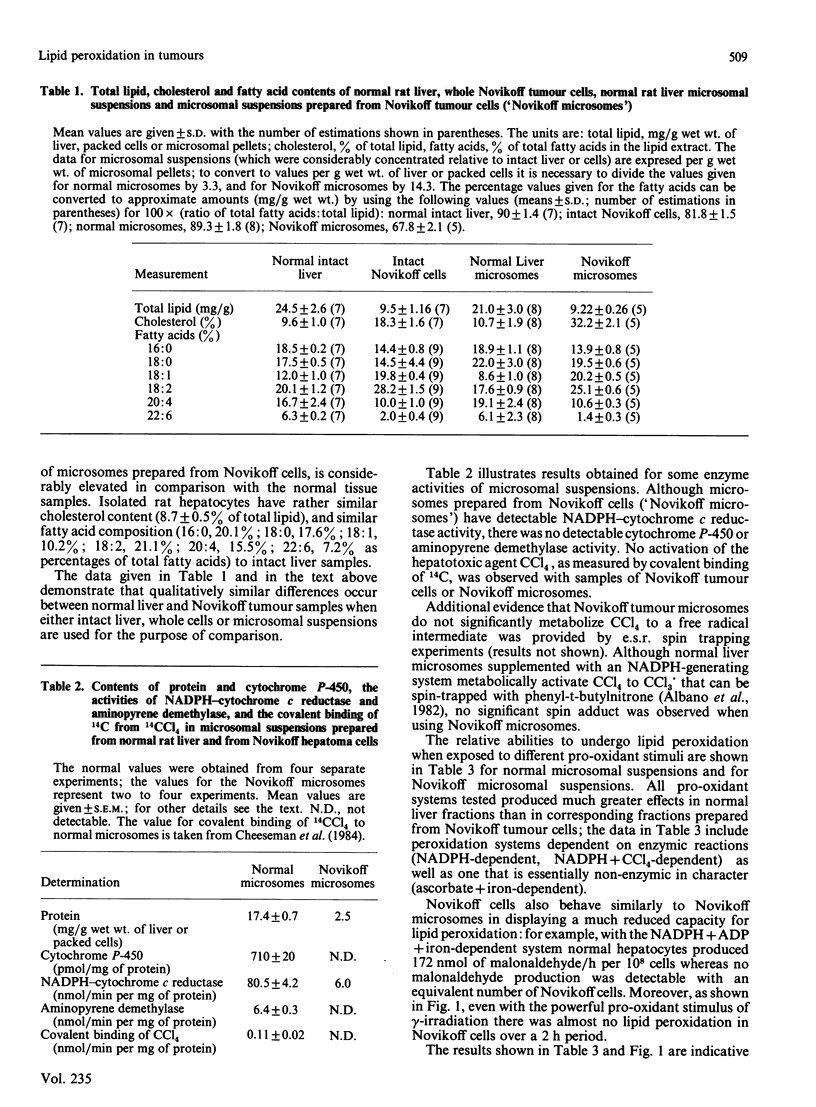
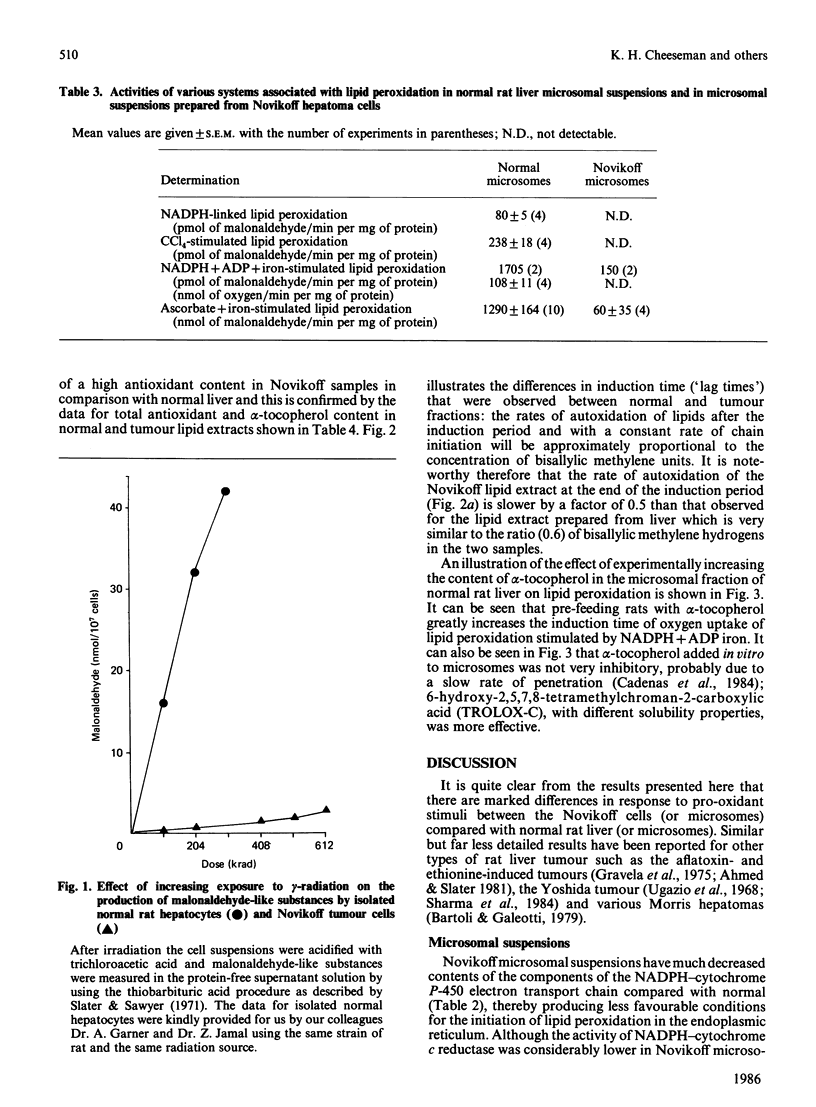
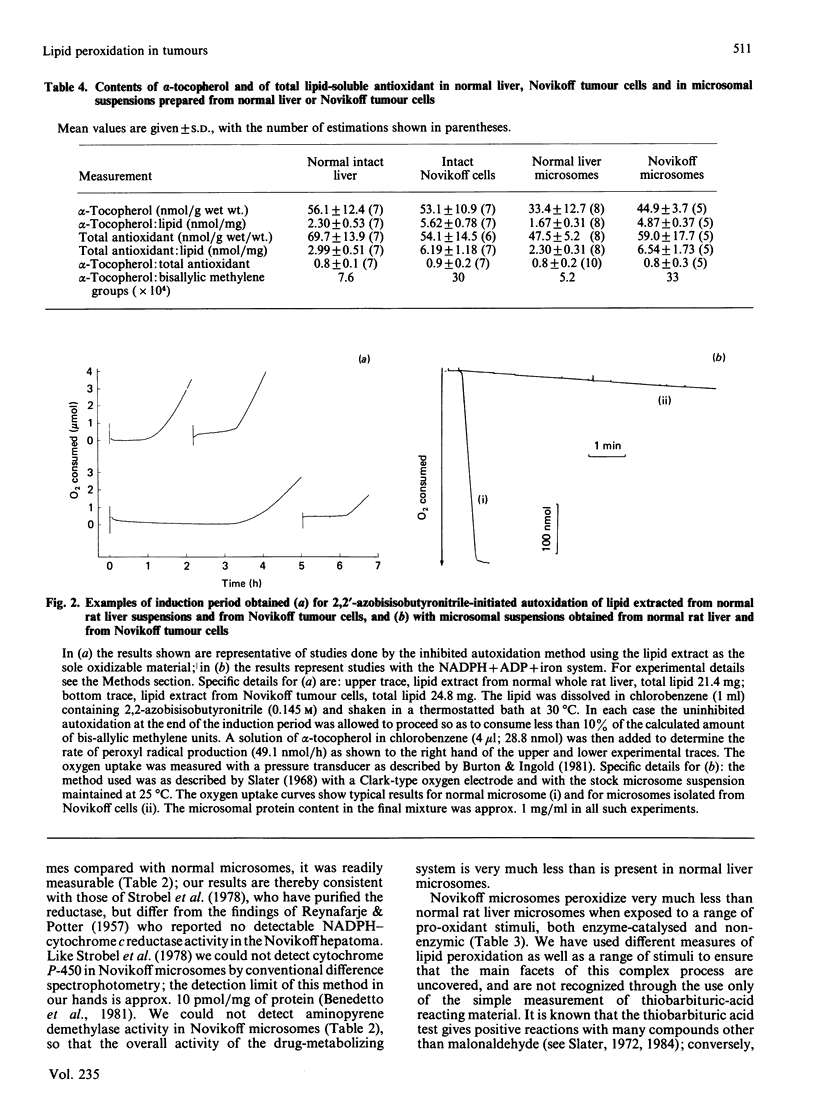
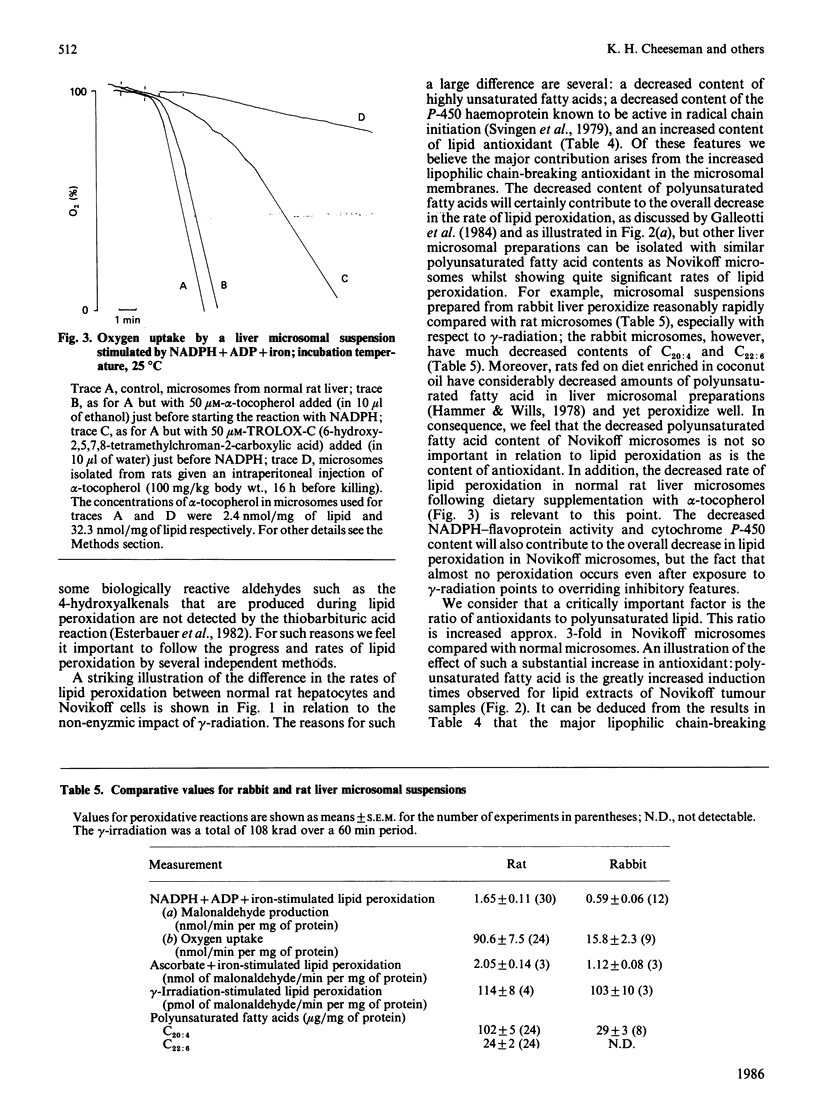
Abstract
A study has been made of the factors that contribute to the decreased rates of lipid peroxidation under different pro-oxidant conditions in intact Novikoff tumour cells, and in microsomal suspensions prepared from Novikoff tumour cells, compared with isolated normal rat hepatocytes and microsomal suspensions prepared from normal rat liver. The pro-oxidant conditions were the addition of either NADPH, NADPH + ADP + iron, NADPH + CCl4 or ascorbate+iron to the experimental systems used, or exposure to gamma-radiation. Contributory factors to the lower rates of lipid peroxidation observed include: a significant decrease in the polyunsaturated fatty acid content of Novikoff cells or Novikoff microsomes; the decreases are especially marked for the C20:4 and C22:6 fatty acids; a very marked reduction in NADPH-cytochrome c reductase; and no detectable content of cytochrome P-450. Another, and in our opinion critical, contribution to the diminished rate of lipid peroxidation in the tumour material is the substantial increase in alpha-tocopherol relative both to total lipid and to methylene-interrupted double bonds in fatty acids. Moreover, the alpha-tocopherol is the major contributor to lipid-soluble chain-breaking antioxidant in lipid extracts of normal liver and of Novikoff tumour material.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano E., Lott K. A., Slater T. F., Stier A., Symons M. C., Tomasi A. Spin-trapping studies on the free-radical products formed by metabolic activation of carbon tetrachloride in rat liver microsomal fractions isolated hepatocytes and in vivo in the rat. Biochem J. 1982 May 15;204(2):593–603. doi: 10.1042/bj2040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli G. M., Galeotti T. Growth-related lipid peroxidation in tumour microsomal membranes and mitochondria. Biochim Biophys Acta. 1979 Sep 28;574(3):537–541. doi: 10.1016/0005-2760(79)90249-2. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Malvaldi G., Fulceri R., Comporti M. Loss of lipid peroxidation as a histochemical marker for preneoplastic hepatocellular foci of rats. Cancer Res. 1984 Dec;44(12 Pt 1):5712–5717. [PubMed] [Google Scholar]
