Abstract
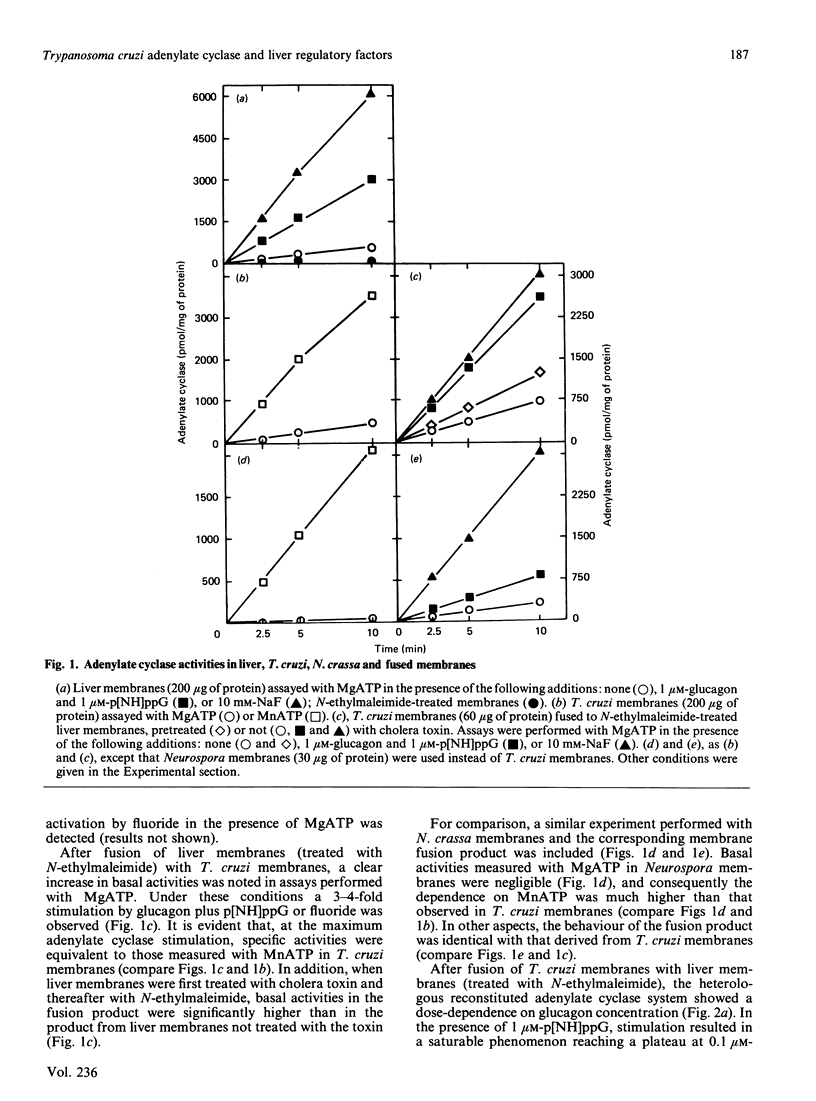
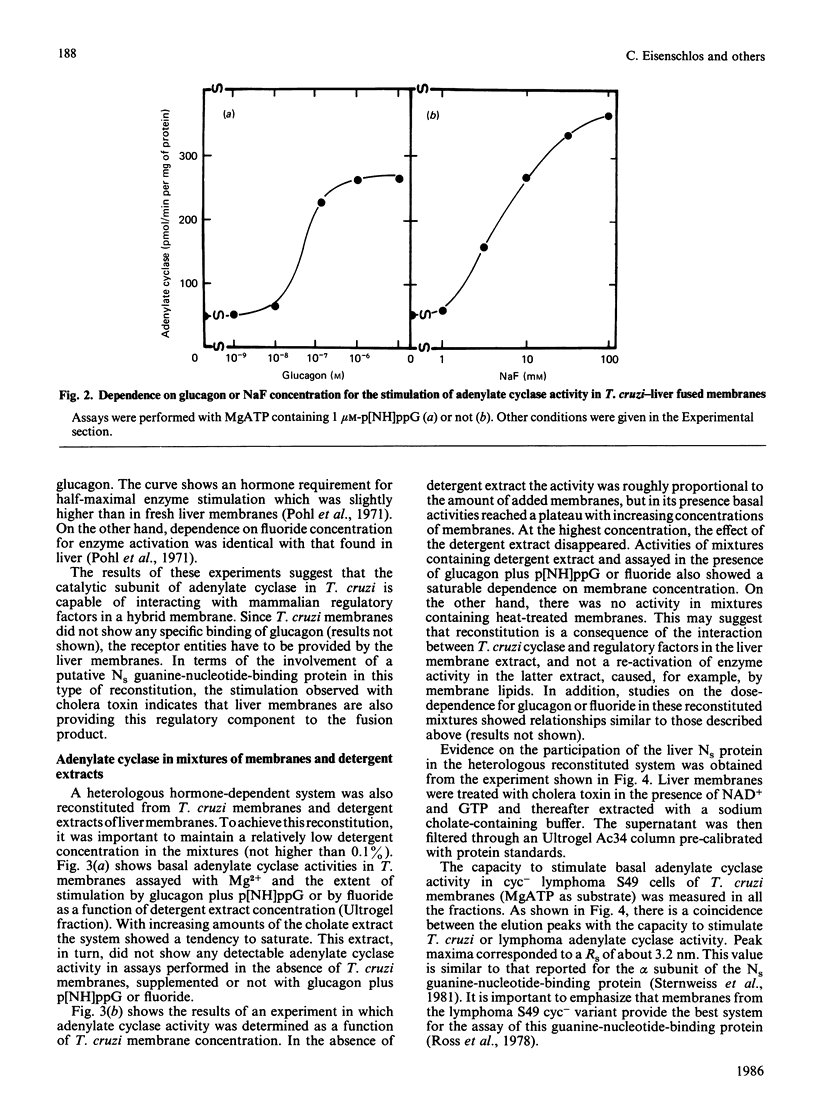
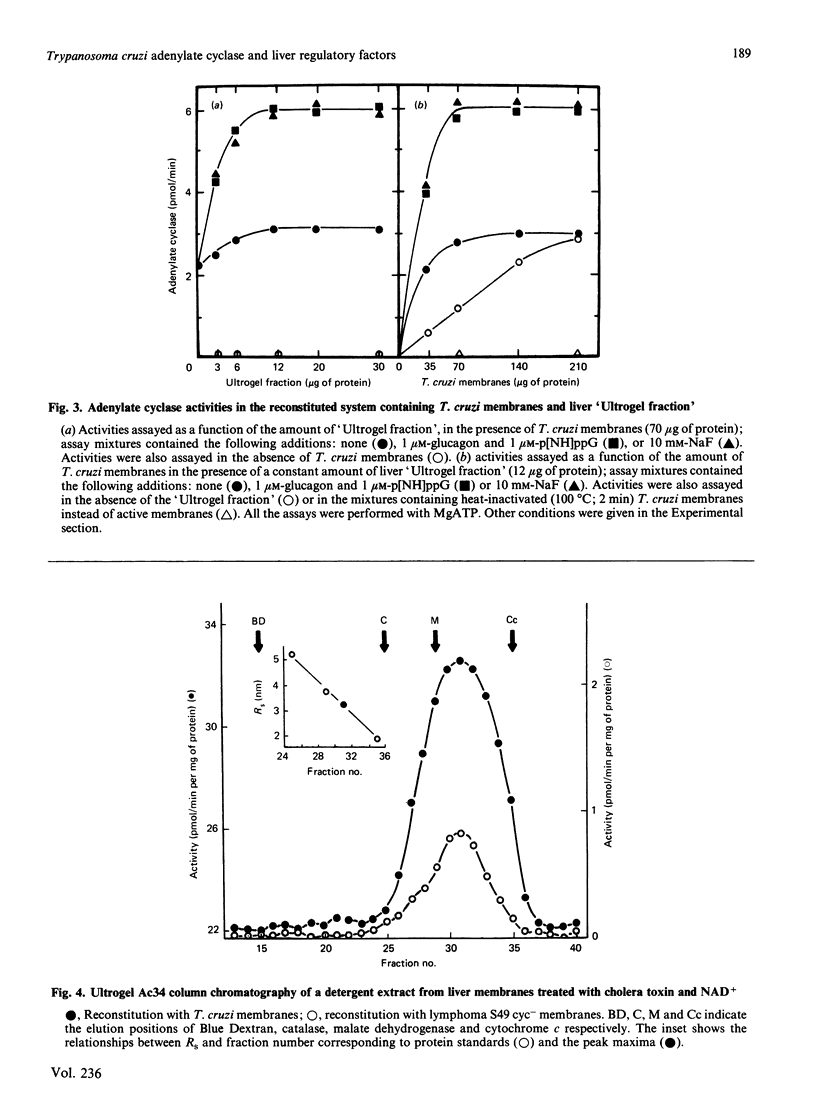
Trypanosoma cruzi adenylate cyclase catalytic subunits may interact with regulatory factors from rat liver membranes, reconstituting heterologous systems which are catalytically active in assay mixtures containing MgATP. The systems show stimulatory responses to glucagon and guanosine 5'-[beta gamma-imido]triphosphate (p[NH]ppG) or fluoride. Reconstitution was obtained by three different methods: fusion of rat liver membranes (pretreated with N-ethylmaleimide) to T. cruzi membranes; interaction of detergent extracts of rat liver membranes with T. cruzi membranes; or interaction of purified preparations of T. cruzi adenylate cyclase and of liver membrane factors in phospholipid vesicles. The liver factors responsible for the guanine nucleotide effect were characterized as the NS protein. Data also indicate that reconstitution requires the presence of a membrane substrate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capon D. J., Seeburg P. H., McGrath J. P., Hayflick J. S., Edman U., Levinson A. D., Goeddel D. V. Activation of Ki-ras2 gene in human colon and lung carcinomas by two different point mutations. Nature. 1983 Aug 11;304(5926):507–513. doi: 10.1038/304507a0. [DOI] [PubMed] [Google Scholar]
- Casperson G. F., Walker N., Brasier A. R., Bourne H. R. A guanine nucleotide-sensitive adenylate cyclase in the yeast Saccharomyces cerevisiae. J Biol Chem. 1983 Jul 10;258(13):7911–7914. [PubMed] [Google Scholar]
