Abstract
Background
Atrial fibrillation (AF) represents a global epidemic. Although international AF practice guidelines indicate weight loss for patients with AF and comorbid obesity (BMI ≥ 30 kg/m2) to alleviate symptom burden and improve prognosis, few cardiac rehabilitation (CR) programs include targeted weight loss treatment.
Aims
This RCT protocol will evaluate the efficacy of a “Small Changes” behavioral weight loss treatment (BWLT) to produce clinically relevant (≥ 10%) weight loss among patients with AF and obesity undergoing CR, relative to CR alone. Secondary aims are to establish efficacy of CR + BWLT for improving AF symptoms, AF risk factors, and health-related quality of life.
Methods
Adults (18 +) with AF and obesity will be recruited and randomized to receive CR + BWLT (intervention) or CR-only (control). Controls will receive CR consisting of supervised exercise and risk factor self-management for 12 weeks. The intervention group will receive CR plus BWLT (12 weekly, group-based virtual sessions, followed by 12 weeks of follow-up support). Weight and AF-risk factors will be assessed at pre-randomization, 12 weeks, 24 weeks, and 52 weeks. AF burden will be assessed using 30-s ECGs recorded bidaily and with AF symptoms. The primary endpoint of weight loss will be calculated from baseline to 52 weeks as a percentage of starting weight. Intention-to-treat analyses will compare the proportion in each group achieving ≥ 10% weight loss. Assuming success rates of 5% and 30% among controls and intervention groups, respectively, and a 30% loss to follow-up, 120 patients (60 per group) will provide 80% power to detect a difference using a two-sided independent test of proportions (alpha = 5%).
Impact
This clinical trial will be the first to demonstrate that adding BWLT to CR promotes clinically meaningful weight loss among patients with AF and comorbid obesity. Findings will inform design and execution of a large efficacy trial of long-term (e.g., 5-year) clinical endpoints (e.g., AF severity, mortality). Implementing weight control interventions designed to target the AF substrate in CR could dramatically reduce morbidity and enhance quality of life among patients living with AF in Canada.
Trial registration
ClinicalTrials.gov registration number: NCT05600829. Registered October 31, 2022.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-024-08527-6.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, affecting 1–2% of the population [1]. Epidemiological studies estimate that true AF prevalence may be much higher considering rates of silent and paroxysmal (intermittent) AF (i.e., up to one million Canadians and 5.2 million Americans) [1–3] AF prevalence is expected to increase three-fold by 2050 owing to an aging population and increased age- and health behavior-associated AF risk factors (e.g., poor cardiorespiratory fitness [CRF], type II diabetes, hypertension, and obesity) [3–5]. AF can cause uncomfortable and distressing symptoms, including dyspnea, palpitations, fatigue, syncope (fainting), and symptoms of depression and anxiety. Patients with AF are at increased risk for severe health outcomes, including stroke, heart failure, dementia, and death [1].
The three pillars of AF management traditionally include stroke prevention, rate control, and rhythm control [6]. Evidence-based rate and rhythm control therapies include medications and ablation procedures, whereas stroke risk is typically managed with anticoagulants [1, 7]. While current treatments reduce symptom burden and improve health-related quality of life (HRQOL) for many patients, they have several limitations. Ablation procedures are invasive and not universally available, and AF recurrence occurs in nearly half of patients in the long-term [8, 9]. Drug therapies are often ineffective in keeping patients in sinus rhythm. Therefore, recent AF clinical guidelines urge practitioners to include risk factor modification as a fourth pillar of AF management [1, 7, 10].
American, Canadian, and European AF clinical guidelines are consistent in highlighting obesity, alcohol and tobacco use, physical inactivity/low cardiorespiratory fitness (CRF), hypertension, diabetes, and sleep apnea as key behavioral intervention targets [1, 7, 10]. In particular, obesity (defined as having a body mass index [BMI] ≥ 30 kg/m2) is a strong predictor of AF incidence—each unit increase in BMI corresponds to a 4–5% increased risk of developing AF [11, 12]. According to the Framingham Heart Study, more than one third of new AF cases present with comorbid obesity [13]. Obesity is also associated with developing more severe AF phenotypes, including persistent AF, defined as AF episodes lasting > 1 week, and permanent AF [14, 15].
Weight loss corresponds to improvements in symptom burden among patients with established AF and comorbid obesity in a dose–response fashion [3, 6, 16, 17]. Furthermore, a recent meta-analysis reported that ≥ 10% weight loss is an important indicator of achieving clinically relevant improvements in AF burden, including decreased AF recurrence (relative risk [RR] = 0.29; 95% CI = 0.19–0.44), event frequency (mean difference [MD] = 1.74; 0.70–2.79), episode duration (MD = 1.80; 1.34–2.45), and symptom severity (MD = 5.36, 3.75–6.97) [18]. In light of these findings, weight loss is now a Class/COR I, Level B-R recommendation for patients with AF and comorbid overweight or obesity [1, 7].
Cardiac rehabilitation
Canadian and International guidelines recommend providing AF risk factor modification within the context of a structured, multidisciplinary health setting [1]. Cardiac rehabilitation (CR) includes supervised aerobic exercise and risk factor management (e.g., dietary counseling, smoking cessation, medication control) and is the gold standard in tertiary prevention of acute coronary syndrome, heart failure with reduced ejection fraction, and post-coronary revascularization [19–21]. Recent findings suggest CR may have important benefits for patients with AF [21]. A systematic review of 13 randomized controlled trials (RCTs; N = 1155 patients) reported that supervised exercise training, including CR (64% of included studies), improved quality of life, reduced AF recurrence, and improved CRF relative to regular care controls [22]. Another systematic review of nine RCTs comparing CR to usual care (N = 959 patients with AF, with or without heart failure) reported that CR participation contributed to improvements in CRF [i.e., standard mean difference peak oxygen consumption (VO2peak) = 1.59 mLO2•kg−1•min−1, 95% CI = 0.11–3.08] and improvements in HRQOL relative to usual care [23]. CR also improves AF burden (i.e., AF recurrence, decreased mean time in AF, fewer self-reported symptoms), total cholesterol, lipids, triglycerides, and blood pressure in some studies [22, 24, 25].
