Abstract
The latency-regulated transmembrane protein LMP2A interferes with signaling from the B-cell antigen receptor by recruiting the tyrosine kinases Lyn and Syk and by targeting them for degradation by binding the cellular E3 ubiquitin ligase AIP4. It has been hypothesized that this constitutive activity of LMP2A requires clustering in the membrane, but molecular evidence for this has been lacking. In the present study we show that LMP2A coclusters with chimeric rat CD2 transmembrane molecules carrying the 27-amino-acid (aa) intracellular C terminus of LMP2A and that this C-terminal domain fused to the glutathione-S-transferase protein associates with LMP2A in cell lysates. This molecular association requires neither the cysteine-rich region between aa 471 and 480 nor the terminal three aa 495 to 497. We also show that the juxtamembrane cysteine repeats in the LMP2A C terminus are the major targets for palmitoylation but that this acylation is not required for targeting of LMP2A to detergent-insoluble glycolipid-enriched membrane microdomains.
Human herpesvirus 4 (Epstein-Barr virus [EBV]) is a ubiquitous gammaherpesvirus carried by over 90% of the human population. The virus remains tightly latent (latency I) in small memory-type B cells that express EBNA-1 and the latency-regulated transmembrane protein LMP2A (3, 10, 35). The latter is believed to safeguard viral latency by abolishing signaling from the B-cell antigen receptor (BCR) (27). Successful activation of the B cell following stimulation of the BCR would lead to concomitant activation of additional viral genes, rendering the EBV-carrying B cell susceptible to immune attack. Thus, LMP2A must have a key role in modulating the phenotype of the EBV-carrying B cell to the advantage of the virus. But LMP2A is also expressed in a majority of EBV-carrying anaplastic nasopharyngeal carcinomas (10) and may contribute to the transformed phenotype of these tumors.
The LMP2A transcription unit spans the termini of the viral genome (23) and consequently can be transcribed from the intracellular viral episome only after circularization of the linear virion DNA. A coterminating transcript, LMP2B, lacks the first exon of LMP2A and is transcribed from a bidirectional promoter, shared with the other major latency-regulated membrane protein, LMP1 (22). The existence of LMP2B as a protein has so far not been shown. The physically separate promoters of LMP2A and LMP2B are subject to different transcriptional controls. While the promoters of LMP2A, LMP2B, and LMP1 depend on the viral nuclear protein EBNA2 for transactivation in B cells, the LMP2A promoter was shown to respond to a murine homologue of the Drosophila melanogaster Notch gene, mNotch IC, which is expressed in hematopoietic cells (39). The expression of LMP2A may thus be independent of other viral factors in latency forms where EBNA2 is not expressed.
The mechanisms by which LMP2A modulates cellular signaling have been the subject of extensive study. The unique first (N-terminal) exon in LMP2A was shown to contain a functional immunotyrosine activation motif similar to those found in the CD79α and CD79β auxiliary chains of the BCR (4), and recent studies show that LMP2A associates with lipid rafts (12, 19) and indicate that LMP2A interferes with raft association of the BCR (12). The phosphorylated tyrosines 74 and 85 in the immunotyrosine activation motif of the LMP2A N-terminal exon are required for the binding of the Syk tyrosine kinase (7, 14, 15), and tyrosine 112 acts as a docking site for the Src family tyrosine kinase Lyn (16). Studies of LMP2A N-terminal deletion mutants indicate, in addition, that the C-terminal Src kinase, Csk, may be responsible for phosphorylation of Y74 in epithelial cells (36). The extracellular regulated kinase (Erk) may further affect phosphorylation of serine residues S15 and S102 in the LMP2A N-terminal domain (30). Two proline-rich (PPPPY) motifs in the N-terminal exon bind WW domains in the cellular E3 ubiquitin ligase AIP4 (34, 44) and other related HECT domain proteins (20, 43).
Unlike the LMP1 membrane protein, which mimics the signaling of an activated CD40/tumor necrosis factor receptor (13), LMP2 proteins are not essential in the growth transformation (immortalization) of B cells by EBV in vitro. However, when introduced as a transgene in mice, LMP2A has been observed to provide a survival signal, allowing immature B cells lacking a functional BCR to populate lymphoid organs and enter the peripheral circulation (8). This may be due to antiapoptotic signals delivered by constitutive activation of the serine-threonine kinase Akt, which has been observed in both EBV-transformed B cells (40) and epithelial cells (37).
Early observations of membrane distribution of LMP1 and LMP2A using immunofluorescence indicated that LMP1 and LMP2A were colocalized in large patches on the membranes of B cells (24). The subsequent demonstration that LMP1 is located in glycosphingolipid-rich domains of the cell membrane provided molecular evidence for the observed patching of LMP1 (11).
In the present study, we show that the C-terminal domain (CT) of LMP2A is sufficient for association between LMP2A molecules as well as other molecules carrying the LMP2A CT. This is the first demonstration that the CT domain of LMP2A participates in molecular association between LMP2A molecules. In addition, we show that two regions of the CT domain are dispensable for LMP2A association, amino acids (aa) 471 to 480 and aa 495 to 497. Also, our experiments show that the cysteine-rich repeats between aa 471 and 480 are targets of palmitoylation, although the localization of LMP2A to glycosphingolipid-rich raft domains in the membrane does not require C-terminal palmitoylation.
MATERIALS AND METHODS
Cells, expression constructs, DNA transfections, and viral gene transduction.
HEK 293 cells are human embryonic kidney cells transformed by the adenovirus type 5 E1A and E1B genes (17). A cDNA clone, pSP64-23TP carrying the LMP2A gene from the B95-8 strain of EBV was generously donated by P. J. Farrell (23). A retroviral vector, pLXPOP, was used to express the LMP2A. This was constructed from the pLNPOX vector (25) by inserting the puromycin resistance gene in the HindIII site following the poliovirus 5′ untranslated region, destroying the BamHI site preceding the 5′ long terminal repeat by Klenow fill and recircularization and replacing the Tn5 aminoglycoside 3′ phosphotransferase (Neo) gene with a multilinker with the sequence 5′-GAATTCACCGGTCGACGTACGGATCCTTAATTAAGCTTATTTAAATTCGAAAGATCTGTTTAAACTCGAG-3′ (G. Winberg and R. Reynolds, unpublished data). The HindIII-to-NsiI fragment from pSP64-23TP was subsequently cloned into the HindIII and BamHI sites of pLXPOP. The rat CD2 (42) was adapted by PCR, and aa 1 through 225 (Phe) were cloned into the EcoRI and BamHI sites of pBKCMV-Sfv (modified from pBKCMV; Stratagene, La Jolla, Calif.), with the amino acids Gly and Ile added in the BamHI site between Phe 224 of the CD2 sequence and the 27-aa LMP2 C terminus. Transfections of DNA from expression plasmids and recombinant retrovirus constructs were done by a modified CaPO4 technique (9), using piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) for buffering instead of N,N-bis(2-hydroxyethyl)-2-ethanesulfonic acid (BES) or using polyethyleneimine precipitation as follows. For a 10-cm-diameter dish of 50% confluent cells, to 66 μl of H2O was added 12 μl of 50% glucose, 24 μl of plasmid DNA (1 mg/ml), and finally, 18 μl of a 50 mM solution of 25-kDa polyethyleneimine (catalog no. 40872-7; Aldrich, Stockholm, Sweden) in water at pH 7.0. The mixture was diluted into 2 ml of growth medium and then added to 10 ml of medium in the dish. Supernatant from the PG13 packaging cell line (26) was filtered through 0.45-μm-pore-size Tuffryn syringe filters (Pall-Gelman, Pall Corporation, Ann Arbor, Mich.) and added to HEK 293 cells in the presence of 8 μg of hexadimethrine bromide (Sigma, St. Louis, Mo.) per ml. The LMP2A-expressing clone C4, as well as the 293P vector control cells, was selected in 2 μg of puromycin (Sigma) per ml after retroviral infection. Clone 3-5, expressing the CD2-LMP2 CT chimeric protein, was selected in 400 μg of G418 (Gibco-BRL, Life Technologies AB, Stockholm, Sweden) per ml after transfection. Expression in individual clones was tested by immunoblotting.
