Abstract
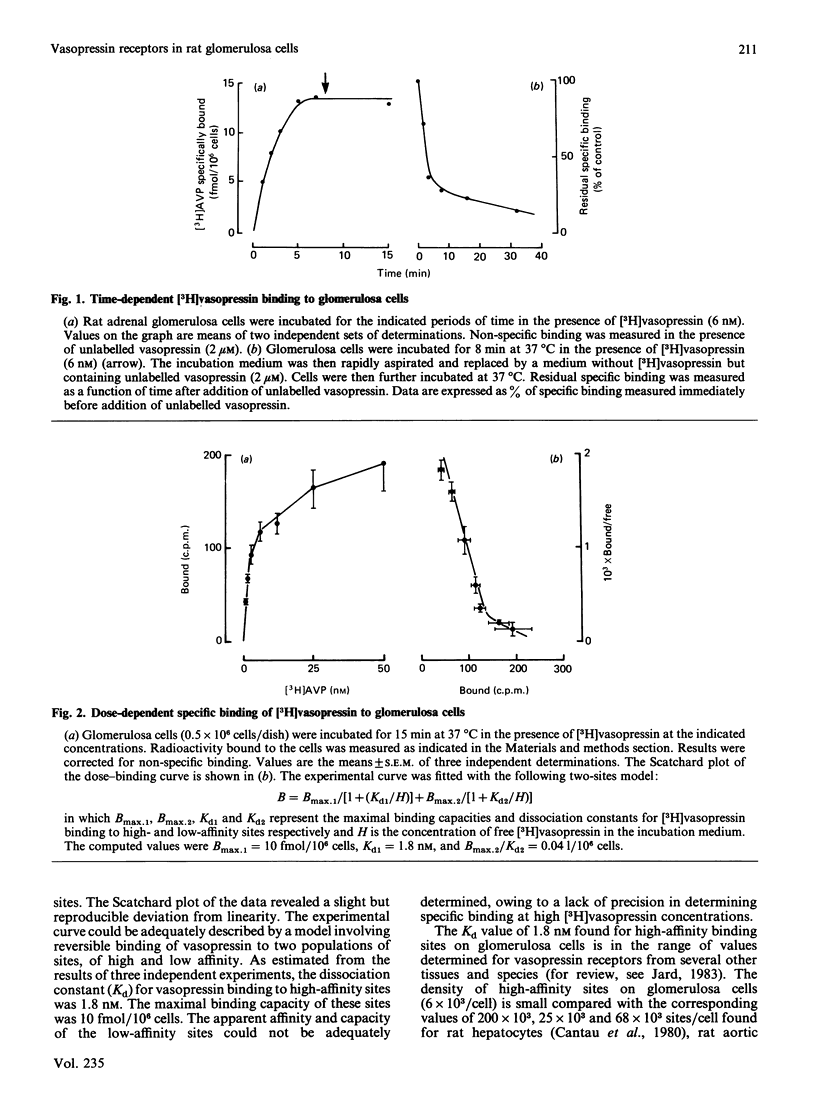
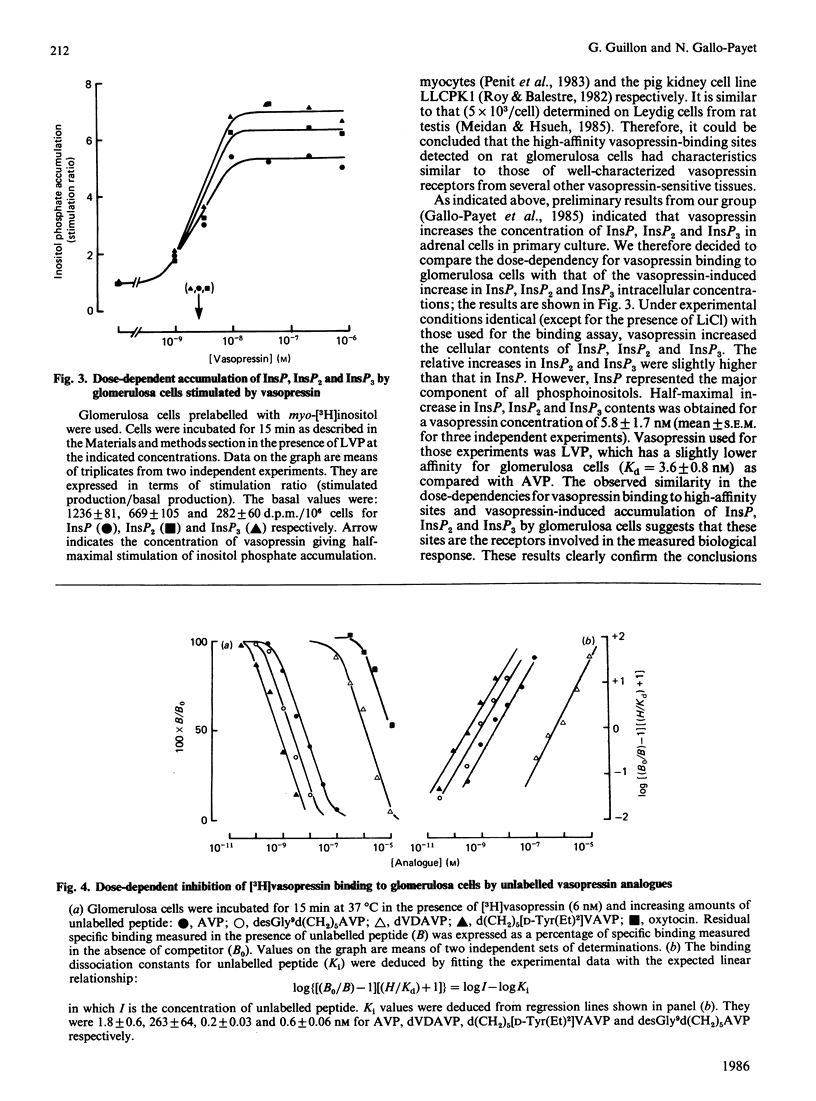
Cells from the zona glomerulosa of rat adrenals were isolated and maintained for 3 days in primary culture. Specific vasopressin binding was determined by using [3H]vasopressin. [3H]Vasopressin binding was time-dependent (half-time of about 2 min for 6 nM free ligand) and reversible on addition of unlabelled vasopressin (80% dissociation within 30 min). Dose-dependent [3H]vasopressin binding at equilibrium indicated that vasopressin interacted with two populations of sites: high-affinity sites (dissociation constant, Kd = 1.8 nM; maximal binding capacity = 10 fmol/10(6) cells) and low-affinity sites. Vasopressin increased the cellular content of labelled inositol mono-, bis- and tris-phosphate in cells prelabelled with myo-[3H]inositol. The vasopressin concentration eliciting half-maximal inositol phosphate accumulation was very close to the Kd value for vasopressin binding to high-affinity sites. Competition experiments using agonists and antagonists with enhanced selectivity for previously characterized vasopressin receptors indicated that vasopressin receptors from rat glomerulosa cells are V1 receptors of the vascular or hepatic subtype. The detected specific vasopressin-binding sites might represent the specific receptors mediating the mitogenic and steroidogenic effects of vasopressin on glomerulosa cells from rat adrenals.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoni F. A. Novel ligand specificity of pituitary vasopressin receptors in the rat. Neuroendocrinology. 1984 Aug;39(2):186–188. doi: 10.1159/000123976. [DOI] [PubMed] [Google Scholar]