While growing evidence suggests CR may provide important benefits to patients with AF, an essential component of comprehensive cardiovascular care is notably absent from CR programs in Canada and other countries: targeted weight loss for patients with comorbid obesity. Traditional CR rarely includes weight loss counseling beyond a general emphasis on increasing physical activity and heart-healthy dietary patterns, resulting in negligible effects on BMI. In a recent review, Ades and Savage reported that only 3 of 36 CR programs surveyed had a formal BWLT either embedded into the program or accessible to patients via referral [26]. This is problematic given that the average BMI of patients referred to CR is 30.1 kg/m2, and obesity is an important risk factor for many cardiac diseases [26, 27].
The addition of behavioral weight loss treatment (BWLT) to CR has been shown to produce greater weight loss among patients with obesity relative to CR alone. For example, Ades and Savage [27] reviewed observational and randomized studies of CR for weight loss among CAD patients and reported that weight changes in traditional CR (i.e., standard programs that did not contain a weight loss component) among patients with overweight and obesity range from − 2% to + 1.5% [27]. In contrast, patients in CR programs that included targeted behavioral support with weight loss achieved − 5.6 to − 7% total body weight loss [27]. These findings suggest that integrating targeted weight loss treatment into CR is feasible and efficacious among patients with overweight or obesity.
A novel “Small Changes” approach to weight management
The ASPIRE (Aspiring for Lifelong Health) Small Changes program is a group-based, manualized BWLT approach consistent with the 2020 Canadian Clinical Practice Guidelines for adult obesity [28] and is informed by prominent behavior change theories (e.g., Social Cognitive Theory, Self-Determination Theory) [29, 30]. ASPIRE emphasizes the adoption of small, cumulative behavioral modifications (dietary changes, increased physical activity, stress reduction, sleep hygiene, and addressing body image dissatisfaction) and realistic weekly goal setting [31, 32]. This is a departure from traditional behavioral approaches that tend to emphasize longer-term goal setting (e.g., setting “goal weights”) and encourage participants to make several drastic lifestyle changes simultaneously. ASPIRE has been validated in various non-CR settings and is shown to produce moderate, sustained (24-month) weight loss in community-based men and women and United States (U.S.) veterans with obesity and obesity-related comorbidities [31–35]. For example, ASPIRE demonstrated superior 12-month weight loss relative to usual care (i.e., 15–30 weekly sessions of the MOVE! Veteran’s Affairs Weight Management Program) in a multi-center randomized pragmatic effectiveness trial of 481 patients with obesity [34]. The ASPIRE-II trial evaluated the 12-week ASPIRE program with 6 months of minimal phone-based follow-up among 25 women with obesity, with average weight loss > 5% at 9-month follow-up [33].
A Small Changes approach may further potentiate the effects of BWLT on achieving sustained and clinically meaningful weight loss in patients with AF and obesity and reduce the risk of offsetting the benefits of weight loss for AF due to fluctuations in weight [2]. Results from a meta-analysis of Small Changes programs from our team (manuscript in preparation) demonstrated an average mean weight loss of 3 kg at 12 months across programs and populations [36]. Furthermore, a recent systematic review comparing results from gradual (e.g., Small Changes) vs. rapid weight loss approaches reports greater total body fat loss and better preservation of resting metabolic rate in gradual weight loss programs [37]. Given the high prevalence of obesity among individuals with AF and its detrimental effect on AF burden and outcomes, there is a critical need for interventions that support weight-loss-promoting behaviors and can be integrated into routine clinical care for AF and that can be feasibly sustained by patients.
The current protocol
CR programs are widely recommended to patients with cardiovascular disease and have a proven track-record of producing clinically relevant improvements in important AF risk factors, including hypertension, lipid profile, and CRF. CR, therefore, represents an ideal setting to promote risk factor management for patients with AF. However, important barriers exist to addressing obesity as a risk factor in AF. Patients may be hesitant to specifically seek weight loss treatment due to stigma or lack of knowledge about the role of obesity in AF outcomes [38]. Furthermore, traditional CR does not produce meaningful weight loss for most patients. There is a clear gap in the ability of traditional CR programming to meet the needs of a growing population of individuals with AF and obesity. The addition of a BWLT component to CR may bridge this gap by providing a venue acceptable for patients to receive evidence-based weight loss treatment along with a comprehensive risk factor management regimen targeting exercise and other behavioral factors relevant to AF outcomes. The BE-WEL in CR-AF study has been designed to provide critical evidence regarding the efficacy of a CR + BWLT program relative to CR alone on weight loss among patients with AF and comorbid obesity. This study has been approved by the Conjoint Health Research Ethics Board at the University of Calgary (CHREB). The trial was registered on ClinicalTrials.gov prior to enrolment of the first patient (NCT05600829); refer to Table 1. This protocol adheres to the SPIRIT guidelines for the reporting of protocols of clinical trials [39].
Table 1.