Construction of CT mutants of the CD2-LMP2 CT chimera and of full-length LMP2A.
Since the LMP2 C terminus is merely 27 aa, or 81 bp, all mutations were incorporated into PCR primers and the C-terminal fragment was ligated into the pBKCMV-Sfv vector between the BamHI site following residue 225 of rat CD2 (42) and the ClaI site at the end of the linker in pBKCMVSfv. The CD2-LMP2 CT chimeric construct is 234 aa (not including the N-terminal signal peptide of CD2), but the protein migrates at about 40 kDa in sodium dodecyl sulfate (SDS) polyacrylamide gels, presumably as a result of glycosylation of the CD2 exodomain. Mutant constructs were verified by sequencing using the Big Dye terminator system (Perkin-Elmer, Stockholm, Sweden). The amino acid sequences of the CT mutants used are listed in Table 1. Of these mutants, the Δ Cys, Δ NTV, and KMN CT mutants were also built back into full-length LMP2A.
TABLE 1.
C-terminal mutations in LMP2A
| Wild type or mutant | C-terminal sequence |
|---|---|
| WT CTa | RCCRYCCYYCLTLESEERPPTPYRNTV |
| T496A | RCCRYCCYYCLTLESEERPPTPYRNAV |
| T496E | RCCRYCCYYCLTLESEERPPTPYRNEV |
| E6 Ter | RCCRYCCYYCLTLESEERPPTPYRETQV |
| Y493F | RCCRYCCYYCLTLESEERPPTPFRNTV |
| Δ NTV | RCCRYCCYYCLTLESEERPPTPYR |
| KMN CT | RCCRYCCYYCLTLESEERPPTPYRKMN |
| SEI CT | RCCRYCCYYCLTLESEERPPTPYRSEI |
| Δ Cysb | KRKKQRSRRNLTLESEERPPTPYRNTV |
WT, wild type.
In the Δ Cys mutation, the cysteine-rich membrane anchor sequence was replaced with the corresponding sequence from the CD2 membrane protein (indicated in boldface).
GST fusion proteins. The C-terminal domain of wild-type (wt) LMP2A was amplified with a 5′ EcoRI site in frame with the glutathione-S-transferase (GST) open reading frame in the pGEX4-T-3 GST expression vector (Amersham Pharmacia Biotech, Uppsala, Sweden) and with a 3′ BamHI site following the termination codon of LMP2A. The Δ NTV and E6 Ter mutants were fused to the GST protein in a similar fashion. The DNA sequences of the constructs were verified by dye terminator sequencing (Big Dye; Perkin-Elmer), and expression was induced in 100-ml cultures of Escherichia coli BL-21 cells (CGSC, New Haven, Conn.) at an optical density at 600 nm of 0.6, by addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 1 mM, followed by a 2-h incubation at 37°C. The pelleted cells were resuspended in 2.5 ml of phosphate-buffered saline–1% Triton X-100 and sonicated with cooling until the lysate cleared. After removal of cell debris by centrifugation at 14,000 rpm in an Eppendorf 5417R centrifuge (Eppendorf-Netheler-Hinz GmbH, Hamburg, Germany) for 30 min at 4°C, 0.5 ml of a 50% suspension of glutathione-Sepharose (Amersham Pharmacia Biotech) was added to the cleared lysates and incubated for 30 min at 4°C. Before use, the GST beads were washed three times in 1% NP-40 lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% NP-40). GST pull-downs were performed by incubating 100 μl of a 10% suspension of GST beads with 1 ml of lysates from 107 LMP2A-expressing C4 cells or control 293 cells for 1 h at 4°C, followed by three washes in 1% NP-40 lysis buffer. Bound proteins were released from the beads by boiling for 5 min at 100°C in 50 μl of SDS sample buffer, of which 25 μl was separated by polyacrylamide gel electrophoresis (PAGE). SDS-PAGE (21), transfer to polyvinylidene difluoride (PVDF) filters, and detection of LMP2A and hDlg proteins on the filters using specific antibodies followed standard procedures (18).
Antibodies, immunofluorescence, immunoprecipitations, and immunoblots.
The rat anti-LMP2A MAbs 8C3, 14B7, and 4E11 (14) were purchased from ITN GmbH, Neuherberg, Germany. The anti-rat CD2 hybridoma OX34 was purchased from American Type Culture Collection, and the MAb was purified from culture supernatant by Protein G Sepharose (Amersham Pharmacia Biotech) chromatography. For use in immunoprecipitation, the LMP2A and CD2 MAbs were covalently coupled to CNBr-activated Sepharose CL-4B (Pharmacia Amersham Biotech) as previously described (18). Immunoprecipitations and immunoblotting were performed using standard techniques, with radioimmunoprecipitation assay (RIPA) buffer (18), for solubilizing of cellular proteins. In short, 10 μl of a 10% suspension of antibody beads was added to 1 ml of lysate from 107 transfected or stably antigen-expressing cells. After three washes in RIPA buffer, captured antigens were released from the beads by boiling in SDS sample buffer and separated by SDS-PAGE as described above. Peroxidase-labeled anti-rat immunoglobulin G (IgG) (heavy plus light chains [H+L]) (P0450; Dako A/S, Glostrup, Denmark), anti mouse IgG (H+L) (PI-2000), anti-goat IgG (H+L) (PI-9500), and anti-rabbit IgG (H+L) (PI-1000) conjugates were from Vector Laboratories (Burlingame, Calif.). Transferrin receptor (sc-7088), hDlg-1 (sc-9961), and caveolin (sc-894) antibodies were from Santa Cruz Biotechnology (Santa Cruz, Calif.).
Insolubilized antibodies to LMP2A and rat CD2 were used in excess over the antigens in immunoprecipitations, since reprecipitation did not yield detectable protein on immunoblots. PVDF filters (Millipore Corporation, Bedford, Mass.) were used throughout for immunoblotting, and detection was done using a luminol-based detection kit (ECL; Amersham Life Science, Little Chalfont, Buckinghamshire, United Kingdom).
