Abstract
Oligonucleotide-directed mutagenesis of cloned Rhodospirillum rubrum ribulose bisphosphate carboxylase/oxygenase with a synthetic 13mer oligonucleotide primer was used to effect a change at Met-330 to Leu-330. The resultant enzyme was kinetically examined in some detail and the following changes were found. The Km(CO2) increased from 0.16 to 2.35 mM, the Km(ribulose bisphosphate) increased from 0.05 to 1.40 mM for the carboxylase reaction and by a similar amount for the oxygenase reaction. The Ki(O2) increased from 0.17 to 6.00 mM, but the ratio of carboxylase activity to oxygenase activity was scarcely affected by the change in amino acid. The binding of the transition state analogue 2-carboxyribitol 1,5-bisphosphate was reversible in the mutant and essentially irreversible in the wild type enzyme. Inhibition by fructose bisphosphate, competitive with ribulose bisphosphate, was slightly increased in the mutant enzyme. These data suggest that the change of the residue from methionine to leucine decreases the stability of the enediol reaction intermediate.
Full text
PDF

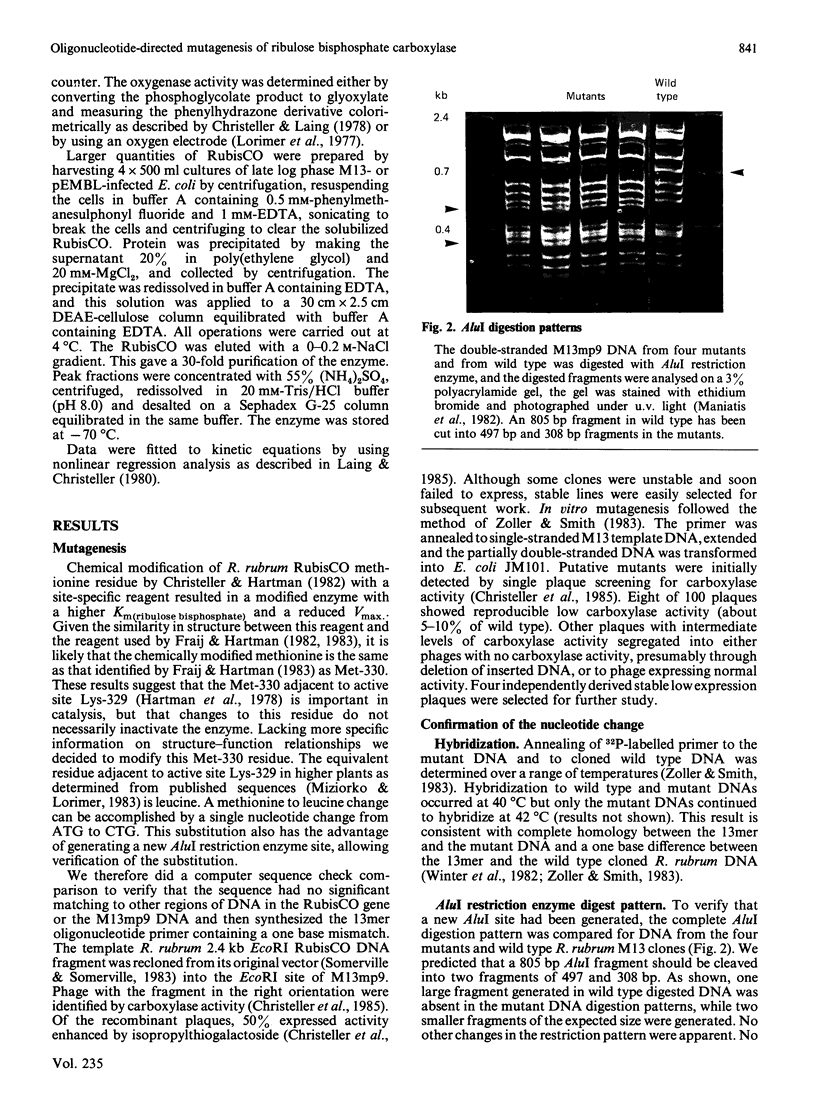
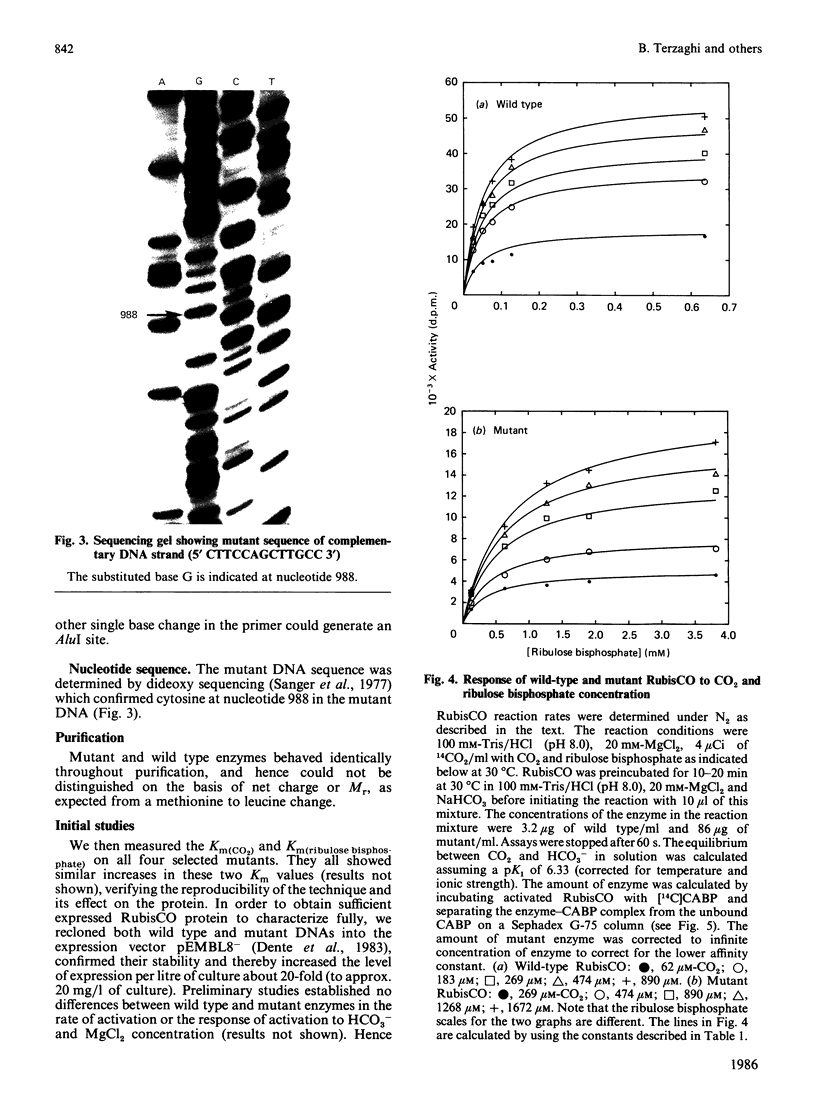



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown H. M., Chollet R. Isolation of a stable enzyme.14CO2.Mg2+.carboxyarabinitol bisphosphate complex with ribulosebisphosphate carboxylase from Chromatium vinosum. J Bacteriol. 1982 Mar;149(3):1159–1161. doi: 10.1128/jb.149.3.1159-1161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALVIN M. Chemical and photochemical reactions of thioctic acid and related disulfides. Fed Proc. 1954 Sep;13(3):697–711. [PubMed] [Google Scholar]
- Christeller J. T., Laing W. A. A kinetic study of ribulose bisphosphate carboxylase from the photosynthetic bacterium Rhodospirillum rubrum. Biochem J. 1978 Aug 1;173(2):467–473. doi: 10.1042/bj1730467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christeller J. T., Laing W. A. Effects of manganese ions and magnesium ions on the activity of soya-bean ribulose bisphosphate carboxylase/oxygenase. Biochem J. 1979 Dec 1;183(3):747–750. doi: 10.1042/bj1830747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraij B., Hartman F. C. 2-Bromoacetylaminopentitol 1,5-bisphosphate as an affinity label for ribulose bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. J Biol Chem. 1982 Apr 10;257(7):3501–3505. [PubMed] [Google Scholar]
