Abstract
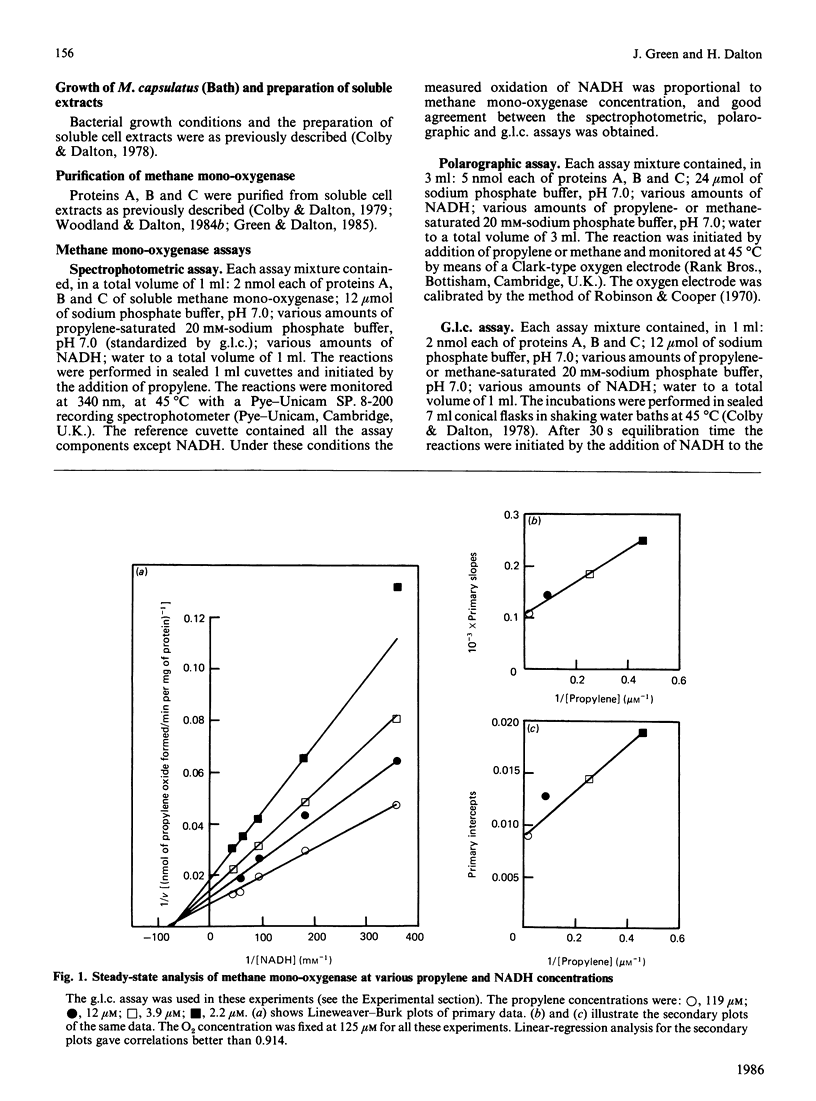
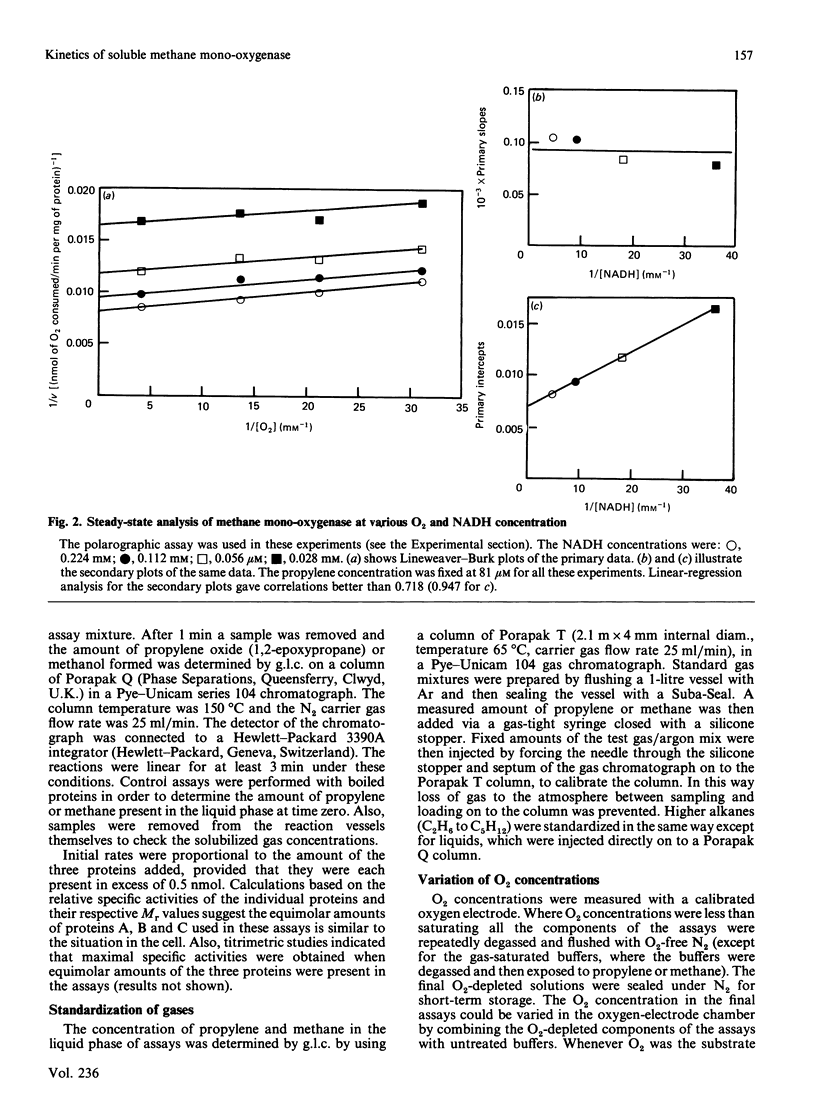
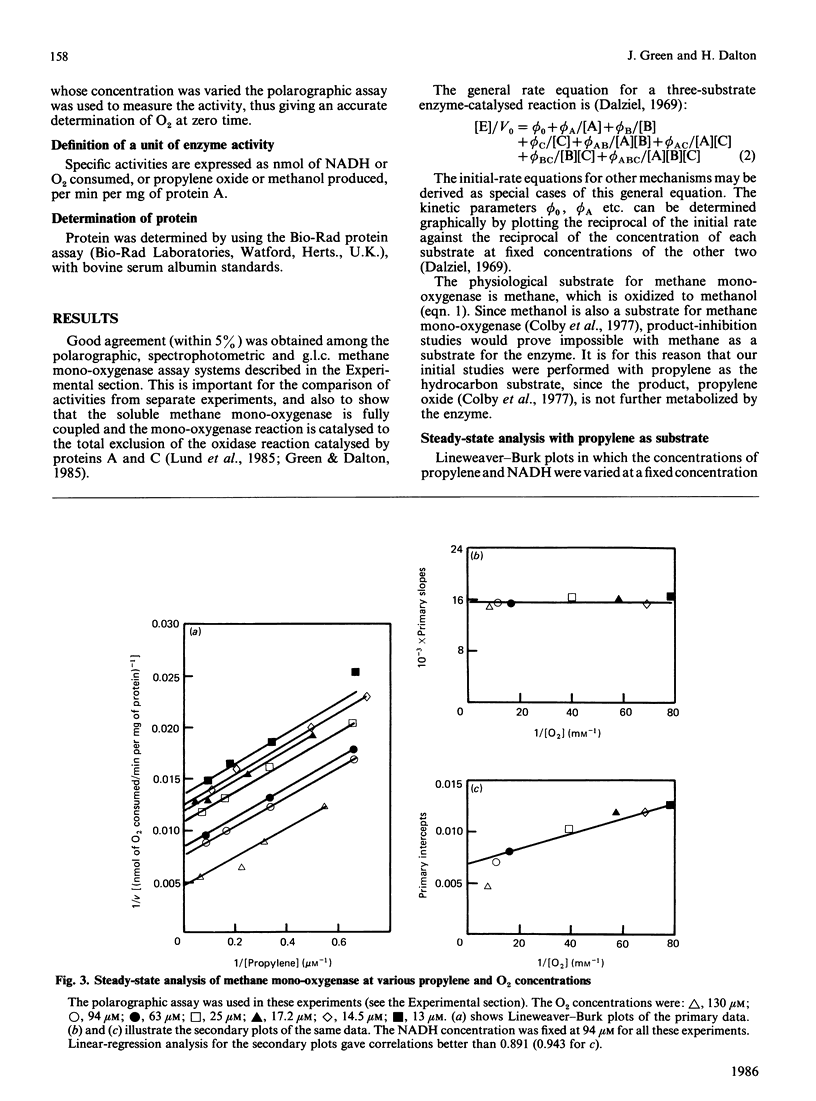
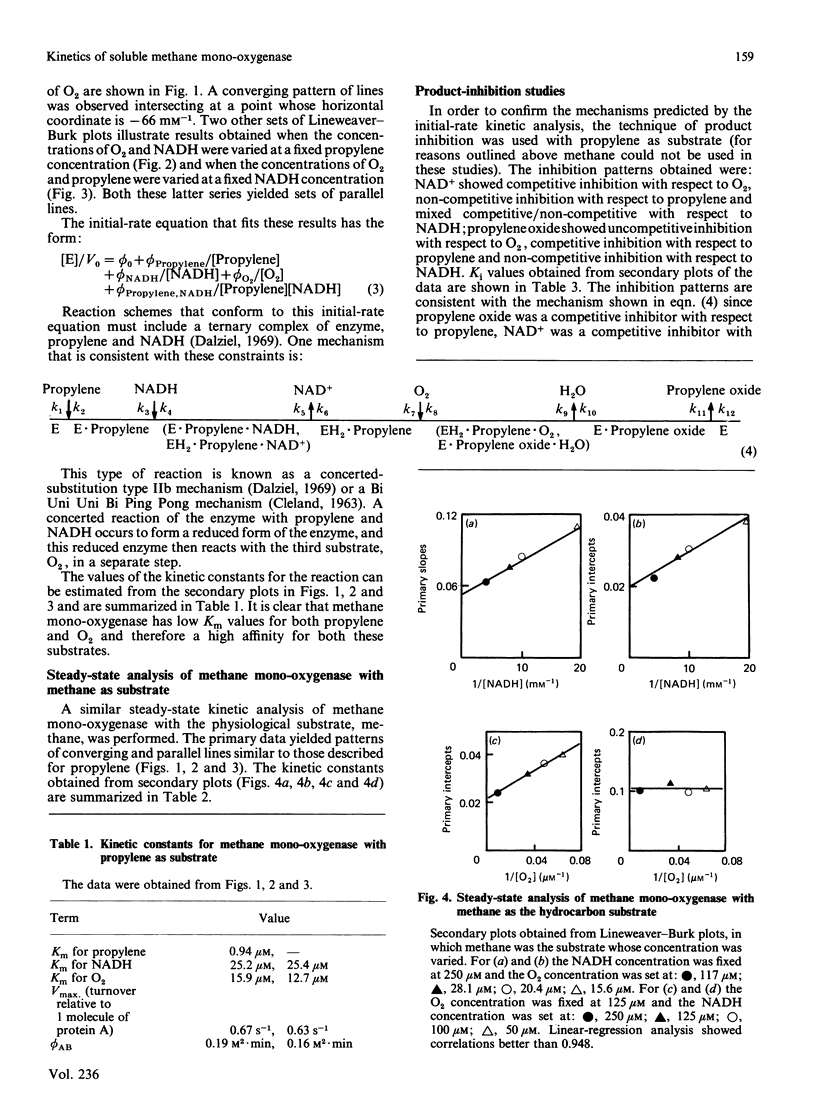
A steady-state kinetic analysis of purified soluble methane mono-oxygenase of Methylococcus capsulatus (Bath) was performed. The enzyme was found to follow a concerted-substitution mechanism. Methane binds to the enzyme followed by NADH, which reacts to yield reduced enzyme and NAD+. The reduced enzyme-methane complex binds O2 to give a second ternary complex, which breaks down to release water and methanol. In this way the enzyme can control the supply of electrons to the active site to coincide with the arrival of methane. Product-inhibition studies (with propylene as substrate) supported the reaction mechanism proposed. Ki values for NAD+ and propylene oxide are reported. The Km for NADH varied from 25 microM to 300 microM, depending on the nature of the hydrocarbon substrate, and thus supports the proposed reaction sequence. With methane as substrate the Km values for methane, NADH and O2 were shown to be 3 microM, 55.8 microM and 16.8 microM respectively. With propylene as substrate the Km values for propylene, NADH and O2 were 0.94 microM, 25.2 microM and 