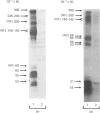
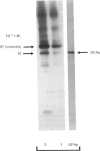
Abstract
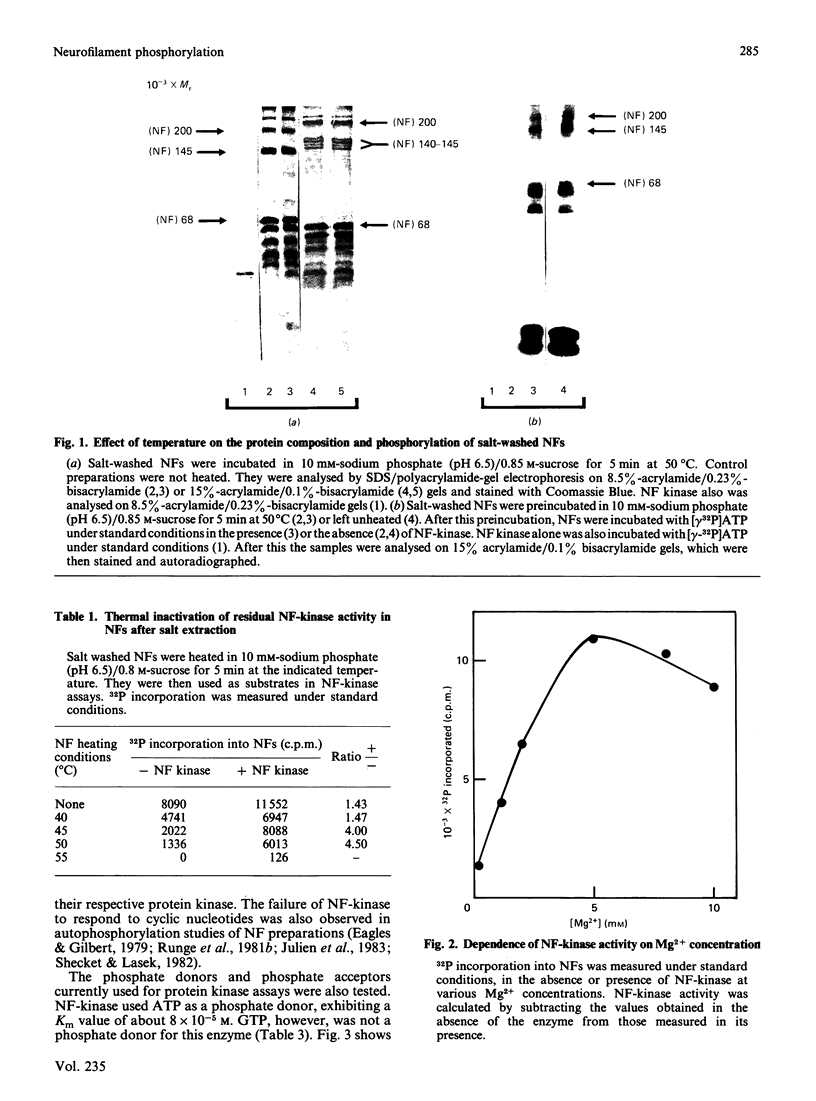
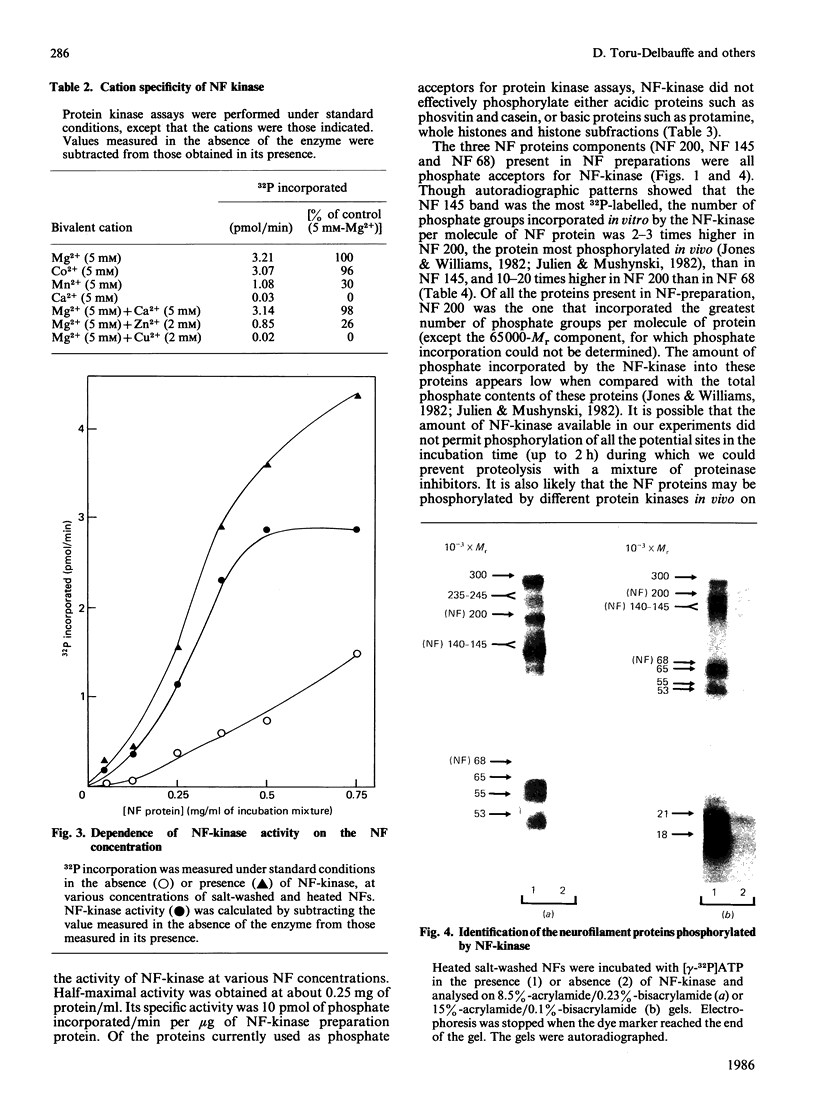
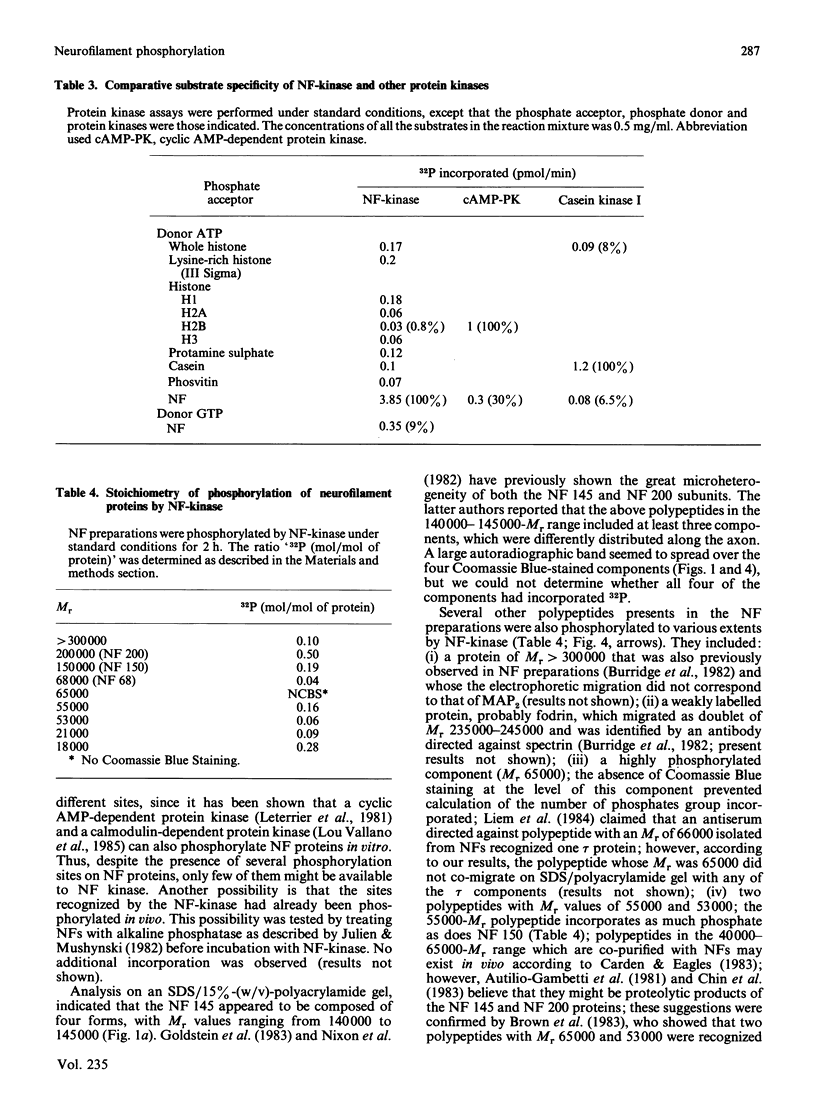
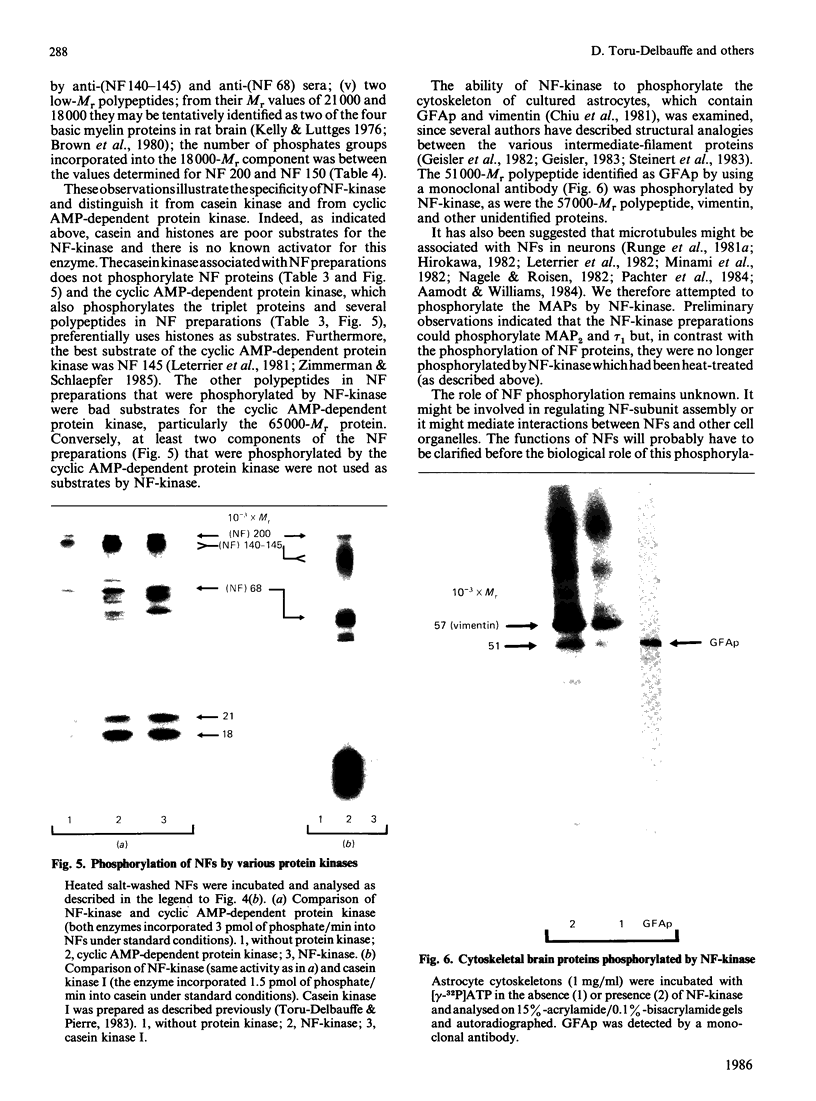
Neurofilament (NF) protein kinase, partially purified from NF preparations [Toru-Delbauffe & Pierre (1983) FEBS Lett. 162, 230-234], was found to be distinct from both the casein kinase present in NFs and the cyclic AMP-dependent protein kinase which is able to phosphorylate NFs. NF-kinase phosphorylated the three NF protein components. The amount of phosphate incorporated per molecule was higher for NF 200 than for NF 145 and NF 68. Other proteins present in the NF preparations were also used as NF-kinase substrates. Two of them might correspond to the myelin basic proteins with Mr values of 18,000 and 21,000. Four other substrates in the NF preparation were not identified (respective Mr values 53,000, 55,000, 65,000 and greater than 300,000). NF kinase also phosphorylated two additional brain-cell cytoskeletal elements: GFAp and vimentin. Casein, histones and phosvitin, currently used as substrates for protein kinase assays, were very poor phosphate acceptors. Half-maximal NF-kinase activity was obtained at an NF protein concentration of about 0.25 mg/ml in heated, salt-washed, NF preparations. The specific activity was about 5 pmol of 32P incorporated/min per microgram of NF kinase preparation protein. ATP was a phospho-group donor (Km 8 X 10(-5) M), but GTP was not. NF-kinase activity remained stable at 65 degrees C for more than 1 h. The enzyme was not degraded by storage at -20 degrees C for several months in a buffer containing 50% (w/v) sucrose. Maximal activity was obtained with 5 mM-Mg2+ (Mg2+ could be replaced by Co2+); Zn2+ and Cu2+ inhibited the reaction. NF-kinase was not dependent on cyclic AMP, cyclic GMP, Ca2+ or Ca2+ plus dioleoylglycerol and phosphatidylserine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aamodt E. J., Williams R. C., Jr Microtubule-associated proteins connect microtubules and neurofilaments in vitro. Biochemistry. 1984 Dec 4;23(25):6023–6031. doi: 10.1021/bi00320a019. [DOI] [PubMed] [Google Scholar]
