Abstract
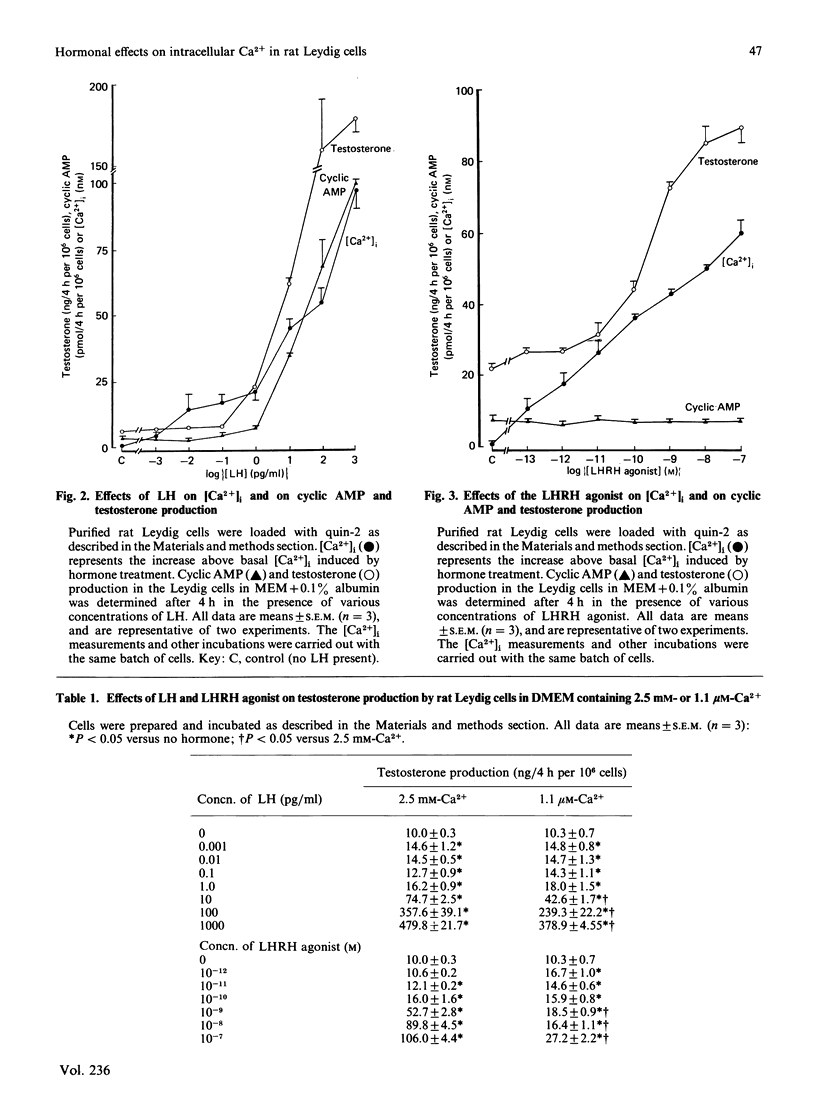
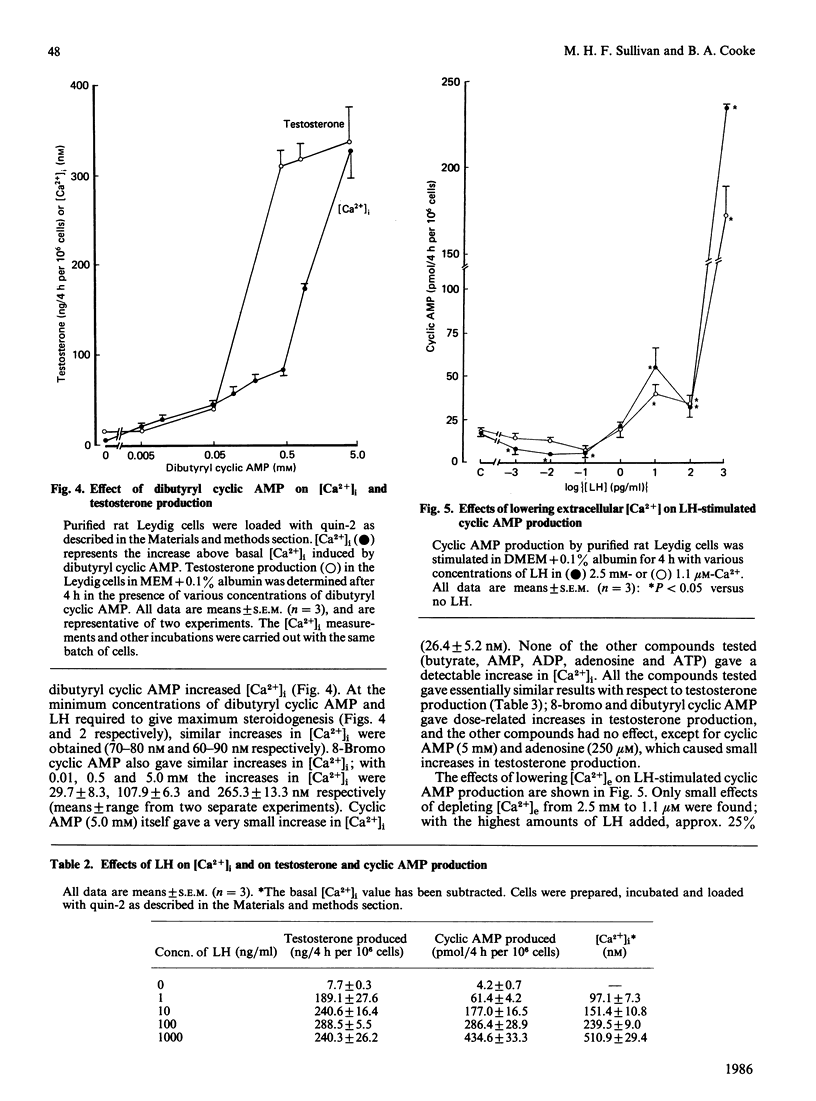
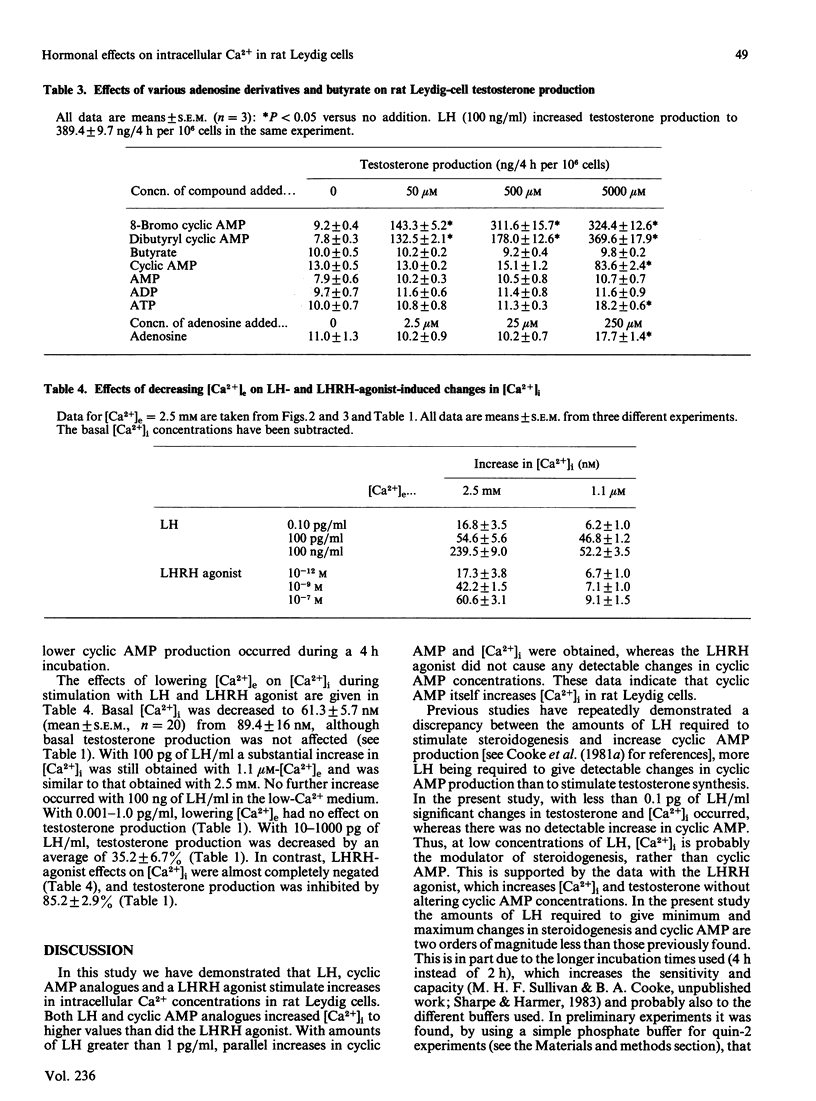
The requirements of purified rat Leydig cells for intra- and extra-cellular Ca2+ during steroidogenesis stimulated by LH (lutropin), cyclic AMP analogues and LHRH (luliberin) agonist were investigated. The intracellular Ca2+ concentrations ([Ca2+]i) were measured by using the fluorescent Ca2+ chelator quin-2. The basal [Ca2+]i was found to be 89.4 +/- 16.6 nM (mean +/- S.D., n = 25). LH, 8-bromo cyclic AMP and dibutyryl cyclic AMP increased [Ca2+]i, by 300-500 nM at the highest concentrations of each stimulator, whereas LHRH agonist only increased [Ca2+]i by a maximum of approx. 60 nM. Low concentrations of LH (less than 1 pg/ml) and all concentrations of LHRH agonist increased testosterone without detectable changes in cyclic AMP. With amounts of LH greater than 1 pg/ml, parallel increases in cyclic AMP and [Ca2+]i occurred. The steroidogenic effect of the LHRH agonist was highly dependent on extracellular Ca2+ concentration ([Ca2+]e), whereas LH effects were only decreased by 35% when [Ca2+]e was lowered from 2.5 nM to 1.1 microM. No increase in [Ca2+]i occurred with the LHRH agonist in the low-[Ca2+]e medium, whereas LH (100 ng/ml) gave an increase of 52 nM. It is concluded that [Ca2+]i can be modulated in rat Leydig cells by LH via mechanisms that are both independent of and dependent on cyclic AMP, whereas LHRH-agonist action on [Ca2+]i is independent of cyclic AMP. The evidence obtained suggests that, at sub-maximal rates of testosterone production, Ca2+, rather than cyclic AMP, is the second messenger, whereas for maximum steroidogenesis both Ca2+- and cyclic-AMP-dependent pathways may be involved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldred L. F., Cooke B. A. The effect of cell damage on the density and steroidogenic capacity of rat testis Leydig cells, using an NADH exclusion test for determination of viability. J Steroid Biochem. 1983 Apr;18(4):411–414. doi: 10.1016/0022-4731(83)90059-6. