Abstract
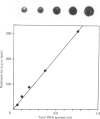
We describe the construction of a recombinant DNA plasmid, consisting of the vector pBR322 and full-length tissue-type plasminogen-activator (t-PA) cDNA, by using polyadenylated RNA from cultured Bowes melanoma cells as substrate. A 1280-base-pair PstI restriction fragment, covering the 3' untranslated region and part of the coding region for the t-PA L-chain, was used as a radiolabelled probe to determine the size and the number of t-PA mRNA molecules in cultured endothelial cells of different origin from the same individual. Northern blotting showed that in all these cells a t-PA mRNA is synthesized of about 2500 nucleotides, indicating that transcriptional initiation, splicing and polyadenylation is similar. The number of t-PA mRNA molecules per cell measured, by using a dot-blotting technique and t-PA mRNA made in vitro, with a plasmid DNA preparation harbouring a specific promotor of the Salmonella typhimurium bacteriophage SP6, t-PA cDNA and SP6 RNA polymerase as standard, is approx. 10,000 in all cultured endothelial cells from adult vessels. However, the amount of t-PA antigen synthesized and/or secreted differs by a factor of 6-20. Relatively large amounts of t-PA antigen secreted were detected in conditioned medium from vena-cava-derived cells, whereas low amounts were found in conditioned medium from arteria-iliaca-derived cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeis J. J., van Hinsbergh V. W., Verheijen J. H., Wijngaards G. Inhibition of tissue-type plasminogen activator by conditioned medium from cultured human and porcine vascular endothelial cells. Biochem Biophys Res Commun. 1983 Jan 27;110(2):392–398. doi: 10.1016/0006-291x(83)91161-0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag T., Cerundolo J., Ilsley S., Kelley P. R., Forand R. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5674–5678. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. L., McConaughy B. L. Selection of functional cDNAs by complementation in yeast. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4412–4416. doi: 10.1073/pnas.80.14.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdenakker G., Billiau A., Volckaert G., de Somer P. Determination of tissue-type plasminogen-activator mRNA in human and non-human cell lines by dot-blot hybridization. Biochem J. 1985 Oct 15;231(2):309–313. doi: 10.1042/bj2310309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., Collen D. Purification and characterization of the plasminogen activator secreted by human melanoma cells in culture. J Biol Chem. 1981 Jul 10;256(13):7035–7041. [PubMed] [Google Scholar]
- Rijken D. C., Wijngaards G., Welbergen J. Relationship between tissue plasminogen activator and the activators in blood and vascular wall. Thromb Res. 1980 Jun 15;18(6):815–830. doi: 10.1016/0049-3848(80)90204-2. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., van Hinsbergh V. W., Sens E. H. Quantitation of tissue-type plasminogen activator in human endothelial cell cultures by use of an enzyme immunoassay. Thromb Res. 1984 Jan 15;33(2):145–153. doi: 10.1016/0049-3848(84)90175-0. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Soeiro R., Birnboim H. C., Girard M., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. I. Identification by zone sedimentation of a heterogeneous fraction separate from ribosomal precursor RNA. J Mol Biol. 1966 Aug;19(2):349–361. doi: 10.1016/s0022-2836(66)80009-8. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh V. W., Havekes L., Emeis J. J., van Corven E., Scheffer M. Low density lipoprotein metabolism by endothelial cells from human umbilical cord arteries and veins. Arteriosclerosis. 1983 Nov-Dec;3(6):547–559. doi: 10.1161/01.atv.3.6.547. [DOI] [PubMed] [Google Scholar]