Abstract
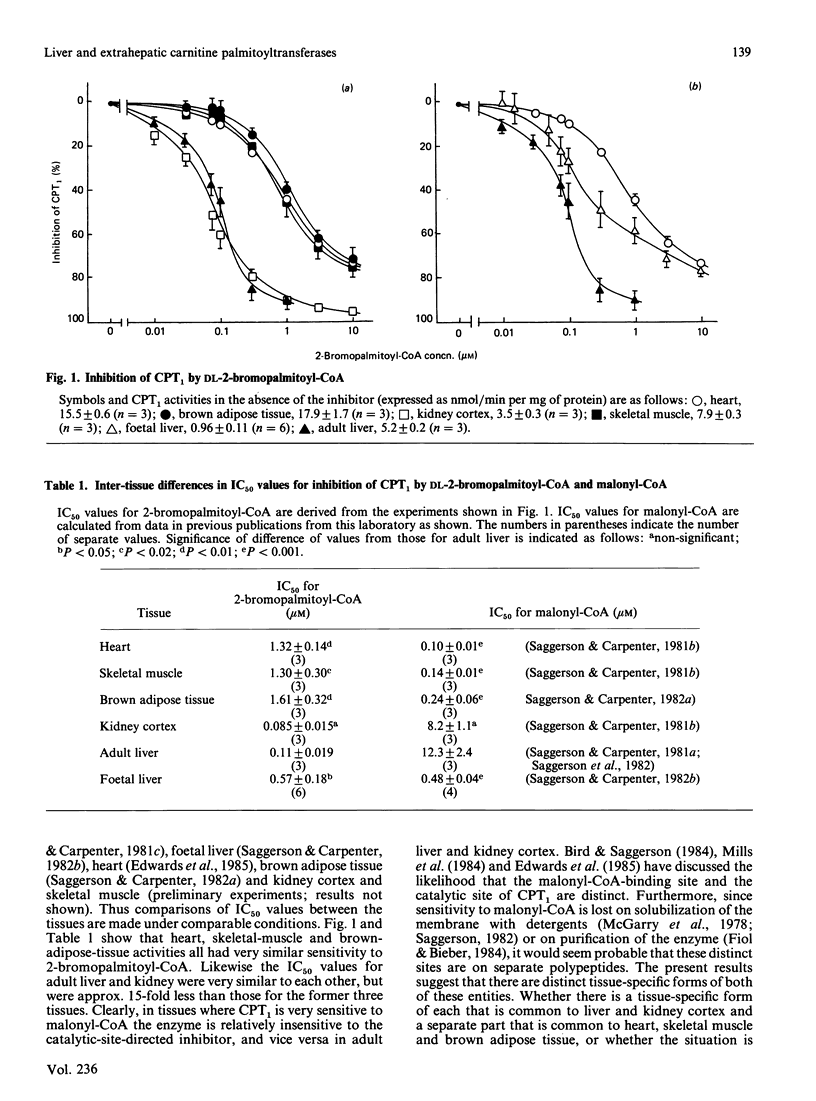
Mitochondria were isolated from rat adult liver, foetal liver, kidney cortex, heart, skeletal muscle and interscapular brown adipose tissue. DL-2-Bromopalmitoyl-CoA inhibited the overt form of carnitine palmitoyltransferase (CPT1) in heart, skeletal muscle and brown adipose tissue, with an IC50 value (concentration giving 50% inhibition) of 1.3-1.6 microM. By contrast, the IC50 value for inhibition of the kidney or adult liver enzyme was 0.08-0.1 microM. CPT1 in near-term foetal liver differed from that in adult liver in that the IC50 for inhibition by 2-bromopalmitoyl-CoA was 0.57 microM. It is suggested that there may be tissue-specific forms of the catalytic entity of CPT1 and that foetal liver may contain a mixture of adult liver- and muscle-type enzymes. In rats made hypothyroid by administration of propylthiouracil and an iodine-deficient diet, hepatic CPT1 activity was decreased by 83%. However, CPT1 activity in extrahepatic tissues showed no adaptive decrease in hypothyroidism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. D., Erwin V. G. Brain acyl-coenzyme A hydrolase: distribution, purification and properties. J Neurochem. 1971 Jul;18(7):1179–1186. doi: 10.1111/j.1471-4159.1971.tb00216.x. [DOI] [PubMed] [Google Scholar]
- Augenfeld J., Fritz I. B. Carnitine palmitolyltransferase activity and fatty acid oxidation by livers from fetal and neonatal rats. Can J Biochem. 1970 Mar;48(3):288–294. doi: 10.1139/o70-050. [DOI] [PubMed] [Google Scholar]
- Bird M. I., Saggerson E. D. Binding of malonyl-CoA to isolated mitochondria. Evidence for high- and low-affinity sites in liver and heart and relationship to inhibition of carnitine palmitoyltransferase activity. Biochem J. 1984 Sep 15;222(3):639–647. doi: 10.1042/bj2220639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J. The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-CoA. Biochim Biophys Acta. 1981 Sep 24;665(3):628–631. doi: 10.1016/0005-2760(81)90282-4. [DOI] [PubMed] [Google Scholar]
- Bremer J., Woldegiorgis G., Schalinske K., Shrago E. Carnitine palmitoyltransferase. Activation by palmitoyl-CoA and inactivation by malonyl-CoA. Biochim Biophys Acta. 1985 Jan 9;833(1):9–16. doi: 10.1016/0005-2760(85)90247-4. [DOI] [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Specific inhibition of mitochondrial fatty acid oxidation by 2-bromopalmitate and its coenzyme A and carnitine esters. Biochem J. 1972 Aug;129(1):55–65. doi: 10.1042/bj1290055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chohan P., Carpenter C., Saggerson E. D. Changes in the anti-lipolytic action and binding to plasma membranes of N6-L-phenylisopropyladenosine in adipocytes from starved and hypothyroid rats. Biochem J. 1984 Oct 1;223(1):53–59. doi: 10.1042/bj2230053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G. A. Differences in the sensitivity of carnitine palmitoyltransferase to inhibition by malonyl-CoA are due to differences in Ki values. J Biol Chem. 1984 Oct 10;259(19):12030–12033. [PubMed] [Google Scholar]
