Abstract
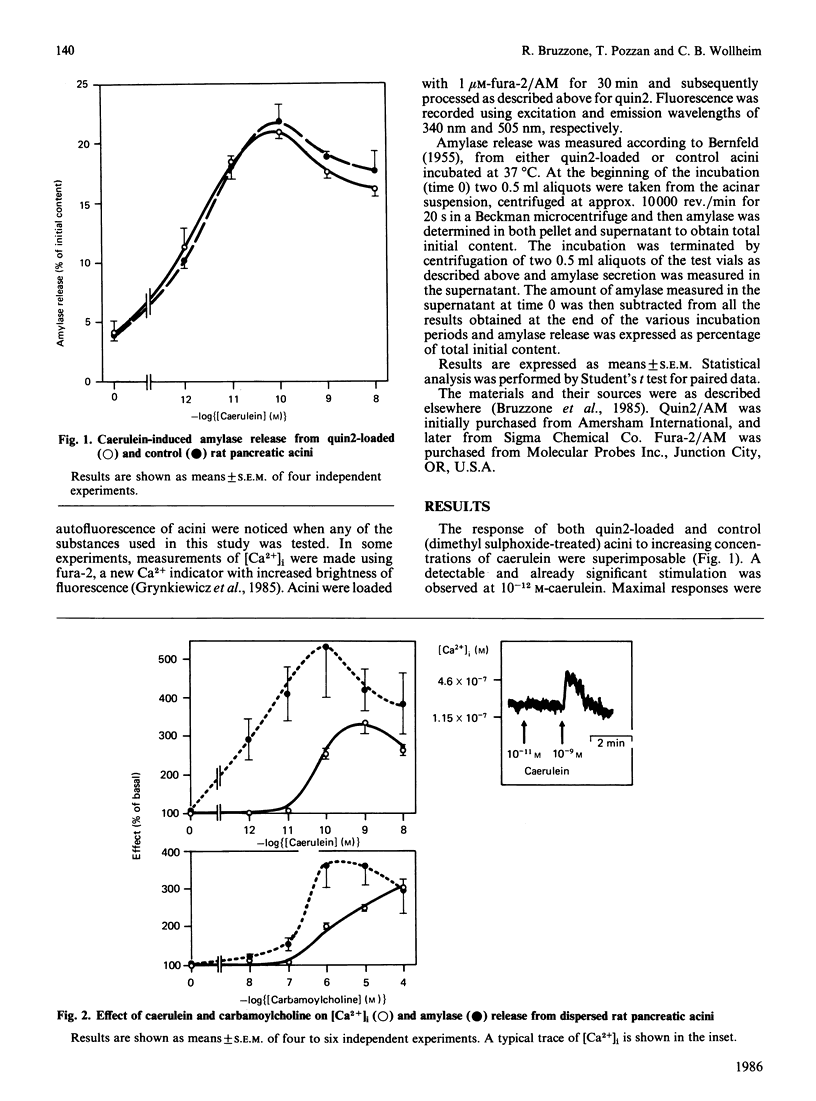
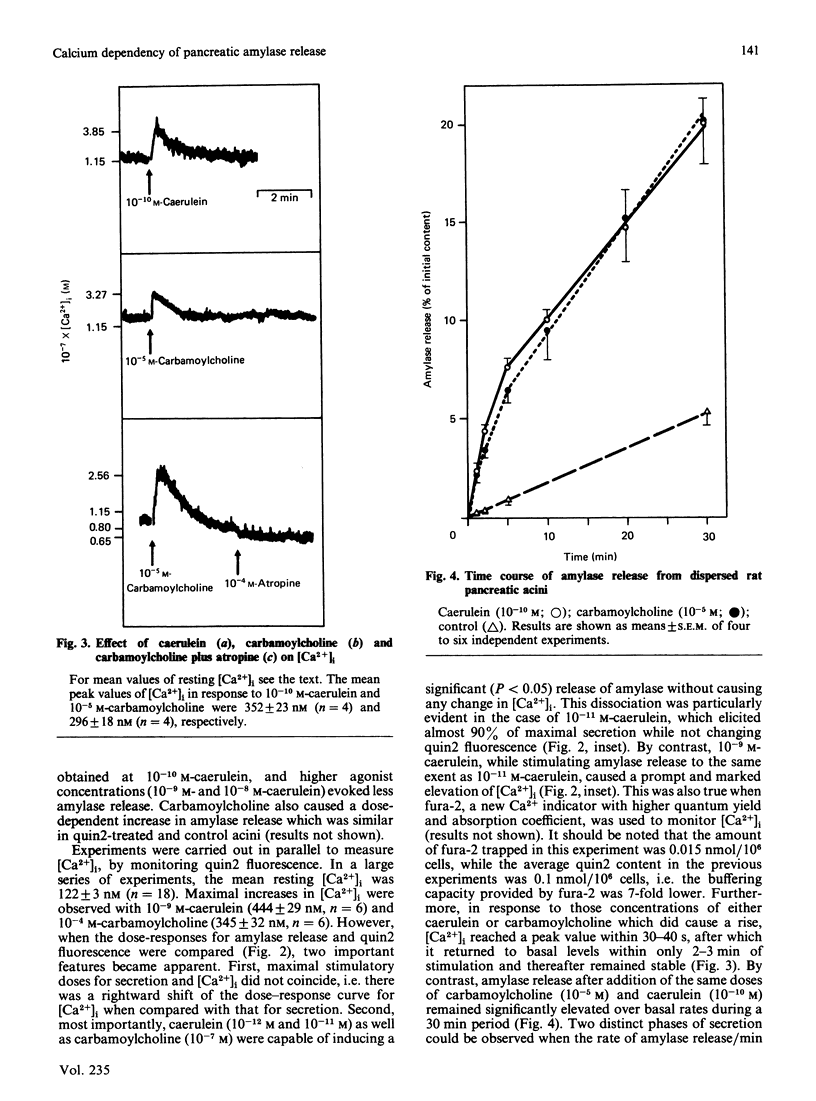
Cytosolic free calcium concentrations ([Ca2+]i) and amylase secretion were measured in isolated rat pancreatic acini loaded with the intracellularly trapped fluorescent indicator quin2. Both caerulein and carbamoylcholine caused a rapid increase in [Ca2+]i, with a maximal 3-fold increase at 10(-9) M-caerulein and 10(-4) M-carbamoylcholine. However, caerulein (10(-12) M and 10(-11) M) as well as carbamoylcholine (10(-7) M) caused a significant stimulation of amylase release, while not inducing any detectable rise in [Ca2+]i. Changes in [Ca2+]i after addition of either secretagogue were transient and did not last more than 2-3 min. By contrast, when amylase secretion was monitored as a function of time, two distinct secretory phases could be observed upon addition of either carbamoylcholine (10(-5) M) or caerulein (10(-10) M). An initial, rapid phase (0-5 min) which caused a 6-7-fold increase above basal, followed by a sustained (5-30 min), but less marked, secretory rate (2-3-fold above basal). Addition of atropine (10(-4) M) 5 min after carbamoylcholine (10(-5) M) (i.e. after termination of the rise in [Ca2+]i and of the first secretory phase) did not cause any significant change in [Ca2+]i, while significantly inhibiting amylase secretion from 5 to 30 min to the same rate observed in the absence of the secretagogue. These results show that caerulein and carbamoylcholine, two agents thought to activate secretion mainly through mobilization of Ca2+ from intracellular stores, are capable of eliciting amylase secretion independently of a concomitant rise in [Ca2+]i. Furthermore, with both secretagogues the rise in [Ca2+]i, when observed, was only transient, while the stimulation of amylase release was sustained.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beglinger C., Fried M., Whitehouse I., Jansen J. B., Lamers C. B., Gyr K. Pancreatic enzyme response to a liquid meal and to hormonal stimulation. Correlation with plasma secretin and cholecystokinin levels. J Clin Invest. 1985 May;75(5):1471–1476. doi: 10.1172/JCI111850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beglinger C., Solomon T. E., Gyr K., Moroder L., Wünsch E. Exocrine pancreatic secretion in response to a new CCK-analog, CCK33 and caerulein in dogs. Regul Pept. 1984 Jul;8(4):291–296. doi: 10.1016/0167-0115(84)90038-7. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bruzzone R., Halban P. A., Gjinovci A., Trimble E. R. A new, rapid, method for preparation of dispersed pancreatic acini. Biochem J. 1985 Mar 1;226(2):621–624. doi: 10.1042/bj2260621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Lew D. P., Pozzan T. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984 Aug 23;310(5979):691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- Erspamer V. Progress report: caerulein. Gut. 1970 Jan;11(1):79–87. doi: 10.1136/gut.11.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Kolesnick R. N., Gershengorn M. C. Direct evidence that burst but not sustained secretion of prolactin stimulated by thyrotropin-releasing hormone is dependent on elevation of cytoplasmic calcium. J Biol Chem. 1985 May 10;260(9):5217–5220. [PubMed] [Google Scholar]
- Long B. W., Gardner J. D. Effects of cholecystokinin on adenylate cyclase activity in dispersed pancreatic acinar cells. Gastroenterology. 1977 Nov;73(5):1008–1014. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Noguchi M., Adachi H., Gardner J. D., Jensen R. T. Calcium-activated, phospholipid-dependent protein kinase in pancreatic acinar cells. Am J Physiol. 1985 Jun;248(6 Pt 1):G692–G701. doi: 10.1152/ajpgi.1985.248.6.G692. [DOI] [PubMed] [Google Scholar]
- Ochs D. L., Korenbrot J. I., Williams J. A. Intracellular free calcium concentrations in isolated pancreatic acini; effects of secretagogues. Biochem Biophys Res Commun. 1983 Nov 30;117(1):122–128. doi: 10.1016/0006-291x(83)91549-8. [DOI] [PubMed] [Google Scholar]
- Orchard J. L., Davis J. S., Larson R. E., Farese R. V. Effects of carbachol and pancreozymin (cholecystokinin-octapeptide) on polyphosphoinositide metabolism in the rat pancreas in vitro. Biochem J. 1984 Jan 1;217(1):281–287. doi: 10.1042/bj2170281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S., Sachs G., Muallem S. Role of free cytosolic calcium in secretagogue-stimulated amylase release from dispersed acini from guinea pig pancreas. J Biol Chem. 1985 Aug 25;260(18):10081–10086. [PubMed] [Google Scholar]