- Benedetto C., Dianzani M. U., Ahmed M., Cheeseman K., Connelly C., Slater T. F. Activation of carbon tetrachloride, and distribution of NADPH-cytochrome c reductase, cytochrome P-450, and other microsomal enzyme activities in rat tissues. Biochim Biophys Acta. 1981 Nov 5;677(3-4):363–372. doi: 10.1016/0304-4165(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Burlakova E. B., Molochkina E. M., Pal'mina N. P. Role of membrane lipid oxidation in control of enzymatic activity in normal and cancer cells. Adv Enzyme Regul. 1980;18:163–179. doi: 10.1016/0065-2571(80)90014-x. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Cheeseman K. H., Doba T., Ingold K. U., Slater T. F. Vitamin E as an antioxidant in vitro and in vivo. Ciba Found Symp. 1983;101:4–18. doi: 10.1002/9780470720820.ch2. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Ingold K. U. beta-Carotene: an unusual type of lipid antioxidant. Science. 1984 May 11;224(4649):569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Joyce A., Ingold K. U. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochem Biophys. 1983 Feb 15;221(1):281–290. doi: 10.1016/0003-9861(83)90145-5. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Webb A., Ingold K. U. A mild, rapid, and efficient method of lipid extraction for use in determining vitamin E/lipid ratios. Lipids. 1985 Jan;20(1):29–39. doi: 10.1007/BF02534359. [DOI] [PubMed] [Google Scholar]
- Cadenas E., Ginsberg M., Rabe U., Sies H. Evaluation of alpha-tocopherol antioxidant activity in microsomal lipid peroxidation as detected by low-level chemiluminescence. Biochem J. 1984 Nov 1;223(3):755–759. doi: 10.1042/bj2230755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman K. H., Burton G. W., Ingold K. U., Slater T. F. Lipid peroxidation and lipid antioxidants in normal and tumor cells. Toxicol Pathol. 1984;12(3):235–239. doi: 10.1177/019262338401200305. [DOI] [PubMed] [Google Scholar]