- Autilio-Gambetti L., Velasco M. E., Sipple J., Gambetti P. Immunochemical characterization of antisera to rat neurofilament subunits. J Neurochem. 1981 Nov;37(5):1260–1265. doi: 10.1111/j.1471-4159.1981.tb04676.x. [DOI] [PubMed] [Google Scholar]
- Brown B. A., Majocha R. E., Staton D. M., Marotta C. A. Axonal polypeptides cross-reactive with antibodies to neurofilament proteins. J Neurochem. 1983 Feb;40(2):299–308. doi: 10.1111/j.1471-4159.1983.tb11283.x. [DOI] [PubMed] [Google Scholar]
- Burridge K., Kelly T., Mangeat P. Nonerythrocyte spectrins: actin-membrane attachment proteins occurring in many cell types. J Cell Biol. 1982 Nov;95(2 Pt 1):478–486. doi: 10.1083/jcb.95.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden M. J., Eagles P. A. Neurofilaments from ox spinal nerves. Isolation, disassembly, reassembly and cross-linking properties. Biochem J. 1983 Nov 1;215(2):227–237. doi: 10.1042/bj2150227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin T. K., Eagles P. A., Maggs A. The proteolytic digestion of ox neurofilaments with trypsin and alpha-chymotrypsin. Biochem J. 1983 Nov 1;215(2):239–252. doi: 10.1042/bj2150239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu F. C., Norton W. T. Bulk preparation of CNS cytoskeleton and the separation of individual neurofilament proteins by gel filtration: dye-binding characteristics and amino acid compositions. J Neurochem. 1982 Nov;39(5):1252–1260. doi: 10.1111/j.1471-4159.1982.tb12562.x. [DOI] [PubMed] [Google Scholar]
- Chiu F. C., Norton W. T., Fields K. L. The cytoskeleton of primary astrocytes in culture contains actin, glial fibrillary acidic protein, and the fibroblast-type filament protein, vimentin. J Neurochem. 1981 Jul;37(1):147–155. doi: 10.1111/j.1471-4159.1981.tb05302.x. [DOI] [PubMed] [Google Scholar]