12.7-15.9 microM respectively. Methane mono-oxygenase was shown to be well adapted to the oxidation of methane compared with other straight-chain alkanes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Colby J., Dalton H. Characterization of the second prosthetic group of the flavoenzyme NADH-acceptor reductase (component C) of the methane mono-oxygenase from Methylococcus capsulatus (Bath). Biochem J. 1979 Mar 1;177(3):903–908. doi: 10.1042/bj1770903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H. Resolution of the methane mono-oxygenase of Methylococcus capsulatus (Bath) into three components. Purification and properties of component C, a flavoprotein. Biochem J. 1978 May 1;171(2):461–468. doi: 10.1042/bj1710461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H. Some properties of a soluble methane mono-oxygenase from Methylococcus capsulatus strain Bath. Biochem J. 1976 Aug 1;157(2):495–497. doi: 10.1042/bj1570495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Stirling D. I., Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J. 1977 Aug 1;165(2):395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel K. The interpretation of kinetic data for enzyme-catalysed reactions involving three substrates. Biochem J. 1969 Sep;114(3):547–556. doi: 10.1042/bj1140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J., Dalton H. Protein B of soluble methane monooxygenase from Methylococcus capsulatus (Bath). A novel regulatory protein of enzyme activity. J Biol Chem. 1985 Dec 15;260(29):15795–15801. [PubMed] [Google Scholar]
- Joergensen L. The methane mono-oxygenase reaction system studied in vivo by membrane-inlet mass spectrometry. Biochem J. 1985 Jan 15;225(2):441–448. doi: 10.1042/bj2250441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J., Woodland M. P., Dalton H. Electron transfer reactions in the soluble methane monooxygenase of Methylococcus capsulatus (Bath). Eur J Biochem. 1985 Mar 1;147(2):297–305. doi: 10.1111/j.1432-1033.1985.tb08750.x. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Ogura Y., Yano K., Higashi N., Arima K. Kinetic studies on the reaction mechanism of p-hydroxybenzoate hydroxylase. Biochemistry. 1970 Aug 4;9(16):3235–3242. doi: 10.1021/bi00818a017. [DOI] [PubMed] [Google Scholar]
- Poulsen L. L., Ziegler D. M. The liver microsomal FAD-containing monooxygenase. Spectral characterization and kinetic studies. J Biol Chem. 1979 Jul 25;254(14):6449–6455. [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- Spector T., Massey V. p-Hydroxybenzoate hydroxylase from Pseudomonas fluorescens. Reactivity with oxygen. J Biol Chem. 1972 Nov 25;247(22):7123–7127. [PubMed] [Google Scholar]