Metabolic labeling of LMP2A- and CD2-LMP2 CT-expressing cells with [14C]palmitate or [3H]palmitate.
HEK 293 cells expressing LMP2A (clone C4), CD2-LMP2 C-terminal chimera (clone 3-5), and vector control cells (293P) were grown to 60 to 70% confluency in 10-cm-diameter dishes with Iscove's modified Eagle's medium and 5% fetal bovine serum. Labeling was in fresh medium with the addition of 0.37 MBq of [14C]palmitic acid per ml for 6 h at 37°C, 5% CO2. In total, each plate received 3 ml of medium with a total of 1.11 MBq of [U-14C]palmitic acid (CFB37; Amersham Pharmacia Biotech). Labeling with [3H]palmitic acid was performed for 4 h at 37°C in 2 ml of medium using a total of 37 MBq per plate of [9,10(n)-3H]palmitic acid (TRK 909; Amersham Pharmacia Biotech). After labeling, cells were lysed on ice in RIPA buffer and subjected to immunoprecipitation (18) using immobilized 8C3, 14B7, and 4E11 rat anti-LMP2A MAbs for lysates from LMP2A-expressing cells and using immobilized anti-CD2 MAb OX34 for the CD2-LMP2 CT-expressing 3-5 cells. Caveolin was immunoprecipitated with the sc-894 antibody (Santa Cruz Biotechnology), while the hDlg protein was specifically precipitated from labeled control 293 cells using the GST-ETQV fusion protein (as described above). The immunoprecipitates were separated by SDS-PAGE. The proteins were transferred to PVDF membranes, and the specific proteins were detected by immunoblotting. Following this, radiolabeled proteins were detected by autofluorography on preflashed film. The dry filters were immersed for 10 min in 22% (wt/vol) 2,5-diphenyloxazole in dimethyl sulfoxide. After precipitating the 2,5-diphenyloxazole in water, the filters were dried and exposed to X-ray film at −70°C as described previously (18).
Membrane fractionation in Triton X-100 flotation gradients.
Cell lysis was in 1 ml of TXNE (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA, 0.1% Triton X-100), with 10 μg of pepstatin A (Sigma) per ml and protease inhibitors (Complete; Roche Diagnostic Systems, Mannheim, Germany) at 4°C for a 10-cm-diameter plate of HEK293, essentially as described previously (6). Optiprep (Nycomed A/S, Oslo, Norway) gradients were made in three steps: the bottom step, containing the cell lysate, was 35% Optiprep (1.2 ml); the middle step was 30% Optiprep (3.1 ml); and the top step was 0.5 ml of TXNE. Gradients were centrifuged at 40,000 rpm for 20 h at 4°C in the SW50Ti rotor (Beckman). Three fractions were harvested: 1 ml from the top fraction, 1 ml from the middle fraction, and 1 ml from the bottom fraction. To ensure quantitative recovery of proteins, the fractions were precipitated with 10% trichloroacetic acid in the presence of 200 μg of insulin per ml as described previously (33). The protein pellets were washed by vortexing with 1 ml of −20°C acetone to remove traces of trichloroacetic acid, before being dissolved in SDS sample buffer (21).
RESULTS
Association between LMP2A and heterologous transmembrane molecules depends on the presence of a clustering signal in the CT of LMP2A.
Previously, no functional role was defined for the CT of the LMP2 proteins; however, it contains a number of amino acid motifs that have similarity to functional motifs in other proteins. The cysteine-rich repeat (RCCRYCCYYC) near the membrane could be a target for acylation or could mediate protein-protein interactions through metal chelates; the C-terminal valine motif (NTV) is reminiscent of motifs that bind PDZ domains; and the glutamic acid- and proline-rich motif (ESEERPPTPY) is reminiscent of a similar motif in the N-terminal exon of LMP2A (EERSNEEPPPPY), which was shown by us and others to mediate binding to a WW domain from several members of a HECT protein family of E3 ubiquitin ligases (20, 43).
To search for functional properties of the CT, we made chimeric expression plasmids where the 27-aa C-terminal domain shared between LMP2A and LMP2B was fused to the transmembrane and extracellular domains of rat CD2 (42). The two cysteines on the cytoplasmic side of the 25-aa CD2 transmembrane domain were not included in the construct. The CD2 chimeric protein was highly expressed in 293 cells.
Expressing these CD2 chimeric proteins in the LMP2A-expressing cell line C4, we noticed that the CD2 chimera was coimmunoprecipitated with LMP2A. Our initial hypothesis was that this might be mediated either by protein-protein interactions mediated by the cysteine-rich motif or by the binding of a scaffolding protein containing PDZ domains to the C-terminal valine motif.
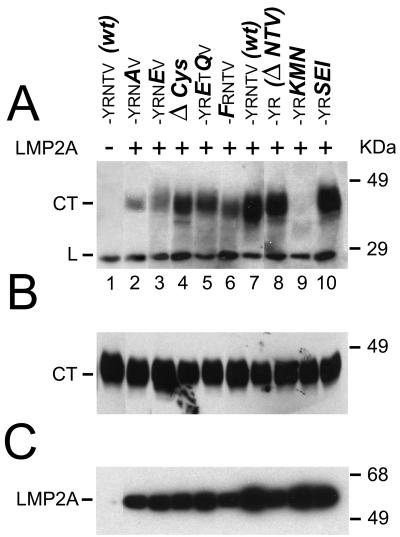
To resolve this question, we created a set of mutants in the CD2 C-terminal chimera and investigated whether they would coimmunoprecipitate with LMP2A after transfection in the LMP2A-expressing cell line C4. The mutants used are shown in Table 1, and the results obtained with these mutants are shown in Fig. 1. In the experiment illustrated in Fig. 1A, immunoprecipitates from C4 cells coexpressing CD2 chimeras with mutant LMP2 C termini were immunoblotted with the OX34 MAb against CD2. Surprisingly, both the cysteine-rich repeat and the terminal valine motif were dispensable for coimmunoprecipitation of the CD2 chimera with LMP2A (Fig. 1A, lanes 4 and 8). As shown in Table 1, the cysteine-rich motif was replaced by the lysine and arginine-rich membrane anchor of CD2, while the C-terminal amino acids NTV were simply deleted.
FIG. 1.
Coimmunoprecipitation of CD2-LMP2 CT mutant chimeras with LMP2A. All CD2-LMP2A chimeras were transiently transfected into the stably LMP2A expressing C4 cell line and lysates were immunoprecipitated with the three rat-anti-LMP2A MAbs 8C3, 14B7 and 4E11 coupled to Sepharose beads (see M&M). Captured antigens were electrophoretically separated, transferred to PVDF membranes and probed with the OX34 anti-CD2 MAb (A), to detect the presence of the chimeric proteins in the LMP2A immunoprecipitates. (B) Whole cell lysates from the transfected C4 cells were probed with the OX34 antibody to determine the relative expression of the chimeric CD2 proteins in the transfected C4 cells. The structure of the 27-aa C-terminal sequence in each wt and mutant CD2 chimera, as well as the nomenclature of the mutants, is explained in Table 1. Each lane contains lysate from 105 cells. (C) Filters with the LMP2A immunoprecipitates were probed with a mixture of the three LMP2A-reactive MAbs to demonstrate that the immunoprecipitates from the C4 cells contained LMP2A. Lane 1 in the three panels is lysate from the LMP2A-negative, CD2 LMP2A CT-expressing cell line 3-5, to show that CD2 is not precipitated unspecifically with the LMP2A MAbs.
