):165–181. doi: 10.1016/0009-8981(68)90354-9. [DOI] [PubMed] [Google Scholar]
- Clarke F. M., Masters C. J. On the association of glycolytic enzymes with structural proteins of skeletal muscle. Biochim Biophys Acta. 1975 Jan 13;381(1):37–46. doi: 10.1016/0304-4165(75)90187-7. [DOI] [PubMed] [Google Scholar]
- Clarke F. M., Masters C. J., Winzor D. J. The different adsorption of aldolase isoenzymes in rat brain. Biochem J. 1970 Jun;118(2):325–327. doi: 10.1042/bj1180325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson R. D., Cardenas J. M. Bovine pyruvate kinases. 3. Hybrids of the liver and skeletal muscle isozymes. J Biol Chem. 1973 Dec 25;248(24):8482–8488. [PubMed] [Google Scholar]
- Dyson R. D., Cardenas J. M., Richards T. C., Garnett M. E. Pyruvate kinase isozymes in cells isolated from fetal and regenerating rat liver. Biochim Biophys Acta. 1977 Mar 15;481(1):115–126. doi: 10.1016/0005-2744(77)90143-7. [DOI] [PubMed] [Google Scholar]
- Eigenbrodt E., Schoner W. Purification and properties of the pyruvate kinase isoenzymes type L and M2 from chicken liver. Hoppe Seylers Z Physiol Chem. 1977 Aug;358(8):1033–1046. doi: 10.1515/bchm2.1977.358.2.1033. [DOI] [PubMed] [Google Scholar]
- Fahimi H. D., Karnovsky M. J. Cytochemical localization of two glycolytic dehydrogenases in white skeletal muscle. J Cell Biol. 1966 Apr;29(1):113–128. doi: 10.1083/jcb.29.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina F. A., Shatton J. B., Morris H. P., Weinhouse S. Isozymes of pyruvate kinase in liver and hepatomas of the rat. Cancer Res. 1974 Jun;34(6):1439–1446. [PubMed] [Google Scholar]
- Faulkner A., Jones C. T. Pyruvate kinase isoenzymes in tissues of the developing guinea pig. Arch Biochem Biophys. 1975 Sep;170(1):228–241. doi: 10.1016/0003-9861(75)90114-9. [DOI] [PubMed] [Google Scholar]
- Faulkner A., Jones C. T. The distribution of enzyme and isoenzyme activities between parenchymal and haematopoietic cells in the liver of the foetal guinea pig. Biochem J. 1979 Jan 15;178(1):89–95. doi: 10.1042/bj1780089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foemmel R. S., Gray R. H., Bernstein I. A. Intracellular localization of fructose 1,6-bisphosphate aldolase. J Biol Chem. 1975 Mar 10;250(5):1892–1897. [PubMed] [Google Scholar]
- Garnett M. E., Dyson R. D., Dost F. N. Pyruvate kinase isozyme changes in parenchymal cells of regenerating rat liver. J Biol Chem. 1974 Aug 25;249(16):5222–5226. [PubMed] [Google Scholar]
- Green D. E., Murer E., Hultin H. O., Richardson S. H., Salmon B., Brierley G. P., Baum H. Association of integrated metabolic pathways with membranes. I. Glycolytic enzymes of the red blood corpuscle and yeast. Arch Biochem Biophys. 1965 Dec;112(3):635–647. doi: 10.1016/0003-9861(65)90107-4. [DOI] [PubMed] [Google Scholar]
- Guguen-Guillouzo C., Marie J., Cottreau D., Pasdeloup N., Kahn A. Isozyme shift in cultured fetal human hepatocytes: a study of pyruvate kinase and phosphofructokinase. Biochem Biophys Res Commun. 1980 Mar 28;93(2):528–534. doi: 10.1016/0006-291x(80)91109-2. [DOI] [PubMed] [Google Scholar]
- HOGEBOOM G. H., SCHNEIDER W. C. Intracellular distribution of enzymes. XI. Glutamic dehydrogenase. J Biol Chem. 1953 Sep;204(1):233–238. [PubMed] [Google Scholar]
- Hall E. R., Cottam G. L. Isozymes of pyruvate kinase in vertebrates: their physical, chemical, kinetic and immunological properties. Int J Biochem. 1978;9(11):785–793. doi: 10.1016/0020-711x(78)90027-7. [DOI] [PubMed] [Google Scholar]
- Hopkirk T. J., Bloxham D. P. Biosynthesis of rat liver pyruvate kinase. Measurement of enzyme lifetime and the rate of synthesis at weaning. Biochem J. 1980 Nov 15;192(2):507–516. doi: 10.1042/bj1920507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibsen K. H., Murray L., Marles S. W. Electrofocusing and kinetic studies of adult and embryonic chicken pyruvate kinases. Biochemistry. 1976 Mar 9;15(5):1064–1073. doi: 10.1021/bi00650a017. [DOI] [PubMed] [Google Scholar]