- Dianzani M. U., Canuto R. A., Rossi M. A., Poli G., Garcea R., Biocca M. E., Cecchini G., Biasi F., Ferro M., Bassi A. M. Further experiments on lipid peroxidation in transplanted and experimental hepatomas. Toxicol Pathol. 1984;12(2):189–199. doi: 10.1177/019262338401200213. [DOI] [PubMed] [Google Scholar]
- Doba T., Burton G. W., Ingold K. U. Antioxidant and co-antioxidant activity of vitamin C. The effect of vitamin C, either alone or in the presence of vitamin E or a water-soluble vitamin E analogue, upon the peroxidation of aqueous multilamellar phospholipid liposomes. Biochim Biophys Acta. 1985 Jul 9;835(2):298–303. doi: 10.1016/0005-2760(85)90285-1. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Cheeseman K. H., Dianzani M. U., Poli G., Slater T. F. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem J. 1982 Oct 15;208(1):129–140. doi: 10.1042/bj2080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fonnesu A., Del Monte U., Olivotto M. Consumo di ossigeno e perossidazione lipidica delle frazioni citoplasmatiche extramitocondriali difegato e di epatoma-ascite di Yoshida. Sperimentale. 1967 Nov-Dec;116(6):353–372. [PubMed] [Google Scholar]
- GLOCK G. E., MCLEAN P. Levels of oxidized and reduced diphosphopyridine nucleotide and triphosphopyridine nucleotide in tumours. Biochem J. 1957 Feb;65(2):413–416. doi: 10.1042/bj0650413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti T., Borrello S., Palombini G., Masotti L., Ferrari M. B., Cavatorta P., Arcioni A., Stremmenos C., Zannoni C. Lipid peroxidation and fluidity of plasma membranes from rat liver and Morris hepatoma 3924A. FEBS Lett. 1984 Apr 24;169(2):169–173. doi: 10.1016/0014-5793(84)80312-9. [DOI] [PubMed] [Google Scholar]
- Gravela E., Feo F., Canuto R. A., Garcea R., Gabriel L. Functional and structural alterations of liver ergastoplasmic membranes during DL-ethionine hepatocarcinogenesis. Cancer Res. 1975 Nov;35(11 Pt 1):3041–3047. [PubMed] [Google Scholar]