Trial registration data
| Data category | Information |
|---|---|
| Primary registry and trial identifying number |
ClinicalTrials.gov |
| Date of registration in primary registry | 01-Nov-2022 |
| Secondary identifying numbers | REB22-0976 |
| Source of monetary or material support | Canadian Institutes of Health Research |
| Primary sponsor | The University of Calgary |
| Secondary sponsor | N/A |
| Contact for public queries |
TW [tamara.williamson@uregina.ca] TC [campbet@ucalgary.ca] BV [behmed@ucalgary.ca] |
| Contact for scientific queries |
TW [tamara.williamson@uregina.ca] TC [campbet@ucalgary.ca] BV [behmed@ucalgary.ca] |
| Public title | A Behavioural Weight Loss Intervention Delivered in Cardiac Rehabilitation for Patients With Atrial Fibrillation and Obesity |
| Scientific title | A Behavioural Weight Loss Intervention Delivered in Cardiac Rehabilitation for Patients With Atrial Fibrillation and Obesity: The BeWEL IN CR-AF Study |
| Countries of recruitment | Canada |
| Health condition(s) or problem(s) studied | Atrial fibrillation (AF) and obesity (BMI ≥ 30 kg/m2) |
| Intervention(s) |
Experimental: Intervention • Patients participate in a traditional 12-week outpatient CR program with added weekly behavioral weight loss sessions (BWLT + CR) Other: Control • Patients participate in a traditional 12-week outpatient CR program (CR-Only) |
| Key inclusion and exclusion criteria |
Ages eligible for study: ≥ 18 years Sexes eligible for study: both Accepts healthy volunteers: no Inclusion criteria: adult patients (≥ 18 years) with symptomatic ECG-documented AF (paroxysmal or persistent type) and obesity Exclusion criteria: Longstanding-persistent (defined as ≥ 3 years continuous AF) or permanent AF, uncontrolled coronary artery disease, completed a CR program within the previous year, currently enrolled in a structured BWLT program, currently scheduled to receive catheter ablation in AF, currently taking GLP-1 receptor agonist or received bariatric surgery in the previous year prior to enrollment OR scheduled for bariatric surgery during the study period |
| Study type |
Interventional Allocation: randomized intervention model Parallel assignment masking: unblinded Primary purpose: tertiary prevention Phase II |
| Date of first enrolment | 11 August, 2023 |
| Target sample size | 120 |
| Recruitment status | Recruiting |
| Primary outcome | Proportion of patients in the intervention group vs. control achieving ≥ 10% body weight change from baseline to 52-week follow-up |
| Key secondary outcomes | Total weight loss (as a percentage of baseline weight), ECG-documented AF symptoms burden, self-reported AF burden, AF-related quality of life, CR exercise session adherence, weekly step counts, cardiorespiratory fitness from baseline to 52-week follow-up |
AF, atrial fibrillation; BMI, body mass index; BWLT, behavioral weight loss treatment; CR, cardiac rehabilitation; ECG, electrocardiogram; GLP-1, glucagon-like peptide 1 receptor agonist
Study aims
The primary aim is to evaluate whether the combination of an AF-specific “Small Changes” BWLT and traditional CR results in a greater proportion of patients with AF and obesity achieving ≥ 10% body weight loss compared to patients who receive standard care (traditional CR alone) at 12-month follow-up. The secondary aims are to evaluate the impact of BWLT + CR on (1) mean % weight loss of controls vs. intervention group, (2) AF burden (i.e., proportion of ECG tracings showing AF during the follow-up period), (3) self-reported AF symptom burden, (4) disease-specific and generic patient-reported outcome measures (e.g., AF-related quality of life and HRQOL; psychological distress), and (5) exercise volume. Exploratory endpoints will include changes in cardiovascular risk factors (e.g., blood pressure, lipid profile, BMI, waist circumference, CRF) collected in standard care by the CR center.
Hypotheses
Primary: A greater proportion of patients in the BWLT + CR group will achieve ≥ 10% weight loss at 12 months post-randomization relative to the CR-only group.
Secondary: Patients in the BWLT + CR group will experience greater improvements in AF burden, AF self-reported symptom burden, increased HRQOL, decreased psychological distress, and increased leisure-time exercise and CR exercise session attendance relative to the CR-only group.
Methods
Study design
This phase II study is a single-center, unblinded, parallel-group RCT testing the efficacy of the BWLT for patients with AF enrolled in CR. As CR has been shown to improve AF burden, symptomology, and HRQOL, this study will control for the effect of CR. Patients will undergo baseline assessments and then be randomized (1:1) to either BWLT + CR or standard care (CR alone).
Participants and recruitment
Adult patients (18 +) with a confirmed diagnosis of paroxysmal or persistent AF and BMI ≥ 30 kg/m2 will be recruited from general cardiology and AF specialty clinics in Calgary, Alberta, Canada (see Table 2 for detailed eligibility criteria). Potentially eligible patients will be informed about the study by their healthcare practitioner (nurse or cardiologist) during a regularly scheduled appointment. Interested patients will be referred to the research assistant by the clinic nurse or physician through the electronic medical records’ internal messaging system. The research assistant will review potential patients’ electronic medical records to determine eligibility, including (1) a doctor’s letter with referral to CR, (2) diagnosis of paroxysmal or persistent atrial fibrillation or atrial flutter within the past year, (3) recent (past 6 months) measurement of BMI, (4) current medications, (5) comorbid diagnoses, (6) bariatric and ablation surgery referral history, and (7) history of participation in other CR programs. Eligible patients will be contacted by the study team, informed about the trial, and given the opportunity to ask any questions about their participation.
Table 2.
Inclusion/exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
1) Symptomatic paroxysmal or persistent atrial fibrillation or atrial flutter 2) Sedentary lifestyle (not currently meeting basic physical activity targets of ≥ 150 min/week) 3) Classified as obese (BMI ≥ 30 kg/m2) 4) At least one (1) of: obstructive sleep apnea; diabetes; hypertension; heart failure; dyslipidemia; CAD; peripheral artery disease; cerebrovascular disease 5) Ability to speak and write in English to complete surveys and participate in the BWLT 6) Willing and able to actively participate in a virtual BWLT program (must own and be able to use a device with webcam and microphone/headset) |
1) Longstanding-persistent (defined as ≥ 3 years continuous AF) or permanent AF 2) Uncontrolled coronary artery disease 3) Completed a CR program within the previous year 4) Currently enrolled in a structured behavioral weight loss program [defined as one-on-one or group support/counseling to aid with weight loss, e.g., Weight Watchers, medical weight loss center, private behavioral counseling 5) Scheduled to receive catheter ablation for AF during the study period (next 1 year) 6) *Currently taking a GLP-1 receptor agonist 7) **Scheduled for bariatric surgery during the study period |
AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CR, cardiac rehabilitation; BWLT, behavioral weight loss treatment
*Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) taken in conjunction with a calorie-reduced BWLT can bolster weight loss in patients with obesity [58]. Given the short follow-up period, patients will be asked to refrain from starting a new GLP-1 RA while in the study
**Bariatric surgery may produce rapid weight loss in the first 12–18 months post-surgically
Informed consent and confidentiality of patient information
The research assistant will verbally review the aspects of informed consent over the phone with patients including limits to confidentiality. Patients will be informed that absolute confidentiality cannot be guaranteed in a virtual environment due to limits to security of digital devices (e.g., computers, smartphones, FitBits) and videoconferencing software (Zoom). Patients who agree to participate in the trial will be sent an electronic informed consent form by e-mail (see the supplementary material).