- Fellous A., Francon J., Lennon A. M., Nunez J. Microtubule assembly in vitro. Purification of assembly-promoting factors. Eur J Biochem. 1977 Aug 15;78(1):167–174. doi: 10.1111/j.1432-1033.1977.tb11726.x. [DOI] [PubMed] [Google Scholar]
- Geisler N., Plessmann U., Weber K. Related amino acid sequences in neurofilaments and non-neural intermediate filaments. Nature. 1982 Apr 1;296(5856):448–450. doi: 10.1038/296448a0. [DOI] [PubMed] [Google Scholar]
- Goldstein M. E., Sternberger L. A., Sternberger N. H. Microheterogeneity ("neurotypy") of neurofilament proteins. Proc Natl Acad Sci U S A. 1983 May;80(10):3101–3105. doi: 10.1073/pnas.80.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Cross-linker system between neurofilaments, microtubules, and membranous organelles in frog axons revealed by the quick-freeze, deep-etching method. J Cell Biol. 1982 Jul;94(1):129–142. doi: 10.1083/jcb.94.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. M., Williams R. C., Jr Phosphate content of mammalian neurofilaments. J Biol Chem. 1982 Sep 10;257(17):9902–9905. [PubMed] [Google Scholar]
- Julien J. P., Mushynski W. E. Multiple phosphorylation sites in mammalian neurofilament polypeptides. J Biol Chem. 1982 Sep 10;257(17):10467–10470. [PubMed] [Google Scholar]
- Julien J. P., Smoluk G. D., Mushynski W. E. Characteristics of the protein kinase activity associated with rat neurofilament preparations. Biochim Biophys Acta. 1983 Jan 4;755(1):25–31. doi: 10.1016/0304-4165(83)90268-4. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Luttges M. W. Mouse brain protein composition during postnatal development: an electrophoretic analysis. J Neurochem. 1976 Nov;27(5):1163–1172. doi: 10.1111/j.1471-4159.1976.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Lennon A. M., Chantoux F., Osty J., Francon J. A high affinity thyroid hormone binding protein in the cytosol of embryonic rat brain cells in primary cultures. Biochem Biophys Res Commun. 1983 Nov 15;116(3):901–908. doi: 10.1016/s0006-291x(83)80227-7. [DOI] [PubMed] [Google Scholar]