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges J. L., Scott D., Kaiser D. L., Evans W. S., Thorner M. O. Ca+2 dependence of gonadotropin-releasing hormone-stimulated luteinizing hormone secretion: in vitro studies using continuously perifused dispersed rat anterior pituitary cells. Endocrinology. 1983 Aug;113(2):557–562. doi: 10.1210/endo-113-2-557. [DOI] [PubMed] [Google Scholar]
- Braley L., Menachery A., Brown E., Williams G. The effects of extracellular K+ and angiotensin II on cytosolic Ca++ and steroidogenesis in adrenal glomerulosa cells. Biochem Biophys Res Commun. 1984 Sep 17;123(2):810–815. doi: 10.1016/0006-291x(84)90302-4. [DOI] [PubMed] [Google Scholar]
- Cachelin A. B., de Peyer J. E., Kokubun S., Reuter H. Ca2+ channel modulation by 8-bromocyclic AMP in cultured heart cells. Nature. 1983 Aug 4;304(5925):462–464. doi: 10.1038/304462a0. [DOI] [PubMed] [Google Scholar]
- Capponi A. M., Lew P. D., Jornot L., Vallotton M. B. Correlation between cytosolic free Ca2+ and aldosterone production in bovine adrenal glomerulosa cells. Evidence for a difference in the mode of action of angiotensin II and potassium. J Biol Chem. 1984 Jul 25;259(14):8863–8869. [PubMed] [Google Scholar]
- Clapper D. L., Conn P. M. Gonadotropin-releasing hormone stimulation of pituitary gonadotrope cells produces an increase in intracellular calcium. Biol Reprod. 1985 Mar;32(2):269–278. doi: 10.1095/biolreprod32.2.269. [DOI] [PubMed] [Google Scholar]
- Cooke B. A., Dix C. J., Magee-Brown R., Janszen F. H., van der Molen H. J. Hormonal control of Leydig cell function. Adv Cyclic Nucleotide Res. 1981;14:593–609. [PubMed] [Google Scholar]
- Cooke B. A., Magee-Brown R., Golding M., Dix C. J. The heterogeneity of Leydig cells from mouse and rat testes--evidence for a Leydig cell cycle? Int J Androl. 1981 Jun;4(3):355–366. doi: 10.1111/j.1365-2605.1981.tb00719.x. [DOI] [PubMed] [Google Scholar]
- Cooke B. A., Sullivan M. H. The mechanisms of LHRH agonist action in gonadal tissues. Mol Cell Endocrinol. 1985 Jul;41(2-3):115–122. doi: 10.1016/0303-7207(85)90013-9. [DOI] [PubMed] [Google Scholar]