- Baertschi A. J., Friedli M. A novel type of vasopressin receptor on anterior pituitary corticotrophs? Endocrinology. 1985 Feb;116(2):499–502. doi: 10.1210/endo-116-2-499. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Cantau B., Keppens S., De Wulf H., Jard S. (3H)-vasopressin binding to isolated rat hepatocytes and liver membranes: regulation by GTP and relation to glycogen phosphorylase activation. J Recept Res. 1980;1(2):137–168. doi: 10.3109/10799898009044096. [DOI] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meidan R., Hsueh A. J. Identification and characterization of arginine vasopressin receptors in the rat testis. Endocrinology. 1985 Jan;116(1):416–423. doi: 10.1210/endo-116-1-416. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Billah M. M. Hormonal stimulation of phosphatidylinositol breakdown with particular reference to the hepatic effects of vasopressin. Biochem Soc Trans. 1979 Oct;7(5):861–865. doi: 10.1042/bst0070861. [DOI] [PubMed] [Google Scholar]
- Payet N., Déziel Y., Lehoux J. G. Vasopressin: a potent growth factor in adrenal glomerulosa cells in culture. J Steroid Biochem. 1984 Jan;20(1):449–454. doi: 10.1016/0022-4731(84)90252-8. [DOI] [PubMed] [Google Scholar]
- Payet N., Lehoux J. G. A comparative study of the role of vasopressin and ACTH in the regulation of growth and function of rat adrenal glands. J Steroid Biochem. 1980 Jan;12:461–467. doi: 10.1016/0022-4731(80)90307-6. [DOI] [PubMed] [Google Scholar]
- Payet N., Lehoux J. G. Aldosterone and corticosterone stimulation by ACTH in isolated rat adrenal glomerulosa cells: interaction with vasopressin. J Physiol (Paris) 1982;78(3):317–321. [PubMed] [Google Scholar]
- Penit J., Faure M., Jard S. Vasopressin and angiotensin II receptors in rat aortic smooth muscle cells in culture. Am J Physiol. 1983 Jan;244(1):E72–E82. doi: 10.1152/ajpendo.1983.244.1.E72. [DOI] [PubMed] [Google Scholar]
- Sawyer W. H., Grzonka Z., Manning M. Neurohypophysial peptides. Design of tissue-specific agonists and antagonists. Mol Cell Endocrinol. 1981 May;22(2):117–134. doi: 10.1016/0303-7207(81)90086-1. [DOI] [PubMed] [Google Scholar]