- Leterrier J. F., Liem R. K., Shelanski M. L. Interactions between neurofilaments and microtubule-associated proteins: a possible mechanism for intraorganellar bridging. J Cell Biol. 1982 Dec;95(3):982–986. doi: 10.1083/jcb.95.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier J. F., Liem R. K., Shelanski M. L. Preferential phosphorylation of the 150,000 molecular weight component of neurofilaments by a cyclic AMP-dependent, microtubule-associated protein kinase. J Cell Biol. 1981 Sep;90(3):755–760. doi: 10.1083/jcb.90.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem R. K., Pachter J. S., Napolitano E. W., Chin S. S., Moraru E., Heimann R. Associated proteins as possible cross-linkers in the neuronal cytoskeleton. Ann N Y Acad Sci. 1985;455:492–508. doi: 10.1111/j.1749-6632.1985.tb50431.x. [DOI] [PubMed] [Google Scholar]
- Minami Y., Murofushi H., Sakai H. Interaction of tubulin with neurofilaments: formation of networks by neurofilament-dependent tubulin polymerization. J Biochem. 1982 Sep;92(3):889–898. doi: 10.1093/oxfordjournals.jbchem.a134003. [DOI] [PubMed] [Google Scholar]
- Nagele R. G., Roisen F. J. Ultrastructure of a new microtubule-neurofilament coupler in nerves. Brain Res. 1982 Dec 16;253(1-2):31–37. doi: 10.1016/0006-8993(82)90670-9. [DOI] [PubMed] [Google Scholar]
- Nestler E. J., Greengard P. Protein phosphorylation in the brain. Nature. 1983 Oct 13;305(5935):583–588. doi: 10.1038/305583a0. [DOI] [PubMed] [Google Scholar]
- Nixon R. A., Brown B. A., Marotta C. A. Posttranslational modification of a neurofilament protein during axoplasmic transport: implications for regional specialization of CNS axons. J Cell Biol. 1982 Jul;94(1):150–158. doi: 10.1083/jcb.94.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge M. S., Laue T. M., Yphantis D. A., Lifsics M. R., Saito A., Altin M., Reinke K., Williams R. C., Jr ATP-induced formation of an associated complex between microtubules and neurofilaments. