Abstract
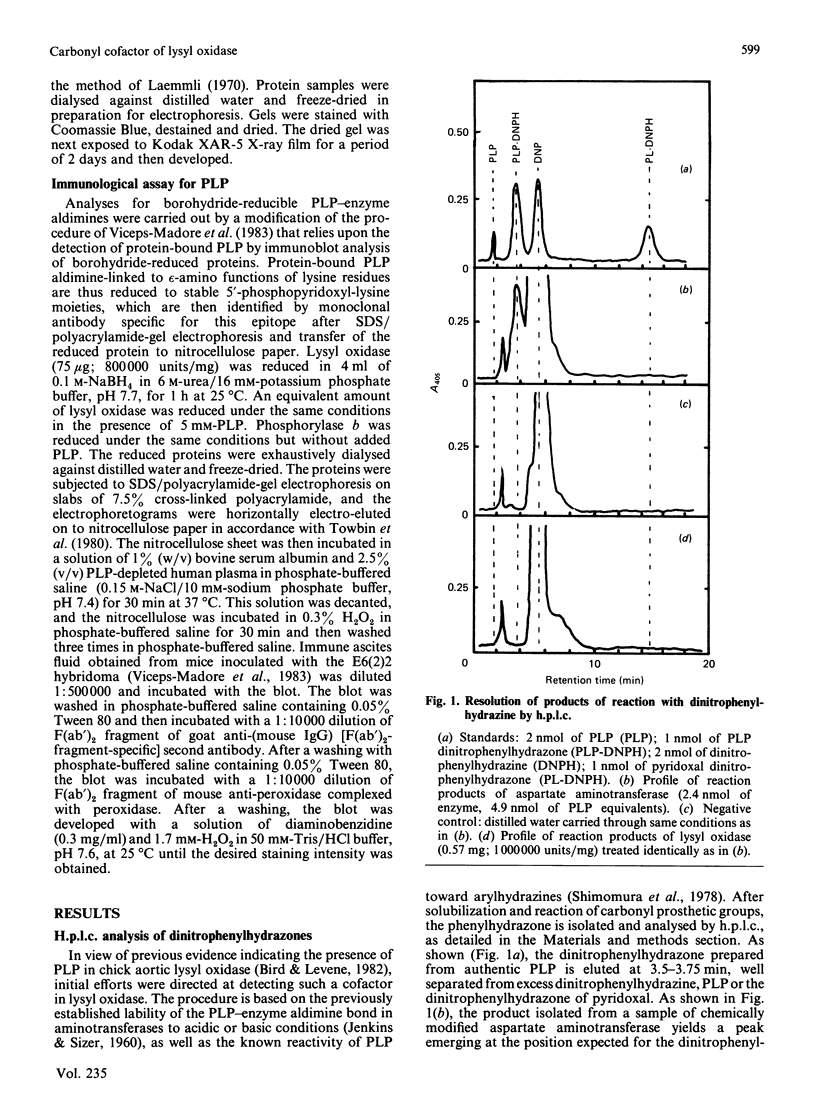
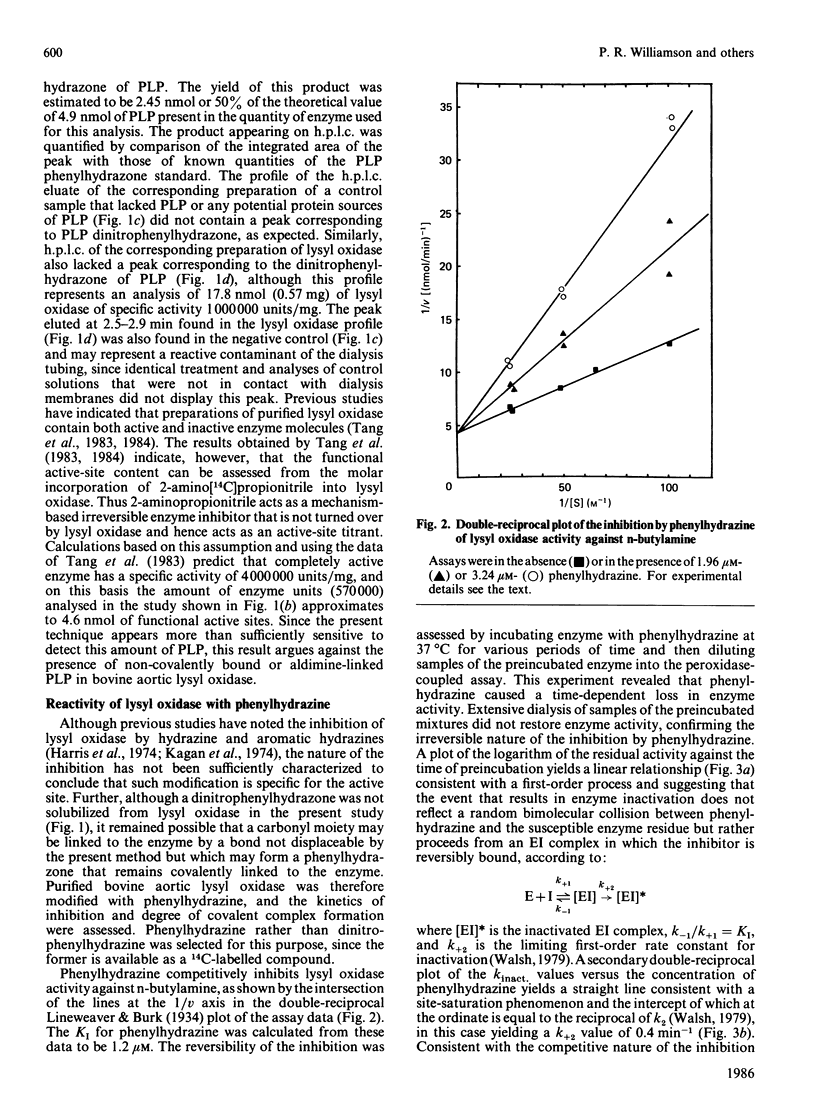
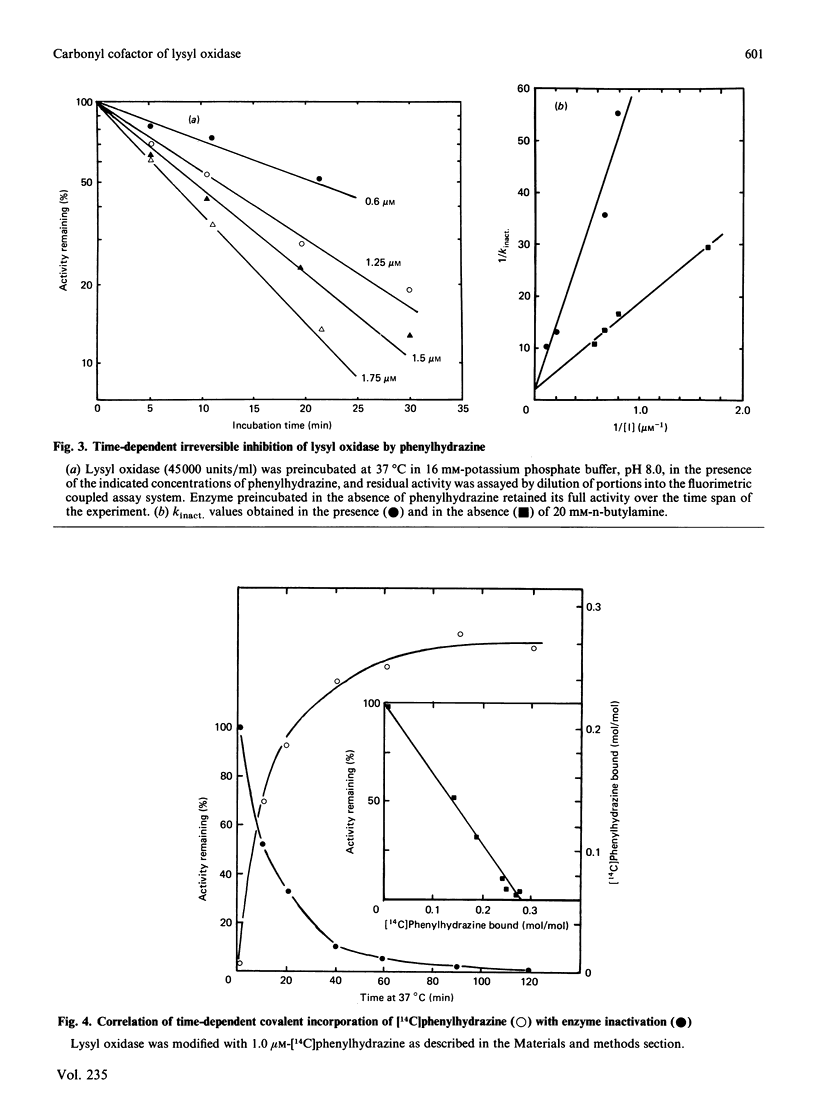
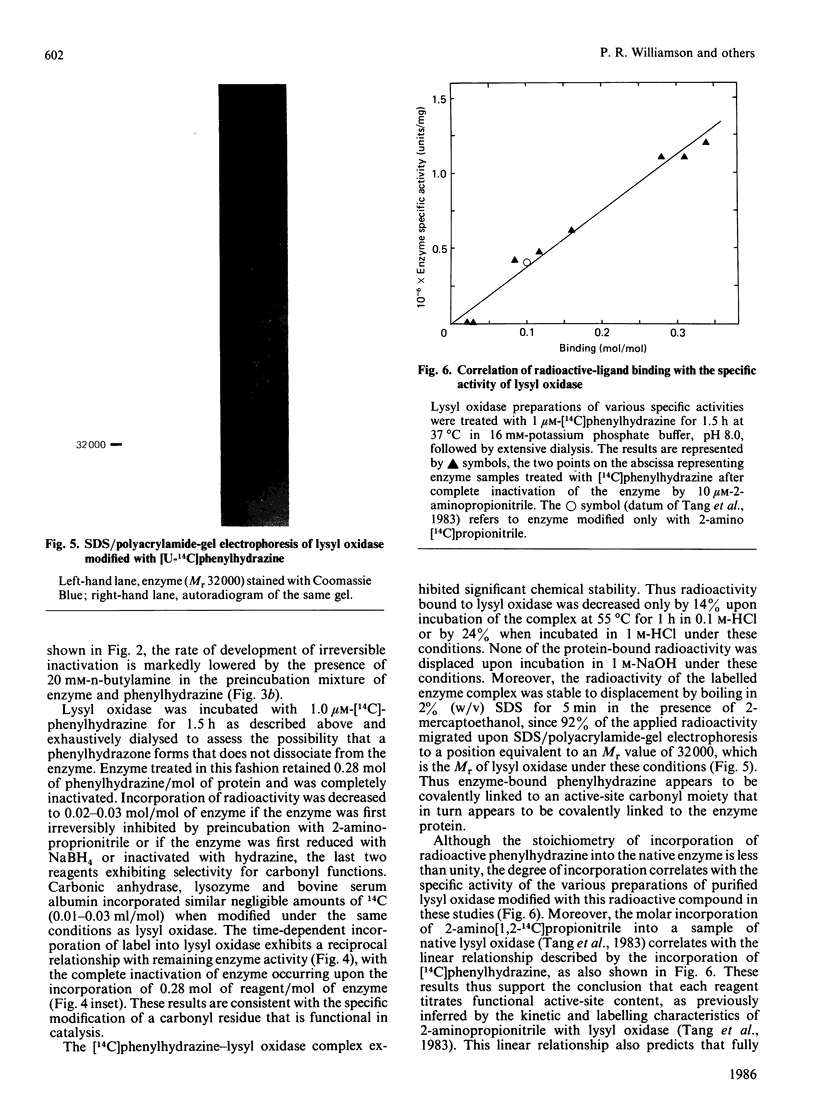
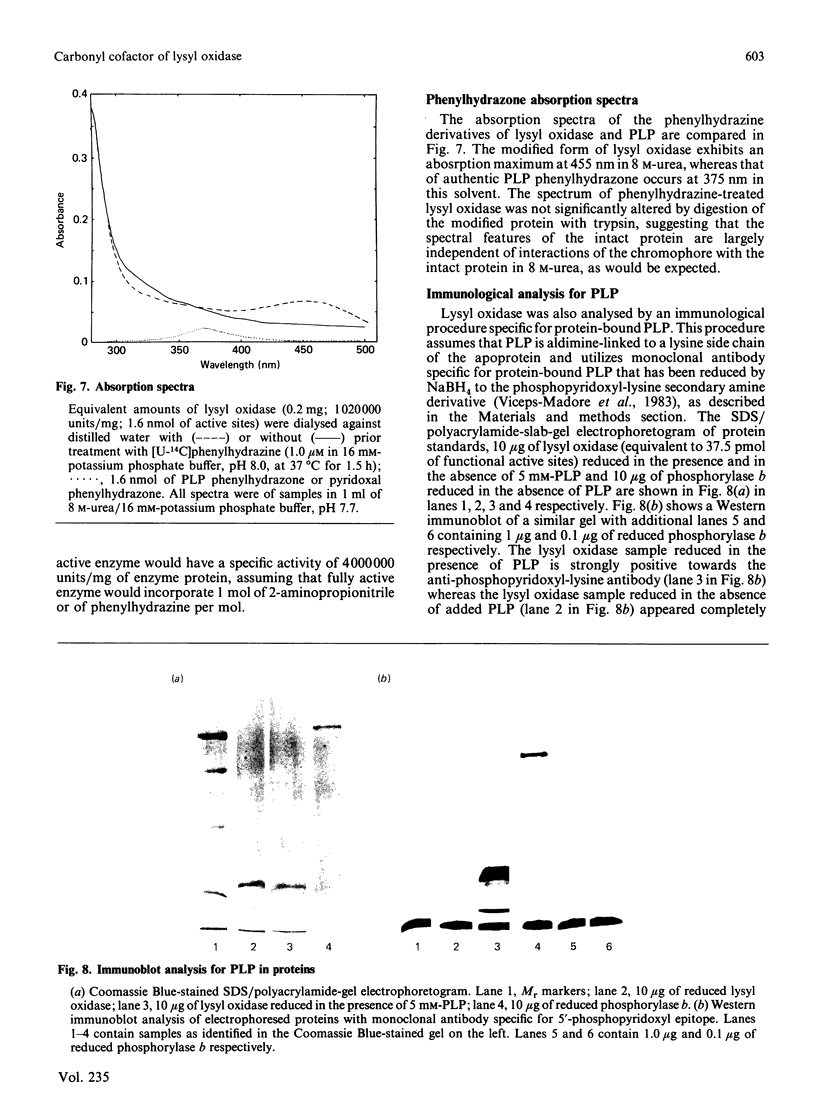
Previous studies have pointed towards a cofactor role for pyridoxal 5'-phosphate (PLP) in lysyl oxidase, the enzyme that generates the peptidyl aldehyde precursor to the lysine-derived cross-linkages in elastin and collagen. The nature of a carbonyl moiety in purified bovine aortic lysyl oxidase was explored in the present study. A PLP dinitrophenylhydrazone could not be isolated from lysyl oxidase, although corresponding preparations of aspartate aminotransferase, a PLP-dependent enzyme, yielded this derivative, as revealed by h.p.l.c. Analysis of lysyl oxidase for PLP after reduction of the enzyme by NaBH4, a procedure that converts PLP-protein aldimines into stable 5'-phosphopyridoxyl functions, also proved negative in tests using monoclonal antibody specific for this epitope. Lysyl oxidase was competitively inhibited by phenylhydrazine, and inhibition became irreversible with time at 37 degrees C, displaying a first-order inactivation rate constant of 0.4 min-1 and KI of 1 microM. [14C]Phenylhydrazine was covalently incorporated into the enzyme in a manner that was prevented by prior modification of the enzyme with beta-aminopropionitrile, a specific active-site inhibitor, and which correlated with