Randomization, allocation, and concealment
After completing the baseline assessment and providing written informed consent, patients will be placed on a waitlist until 20 patients have been recruited (10 controls; 10 intervention) or until a patient has been on the waitlist for more than 3 months and at least 8 patients (4 experimental) have been recruited. When sufficient patients have been recruited, patients will be randomized using a 1:1 allocation schedule stratified by sex to ensure equal representation between conditions. The commercially available software, www.studyrandomizer.com, will be used to generate a list of randomly sequenced numbers for assigning patients to a condition as outlined in the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines [40]. A research assistant will access the randomization website through a closed system, and a randomization code will be assigned to the participant. Automated audit trails will document the patient allocation number, assigned condition, and the date and time of transaction. Following randomization, patients will be emailed baseline questionnaires and scheduled for their baseline exercise stress test at TCR. All TCR staff (e.g., patient education facilitators, exercise specialists, and staff assessing baseline and follow-up measures) will be blinded to study condition.
Intervention
Traditional CR components will be identical across conditions (see “control group” description below). All patients will be provided with a portable ECG monitor (KardiaMobile device by AliveCor™ Inc.) and trained in its use. As part of standard clinical flow, all patients enrolled in CR complete a baseline, 12-week, and 1-year assessment, which includes a symptom-limited exercise test, anthropomorphic measurements (weight and waist circumference), blood pressure, and bloodwork (see Fig. 1 and below for details). For the purposes of this study, patients will receive an additional exercise test at the study halfway point (24 weeks). Patients will receive an email containing a link to complete an online questionnaire battery via REDCap’s survey system at baseline (immediately post-randomization but prior to their baseline TCR assessment; T1) and 12 weeks (T2), 24 weeks (T3), and 52 weeks (T4) post-randomization. The questionnaire batteries will assess self-reported AF symptom burden, AF-related quality of life, and psychological distress (Table 4). Body mass (kg) and AF risk factors (blood pressure, lipids) will be assessed by CR staff at all timepoints. Patients in the intervention group (BWLT + CR) will be provided with a FitBit Charge activity monitor and FitBit Bluetooth scale to record daily steps and weekly body weight.
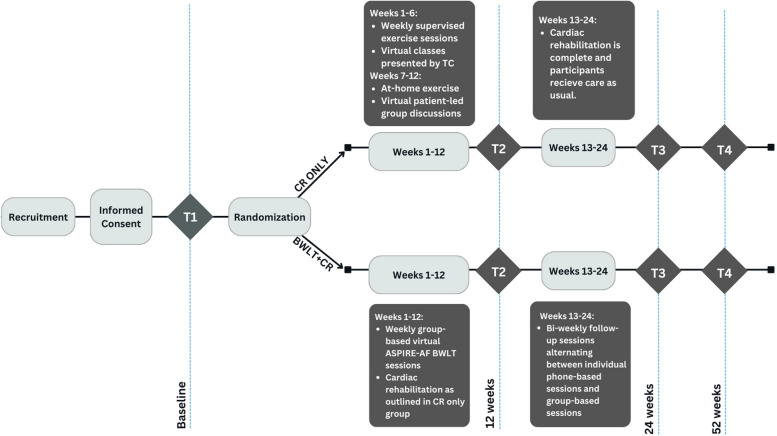
Fig. 1.
Patient flow diagram. ASPIRE-AF, the ASPIRE Small Changes Program; BWLT, behavioral weight loss treatment; CR, cardiac rehabilitation; TC, TotalCardiology Rehabilitation CR program
Table 4.
Description of validated self-report questionnaires
| Outcome | Psychometric measures | Description |
|---|---|---|
| AF symptom burden | University of Toronto AF Symptom Severity Scale (AFSS) scores [59, 60] | Assesses global wellbeing, AF frequency, AF duration, AF severity, and healthcare utilization |
| AF-related quality of life | AF Effect on Quality of Life (AFEQL) Scale [61] | Measures subjective concerns about AF symptoms, physical limitations, satisfaction with treatment, and AF-related anxiety/worry |
| Health-related quality of life | Short Form Health Status Questionnaire (SF-36) [62] | Measures physical functioning, role limitations, energy/fatigue, emotional/social functioning, and pain |
| Psychological distress | Hospital Anxiety and Depression Scales (HADS) [63] | Validated and reliable tool measuring symptoms of anxiety and depression in chronic health populations with established clinical cut-offs |
Control group: TotalCardiology™ Rehabilitation (TCR) program
A traditional, 12-week exercise-based CR program will be used as the comparator to control for the role of CR in weight loss. TCR is the largest outpatient CR program in Alberta, with an annual clinical load of ~ 2100 patients (see https://tcrehab.totalcardiology.ca). The TCR program consists of virtual patient education, medication management, risk factor modification, and 12 weeks of supervised cardiovascular exercise. Participants can complete up to six 60-min supervised exercise sessions onsite at TCR during the first 6 weeks of their program or choose exclusively home-based exercise. During the second half of the TCR program, participants attend weekly group-based virtual sessions designed to offer peer support with their continued exercise program. Patients who choose the entirely home-based program receive biweekly phone calls from a health coach during the first 6 weeks and attend virtual group sessions during the subsequent 6 weeks. Additionally, the TCR program provides online educational classes (e.g., nutrition, managing stress and sleep, medications, and managing cardiac conditions).