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1431–1435. doi: 10.1073/pnas.78.3.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge M. S., el-Maghrabi M. R., Claus T. H., Pilkis S. J., Williams R. C., Jr A MAP-2-stimulated protein kinase activity associated with neurofilaments. Biochemistry. 1981 Jan 6;20(1):175–180. doi: 10.1021/bi00504a029. [DOI] [PubMed] [Google Scholar]
- Shecket G., Lasek R. J. Neurofilament protein phosphorylation. Species generality and reaction characteristics. J Biol Chem. 1982 May 10;257(9):4788–4795. [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Rice R. H., Roop D. R., Trus B. L., Steven A. C. Complete amino acid sequence of a mouse epidermal keratin subunit and implications for the structure of intermediate filaments. Nature. 1983 Apr 28;302(5911):794–800. doi: 10.1038/302794a0. [DOI] [PubMed] [Google Scholar]
- Toru-Delbauffe D., Pierre M. A rat brain protein kinase phosphorylating specifically neurofilaments. FEBS Lett. 1983 Oct 17;162(2):230–234. doi: 10.1016/0014-5793(83)80761-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallano M. L., Buckholz T. M., DeLorenzo R. J. Phosphorylation of neurofilament proteins by endogenous calcium/calmodulin-dependent protein kinase. Biochem Biophys Res Commun. 1985 Aug 15;130(3):957–963. doi: 10.1016/0006-291x(85)91708-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Shaw G., Osborn M., Debus E., Geisler N. Neurofilaments, a subclass of intermediate filaments: structure and expression. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):717–729. doi: 10.1101/sqb.1983.048.01.075. [DOI] [PubMed] [Google Scholar]
- Zimmerman U. J., Schlaepfer W. W. Characterization of calcium-activated neutral protease (CANP)-associated protein kinase from bovine brain and its phosphorylation of neurofilaments. Biochem Biophys Res Commun. 1985 Jun 28;129(3):804–811. doi: 10.1016/0006-291x(85)91963-1. [DOI] [PubMed] [Google Scholar]