- Pandol S. J., Thomas M. W., Schoeffield M. S., Sachs G., Muallem S. Role of calcium in cholecystokinin-stimulated phosphoinositide breakdown in exocrine pancreas. Am J Physiol. 1985 May;248(5 Pt 1):G551–G560. doi: 10.1152/ajpgi.1985.248.5.G551. [DOI] [PubMed] [Google Scholar]
- Powers R. E., Johnson P. C., Houlihan M. J., Saluja A. K., Steer M. L. Intracellular Ca2+ levels and amylase secretion in Quin 2-loaded mouse pancreatic acini. Am J Physiol. 1985 May;248(5 Pt 1):C535–C541. doi: 10.1152/ajpcell.1985.248.5.C535. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Gatti G., Dozio N., Vicentini L. M., Meldolesi J. Ca2+-dependent and -independent release of neurotransmitters from PC12 cells: a role for protein kinase C activation? J Cell Biol. 1984 Aug;99(2):628–638. doi: 10.1083/jcb.99.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Rubin R. P., Godfrey P. P., Chapman D. A., Putney J. W., Jr Secretagogue-induced formation of inositol phosphates in rat exocrine pancreas. Implications for a messenger role for inositol trisphosphate. Biochem J. 1984 Apr 15;219(2):655–659. doi: 10.1042/bj2190655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. P. Stimulation of inositol trisphosphate accumulation and amylase secretion by caerulein in pancreatic acini. J Pharmacol Exp Ther. 1984 Dec;231(3):623–627. [PubMed] [Google Scholar]
- Schulz I. Messenger role of calcium in function of pancreatic acinar cells. Am J Physiol. 1980 Nov;239(5):G335–G347. doi: 10.1152/ajpgi.1980.239.5.G335. [DOI] [PubMed] [Google Scholar]
- Streb H., Bayerdörffer E., Haase W., Irvine R. F., Schulz I. Effect of inositol-1,4,5-trisphosphate on isolated subcellular fractions of rat pancreas. J Membr Biol. 1984;81(3):241–253. doi: 10.1007/BF01868717. [DOI] [PubMed] [Google Scholar]
- Streb H., Heslop J. P., Irvine R. F., Schulz I., Berridge M. J. Relationship between secretagogue-induced Ca2+ release and inositol polyphosphate production in permeabilized pancreatic acinar cells. J Biol Chem. 1985 Jun 25;260(12):7309–7315. [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A. Regulation of pancreatic acinar cell function by intracellular calcium. Am J Physiol. 1980 Apr;238(4):G269–G279. doi: 10.1152/ajpgi.1980.238.4.G269. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Pozzan T. Correlation between cytosolic free Ca2+ and insulin release in an insulin-secreting cell line. J Biol Chem. 1984 Feb 25;259(4):2262–2267. [PubMed] [Google Scholar]