- Ibsen K. H., Schiller K. W., Haas T. A. Interconvertible kinetic and physical forms of human erythrocyte pyruvate kinase. J Biol Chem. 1971 Mar 10;246(5):1233–1240. [PubMed] [Google Scholar]
- Ibsen K. H., Trippet P. A comparison of kinetic parameters obtained with three major non-interconvertible isozymes of rat pyruvate kinase. Arch Biochem Biophys. 1973 Jun;156(2):730–744. doi: 10.1016/0003-9861(73)90326-3. [DOI] [PubMed] [Google Scholar]
- Imamura K., Tanaka T. Multimolecular forms of pyruvate kinase from rat and other mammalian tissues. I. Electrophoretic studies. J Biochem. 1972 Jun;71(6):1043–1051. doi: 10.1093/oxfordjournals.jbchem.a129852. [DOI] [PubMed] [Google Scholar]
- Jaussi R., Sonderegger P., Flückiger J., Christen P. Biosynthesis and topogenesis of aspartate aminotransferase isoenzymes in chicken embryo fibroblasts. The precursor of the mitochondrial isoenzyme is either imported into mitochondria or degraded in the cytosol. J Biol Chem. 1982 Nov 25;257(22):13334–13340. [PubMed] [Google Scholar]
- Kahn A., Marie J., Garreau H., Sprengers E. D. The genetic system of the L-type pyruvate kinase forms in man. Subunit structure, interrelation and kinetic characteristics of the pyruvate kinase enzymes from erythrocytes and liver. Biochim Biophys Acta. 1978 Mar 14;523(1):59–74. doi: 10.1016/0005-2744(78)90009-8. [DOI] [PubMed] [Google Scholar]
- Kawajiri K., Harano T., Omura T. Biogenesis of the mitochondrial matrix enzyme, glutamate dehydrogenase, in rat liver cells. II. Significance of binding of glutamate dehydrogenase to microsomal membrane. J Biochem. 1977 Nov;82(5):1417–1423. doi: 10.1093/oxfordjournals.jbchem.a131829. [DOI] [PubMed] [Google Scholar]
- Knull H. R., Taylor W. F., Wells W. W. Insulin effects on brain energy metabolism and the related hexokinase distribution. J Biol Chem. 1974 Nov 10;249(21):6930–6935. [PubMed] [Google Scholar]
- Lakomek M., Scharnetzky M., Tillmann W., Schröter W., Winkler H. On the temperature- and salt-dependent conformation change in human erythrocyte pyruvate kinase. Hoppe Seylers Z Physiol Chem. 1983 Jul;364(7):787–792. doi: 10.1515/bchm2.1983.364.2.787. [DOI] [PubMed] [Google Scholar]
- Leffert H. L., Paul D. Studies on primary cultures of differentiated fetal liver cells. J Cell Biol. 1972 Mar;52(3):559–568. doi: 10.1083/jcb.52.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie J., Kahn A., Boivin P. Human erythrocyte pyruvate kinase. Total purification and evidence for its antigenic identity with L-type enzyme. Biochim Biophys Acta. 1977 Mar 15;481(1):96–104. doi: 10.1016/0005-2744(77)90141-3. [DOI] [PubMed] [Google Scholar]
- Marie J., Simon M. P., Dreyfus J. C., Kahn A. One gene, but two messenger RNAs encode liver L and red cell L' pyruvate kinase subunits. Nature. 1981 Jul 2;292(5818):70–72. doi: 10.1038/292070a0. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Hawthorne J. N. The site of diphosphoinositide synthesis in rat liver. Biochem Biophys Res Commun. 1965 Nov 22;21(4):333–338. doi: 10.1016/0006-291x(65)90198-1. [DOI] [PubMed] [Google Scholar]
- Middleton M. C., Walker D. G. Comparison of the properties of two forms of pyruvate kinase in rat liver and determination of their separate activities during development. Biochem J. 1972 May;127(4):721–731. doi: 10.1042/bj1270721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton D. J., Clarke F. M., Masters C. J. An electron microscope study of the interaction between fructose diphosphate aldolase and actin-containing filaments. J Cell Biol. 1977 Sep;74(3):1016–1023. doi: 10.1083/jcb.74.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman J., Fritz P. J., Wuntch T. Pyruvate kinase isozymes from rat tissues. Developmental studies. J Biol Chem. 1973 Feb 10;248(3):1011–1018. [PubMed] [Google Scholar]