- Fraij B., Hartman F. C. Isolation and sequencing of an active-site peptide from Rhodospirillum rubrum ribulosebisphosphate carboxylase/oxygenase after affinity labeling with 2-[(bromoacetyl)amino]pentitol 1,5-bisphosphate. Biochemistry. 1983 Mar 15;22(6):1515–1520. doi: 10.1021/bi00275a028. [DOI] [PubMed] [Google Scholar]
- Gait M. J., Matthes H. W., Singh M., Sproat B. S., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides. VII. Solid phase synthesis of oligodeoxyribonucleotides by a continuous flow phosphotriester method on a kieselguhr-polyamide support. Nucleic Acids Res. 1982 Oct 25;10(20):6243–6254. doi: 10.1093/nar/10.20.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge S., Sigal I., Thomas B., Arentzen R., Cordova A., Lorimer G. A site-specific mutation within the active site of ribulose-1,5-bisphosphate carboxylase of Rhodospirillum rubrum. EMBO J. 1984 Dec 1;3(12):2737–2743. doi: 10.1002/j.1460-2075.1984.tb02204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSCH C., DEUTSCH E. W., SMITH R. N. THE USE OF SUBSTITUENT CONSTANTS AND REGRESSION ANALYSIS IN THE STUDY OF ENZYMATIC REACTION MECHANISMS. J Am Chem Soc. 1965 Jun 20;87:2738–2742. doi: 10.1021/ja01090a035. [DOI] [PubMed] [Google Scholar]
- Hall N. P., Pierce J., Tolbert N. E. Formation of a carboxyarabinitol bisphosphate complex with ribulose bisphosphate carboxylase/oxygenase and theoretical specific activity of the enzyme. Arch Biochem Biophys. 1981 Nov;212(1):115–119. doi: 10.1016/0003-9861(81)90349-0. [DOI] [PubMed] [Google Scholar]
- Hartman F. C., Stringer C. D., Lee E. H. Complete primary structure of ribulosebisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. Arch Biochem Biophys. 1984 Jul;232(1):280–295. doi: 10.1016/0003-9861(84)90544-7. [DOI] [PubMed] [Google Scholar]
- Herndon C. S., Hartman F. C. 2-(4-Bromoacetamido)anilino-2-deoxypentitol 1,5-bisphosphate, a new affinity label for ribulose bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. Determination of reaction parameters and characterization of an active site peptide. J Biol Chem. 1984 Mar 10;259(5):3102–3110. [PubMed] [Google Scholar]
- Jordan D. B., Ogren W. L. A Sensitive Assay Procedure for Simultaneous Determination of Ribulose-1,5-bisphosphate Carboxylase and Oxygenase Activities. Plant Physiol. 1981 Feb;67(2):237–245. doi: 10.1104/pp.67.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D. B., Ogren W. L. Species variation in kinetic properties of ribulose 1,5-bisphosphate carboxylase/oxygenase. Arch Biochem Biophys. 