- Flawiá M. M., Kornblihtt A. R., Reig J. A., Torruella M., Torres H. N. Reconstitution of a hormone-sensitive adenylate cyclase with membrane extracts from Neurospora and avian erythrocytes. J Biol Chem. 1983 Jul 10;258(13):8255–8259. [PubMed] [Google Scholar]
- Flawiá M. M., Terenzi H. F., Torres H. N. Characterization of Neurospora crassa mutant stratins deficient in adenylate cyclase activity. Arch Biochem Biophys. 1977 Apr 30;180(2):334–342. doi: 10.1016/0003-9861(77)90046-7. [DOI] [PubMed] [Google Scholar]
- Flawiá M. M., Torres H. N. Adenylate cyclase activity in Neurospora crassa. I. General properties. J Biol Chem. 1972 Nov 10;247(21):6873–6879. [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers S., McGill C., Fasano O., Strathern J., Broach J., Wigler M. Genetic analysis of yeast RAS1 and RAS2 genes. Cell. 1984 Jun;37(2):437–445. doi: 10.1016/0092-8674(84)90374-x. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Powers S., Kataoka T., Fasano O., Goldfarb M., Strathern J., Broach J., Wigler M. Genes in S. cerevisiae encoding proteins with domains homologous to the mammalian ras proteins. Cell. 1984 Mar;36(3):607–612. doi: 10.1016/0092-8674(84)90340-4. [DOI] [PubMed] [Google Scholar]
- Reig J. A., Kornblihtt A. R., Flawiá M. M., Torres H. N. Soluble adenylate cyclase activity in Neurospora crassa. Biochem J. 1982 Oct 1;207(1):43–49. doi: 10.1042/bj2070043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond C. D., Gomer R. H., Mehdy M. C., Firtel R. A. Developmental regulation of a Dictyostelium gene encoding a protein homologous to mammalian ras protein. Cell. 1984 Nov;39(1):141–148. doi: 10.1016/0092-8674(84)90199-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg G. B., Pall M. L. Reconstitution of adenylate cyclase in Neurospora from two components of the enzyme. Arch Biochem Biophys. 