- Cook G. A., Stephens T. W., Harris R. A. Altered sensitivity of carnitine palmitoyltransferase to inhibition by malonyl-CoA in ketotic diabetic rats. Biochem J. 1984 Apr 1;219(1):337–339. doi: 10.1042/bj2190337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. R., Bird M. I., Saggerson E. D. Effects of DL-2-bromopalmitoyl-CoA and bromoacetyl-CoA in rat liver and heart mitochondria. Inhibition of carnitine palmitoyltransferase and displacement of [14C]malonyl-CoA from mitochondrial binding sites. Biochem J. 1985 Aug 15;230(1):169–179. doi: 10.1042/bj2300169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiol C. J., Bieber L. L. Sigmoid kinetics of purified beef heart mitochondrial carnitine palmitoyltransferase. Effect of pH and malonyl-CoA. J Biol Chem. 1984 Nov 10;259(21):13084–13088. [PubMed] [Google Scholar]
- Foster P. C., Bailey E. Changes in the activities of the enzymes of hepatic fatty acid oxidation during development of the rat. Biochem J. 1976 Jan 15;154(1):49–56. doi: 10.1042/bj1540049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble M. S., Cook G. A. Alteration of the apparent Ki of carnitine palmitoyltransferase for malonyl-CoA by the diabetic state and reversal by insulin. J Biol Chem. 1985 Aug 15;260(17):9516–9519. [PubMed] [Google Scholar]
- Harano Y., Kowal J., Yamazaki R., Lavine L., Miller M. Carnitine palmitoyltransferase activities (1 and 2) and the rate of palmitate oxidation in liver mitochondria from diabetic rats. Arch Biochem Biophys. 1972 Dec;153(2):426–437. doi: 10.1016/0003-9861(72)90360-8. [DOI] [PubMed] [Google Scholar]
- Harper R. D., Saggerson E. D. Some aspects of fatty acid oxidation in isolated fat-cell mitochondria from rat. Biochem J. 1975 Dec;152(3):485–494. doi: 10.1042/bj1520485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer T. E. Factors affecting the activity and stability of the palmitoyl-coenzyme A hydrolase of rat brain. Biochem J. 1979 Jun 1;179(3):515–523. doi: 10.1042/bj1790515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Mills S. E., Long C. S., Foster D. W. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983 Jul 15;214(1):21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Effects of pH on the interaction of substrates and malonyl-CoA with mitochondrial carnitine palmitoyltransferase I. Biochem J. 1984 Apr 15;219(2):601–608. doi: 10.1042/bj2190601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Interaction of malonyl-CoA and related compounds with mitochondria from different rat tissues. Relationship between ligand binding and inhibition of carnitine palmitoyltransferase I. Biochem J. 1983 Jul 15;214(1):83–91. doi: 10.1042/bj2140083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkilä E. A., Kekki M. Plasma triglyceride metabolism in thyroid disease. J Clin Invest. 1972 Aug;51(8):2103–2114. doi: 10.1172/JCI107017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson D. J., Ward K. M., Shug A. L. Malonyl CoA inhibition of carnitine palmityltransferase in rat heart mitochondria. FEBS Lett. 1984 Oct 29;176(2):381–384. doi: 10.1016/0014-5793(84)81201-6. [DOI] [PubMed] [Google Scholar]
- Robinson I. N., Zammit V. A. Sensitivity of carnitine acyltransferase I to malonly-CoA inhibition in isolated rat liver mitochondria is quantitatively related to hepatic malonyl-CoA concentration in vivo. Biochem J. 1982 Jul 15;206(1):177–179. doi: 10.1042/bj2060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Bird M. I., Carpenter C. A., Winter K. A., Wright J. J. Cycloheximide blocks changes in rat liver carnitine palmitoyltransferase 1 activity in starvation. Biochem J. 1984 Nov 15;224(1):201–206. doi: 