functional active-site content. The chemical stability of the enzyme-bound phenylhydrazine exceeded that expected of linkages between PLP and proteins. The absorption spectrum of the phenylhydrazine derivative of lysyl oxidase was clearly distinct from that of the phenylhydrazone of PLP. It is concluded that lysyl oxidase contains a carbonyl cofactor that is not identical with PLP and that is bound to the enzyme by a stable chemical bond.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arem A. J., Misiorowski R. Lathyritic activity of isoniazid. J Med. 1976;7(3-4):239–248. [PubMed] [Google Scholar]
- Bird T. A., Levene C. I. Lysyl oxidase: evidence that pyridoxal phosphate is a cofactor. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1172–1180. doi: 10.1016/0006-291x(82)92124-6. [DOI] [PubMed] [Google Scholar]
- Carrington M. J., Bird T. A., Levene C. I. The inhibition of lysyl oxidase in vivo by isoniazid and its reversal by pyridoxal. Effect on collagen cross-linking in the chick embryo. Biochem J. 1984 Aug 1;221(3):837–843. doi: 10.1042/bj2210837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K., Kajiwara T., Kurosu H. Effect of vitamin B6 deficiency on the crosslink formation of collagen. FEBS Lett. 1979 Jan 1;97(1):193–195. doi: 10.1016/0014-5793(79)80082-4. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Gonnerman W. A., Savage J. E., O'Dell B. L. Connective tissue amine oxidase. II. Purification and partial characterization of lysyl oxidase from chick aorta. Biochim Biophys Acta. 1974 Apr 25;341(2):332–344. doi: 10.1016/0005-2744(74)90226-5. [DOI] [PubMed] [Google Scholar]