- Dix C. J., Habberfield A. D., Sullivan M. H., Cooke B. A. Inhibition of steroid production in Leydig cells by non-steroidal anti-inflammatory and related compounds: evidence for the involvement of lipoxygenase products in steroidogenesis. Biochem J. 1984 Apr 15;219(2):529–537. doi: 10.1042/bj2190529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein M. B., Egan J. J., Sha'afi R. I., White J. The cytoplasmic concentration of free calcium in platelets is controlled by stimulators of cyclic AMP production (PGD2, PGE1, forskolin). Biochem Biophys Res Commun. 1983 Jun 15;113(2):598–604. doi: 10.1016/0006-291x(83)91768-0. [DOI] [PubMed] [Google Scholar]
- Hall P. F., Osawa S., Mrotek J. The influence of calmodulin on steroid synthesis in leydig cells from rat testis. Endocrinology. 1981 Nov;109(5):1677–1682. doi: 10.1210/endo-109-5-1677. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Dibutyryl cyclic AMP triggers Ca2+ influx and Ca2+-dependent electrical activity in pancreatic B cells. Biochem Biophys Res Commun. 1983 Apr 29;112(2):614–620. doi: 10.1016/0006-291x(83)91508-5. [DOI] [PubMed] [Google Scholar]
- Hunter M. G., Sullivan M. H., Dix C. J., Aldred L. F., Cooke B. A. Stimulation and inhibition by LHRH analogues of cultured rat Leydig cell function and lack of effect on mouse Leydig cells. Mol Cell Endocrinol. 1982 Jun;27(1):31–44. doi: 10.1016/0303-7207(82)90060-0. [DOI] [PubMed] [Google Scholar]
- Janszen F. H., Cooke B. A., Van Driel M. J., Van Der Molen H. J. The effect of calcium ions on testosterone production in Leydig cells from rat testis. Biochem J. 1976 Dec 15;160(3):433–437. doi: 10.1042/bj1600433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowitt S., Farese R. V., Sabir M. A., Root A. W. Rat Leydig cell phospholipid content is increased by luteinizing hormone and 8-bromo-cyclic AMP. Endocrinology. 1982 Oct;111(4):1415–1417. doi: 10.1210/endo-111-4-1415. [DOI] [PubMed] [Google Scholar]
- Molcho J., Zakut H., Naor Z. Gonadotropin-releasing hormone stimulates phosphatidylinositol labeling and prostaglandin E production in Leydig cells. Endocrinology. 1984 Mar;114(3):1048–1050. doi: 10.1210/endo-114-3-1048. [DOI] [PubMed] [Google Scholar]