We also investigated whether binding of a putative PDZ-domain protein might be regulated by phosphorylation of the penultimate threonine (Fig. 1A, lanes 2 and 3) and whether phosphorylation of the tyrosine in the context of the proline motif PPTPY might be required for the association of CD2 chimeras with LMP2A (lane 6). Mutation of these hydroxyamino acids did not abolish binding to LMP2A, but substituting the three terminal amino acids NTV with KMN (lane 9) did inhibit binding. Substituting consensus sequences for PDZ proteins, one with terminal ETQV from the HPV16 E6 protein and one with a terminal isoleucine instead of valine, gave two mutants which were both active in binding LMP2A (lanes 5 and 10). In all experiments, the expressions of both LMP2A and the CD2 chimeras were similar between samples, as demonstrated for the CD2 chimeric proteins in Fig. 1B, showing whole-cell lysates from the transfected cells immunoblotted with the OX34 MAb against CD2, and for LMP2A in Fig. 1C, which shows an immunoblot of immunoprecipitated LMP2A from the CD2-transfected C4 cells. Lane 1 is a lysate from the CD2-LMP2 CT-expressing cell line 3-5, showing that the CD2 chimera is not precipitated by the rat-anti LMP2A MAbs 8C3, 14B7, and 4E11 (14). In the coimmunoprecipitations shown in Fig. 1, the CD2 constructs are expressed in excess over LMP2A (see also the radioimmunoprecipitation experiment illustrated in Fig. 4A). Moreover, LMP2A is quantitatively precipitated from the cell lysates, while only a fraction of the available CD2 constructs was coimmunoprecipitated with LMP2A, since a second round of immunoprecipitation with LMP2A antibodies did not bring down additional LMP2A protein, while reprecipitation with CD2 antibodies recovered residual CD2 construct.
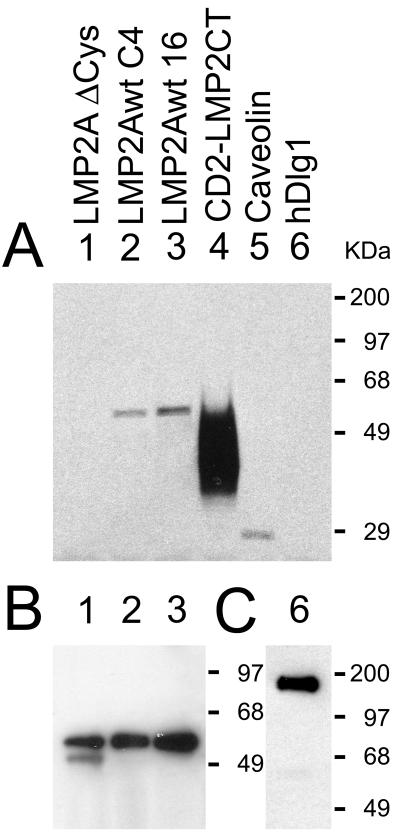
FIG. 4.
Metabolic labeling of LMP2A and CD2-LMP2CT with [3H]palmitate. (A) Autofluorogram of a PVDF filter with appropriate immunoprecipitates from the labeled cell lysates. Lanes: 1 and 3, immunoprecipitates of the LMP2A Δ Cys mutant and the wt LMP2A after transient transfection into 293 cells; 2, LMP2A immunoprecipitate from the constitutively LMP2A expressing cell line C4; 4, CD2 immunoprecipitate from the stable cell line 3-5, expressing the CD2-LMP2 CT construct. The caveolin (lane 5) and hDlg1 (lane 6) immunoprecipitates represent expression of the respective endogenous proteins in control 293 cells. (B) LMP2A immunoblot of the same filter as in panel A, demonstrating that all three LMP2A constructs were expressed. (C) Same filter as in panel A, after stripping and reprobing with hDlg antibody. Immunoblots of CD2 and caveolin immunoprecipitates on the filter in panel A are not shown, since the autofluorogram shows that label was incorporated into these proteins.
The C-terminal clustering signal in the LMP2 C-terminal domain does not require membrane association.
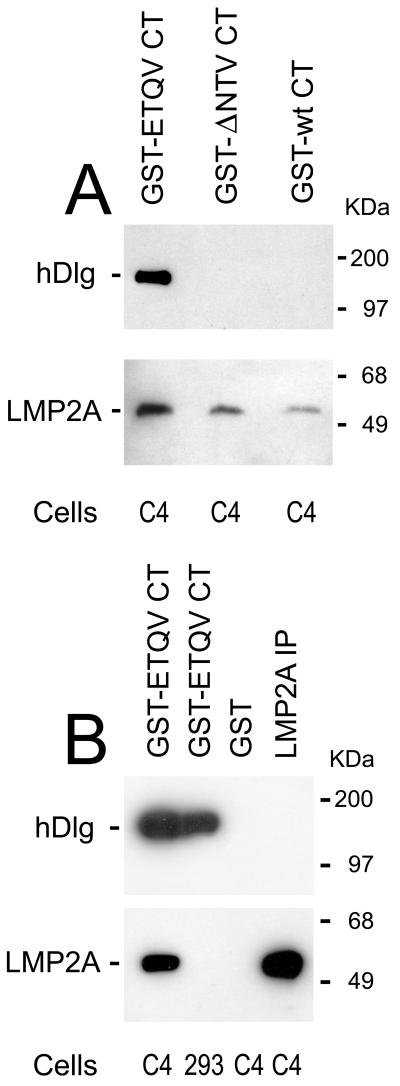
To rule out the possibility that association of CD2 chimeric constructs with LMP2A might depend on interactions between the transmembrane domains of LMP2A and CD2, we decided to test whether LMP2A might also associate with a GST fusion protein carrying the wt LMP2 CT domain. Figure 2 shows that this is the case and, in addition, that the CT domain with deletion of the terminal NTV motif is also active in the GST pull-downs of LMP2A (Fig. 2A). Remarkably, the GST construct carrying the mutation with terminal ETQV pulls down not only LMP2A but also hDlg, in keeping with its function in the HPV16 E6 protein. Figure 2B shows that the ETQV construct pulls down hDlg from control 293 cells while GST protein alone is inactive. These experiments demonstrate that the association between LMP2A and the C-terminal domain of LMP2 is independent of hydrophobic effects from transmembrane domains. This experiment also shows that the sequences required for association of LMP2A with the LMP2 CT domain are separate from those involved in binding a putative PDZ protein to the C-terminal valine motif, since both proteins can bind to the C terminus.
FIG. 2.