- Huynh Q. K., Recsei P. A., Vaaler G. L., Snell E. E. Histidine decarboxylase of Lactobacillus 30a. Sequences of the overlapping peptides, the complete alpha chain, and prohistidine decarboxylase. J Biol Chem. 1984 Mar 10;259(5):2833–2839. [PubMed] [Google Scholar]
- JENKINS W. T., SIZER I. W. Glutamic aspartic transaminase. IV. The mechanism of transamination. J Biol Chem. 1960 Mar;235:620–624. [PubMed] [Google Scholar]
- Kagan H. M., Hewitt N. A., Salcedo L. L., Franzblau C. Catalytic activity of aortic lysyl oxidase in an insoluble enzyme-substrate complex. Biochim Biophys Acta. 1974 Sep 13;365(1):223–234. doi: 10.1016/0005-2795(74)90267-0. [DOI] [PubMed] [Google Scholar]
- Kagan H. M., Sullivan K. A., Olsson T. A., 3rd, Cronlund A. L. Purification and properties of four species of lysyl oxidase from bovine aorta. Biochem J. 1979 Jan 1;177(1):203–214. doi: 10.1042/bj1770203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan H. M., Tseng L., Trackman P. C., Okamoto K., Rapaka R. S., Urry D. W. Repeat polypeptide models of elastin as substrates for lysyl oxidase. J Biol Chem. 1980 Apr 25;255(8):3656–3659. [PubMed] [Google Scholar]