10.1042/bj2240201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. Carnitine acyltransferase activities in rat liver and heart measured with palmitoyl-CoA and octanoyl-CoA. Latency, effects of K+, bivalent metal ions and malonyl-CoA. Biochem J. 1982 Feb 15;202(2):397–405. doi: 10.1042/bj2020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Carnitine palmitoyltransferase and carnitine octanoyltransferase activities in liver, kidney cortex, adipocyte, lactating mammary gland, skeletal muscle and heart. FEBS Lett. 1981 Jul 6;129(2):229–232. doi: 10.1016/0014-5793(81)80171-8. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Effects of fasting and malonyl CoA on the kinetics of carnitine palmitoyltransferase and carnitine octanoyltransferase in intact rat liver mitochondria. FEBS Lett. 1981 Sep 28;132(2):166–168. doi: 10.1016/0014-5793(81)81152-0. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Effects of fasting, adrenalectomy and streptozotocin-diabetes on sensitivity of hepatic carnitine acyltransferase to malonyl CoA. FEBS Lett. 1981 Jul 6;129(2):225–228. doi: 10.1016/0014-5793(81)80170-6. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Regulation of hepatic carnitine palmitoyltransferase activity during the foetal-neonatal transition. FEBS Lett. 1982 Dec 13;150(1):177–180. doi: 10.1016/0014-5793(82)81329-x. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Sensitivity of brown-adipose-tissue carnitine palmitoyltransferase to inhibition by malonyl-CoA. Biochem J. 1982 Apr 15;204(1):373–375. doi: 10.1042/bj2040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. The effect of malonyl-CoA on overt and latent carnitine acyltransferase activities in rat liver and adipocyte mitochondria. Biochem J. 1983 Feb 15;210(2):591–597. doi: 10.1042/bj2100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A., Tselentis B. S. Effects of thyroidectomy and starvation on the activity and properties of hepatic carnitine palmitoyltransferase. Biochem J. 1982 Dec 15;208(3):667–672. doi: 10.1042/bj2080667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux J., Giacobino J. P., Girardier L. Impaired metabolic response to nerve stimulation in brown adipose tissue of hypothyroid rats. Mol Cell Endocrinol. 1982 Feb;25(2):213–226. doi: 10.1016/0303-7207(82)90054-5. [DOI] [PubMed] [Google Scholar]
- Stakkestad J. A., Bremer J. The outer carnitine palmitoyltransferase and regulation of fatty acid metabolism in rat liver in different thyroid states. Biochim Biophys Acta. 1983 Feb 7;750(2):244–252. doi: 10.1016/0005-2760(83)90025-5. [DOI] [PubMed] [Google Scholar]
- Sundin U., Mills I., Fain J. N. Thyroid-catecholamine interactions in isolated rat brown adipocytes. Metabolism. 1984 Nov;33(11):1028–1033. doi: 10.1016/0026-0495(84)90232-4. [DOI] [PubMed] [Google Scholar]
- Veerkamp J. H., Van Moerkerk H. T. The effect of malonyl-CoA on fatty acid oxidation in rat muscle and liver mitochondria. Biochim Biophys Acta. 1982 Feb 15;710(2):252–255. doi: 10.1016/0005-2760(82)90157-6. [DOI] [PubMed] [Google Scholar]
- Yeh Y. Y., Zee P. Fatty acid oxidation in isolated rat liver mitochondria. Developmental changes and their relation to hepatic levels of carnitine and glycogen and to carnitine acyltransferase activity. Arch Biochem Biophys. 1979 Oct 15;197(2):560–569. doi: 10.1016/0003-9861(79)90280-7. [DOI] [PubMed] [Google Scholar]