Patients undergo a symptom-limited graded exercise test at baseline/CR intake, 12 weeks, 24 weeks, and 52 weeks to assess CRF (i.e., peak metabolic equivalents [METS]). Cardiometabolic risk factors (blood pressure, blood lipid profile, BMI, waist circumference, depression and anxiety symptoms, tobacco use,) are also collected at baseline, 12 weeks, 24 weeks, and 52 weeks as part of usual care. CR programs in Canada vary in funding models, with many CR programs having a user fee to cover costs. There is a 375 CAD user fee to participate in the TCR program, which is provided on a sliding-scale or waived entirely so that patients are not declined CR for financial reasons. The program fee will be waived for all study participants in both control and intervention groups. The TCR program has been demonstrated to improve cardiovascular risk factors and decrease cardiac events and mortality in patients with heterogenous cardiac diagnoses and comorbidities [41–45].
Intervention group: ASPIRE-AF plus CR
Patients randomized to receive BWLT will attend twelve virtual, 90-min, weekly group-based sessions via Zoom in addition to their twice-weekly CR exercise sessions and virtual education classes. The empirically validated ASPIRE BWLT was adapted for an AF population with obesity (i.e., ASPIRE-AF) based on the results of recent early-phase work completed by our team (manuscript in preparation). Briefly, the ASPIRE treatment manual was modified to incorporate patient education and practical strategies requested by patients in our preliminary qualitative and prospective observational pilot studies (see Table 3 for details). These included information on AF risk factor management, pathophysiology, medications, disease course, exercising with AF, and coping with difficult emotions regarding an AF diagnosis. Patients set SMALL [Self-selected, Measurable, Action-Oriented, Linked to your Life, and (Time) Limited] goals at the end of each session based on that week’s topic (see supplementary materials for full intervention details). Weekly session goals focus on healthy food choices and moderate calorie reduction (e.g., 1–2 changes amounting to 100–200 fewer consumed calories per day) and gradual increases in physical activity (e.g., 100–500 additional steps per day). Following the initial 12 weeks, patients will attend bi-weekly group-based follow-up sessions for an additional 12 weeks. Follow-up sessions alternate between individual telephone sessions and group-based sessions to support continued weight loss/maintenance, relapse prevention, and troubleshoot barriers that arise.
Table 3.
Intervention modifications and adaptations according to qualitative patient-reported themes
| Qualitative theme | Intervention component |
|---|---|
| AF symptoms are manageable |
• Interventionists receive training in AF pathophysiology and symptom profiles, and concurrent treatments (medications) to facilitate group discussions regarding overcoming AF-related barriers to behavior change • Interventionists support patient self-efficacy in managing AF symptoms while adhering to CR program and setting weekly, “SMALL” behavioral health goals |
| Frustration with Prior Weight Loss Attempts and “Diet” Programs |
• Removed pre-existing material on calorie math and calorie counting from the ASPIRE manual • Facilitators and handouts emphasize differences between the Small Changes approach and “diets” • Facilitators and intervention materials repeatedly emphasize the importance of making changes relative to patient’s unique baseline behaviors (i.e., this is not a one-size-fits-all approach) • Instruct patients on the difference between setting behavioral goals (e.g., “I will add 500 daily steps to my total steps next week”) vs. having a “goal weight” (e.g., “I want to be XX pounds). The latter is discouraged throughout the program • Ongoing psychoeducation re: benefits of WL for AF vs. WL for body image or societal reasons |
| Information Needs: Patients Want More Information about Atrial Fibrillation |
• AF psychoeducation incorporated throughout modules with a specific focus in Module 1 • AF-specific handouts (e.g., exercising with AF) |
| Flexibility |
• Virtual delivery model allows patient to participate from work or home • Daytime and evening sessions available |
ASPIRE-AF will be facilitated by senior clinical psychology PhD students, supervised by a registered clinical health psychologist. The health psychologist has extensive experience in health behavior change strategies and CR. The facilitators will be recruited from the University of Calgary’s Clinical psychology program. These trainees have 3 + years of advanced training in cognitive behavioral therapy and motivational communication principles that comprise BWLT [46, 47].
Treatment fidelity monitoring
To ensure fidelity to the intervention manual, a research assistant will review a proportion of session video recordings and complete a fidelity checklist. Sessions are recorded on Zoom with patients’ consent and stored on an encrypted drive. Recordings are deleted when fidelity checks are completed. Compliance with the treatment protocol will be reported with the trial outcomes.
Measures
Refer to Fig. 2. Baseline sociodemographic and clinical variables will include age, sex, gender identity, race/ethnicity, income, and education obtained through a baseline sociodemographic questionnaire. Clinical variables (medications, medical comorbidities [e.g., diabetes, coronary artery disease, hypertension, etc.] and cardiometabolic risk factors [blood pressure, lipids, resting heart rate, waist circumference, and BMI]) are collected as a standard of care at TCR and will be determined by medical chart review at baseline and at 12 months.
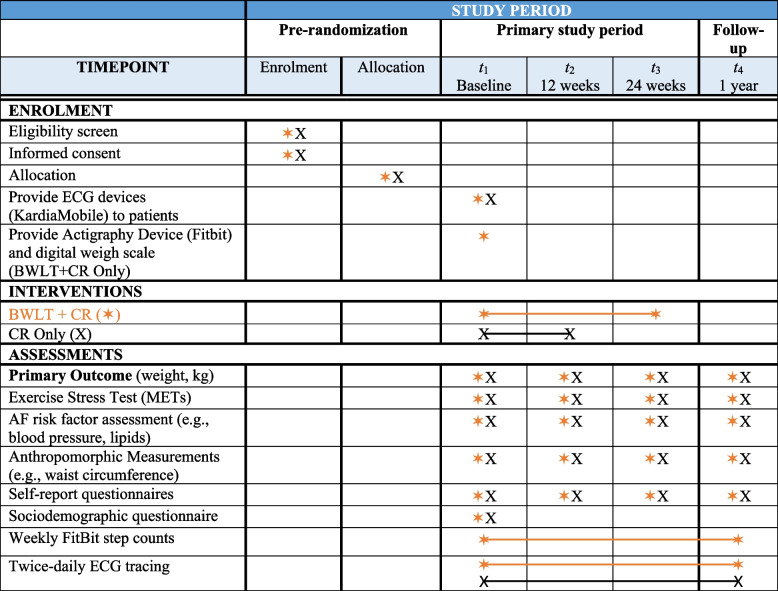
Fig. 2.