- Kagan H. M., Williams M. A., Calaman S. D., Berkowitz E. M. Histone H1 is a substrate for lysyl oxidase and contains endogenous sodium borotritide-reducible residues. Biochem Biophys Res Commun. 1983 Aug 30;115(1):186–192. doi: 10.1016/0006-291x(83)90987-7. [DOI] [PubMed] [Google Scholar]
- Kagan H. M., Williams M. A., Williamson P. R., Anderson J. M. Influence of sequence and charge on the specificity of lysyl oxidase toward protein and synthetic peptide substrates. J Biol Chem. 1984 Sep 25;259(18):11203–11207. [PubMed] [Google Scholar]
- LEVENE C. I. Structural requirements for lathyrogenic agents. J Exp Med. 1961 Sep 1;114:295–310. doi: 10.1084/jem.114.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lobenstein-Verbeek C. L., Jongejan J. A., Frank J., Duine J. A. Bovine serum amine oxidase: a mammalian enzyme having covalently bound PQQ as prosthetic group. FEBS Lett. 1984 May 21;170(2):305–309. doi: 10.1016/0014-5793(84)81333-2. [DOI] [PubMed] [Google Scholar]
- Murray J. C., Fraser D. R., Levene C. I. The effect of pyridoxine deficiency on lysyl oxidase activity in the chick. Exp Mol Pathol. 1978 Jun;28(3):301–308. doi: 10.1016/0014-4800(78)90004-7. [DOI] [PubMed] [Google Scholar]
- Murray J. C., Levene C. I. Evidence for the role of vitamin C-6 as a cofactor of lysyl oxidase. Biochem J. 1977 Nov 1;167(2):463–467. doi: 10.1042/bj1670463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B. A., Dubick M. A., Reynolds R. D., Rucker R. B. Effect of vitamin B-6 (pyridoxine) deficiency on lung elastin