Abstract
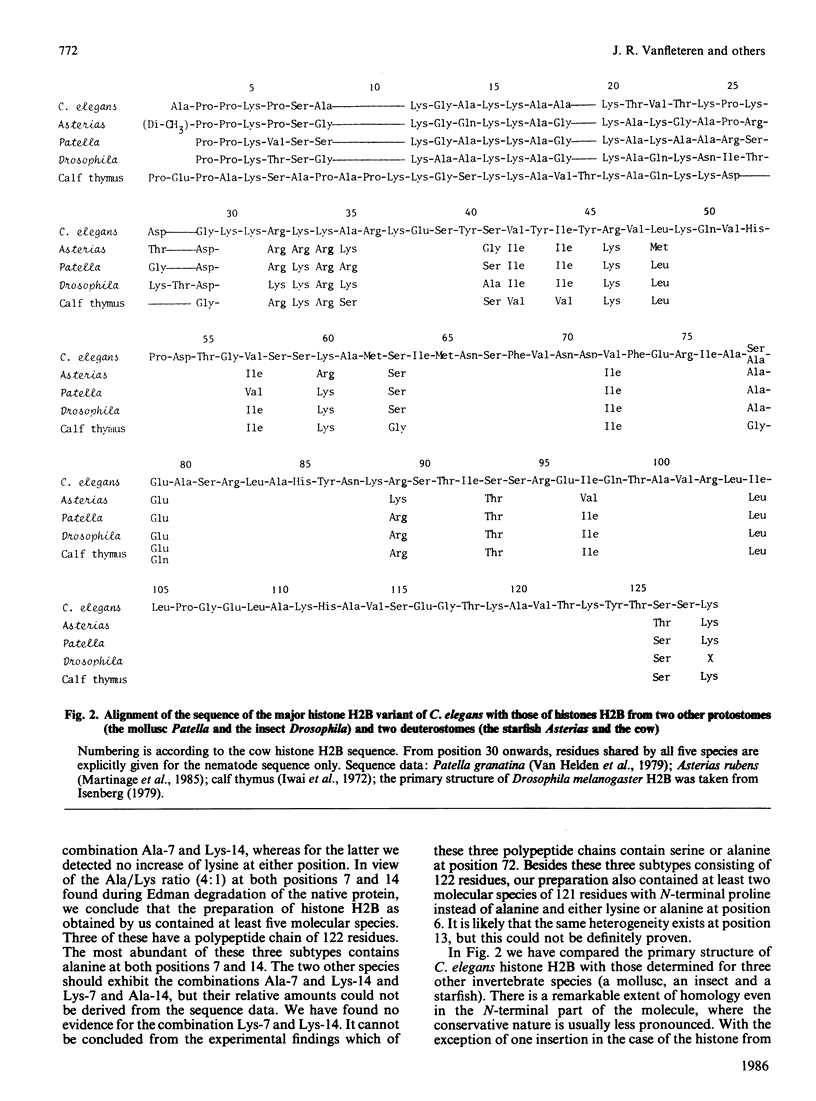
The complete amino acid sequence of histone H2B from the nematode Caenorhabditis elegans was determined. The protein as obtained by us is a mixture of multiple forms. Approx. 90% of the molecules consist of a polypeptide chain of 122 amino acids with alanine as N-terminal residue and proline at the second position. In the remaining 10% alanine is lacking and the chain starts with proline. In addition to the heterogeneity of chain length, polymorphism occurs at the positions 7 (Ala/Lys), 14 (Ala/Lys) and 72 (Ala/Ser) of the major chain and at position 6 (Ala/Lys) of the shorter chain. In the N-terminal third of the molecule there is a high degree of sequence homology to the corresponding region in H2B from Drosophila (insect), Patella (mollusc) and Asterias (starfish). In contrast, this part of the molecule differs considerably from mammalian histone H2B.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Brandt W. F., Strickland W. N., Strickland M., Carlisle L., Woods D., von Holt C. A histone programme during the life cycle of the sea urchin. Eur J Biochem. 1979 Feb 15;94(1):1–10. doi: 10.1111/j.1432-1033.1979.tb12864.x. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. The sequence determination of a protein in a micro scale: the sequence analysis of ribosomal protein L34 of Escherichia coli. Hoppe Seylers Z Physiol Chem. 1976 Jun;357(6):873–886. doi: 10.1515/bchm2.1976.357.1.873. [DOI] [PubMed] [Google Scholar]
- Childs G., Maxson R., Kedes L. H. Histone gene expression during sea urchin embryogenesis: isolation and characterization of early and late messenger RNAs of Strongylocentrotus purpuratus by gene-specific hybridization and template activity. Dev Biol. 1979 Nov;73(1):153–173. doi: 10.1016/0012-1606(79)90144-1. [DOI] [PubMed] [Google Scholar]
- Cohen L. H., Newrock K. M., Zweidler A. Stage-specific switches in histone synthesis during embryogenesis of the sea urchin. Science. 1975 Dec 5;190(4218):994–997. doi: 10.1126/science.1237932. [DOI] [PubMed] [Google Scholar]
- Franklin S. G., Zweidler A. Non-allelic variants of histones 2a, 2b and 3 in mammals. Nature. 1977 Mar 17;266(5599):273–275. doi: 10.1038/266273a0. [DOI] [PubMed] [Google Scholar]
- Grunstein M. Hatching in the sea urchin Lytechinus pictus is accompanied by a shift in histone H4 gene activity. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4135–4139. doi: 10.1073/pnas.75.9.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieter P. A., Hendricks M. B., Hemminki K., Weinberg E. S. Histone gene switch in the sea urchin embryo. Identification of late embryonic histone messenger ribonucleic acids and the control of their synthesis. Biochemistry. 1979 Jun 26;18(13):2707–2716. doi: 10.1021/bi00580a004. [DOI] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Analysis of phenylthiohydantoins by ultrasensitive gradient high-performance liquid chromatography. Methods Enzymol. 1983;91:486–493. doi: 10.1016/s0076-6879(83)91045-5. [DOI] [PubMed] [Google Scholar]
- Isenberg I. Histones. Annu Rev Biochem. 1979;48:159–191. doi: 10.1146/annurev.bi.48.070179.001111. [DOI] [PubMed] [Google Scholar]