SPIRIT figure of study enrolment, intervention, and assessment period. AF, atrial fibrillation, BWLT, behavioral weight loss treatment, CR, Cardiac rehabilitation, METs, metabolic equivalent of tasks
Primary outcome
Clinically meaningful weight loss will be defined as the proportion of patients achieving ≥ 10% body weight loss at 52 weeks post-randomization. Weight loss will be measured by TCR staff at all study timepoints using a digital scale. Weight loss will be assessed by dividing the number of kilograms lost at 52 weeks by initial (T1) kg and multiplying by 100%. Percent weight loss scores will be dichotomized according to the ≥ 10% cut-off [18, 48] to determine the proportion of patients in each group that achieve this target.
Secondary outcomes
Mean total weight loss will be assessed by dividing the number of kilograms lost at 52 weeks by initial (T1) kg, multiplying by 100% and comparing across conditions.
AF burden will be assessed by daily ECG monitoring with the AliveCor™ device [49]. Patients in both groups will be provided with a KardiaMobile (and compatible smartphone if they do not have one) and instructed on its use. Patients will record 30-s ECGs twice daily and with AF symptoms for the duration of the study and follow-up period (12 months). Patients will be instructed to program the KardiaMobile smartphone app to remind them to record two ECG tracings daily. The study team will monitor patients’ adherence to recording ECG tracings bi-weekly and provide reminder calls when necessary. In recent clinical trials, the KardiaMobile device has demonstrated 91–100% sensitivity and 94–99% specificity in detecting episodes of AF [50–52]. The KardiaMobile device includes access to an online physician portal which stores and organizes all ECG tracings transmitted by patients. All ECG tracings classified as possible AF, and all tracings with potentially clinically important findings will be over-read by a study cardiologist who is unaware of randomized group. Total AF burden will be calculated as the percentage of all ECG tracings showing AF from weeks 12 to 52 to allow for latency of intervention effect on AF pathophysiology. Multiple imputation will be used to handle missing ECG data; a biostatistician will be consulted to assist with statistical procedures.
Self-reported disease burden, psychological distress, and quality of life will be assessed by validated and reliable psychometric instruments administered online via REDCAP. These are described in Table 4.
CR exercise prescription adherence will be measured by TCR chart review of participants’ attendance to six scheduled supervised onsite exercise sessions. Patients will also be asked to self-report their daily participation (duration, intensity) in moderate intensity aerobic exercise through weekly exercise logs submitted to the research team.
Exploratory outcomes will include AF risk factors and CRF assessed by TCR staff as part of the usual course of care in CR at baseline, 12, 24, and 52 weeks. AF risk factors to be measured include resting blood pressure, lipid profile, waist circumference, and smoking status. CRF is assessed using a symptom-limited exercise test to determine peak METs. Exercise testing procedures at TCR have been previously described [53].
Statistical analysis plan
Analysis will be by intention to treat. The primary analysis will compare the proportion in each group achieving ≥ 10% weight loss between baseline and 52 weeks post-randomization. Dropouts/lost to follow-up will be classified as non-responders (i.e., < 10% weight loss). A secondary per-protocol analysis will be performed, including only participants who complete at least the initial 12 weeks of the BWLT. AF burden will be calculated as a % of total ECG tracings with AF and compared between treatment and controls. Self-reported secondary outcomes will be evaluated using linear mixed modeling. Analyses will be stratified by AF pattern (paroxysmal vs persistent). Sensitivity analyses will be performed to compare dropouts vs those who remained in the trial on baseline characteristics, including AF severity. Finally, patients will be grouped according to intervention adherence (i.e., poor adherers [< 80% of BWLT sessions attended] compared with good adherers [≥ 80%]).
Sample size
Assuming a 5% rate of clinically significant improvement in the control group (quality assurance data from TCR shows < 2% of patients with obesity achieve ≥ 10% weight loss in traditional CR; RCTs of BWLTs typically report < 5% total body weight loss among controls [54]) and a 30% rate of clinically significant improvement in the intervention group (consistent with results from weight loss trials for patients with AF and obesity [48, 54] and systematic reviews of BWLT in general [54, 55]), 78 patients (39 per group) will provide 80% power to detect a difference using a two-sided independent test of proportions with a 5% alpha level. We estimate loss to follow-up and drop-outs of 20% and 10%, respectively; therefore, 120 patients will be recruited in total (60 per group).
Data management plan
Electronic data will be both encrypted and password protected and stored on a secure university server. Data from both the FitBit Charge 5 fitness trackers and FitBit Aria Air scales will be synced to online password-protected accounts managed by the study team. Geolocation and heart rate monitoring functions will be disabled on FitBit devices, and participants will be instructed to turn off geolocation when connecting the devices to a mobile application on their smartphones. Weight and step-count data will be exported and saved on a secure REDCap database, while other data will be discarded. Participants’ FitBit accounts will be deleted after the completion of the study. Data from KardiaMobile devices will be synced to online password-protected accounts created and managed by the study team. AliveCor has high-level security and privacy precautions in place to ensure that patients' confidentiality is protected. No identifiable information will be linked to the accounts, and ECG tracings will be downloaded and stored on secure password-protected university servers.
Trial management
Trial steering and DMC committee
Drs. Campbell and Wilton from the University of Calgary and Drs. Rouleau and Aggarwal from TotalCardiology comprise the steering committee. The committee meets quarterly to monitor progress including the study budget. A bi-annual report of randomization allocations, drop-outs, and adverse events will be reviewed by two external monitors unaffiliated with the study (data monitoring committee).
Protocol amendments
All amendments to the study protocol due to safety or operational issues will be submitted to the CHREB for approval prior to implementation. Consent addendums will then be disseminated to all enrolled patients.