cross-linking in perinatal and weanling rat pups. Biochem J. 1985 Jul 1;229(1):153–160. doi: 10.1042/bj2290153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimni M. E. A defect in the intramolecular and intermolecular cross-linking of collagen caused by penicillamine. I. Metabolic and functional abnormalities in soft tissues. J Biol Chem. 1968 Apr 10;243(7):1457–1466. [PubMed] [Google Scholar]
- Pinnell S. R., Martin G. R. The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc Natl Acad Sci U S A. 1968 Oct;61(2):708–716. doi: 10.1073/pnas.61.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura S., Nakano K., Fukui T. Affinity labeling of the cofactor site in glycogen phosphorylase b with a pyridoxal 5'-phosphate analog. Biochem Biophys Res Commun. 1978 May 30;82(2):462–468. doi: 10.1016/0006-291x(78)90897-5. [DOI] [PubMed] [Google Scholar]
- Siegel R. C. Collagen cross-linking. Effect of D-penicillamine on cross-linking in vitro. J Biol Chem. 1977 Jan 10;252(1):254–259. [PubMed] [Google Scholar]
- Siegel R. C. Lysyl oxidase. Int Rev Connect Tissue Res. 1979;8:73–118. doi: 10.1016/b978-0-12-363708-6.50009-6. [DOI] [PubMed] [Google Scholar]
- Starcher B. C. The effect of pyridoxine deficiency on aortic elastin biosynthesis. Proc Soc Exp Biol Med. 1969 Oct;132(1):379–382. doi: 10.3181/00379727-132-34219. [DOI] [PubMed] [Google Scholar]