- Samli M. H., Geschwind I. I. Some effects of energy-transfer inhibitors and of Ca++-free or K+-enhanced media on the release of luteinizing hormone (LH) from the rat pituitary gland in vitro. Endocrinology. 1968 Feb;82(2):225–231. doi: 10.1210/endo-82-2-225. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Sullivan M. H., Cooke B. A. Effects of calmodulin and lipoxygenase inhibitors on LH (lutropin)- and LHRH (luliberin)-agonist-stimulated steroidogenesis in rat Leydig cells. Biochem J. 1985 Nov 15;232(1):55–59. doi: 10.1042/bj2320055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M. H., Cooke B. A. LHRH agonist decreases LH- but not forskolin-stimulated cyclic AMP levels in rat Leydig cells in vitro. Mol Cell Endocrinol. 1984 Jun;36(1-2):115–122. doi: 10.1016/0303-7207(84)90090-x. [DOI] [PubMed] [Google Scholar]
- Sullivan M. H., Cooke B. A. The effect of calcium on the potentiation of LH-stimulated steroidogenesis and inhibition of LH-stimulated cyclic AMP production by LHRH agonist (ICI 118630) in rat Leydig cells. Mol Cell Endocrinol. 1984 Jan;34(1):17–22. doi: 10.1016/0303-7207(84)90154-0. [DOI] [PubMed] [Google Scholar]
- Sullivan M. H., Cooke B. A. The role of calcium in luteinizing hormone-releasing hormone agonist (ICI 118630)-stimulated steroidogenesis in rat Leydig cells. Biochem J. 1984 Mar 1;218(2):621–624. doi: 10.1042/bj2180621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta K., Grewe C. W., Cote T. E., Eskay R. L., Kebabian J. W. Coordinated action of calcium ion and adenosine 3',5'-monophosphate upon the release of alpha-melanocyte-stimulating hormone from the intermediate lobe of the rat pituitary gland. Endocrinology. 1982 Apr;110(4):1133–1140. doi: 10.1210/endo-110-4-1133. [DOI] [PubMed] [Google Scholar]
- Verjans H. L., Cooke B. A., de Jong F. H., de Jong C. M., van der Molen H. J. Evaluation of a radioimmunoassay for testosterone estimation. J Steroid Biochem. 1973 Nov;4(6):665–676. doi: 10.1016/0022-4731(73)90042-3. [DOI] [PubMed] [Google Scholar]