Discussion
It is now evident that targeting durable risk factor modification is key to developing and implementing innovative treatments to reduce AF-related morbidity and healthcare utilization [2, 6], but the most effective treatment components and delivery mechanisms remain to be elucidated. This rigorous clinical trial aims to establish the efficacy of adding BWLT for weight loss to traditional CR in this population. It will also provide initial data on the potential of this approach for achieving AF control.
Anticipated results
It is anticipated that patients with AF and obesity who receive ASPIRE-AF as part of their CR program will lose more weight on average and be more likely to achieve a clinically significant reduction in weight of ≥ 10% than patients who attend a traditional CR program. This additional weight loss is predicted to contribute to greater improvements in secondary and exploratory outcomes including AF symptoms, HRQOL, psychological symptoms, and cardiometabolic risk factors (e.g., lipids, CRF). It is also predicted that the addition of an ASPIRE-AF component will be relatively low-burden and feasible for both patients and CR staff to include as a required or optional “add on” to standard CR. Diverse patient groups benefit from participating in CR; however, programs must be tailored to the unique needs of specific disease populations. Our findings will therefore inform recommendations for CR program delivery for patients with AF and comorbid obesity. CR programs are ubiquitous in Canadian cities, familiar to referring practitioners, and already provide evidence-based management of some AF risk factors. Therefore, enhancing CR with weight loss interventions designed specifically to target the AF substrate has the potential for rapid translation into practice.
Trial status
Protocol version number: 1.5. Protocol version date: July 11, 2023. Recruitment for the present trial began in July 2023. At the time of writing, 16 patients have been recruited in total and 13 have been randomized to condition. The first ASPIRE-AF intervention group started on November 27, 2023, and patients in that group completed their 24-week (T3) assessment in June 2024. Given the current average recruitment rate of three patients per month, we anticipate that recruitment will continue until 2026.
Dissemination plan
Knowledge translation activities will be aimed at Canadian CR programs, the key end users for this intervention. If the findings support the use of ASPIRE-AF, the study team will disseminate the results through the Canadian Association of Cardiovascular Prevention and Rehabilitation (CACPR) via webinar series’ and bi-annual meetings. Webinars and workshops will be part of a larger implementation toolkit that will include all program materials (BWLT manuals, slides, worksheets, recruitment materials), needs assessment tools, implementation planning grid, information on key components and adaptations, planning and training tools, and online fidelity measures. Program consultation will also be available from the PI to facilitate translation to unique needs of specific CR settings. Furthermore, our CR + BWLT model for patients with AF and obesity will be incorporated to the next edition of the CACPR clinical practice guidelines.
Strength and limitations
Strengths of the present trial include a randomized design allowing the investigators to control for the effect of standard CR programming on the primary outcome of weight loss. Furthermore, the trial is supported by substantial pilot and preliminary work including a qualitative investigation of patients’ needs and perspectives on barriers and facilitators to participating in the ASPIRE-AF intervention. The protocol and patient materials from the validated ASPIRE program were adapted using these results to ensure the program would be acceptable to the target population, thereby optimizing patient “buy-in” for the present trial. ASPIRE-AF is also facilitated by rigorously trained clinical psychology graduate students supervised by clinical psychologists, and fidelity is being monitored through session recordings and checks against an intervention fidelity checklist. Finally, while previous work in the CR population tends to suffer from a predominantly male sample, the present study has ensured equal gender representation by recruiting through cardiology and atrial fibrillation speciality clinics, rather than at the CR center itself.
Limitations to the present protocol should also be considered. Consistent with procedural limitations for all behavioral intervention trials, blinding participants and interventionists to experimental condition is not entirely possible. This increases the risk of investigator and patient expectancy effects biasing the trial results. To mitigate this risk, patients in both conditions will be blinded to the study hypotheses. Furthermore, CR staff at TCR will be blinded to patients’ condition, although it is possible that a participant could disclose their condition during regular interactions with CR staff. In addition, the average recruitment rate over the first 6 months of the trial was only 3 patients/month. This is certainly an underrepresentation of the number of eligible patients in Calgary who are seen by AF specialty clinics and cardiology clinics in the city. Preliminary data from our team suggests that several prospective patients declined to participate because they perceived the BWLT + CR program to be too much of a time commitment. Of note, most of the patients who declined to participate were of working age (between 18 and 64) with a greater burden of work and family commitments. Finally, the inclusion criterion that patients must speak English and have the necessary equipment to participate in virtual BWLT sessions may inadvertently exclude certain populations (e.g., low socioeconomic status, newcomers to Canada, older adults) and limit the representativeness of the sample.
Future research directions
If the results of this trial support the efficacy of an ASPIRE-AF component in CR for weight loss in patients with AF, next steps include a larger multi-site efficacy trial of the intervention on primary clinical endpoints including AF recurrence, AF remission, hospitalization, and AF-related major adverse cardiac events. Beyond this, effectiveness research will be conducted to evaluate ASPIRE-AF delivered by multidisciplinary CR staff (e.g., nurse clinicians, exercise physiologists, dietitians). While experienced, senior PhD students in clinical psychology are facilitating ASPIRE-AF in the present study, this program will be more sustainable in clinical practice if provided by existing CR staff members and integrated into the regular CR programming. There are numerous examples of successful training and implementation of evidence-based behavior change interventions by primary care providers and frontline medical staff that preserve intervention fidelity and treatment outcomes (e.g., [56, 57]). Providing a rigorously tested, feasible, and effective BWLT for patients with AF who would benefit from weight loss while participating in CR is an important step towards closing the existing gap in risk factor modification approaches for this growing patient population.
Supplementary Information
Supplementary Material 1. SPIRIT Checklist for Trials.
Acknowledgements
The authors acknowledge the contributions of Jasleen Kaur and Thomas Qiao to the preparation of this protocol and delivery of the intervention.