GST pull-downs of LMP2A and hDlg. Lysates from LMP2A-expressing C4 cells or control 293 cells were incubated with GST fusion proteins containing the wt and two mutant LMP2 C termini. (A) GST fusion proteins with the wt or Δ NTV mutant pull down LMP2A but not hDlg from C4 cells, while the GST fusion protein with the HPV16 E6 C terminus (ETQV) pulls down both LMP2A and hDlg. (B) The GST-ETQV CT construct is shown to pull down hDlg from the LMP2A negative control cell 293, while the GST protein alone pulls down neither LMP2A nor hDlg from the C4 cell line. This shows that the binding site for the PDZ protein is physically separate from that of LMP2A. The two immunoblots in each panel were first blotted with hDlg antibody, and then they were stripped and reblotted with the anti-LMP2A MAbs.
Molecular association between the C-terminal clustering domains.
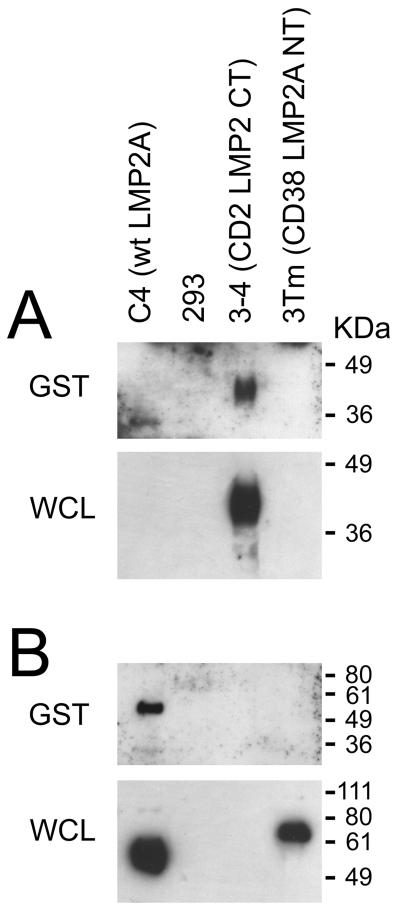
To resolve the question as to what part of wt LMP2A associates with the CD2- and GST-fusion proteins used in the above experiments, we performed pull-down experiments with the GST-LMP2CT fusion protein from lysates of the 3-4 cell line, which constitutively expresses the CD2-LMP2CT construct, from the C4 cell line expressing the wt LMP2A protein, and from 293 cells stably expressing the chimeric CD38 transmembrane protein 3Tm (43), carrying the LMP2A N-terminal exon in the appropriate orientation of a type II membrane protein. Figure 3 shows that the C-terminal domain of LMP2A can mediate association with another C-terminal domain, and that no other sequences from LMP2A are required for this interaction (Fig. 3A). It is conceivable that this association between C-terminal domains is not a direct interaction but is mediated by an as yet unidentified adapter protein.
FIG. 3.
Interactions of the isolated C-terminal domain of LMP2 with membrane bound C- or N-terminal domains of LMP2A. (A) Immunoblots with the OX34 MAb, directed against rat CD2. The top panel shows GST pull-downs from lysates of the stable expressing cell lines indicated above the figure, using the GST-LMP2 CT fusion protein, while the lower panel shows expression of proteins in whole-cell lysates (WCL). Approximately 2% of each WCL was loaded on the gel. (B) Immunoblots in panel A were stripped and reprobed with MAb directed against the N-terminal domain of LMP2A to detect whether the C-terminal domain associates with the N-terminal domain of LMP2A (see Materials and Methods).
While the wt LMP2A protein clearly associates with the GST-LMP2CT construct (Fig. 3B), we were unable to detect association between the GST construct and the N-terminal exon of LMP2A in the background of the 3Tm construct. The secondary structure of the LMP2A N-terminal domain in the context of the chimeric 3Tm construct might, however, deviate sufficiently from that of the wt LMP2A molecule to prevent its association with the C-terminal sequence in the GST fusion protein.
The C-terminal cysteine residues are the major target of LMP2A palmitoylation.
LMP2A is an integral transmembrane protein of 497 aa with type III topology, with intracellular N and C termini, tentatively assigned 12 transmembrane domains. The C-terminal domain is merely 27 aa in length and has a prominent cysteine repeat interspersed with tyrosines and arginines located at the junction between the last transmembrane and the intracellular domain. The structure is highly conserved between the LMP2A proteins of gammaherpesviruses EBV (human herpesvirus 4), herpesvirus papio (baboon), and ceropithecine herpesvirus 15 (rhesus monkey).
A recent paper reports that LMP2A is palmitoylated (19). In the experiments described above we established that the C-terminal cysteine repeats are dispensable for protein-protein association between LMP2A and CD2 chimeras carrying the LMP2 CT domain (Fig. 1A, lane 4). To clarify whether the C-terminal cysteine repeats are targets of this palmitoylation, we performed metabolic labeling, with [14C]palmitic acid or [3H] palmitic acid, of 293 cells expressing wt LMP2A, LMP2A Δ Cys (where the cysteine repeats in the CT were replaced with the arginine- and lysine-rich membrane anchor from the CD2 molecule), and finally, a CD2-LMP2 CT chimera carrying the wt LMP2A CT domain. The results of labeling experiments using [14C]palmitic acid or [3H]palmitic acid did not differ.
Figure 4A) shows an autofluorogram of a PVDF filter with [3H]palmitic acid labeled immunoprecipitates from the labeled cell lysates. The LMP2A Δ Cys and LMP2A wt 16 lysates were from 293 cells, transfected with expression constructs in the pBKCMV-Sfv vector. The LMP2A wt C4 lysate was from the C4 clone of 293 cells, stably expressing LMP2A. There was no significant difference in palmitoylation between the two wt LMP2A samples; however, the Δ Cys mutation showed no labeling with palmitate, although the expressions of the three full-length LMP2A constructs were found to be similar by immunoblotting (Fig. 4B). The strong palmitoylation of the CD2-LMP2 CT chimera (Fig. 4A, lane 4) is explained by a very high expression level of this construct in the stably expressing 293 cell line 3-5. Palmitoylation of the raft protein caveolin is shown in lane 5, and hDlg1 was unlabeled, although its expression was detected by immunoblotting (Fig. 4C, lane 6), indicating that the label was incorporated in the form of palmitate and not as recycled 14C or 3H from metabolized lipid. The failure to detect palmitate label in the LMP2A Δ Cys construct indicates that acylation of cysteine residues outside the C-terminal domain of LMP2A must be low compared to the C-terminal cysteines. The detection limit can be roughly estimated by comparison to the endogenously expressed caveolin (lane 5). Caveolin is irreversibly palmitoylated posttranslationally (31) on at least two of the three juxtamembrane cysteines in its C terminus (41) to allow its raft association and cholesterol transport. Therefore, it is reasonable to assume that a single acylated cysteine outside the C-terminal domain might remain undetected. It is also worth mentioning that the two juxtamembrane cysteines in the cytosolic part of the wt CD2 molecule were excluded from the constructs used here, so palmitoylation of the CD2-LMP2A CT chimera depends solely on palmitoylation of one or more of the five cysteines in the LMP2 CT.
Localization of LMP2A in a Triton X-100-insoluble glycolipid-rich membrane domain does not depend on C-terminal palmitoylation.