- Iwai K., Hayashi H., Ishikawa K. Calf thymus lysine- and serine-rich histone. 3. Complete amino acid sequence and its implication for interactions of histones with DNA. J Biochem. 1972 Aug;72(2):357–367. doi: 10.1093/oxfordjournals.jbchem.a129911. [DOI] [PubMed] [Google Scholar]
- Martinage A., Briand G., Van Dorsselaer A., Turner C. H., Sautiere P. Primary structure of histone H2B from gonads of the starfish Asterias rubens. Identification of an N-dimethylproline residue at the amino-terminal. Eur J Biochem. 1985 Mar 1;147(2):351–359. doi: 10.1111/j.1432-1033.1985.tb08757.x. [DOI] [PubMed] [Google Scholar]
- Newrock K. M., Freedman N., Alfageme C. R., Cohen L. H. Isolation of sea urchin embryo histone H2A and immunological identification of other stage-specific H2A proteins. Dev Biol. 1982 Jan;89(1):248–253. doi: 10.1016/0012-1606(82)90311-6. [DOI] [PubMed] [Google Scholar]
- Poccia D. L., Hinegardner R. T. Developmental changes in chromatin proteins of the sea urchin from blastula to mature larva. Dev Biol. 1975 Jul;45(1):81–89. doi: 10.1016/0012-1606(75)90243-2. [DOI] [PubMed] [Google Scholar]
- Poccia D., Salik J., Krystal G. Transitions in histone variants of the male pronucleus following fertilization and evidence for a maternal store of cleavage-stage histones in the sera urchin egg. Dev Biol. 1981 Mar;82(2):287–296. doi: 10.1016/0012-1606(81)90452-8. [DOI] [PubMed] [Google Scholar]
- Strickland M. S., Strickland W. N., von Holt C. The histone H2B from the sperm cell of the starfish Marthasterias glacialis. Eur J Biochem. 1980 May;106(2):541–548. doi: 10.1111/j.1432-1033.1980.tb04601.x. [DOI] [PubMed] [Google Scholar]
- Strickland M., Strickland W. N., Brandt W. F., Von Holt C. The complete amino-acid sequence of histone H2B(1) from sperm of the sea urchin Parechinus angulosus. Eur J Biochem. 1977 Jul 15;77(2):263–275. doi: 10.1111/j.1432-1033.1977.tb11665.x. [DOI] [PubMed] [Google Scholar]
- Strickland M., Strickland W. N., Brandt W. F., Von Holt C., Wittmann-Liebold B. The complete amino-acid sequence of histone H2B(3) from sperm of the sea urchin Parechinus angulosus. Eur J Biochem. 1978 Sep 1;89(2):443–452. doi: 10.1111/j.1432-1033.1978.tb12547.x. [DOI] [PubMed] [Google Scholar]
- Strickland W. N., Strickland M., Brandt W. F., Von Holt C. The complete amino-acid sequence of histone H2B(2) from sperm of the sea urchin Parechinus angulosus. Eur J Biochem. 1977 Jul 15;77(2):277–286. doi: 10.1111/j.1432-1033.1977.tb11666.x. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanfleteren J. R. Nematode chromosomal proteins--II. Fractionation and identification of the histones of Caenorhabditis elegans. Comp Biochem Physiol B. 1982;73(3):709–718. doi: 10.1016/0305-0491(82)90101-8. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Walter R., Tsuru D. Proline-specific endopeptidase from Flavobacterium. Purification and properties. J Biol Chem. 1980 May 25;255(10):4786–4792. [PubMed] [Google Scholar]
- van Helden P. D., Strickland W. N., Brandt W. F., von Holt C. The complete amino-acid sequence of histone H2B from the mollusc Patella granatina. Eur J Biochem. 1979 Jan 2;93(1):71–78. doi: 10.1111/j.1432-1033.1979.tb12796.x. [DOI] [PubMed] [Google Scholar]
- van der Westhuyzen D. R., von Holt C. A new procedure for the isolation and fractionation of histones. FEBS Lett. 1971 May 20;14(5):333–337. doi: 10.1016/0014-5793(71)80294-6. [DOI] [PubMed] [Google Scholar]