1983 Feb 15;221(1):254–260. doi: 10.1016/0003-9861(83)90142-x. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Howlett A. C., Ferguson K. M., Gilman A. G. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978 Sep 25;253(18):6401–6412. [PubMed] [Google Scholar]
- Scolnick E. M., Papageorge A. G., Shih T. Y. Guanine nucleotide-binding activity as an assay for src protein of rat-derived murine sarcoma viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5355–5359. doi: 10.1073/pnas.76.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis P. C., Northup J. K., Smigel M. D., Gilman A. G. The regulatory component of adenylate cyclase. Purification and properties. J Biol Chem. 1981 Nov 25;256(22):11517–11526. [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Torruella M., Flawiá M. M., Eisenschlos C., Molina y Vedia L., Rubinstein C. P., Torres H. N. Trypanosoma cruzi adenylate cyclase activity. Purification and characterization. Biochem J. 1986 Feb 15;234(1):145–150. doi: 10.1042/bj2340145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker R. H., Margulis L. Protist classification and the kingdoms of organisms. Biosystems. 1978 Apr;10(1-2):3–18. doi: 10.1016/0303-2647(78)90023-0. [DOI] [PubMed] [Google Scholar]
- Yuasa Y., Srivastava S. K., Dunn C. Y., Rhim J. S., Reddy E. P., Aaronson S. A. Acquisition of transforming properties by alternative point mutations within c-bas/has human proto-oncogene. Nature. 1983 Jun 30;303(5920):775–779. doi: 10.1038/303775a0. [DOI] [PubMed] [Google Scholar]