- Stirling D. I., Dalton H. Effect of metal-binding and other compounds on methane oxidation by two strains of Methylococcus capsulatus. Arch Microbiol. 1977 Jul 26;114(1):71–76. doi: 10.1007/BF00429633. [DOI] [PubMed] [Google Scholar]
- Strickland S., Massey V. The mechanism of action of the flavoprotein melilotate hydroxylase. J Biol Chem. 1973 Apr 25;248(8):2953–2962. [PubMed] [Google Scholar]
- White-Stevens R. H., Kamin H., Gibson Q. H. Studies of a flavoprotein, salicylate hydroxylse. I. Enzyme mechanism. J Biol Chem. 1972 Apr 25;247(8):2371–2381. [PubMed] [Google Scholar]
- Woodland M. P., Dalton H. Purification and characterization of component A of the methane monooxygenase from Methylococcus capsulatus (Bath). J Biol Chem. 1984 Jan 10;259(1):53–59. [PubMed] [Google Scholar]
- Woodland M. P., Dalton H. Purification of component A of the soluble methane monooxygenase of Methylococcus capsulatus (Bath) by high-pressure gel permeation chromatography. Anal Biochem. 1984 Jun;139(2):459–462. doi: 10.1016/0003-2697(84)90034-4. [DOI] [PubMed] [Google Scholar]