- Hammer C. T., Wills E. D. The role of lipid components of the diet in the regulation of the fatty acid composition of the rat liver endoplasmic reticulum and lipid peroxidation. Biochem J. 1978 Aug 15;174(2):585–593. doi: 10.1042/bj1740585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landi L., Cabrini L., Sechi A. M., Pasquali P. Antioxidative effect of ubiquinones on mitochondrial membranes. Biochem J. 1984 Sep 1;222(2):463–466. doi: 10.1042/bj2220463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash E. D. The antioxidant and prooxidant activity in ascites tumors. Arch Biochem Biophys. 1966 Aug;115(2):332–336. doi: 10.1016/0003-9861(66)90283-9. [DOI] [PubMed] [Google Scholar]
- Morisaki N., Lindsey J. A., Stitts J. M., Zhang H., Cornwell D. G. Fatty acid metabolism and cell proliferation. V. Evaluation of pathways for the generation of lipid peroxides. Lipids. 1984 Jun;19(6):381–394. doi: 10.1007/BF02537399. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B. A transplantable rat liver tumor induced by 4-dimethylaminoazobenzene. Cancer Res. 1957 Nov;17(10):1010–1027. [PubMed] [Google Scholar]
- Packer J. E., Slater T. F., Willson R. L. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature. 1979 Apr 19;278(5706):737–738. doi: 10.1038/278737a0. [DOI] [PubMed] [Google Scholar]
- Player T. J., Mills D. J., Horton A. A. Lipid peroxidation of the microsomal fraction and extracted microsomal lipids from DAB-induced hepatomas. Br J Cancer. 1979 Jun;39(6):773–778. doi: 10.1038/bjc.1979.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Cheeseman K., Slater T. F., Dianzani M. U. The role of lipid peroxidation in CCl4-induced damage to liver microsomal enzymes: comparative studies in vitro using microsomes and isolated liver cells. Chem Biol Interact. 1981 Oct;37(1-2):13–24. doi: 10.1016/0009-2797(81)90162-9. [DOI] [PubMed] [Google Scholar]