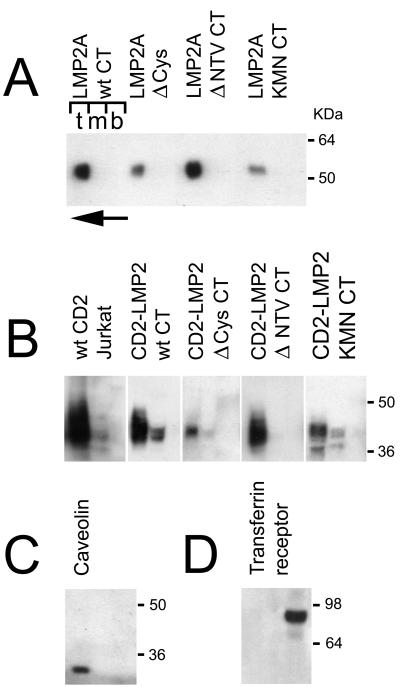
Since acylation is often a signal for apical sorting into glycolipid-rich rafts, it was of interest to investigate whether the raft localization of LMP2A (12) was dependent on palmitoylation. It was also important to establish whether the mutant CD2-CT chimeric constructs used to demonstrate association between the CT domain and full-length LMP2A were expressed in the same membrane compartment as LMP2A. Figure 5 shows the results of Optiprep flotation gradients. Endogenous transferrin receptor and caveolin in lysates of 293P vector control cells (Fig. 5C and D, respectively) were used as markers for the solubilized and Triton X-100-insoluble membrane compartments, respectively (6). The endogenous CD2 in Jurkat T cells also serves as a control since CD2 is located in rafts (45). In Fig. 5A, wt and three LMP2A mutants are shown. The Δ Cys mutant was of interest because it lacks cysteines in the CT domain, and thus lacks C-terminal palmitoylation (Fig. 4, lane 1). The Δ NTV mutant, which lacks the PDZ-like terminal valine motif, was included because it might fail to localize to rafts if clustering by a PDZ protein would be essential for apical sorting (28, 29). The KMN CT mutant was investigated because its failure to cluster with other CT constructs (Fig. 1) might depend on inappropriate expression in the membrane. In all cases, however, the full-length LMP2A constructs were recovered from the Triton X-100-insoluble top fraction of the gradients (Fig. 4A).
FIG. 5.
Optiprep gradient fractionation of different membrane compartments from HEK 293 cells expressing either LMP2A (A) or CD2-LMP2 CT chimeric membrane proteins (B). Three fractions were collected, one each from the top, middle, and bottom layers of the gradient (indicated by the letters t, m, and b above the left gradient shown in panel A). The direction of flotation of the lighter membrane fraction, not solubilized in Triton X-100, is shown with an arrow, pointing from the bottom to the top of the gradient at the top left of panel A. Fractions were collected, treated as described in Materials and Methods, and blotted on PVDF membranes for immunoblotting. (A) Distribution of LMP2A protein in the gradients is shown for the LMP2A wild type and the Δ Cys, Δ NTV, and KMN CT mutants in the full-length LMP2A background. (B) Distribution of CD2-LMP2A CT constructs between Triton X-100-soluble (bottom) and -insoluble (top) membrane fractions is shown. A lysate from the human T-cell line Jurkat was included, to demonstrate that the native CD2 molecule distributes similarly to the chimeric constructs used in this study. (C and D) Locations of two control proteins, endogenously expressed in the 293 cells; caveolin which is a marker for the detergent-insoluble glycolipid-enriched membranes (rafts) (C) and transferrin receptor, which is known to distribute in the Triton X-100 soluble phosphatidyl-etanolamine rich part of the membrane (D). Immunoblotting was performed with the appropriate antibodies as described in Materials and Methods.
The CD2-CT chimeric constructs shown in Fig. 5B are also located in the top fraction of the gradients, showing that they are appropriately expressed in the same membrane compartment as LMP2A and that the failure of the CD2-LMP2 KMN CT construct to associate with wt LMP2A (Fig. 1) is not a result of expression in a separate membrane compartment.
The raft association of the CD2 constructs is likely to depend on properties of the CD2 backbone in these constructs (including the transmembrane domain), since the native CD2 molecule is located in rafts (Fig. 4B). A fine punctate membrane-staining pattern in 293 cells expressing the CD2 constructs also supports the raft localization (data not shown).
DISCUSSION
It is known that molecular clustering is required for activation of the LMP2A N-terminal domain by tyrosine phosphorylation. In the initial demonstration of signal transduction activity by the LMP2A N-terminal domain (2), antibody ligation of a CD8α-LMP2A N-terminal chimera resulted in calcium signals and interleukin 2 production by IIA1.6 lymphoma B cells, while the nonligated chimeric molecule was inactive. In addition, our demonstration that the E3 ubiquitin ligase binds to nonphosphorylated tyrosines in the two PPPPY motifs in the LMP2A N-terminal exon relied on the demonstration that AIP4 binds to a CD38-LMP2A chimeric transmembrane construct (3Tm), where all tyrosines remain in the unphosphorylated state as long as the construct is not clustered by antibody to the CD38 exodomain (43). Since wt LMP2A is constitutively phosphorylated on tyrosine 112 (16), it is reasonable to hypothesize that LMP2A molecules spontaneously associate in the membrane. This could be the result of direct interaction between LMP2A molecules or be mediated by a scaffolding protein (32) as described for the cystic fibrosis transmembrane conductance receptor and many other ion channels (5). One purpose of this investigation was to decide if and by what mechanism association between LMP2A molecules might take place. Our finding is that transmembrane chimeras carrying the rat CD2 exodomain and transmembrane domain fused to the LMP2 CT domain as well as GST fusion proteins carrying the LMP2CT domain interact with wt LMP2A. Furthermore, we show that the C-terminal domain is capable of association with itself, since the CD2-LMP2CT chimera efficiently interacts with the GST-LMP2CT fusion protein. Our mutation analysis shows that neither the cysteine-rich repeat nor the PDZ-like motif at the LMP2 C terminus is essential for this protein-protein interaction.
If LMP2A molecules required a PDZ-containing scaffolding protein for clustering, then such a protein would be likely to bind to the terminal valine motif. However, this protein is not hDlg, since hDlg cannot be detected on an immunoblot of the GST-wt CT pull-down in Fig. 2A. On the other hand, the hDlg binding to the GST-ETQV CT construct, shown in the same immunoblot, would then block binding of the hypothetical PDZ scaffolding protein that normally would cluster LMP2A molecules. This would also prevent binding of LMP2A. Since both hDlg and LMP2A bind to the ETQV construct, and since LMP2A also binds to the GST Δ NTV construct, it is unlikely that the terminal NTV motif is involved in LMP2A clustering. Instead, it is probable that there is a protein interaction motif located between the cysteine-rich repeat and the terminal NTV tripeptide. This would correspond to all or part of the sequence LTLESEERPPTPYR, located between the cysteine-rich repeat and the terminal NTV tripeptide.