1983 Dec;227(2):425–433. doi: 10.1016/0003-9861(83)90472-1. [DOI] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T. A model for the kinetics of activation and catalysis of ribulose 1,5-bisphosphate carboxylase. Biochem J. 1976 Dec 1;159(3):563–570. doi: 10.1042/bj1590563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T. A steady-state kinetic study on the catalytic mechanism of ribulose bisphosphate carboxylase from soybean. Arch Biochem Biophys. 1980 Jul;202(2):592–600. doi: 10.1016/0003-9861(80)90466-x. [DOI] [PubMed] [Google Scholar]
- Laing W. A. Regulation of Soybean Net Photosynthetic CO(2) Fixation by the Interaction of CO(2), O(2), and Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1974 Nov;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Petersen G. B., Reeves J. M. The occurrence of long sequences of consecutive pyrimidine deoxyribonucleotides in the DNA of bacteriophage f1. Biochim Biophys Acta. 1969 Apr 22;179(2):510–512. doi: 10.1016/0005-2787(69)90062-8. [DOI] [PubMed] [Google Scholar]
- Pierce J., Tolbert N. E., Barker R. Interaction of ribulosebisphosphate carboxylase/oxygenase with transition-state analogues. Biochemistry. 1980 Mar 4;19(5):934–942. doi: 10.1021/bi00546a018. [DOI] [PubMed] [Google Scholar]
- Roeske C. A., O'Leary M. H. Carbon isotope effect on carboxylation of ribulose bisphosphate catalyzed by ribulosebisphosphate carboxylase from Rhodospirillum rubrum. Biochemistry. 1985 Mar 26;24(7):1603–1607. doi: 10.1021/bi00328a005. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver B. G., Knowles J. R. Ribulose-1,5-bisphosphate carboxylase: enzyme-catalyzed appearance of solvent tritium at carbon 3 of ribulose 1,5-bisphosphate reisolated after partial reaction. Biochemistry. 1982 Oct 26;21(22):5398–5403. doi: 10.1021/bi00265a003. [DOI] [PubMed] [Google Scholar]
- Sue J. M., Knowles J. R. Ribulose-1,5-bisphosphate carboxylase: fate of the tritium label in [3]3H]ribulose 1,5-bisphosphate during the enzyme-catalyzed reaction. Biochemistry. 1982 Oct 26;21(22):5404–5410. doi: 10.1021/bi00265a004. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., Caruso P., Whitman W. Facile assay of enzymes unique to the Calvin cycle in intact cells, with special reference to ribulose 1,5-bisphosphate carboxylase. Anal Biochem. 1978 Feb;84(2):462–472. doi: 10.1016/0003-2697(78)90064-7. [DOI] [PubMed] [Google Scholar]
- Winter G., Fersht A. R., Wilkinson A. J., Zoller M., Smith M. Redesigning enzyme structure by site-directed mutagenesis: tyrosyl tRNA synthetase and ATP binding. Nature. 1982 Oct 21;299(5885):756–758. doi: 10.1038/299756a0. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]