- Poli G., Dianzani M. U., Cheeseman K. H., Slater T. F., Lang J., Esterbauer H. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions. Biochem J. 1985 Apr 15;227(2):629–638. doi: 10.1042/bj2270629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNAFARJE B., POTTER V. R. Comparison of transhydrogenase and pyridine nucleotide-cytochrome c reductase activities in rat liver and Novikoff hepatoma. Cancer Res. 1957 Dec;17(11):1112–1119. [PubMed] [Google Scholar]
- Roomi M. W., Ho R. K., Sarma D. S., Farber E. A common biochemical pattern in preneoplastic hepatocyte nodules generated in four different models in the rat. Cancer Res. 1985 Feb;45(2):564–571. [PubMed] [Google Scholar]
- Ross D. A., Jackson R. C., Weber G., Morris H. P. Decreased content of reduced and oxidized nicotinamide-adenine dinucleotide phosphate in rat hepatomas. Cancer Biochem Biophys. 1982;6(2):61–64. [PubMed] [Google Scholar]
- SWICK R. W., BAUMANN C. A., MILLER W. L., Jr, RUMSFELD H. W., Jr Tocopherol in tumor tissues and effects of tocopherol on the development of liver tumors. Cancer Res. 1951 Dec;11(12):948–953. [PubMed] [Google Scholar]
- Saine S. E., Fang W. F., Strobel H. W. Drug metabolism in the Novikoff hepatoma: evidence for a mixed function oxidase system and partial purification of cytochrome P-450 reductase. Biochim Biophys Acta. 1978 Oct 12;526(2):345–358. doi: 10.1016/0005-2744(78)90126-2. [DOI] [PubMed] [Google Scholar]
- Slater T. F. Free-radical mechanisms in tissue injury. Biochem J. 1984 Aug 15;222(1):1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C. The effects of carbon tetrachloride on rat liver microsomes during the first hour of poisoning in vivo, and the modifying actions of promethazine. Biochem J. 1969 Feb;111(3):317–324. doi: 10.1042/bj1110317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem J. 1971 Aug;123(5):805–814. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F. The inhibitory effects in vitro of phenothiazines and other drugs on lipid-peroxidation systems in rat liver microsomes, and their relationship to the liver necrosis produced by carbon tetrachloride. Biochem J. 1968 Jan;106(1):155–160. doi: 10.1042/bj1060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel H. W., Dignam J. D., Saine S. E., Fang W. F., Fennell P. M. The drug metabolism systems of liver and liver tumors: a comparison of activities and characteristics. Mol Cell Biochem. 