This prompted us to ask whether the cysteine-rich repeats might have a different function. We demonstrate that LMP2A and the corresponding CD2 chimera are palmitoylated, while in a mutant LMP2A (Δ Cys), with an arginine- and lysine-rich membrane anchor in place of the cysteine-rich repeat, palmitoylation was not detected. This shows that the C-terminal cysteines are the major site of LMP2A palmitoylation. As previously described, palmitoylation of membrane proteins may regulate their rate of internalization (1), which may be relevant in the context of recent studies indicating that LMP2A interferes with internalization of antigen-activated B-cell receptor (12).
Our studies of LMP2A localization in rafts (38) also confirm previous findings (12, 19) and demonstrate, additionally, that C-terminal LMP2A palmitoylation is nonessential for the localization of LMP2A in detergent-insoluble glycolipid-enriched membrane microdomains (rafts). These experiments also verify that the molecular interactions between LMP2A and C-terminal mutants in the CD2 chimeric background take place between proteins that are expressed in the same membrane compartment. This shows, specifically, that the failure of the CD2-KMN CT mutant to associate with LMP2A is not due to a defect in raft association.
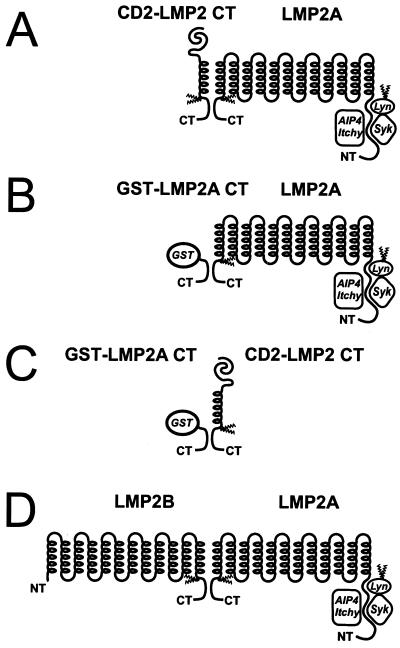
Figure 6A, B, and C illustrate our findings regarding molecular association of LMP2A molecules. In Fig. 6D, finally, we show a model of the hypothetical interaction between LMP2 proteins suggested by our demonstration of a C-terminal association motif. The demonstration of this interaction is biologically meaningful only if the existence of the LMP2B protein can be shown in EBV-infected cells. We are currently using the GST-LMP2CT fusion protein to isolate this protein from B cells in latency III and from EBV-positive NPC cells in culture.
FIG. 6.
Models of C-terminal interactions between LMP2 proteins. (A) Association between LMP2A and the CD2 chimeric transmembrane proteins with the LMP2 C-terminal domain. (B) Interaction of the GST-LMP2 CT constructs. (C) The interactions involve two C-terminal domains, since the GST-LMP2A CT fusion protein can pull down the CD2-LMP2A C-terminal chimera from cell lysates of 3-5 cells. While we show that these interactions occur as tail-tail interactions, we cannot exclude that head-tail interactions might also occur. (D) Hypothetical interaction between LMP2 proteins through their shared C-terminal domains.
Our present finding that a C-terminal motif, hypothetically located between aa 481 and 494, is sufficient for the physical association between LMP2A and heterologous transmembrane molecules or soluble GST fusion proteins carrying this motif may help explain how LMP2A is normally kept in a clustered, constitutively active state and may be of particular importance for understanding the role of the LMP2B in regulating the latency state of EBV-carrying cells. Although the existence of the LMP2B protein remains to be demonstrated, it has been hypothesized that the stringent control of B-cell phenotype maintained by LMP2A in type I latency might be relaxed as the cell progresses through latency II and III, perhaps in part as a result of LMP2B expression. The LMP2B protein, which lacks the N-terminal exon of LMP2A, might cocluster with LMP2A and reduce the local concentration of cellular proteins binding to the N-terminal exon of LMP2A. This would reduce the effects of LMP2A on signal transduction in the B cell.
ACKNOWLEDGMENTS
This work was supported in part by grant No K1999-06X-012622-02B from the Swedish Medical Research Council to G.W. and L.M., by grants from the Swedish Cancer Society to I.E., and by grants from the Canadian Institutes of Health Research and the National Cancer Institute of Canada to T.P.
REFERENCES
- 1.Alvarez E, Girones N, Davis R J. Inhibition of the receptor-mediated endocytosis of diferric transferrin is associated with the covalent modification of the transferrin receptor with palmitic acid. J Biol Chem. 1990;265:16644–16655. [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1988. [Google Scholar]
- 3.Babcock G J, Decker L L, Volk M, Thorley-Lawson D A. EBV persistence in memory B cells in vivo. Immunity. 1998;9:395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 4.Beaufils P, Choquet D, Mamoun R Z, Malissen B. The (YXXL/I) 2 signalling motif found in the cytoplasmic segments of the bovine leukaemia virus envelope protein and Epstein-Barr virus latent membrane protein 2A can elicit early and late lymphocyte activation events. EMBO J. 1993;12:5105–5112. doi: 10.1002/j.1460-2075.1993.tb06205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bezprozvanny I, Maximov A. PDZ domains: more than just a glue. Proc Natl Acad Sci USA. 2001;98:787–789. doi: 10.1073/pnas.98.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruckner K, Pablo Labrador J, Scheiffele P, Herb A, Seeburg P H, Klein R. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron. 1999;22:511–524. doi: 10.1016/s0896-6273(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 7.Burkhardt A L, Bolen J B, Kieff E, Longnecker R. An Epstein-Barr virus transformation-associated membrane protein interacts with family tyrosine kinases. J Virol. 1992;66:5161–5167. doi: 10.1128/jvi.66.8.5161-5167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldwell R G, Brown R C, Longnecker R. Epstein-Barr virus LMP2A-induced B-cell survival in two unique classes of EmuLMP2A transgenic mice. J Virol. 2000;74:1101–1113. doi: 10.1128/jvi.74.3.1101-1113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F, Hu L F, Ernberg I, Klein G, Winberg G. Coupled transcription of Epstein-Barr virus latent membrane protein (LMP)-1 and LMP-2B genes in nasopharyngeal carcinomas. J Gen Virol. 1995;76:131–138. doi: 10.1099/0022-1317-76-1-131. [DOI] [PubMed] [Google Scholar]
- 11.Clausse B, Fizazi K, Walczak V, Tetaud C, Wiels J, Tursz T, Busson P. High concentration of the EBV latent membrane protein 1 in glycosphingolipid-rich complexes from both epithelial and lymphoid cells. Virology. 1997;228:285–293. doi: 10.1006/viro.1996.8380. [DOI] [PubMed] [Google Scholar]
- 12.Dykstra M L, Longnecker R, Pierce S K. Epstein-Barr virus coopts lipid rafts to block the signaling and antigen transport functions of the BCR. Immunity. 2001;14:57–67. doi: 10.1016/s1074-7613(01)00089-9. [DOI] [PubMed] [Google Scholar]
- 13.Eliopoulos A G, Dawson C W, Mosialos G, Floettmann J E, Rowe M, Armitage R J, Dawson J, Zapata J M, Kerr D J, Wakelam M J, Reed J C, Kieff E, Young L S. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr Virus-encoded LMP1, involvement of TRAF3 as a common mediator. Oncogene. 1996;13:2243–2254. [PubMed] [Google Scholar]