- Papahadjopoulos D., Moscarello M., Eylar E. H., Isac T. Effects of proteins on thermotropic phase transitions of phospholipid membranes. Biochim Biophys Acta. 1975 Sep 2;401(3):317–335. doi: 10.1016/0005-2736(75)90233-3. [DOI] [PubMed] [Google Scholar]
- Parker J. C., Hoffman J. F. The role of membrane phosphoglycerate kinase in the control of glycolytic rate by active cation transport in human red blood cells. J Gen Physiol. 1967 Mar;50(4):893–916. doi: 10.1085/jgp.50.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. H., Cardenas J. M. Analysis of the renaturation kinetics of bovine muscle pyruvate kinase. Biochemistry. 1980 Jul 22;19(15):3447–3452. doi: 10.1021/bi00556a007. [DOI] [PubMed] [Google Scholar]
- SCHRIER S. L. Studies of the metabolism of human erythrocyte membranes. J Clin Invest. 1963 Jun;42:756–766. doi: 10.1172/JCI104768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki S., Harada K., Sanno Y., Tanaka T. Hybrid isozymes of rat pyruvate kinase. Their subunit structure and developmental changes in the liver. Biochim Biophys Acta. 1978 Sep 11;526(1):116–128. doi: 10.1016/0005-2744(78)90296-6. [DOI] [PubMed] [Google Scholar]
- Schrier S. L., Ben-Bassat I., Junga I., Seeger M., Grumet F. C. Characterization of erythrocyte membrane-associated enzymes (glyceraldehyde-3-phosphate dehydrogenase and phosphoglyceric kinase). J Lab Clin Med. 1975 May;85(5):797–810. [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandholm J. J., Dyson R. D., Cardenas J. M. Bovine pyruvate kinase isozymes and hybrid isozymes. Electrophoretic studies and tissue distribution. Arch Biochem Biophys. 1976 Mar;173(1):125–131. doi: 10.1016/0003-9861(76)90242-3. [DOI] [PubMed] [Google Scholar]
- Susor W. A., Rutter W. J. Some distinctive properties of pyruvate kinase purified from rat liver. Biochem Biophys Res Commun. 1968 Jan 11;30(1):14–20. doi: 10.1016/0006-291x(68)90705-5. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Harano Y., Sue F., Morimura H. Crystallization, characterization and metabolic regulation of two types of pyruvate kinase isolated from rat tissues. J Biochem. 1967 Jul;62(1):71–91. doi: 10.1093/oxfordjournals.jbchem.a128639. [DOI] [PubMed] [Google Scholar]
- Tillman W., Cordua A., Schröter W. Organization of enzymes of glycolysis and of glutathione metabolism in human red cell membranes. Biochim Biophys Acta. 1975 Mar 13;382(2):157–171. doi: 10.1016/0005-2736(75)90174-1. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T. L., Zieske J. D., Bernstein I. A. Reversible microsomal binding of hepatic aldolase. Biochim Biophys Acta. 1981 Oct 13;661(2):221–229. doi: 10.1016/0005-2744(81)90007-3. [DOI] [PubMed] [Google Scholar]
- Whittell N. M., Ng D. O., Prabhakararao K., Holmes R. S. A comparative electrophoretic analysis of mammalian pyruvate kinase isozymes. Comp Biochem Physiol B. 1973 Sep 15;46(1):71–80. doi: 10.1016/0305-0491(73)90046-1. [DOI] [PubMed] [Google Scholar]
- Wilson J. E. Brain hexokinase. A proposed relation between soluble-particulate distribution and activity in vivo. J Biol Chem. 1968 Jul 10;243(13):3640–3647. [PubMed] [Google Scholar]
- de Domenech E. M., Domenech C. E., Blanco A. Distribution of lactate dehydrogenase isozymes in subcellular fractions of rat tissues. Arch Biochem Biophys. 1970 Nov;141(1):147–154. doi: 10.1016/0003-9861(70)90117-7. [DOI] [PubMed] [Google Scholar]
- van Berkel T. J., Koster J. F., Hülsmann W. C. Distribution of L- and M-type pyruvate kinase between parenchymal and Kupffer cells of rat liver. Biochim Biophys Acta. 1972 Aug 28;276(2):425–429. doi: 10.1016/0005-2744(72)91003-0. [DOI] [PubMed] [Google Scholar]