1978 Dec 22;22(2-3):79–91. doi: 10.1007/BF00496236. [DOI] [PubMed] [Google Scholar]
- Svingen B. A., Buege J. A., O'Neal F. O., Aust S. D. The mechanism of NADPH-dependent lipid peroxidation. The propagation of lipid peroxidation. J Biol Chem. 1979 Jul 10;254(13):5892–5899. [PubMed] [Google Scholar]
- Tappel A. L. Will antioxidant nutrients slow aging processes? Geriatrics. 1968 Oct;23(10):97–105. [PubMed] [Google Scholar]
- Tisdale M. J., Mahmoud M. B. Activities of free radical metabolizing enzymes in tumours. Br J Cancer. 1983 Jun;47(6):809–812. doi: 10.1038/bjc.1983.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugazio G., Gabriel L., Burdino E. Ricerche sugli inibitori della perossidazione lipidica presenti nelle cellule dell'epatoma ascite di Yoshida. Boll Soc Ital Biol Sper. 1968 Jan 15;44(1):30–34. [PubMed] [Google Scholar]
- Utsumi K., Yamamoto G., Inaba K. Failure of FE2+-induced lipid peroxidation and swelling in the mitochondria isolated from ascites tumor cells. Biochim Biophys Acta. 1965 Aug 24;105(2):368–371. doi: 10.1016/s0926-6593(65)80160-6. [DOI] [PubMed] [Google Scholar]
- Wayner D. D., Burton G. W., Ingold K. U., Locke S. Quantitative measurement of the total, peroxyl radical-trapping antioxidant capability of human blood plasma by controlled peroxidation. The important contribution made by plasma proteins. FEBS Lett. 1985 Jul 22;187(1):33–37. doi: 10.1016/0014-5793(85)81208-4. [DOI] [PubMed] [Google Scholar]
- Weber G. Biochemical strategy of cancer cells and the design of chemotherapy: G. H. A. Clowes Memorial Lecture. Cancer Res. 1983 Aug;43(8):3466–3492. [PubMed] [Google Scholar]
- Wirth P. J., Thorgeirsson S. S. Glutathione synthesis and degradation in fetal and adult rat liver and Novikoff hepatoma. Cancer Res. 1978 Sep;38(9):2861–2865. [PubMed] [Google Scholar]