- 14.Fruehling S, Lee S K, Herrold R, Frech B, Laux G, Kremmer E, Grässer F A, Longnecker R. Identification of latent membrane protein 2A (LMP2A) domains essential for the dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. J Virol. 1996;70:6216–6226. doi: 10.1128/jvi.70.9.6216-6226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fruehling S, Longnecker R. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A essential for blocking BCR-mediated signal transduction. Virology. 1997;235:241–251. doi: 10.1006/viro.1997.8690. [DOI] [PubMed] [Google Scholar]
- 16.Fruehling S, Swart R, Dolwick K M, Kremmer E, Longnecker R. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J Virol. 1998;72:7796–7806. doi: 10.1128/jvi.72.10.7796-7806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 18.Harlowe E, Lane D. Antibodies, a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 19.Higuchi M, Izumi K M, Kieff E. Epstein-Barr virus latent-infection membrane proteins are palmitoylated and raft-associated: Protein 1 binds to the cytoskeleton through TNF receptor cytoplasmic factors. Proc Natl Acad Sci USA. 2001;98:4675–4680. doi: 10.1073/pnas.081075298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda M, Ikeda A, Longan L C, Longnecker R. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology. 2000;268:178–191. doi: 10.1006/viro.1999.0166. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Laux G, Dugrillon F, Eckert C, Adam B, Zimber-Strobl U, Bornkamm G W. Identification and characterization of an Epstein-Barr virus nuclear antigen 2-responsive cis element in the bidirectional promoter region of latent membrane and terminal protein 2 genes. J Virol. 1994;68:6947–6958. doi: 10.1128/jvi.68.11.6947-6958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laux G, Perricaudet M, Farrell P J. A spliced Epstein-Barr virus gene expressed in immortalized lymphocytes is by circularization of the linear viral genome. EMBO J. 1988;7:769–774. doi: 10.1002/j.1460-2075.1988.tb02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longnecker R, Kieff E. A second Epstein-Barr virus membrane protein (LMP2.) is expressed in latent infection and colocalizes with LMP1. J Virol. 1990;64:2319–2326. doi: 10.1128/jvi.64.5.2319-2326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller A D, Rosman G J. Improved retroviral vectors for gene transfer and expression. BioTechniques. 1989;7:980–982. , 984–986, 989–990. [PMC free article] [PubMed] [Google Scholar]
- 26.Miller A D, Garcia J V, von Suhr N, Lynch C M, Wilson C, Eiden M V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991;65:2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller C L, Burkhardt A L, Lee J H, Stealey B, Longnecker R, Bolen J B, Kieff E. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity. 1995;2:155–166. doi: 10.1016/s1074-7613(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 28.Mohler P J, Kreda S M, Boucher R C, Sudol M, Stutts M J, Milgram S L. Yes-associated protein 65 localizes p62(c-Yes) to the apical compartment of airway epithelia by association with EBP50. J Cell Biol. 1999;147:879–890. doi: 10.1083/jcb.147.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moyer B D, Denton J, Karlson K H, Reynolds D, Wang S, Mickle J E, Milewski M, Cutting G R, Guggino W B, Li M, Stanton B A. A PDZ-interacting domain in CFTR is an apical membrane polarization signal. J Clin Investig. 1999;104:1353–1361. doi: 10.1172/JCI7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panousis C G, Rowe D T. Epstein-Barr virus latent membrane protein 2 associates with and is a substrate for mitogen-activated protein kinase. J Virol. 1997;71:4752–4760. doi: 10.1128/jvi.71.6.4752-4760.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parat M O, Fox P L. Palmitoylation of caveolin-1 in endothelial cells is posttranslational but irreversible. J Biol Chem. 2001;276:15776–15782. doi: 10.1074/jbc.M006722200. [DOI] [PubMed] [Google Scholar]
- 32.Pawson T, Scott J D. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 33.Payne L. Polypeptide composition of extracellular enveloped vaccinia virus. J Virol. 1978;27:28–37. doi: 10.1128/jvi.27.1.28-37.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perry W L, Hustad C M, Swing D A, O'Sullivan T N, Jenkins N A, Copeland N G. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice. Nat Genet. 1998;18:143–146. doi: 10.1038/ng0298-143. [DOI] [PubMed] [Google Scholar]
- 35.Qu L, Rowe D T. Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes. J Virol. 1992;66:3715–3724. doi: 10.1128/jvi.66.6.3715-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scholle F, Longnecker R, Raab-Traub N. Epithelial cell adhesion to extracellular matrix proteins induces tyrosine phosphorylation of the Epstein-Barr virus latent membrane protein 2, a role for C-terminal Src kinase. J Virol. 1999;73:4767–4775. doi: 10.1128/jvi.73.6.4767-4775.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholle F, Bendt K M, Raab-Traub N. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol. 2000;74:10681–10689. doi: 10.1128/jvi.74.22.10681-10689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 39.Strobl L J, Hofelmayr H, Marschall G, Brielmeier M, Bornkamm G W, Zimber-Strobl U. Activated Notch1 modulates gene expression in B cells similarly to Epstein-Barr nuclear antigen 2. J Virol. 2000;74:1727–1735. doi: 10.1128/jvi.74.4.1727-1735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swart R, Ruf I K, Sample J, Longnecker R. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-Kinase/Akt pathway. J Virol. 2000;74:10838–10845. doi: 10.1128/jvi.74.22.10838-10845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uittenbogaard R, Smart E J. Palmitoylation of caveolin-1 is required for cholesterol binding, chaperone complex formation, and rapid transport of cholesterol to caveolae. J Biol Chem. 2000;275:25595–25599. doi: 10.1074/jbc.M003401200. [DOI] [PubMed] [Google Scholar]
- 42.Williams A F, Barclay A N, Clark S J, Paterson D J, Willis A C. Similarities in sequences and cellular expression between rat CD2 and CD4 antigens. J Exp Med. 1987;165:368–380. doi: 10.1084/jem.165.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winberg G, Matskova L, Chen F, Plant P, Rotin D, Gish G, Ingham R, Ernberg I, Pawson T. Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol Cell Biol. 2000;20:8526–8535. doi: 10.1128/mcb.20.22.8526-8535.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood J D, Yuan J, Margolis R L, Colomer V, Duan K, Kushi J, Kaminsky Z, Kleiderlein J J, Sharp A H, Ross C A. Atrophin-1, the DRPLA gene product, interacts with two families of WW domain-containing proteins. Cell Neurosci. 1998;11:149–160. doi: 10.1006/mcne.1998.0677. [DOI] [PubMed] [Google Scholar]
- 45.Yashiro-Ohtani Y, Zhou X Y, Toyo-Oka K, Tai X G, Park C S, Hamaoka T, Abe R, Miyake K, Fujiwara H. Non-CD28 costimulatory molecules present in T cell rafts induce T cell costimulation by enhancing the association of TCR with rafts. J Immunol. 2000;164:1251–1259. doi: 10.4049/jimmunol.164.3.1251. [DOI] [PubMed] [Google Scholar]