- Sullivan K. A., Kagan H. M. Evidence for structural similarities in the multiple forms of aortic and cartilage lysyl oxidase and a catalytically quiescent aortic protein. J Biol Chem. 1982 Nov 25;257(22):13520–13526. [PubMed] [Google Scholar]
- Tane N., Takeda T., Shioji T., Ohyama H., Itoh H. Effect of vitamin B6 deficiency on collagen metabolism in rats. J Nutr Sci Vitaminol (Tokyo) 1976;22(2):105–114. doi: 10.3177/jnsv.22.105. [DOI] [PubMed] [Google Scholar]
- Tang S. S., Simpson D. E., Kagan H. M. Beta-substituted ethylamine derivatives as suicide inhibitors of lysyl oxidase. J Biol Chem. 1984 Jan 25;259(2):975–979. [PubMed] [Google Scholar]
- Tang S. S., Trackman P. C., Kagan H. M. Reaction of aortic lysyl oxidase with beta-aminopropionitrile. J Biol Chem. 1983 Apr 10;258(7):4331–4338. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trackman P. C., Kagan H. M. Nonpeptidyl amine inhibitors are substrates of lysyl oxidase. J Biol Chem. 1979 Aug 25;254(16):7831–7836. [PubMed] [Google Scholar]
- Viceps-Madore D., Cidlowski J. A., Kittler J. M., Thanassi J. W. Preparation, characterization, and use of monoclonal antibodies to vitamin B6. J Biol Chem. 1983 Feb 25;258(4):2689–2696. [PubMed] [Google Scholar]
- Williams M. A., Kagan H. M. Assessment of lysyl oxidase variants by urea gel electrophoresis: evidence against disulfide isomers as bases of the enzyme heterogeneity. Anal Biochem. 1985 Sep;149(2):430–437. doi: 10.1016/0003-2697(85)90594-9. [DOI] [PubMed] [Google Scholar]