Abbreviations
- AF
Atrial fibrillation
- ASPIRE
Aspiring for Lifelong Health Small Changes Program
- BWLT
Behavioral weight loss treatment
- BMI
Body mass index
- CACPR
Canadian Association of Cardiovascular Prevention and Rehabilitation
- CAD
Coronary artery disease
- CONSORT
Consolidated Standards of Reporting Trials
- CR
Cardiac rehabilitation
- CRF
Cardiorespiratory fitness
- ECG
Electrocardiogram
- HRQOL
Health-related quality of life
- METS
Peak metabolic equivalents
- MD
Mean difference
- RCT
Randomized controlled trial
- RR
Relative risk
- SMALL goals
Self-selected, Measurable, Action-Oriented, Linked to your Life, and (Time) Limited
- TCR
TotalCardiology Rehabilitation CR program
- VO2peak
Standard mean difference peak oxygen consumption
Authors’ contributions
TW is a co-primary investigator who co-designed the study, supported the funding application, and prepared the protocol and was the primary writer of this manuscript. CR is a co-investigator who supported the funding application and the conceptualization of the study, supervised the first-author, and provided revisions throughout the writing process. SW is a co-investigator who co-conceptualized the study, supported the funding application, and reviewed and provided revisions throughout the manuscript. BV is a research coordinator who contributed to the writing and revision of the protocol and supporting documentation. CM contributed to the design of the trial and writing/revision of the manuscript. SP contributed to writing and revision of the protocol and supporting documentation including figures and tables. LL is a co-investigator who contributed to the study design and the development of intervention materials. SA is a co-investigator who contributed to trial design and revisions to the protocol. RA is a co-investigator who contributed to the trial design, protocol revisions, and manuscript writing. TC is the senior author and primary investigator. He supervised the first author, secured funding, and co-designed the protocol. All authors have read and approved of this manuscript.
Funding
The trial is funded by a Canadian Institutes of Health Research Project Grant (Application 469337). The funder had no role in the design, execution, analyses, interpretation of the data, or decision to submit results for this study. The study is sponsored by the University of Calgary (2500 University Drive NW, Calgary, Alberta, CANADA, T2N 1N4). The sponsor will ensure there is appropriate indemnity and arrangements in place to store confidential data and maintain a research ethics board.
Data availability
The intervention materials that will be used for the described protocol are available from the corresponding author on reasonable request. No data is associated with the present manuscript.
Declarations
Ethics approval and consent to participate
This study was approved by the University of Calgary Conjoint Ethics Board (REB22-0976). Patients who participate in this trial provide their full informed consent using an online form emailed by the research assistant. The participant information materials and informed consent form are attached as supplementary materials.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36(12):1847–948. [DOI] [PubMed] [Google Scholar]
- 2.Shu H, Cheng J, Li N, Zhang Z, Nie J, Peng Y, Wang Y, Wang DW, Zhou N. Obesity and atrial fibrillation: a narrative review from arrhythmogenic mechanisms to clinical significance. Cardiovasc Diabetol. 2023;22(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. 2017;70(16):2022–35. [DOI] [PubMed] [Google Scholar]
- 4.Morin DP, Bernard ML, Madias C, Rogers PA, Thihalolipavan S, Estes NAM. The state of the art: atrial fibrillation epidemiology, prevention, and treatment. Vol. 91, Mayo Clinic Proceedings. Elsevier Ltd; 2016. p. 1778–810. [DOI] [PubMed]
- 5.Wingerter R, Steiger N, Burrows A, Estes NM III. Impact of lifestyle modification on atrial fibrillation. Am J Cardiol. 2020;125(2):289–97. [DOI] [PubMed] [Google Scholar]
- 6.Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. 2017;136(6):583–96. [DOI] [PubMed] [Google Scholar]
- 7.Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, Deswal A, Eckhardt LL, Goldberger ZD, Gopinathannair R, Gorenek B. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149(1):e1–56. [DOI] [PMC free article] [PubMed]
- 8.Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, Sullivan T, Roberts-Thomson KC, Sanders P. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2(2): e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mujović N, Marinković M, Lenarczyk R, Tilz R, Potpara TS. Catheter ablation of atrial fibrillation: an overview for clinicians. Adv Ther. 2017;34:1897–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hindricks G, Potpara T, Kirchhof P, Kühne M, Ahlsson A, Balsam P, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Vol. 42, European Heart Journal. Oxford University Press; 2021. p. 373–498.
- 11.Wang TJ, Parise H, Levy D, D’Agostino RB, Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. J Am Med Assoc. 2004;292(20):2471–7. [DOI] [PubMed] [Google Scholar]
- 12.Homan EA, Reyes M V., Hickey KT, Morrow JP. Clinical overview of obesity and diabetes mellitus as risk factors for atrial fibrillation and sudden cardiac death. Front Physiol. 2019;10(JAN). [DOI] [PMC free article] [PubMed]
- 13.Vasan RS, Magnani JW, Lubitz SA, McManus DD, Larson MG, Newton-Cheh C, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. The Lancet. 2015 Jul 11;386(9989):154–62. Available from: https://www-sciencedirect-com.ezproxy.lib.ucalgary.ca/science/article/pii/S0140673614617748. [cited 2018 Nov 26]. [DOI] [PMC free article] [PubMed]
- 14.Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, Leong DP, Lau DH, Middeldorp ME, Roberts-Thomson KC, Wittert GA. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. 2011;57(17):1745–51. [DOI] [PubMed] [Google Scholar]
- 15.Sandhu RK, Conen D, Tedrow UB, Fitzgerald KC, Pradhan AD, Ridker PM, et al. Predisposing factors associated with development of persistent compared with paroxysmal atrial fibrillation. J Am Heart Assoc. 2014 May 1;3(3):e000916. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24786144. [cited 2020 Jan 24]. [DOI] [PMC free article] [PubMed]
- 16.Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA - Journal of the American Medical Association. 2013;310(19):2050–60. [DOI] [PubMed] [Google Scholar]
- 17.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64(21):2222–31. [DOI] [PubMed] [Google Scholar]
- 18.Aldaas OM, Lupercio F, Han FT, Hoffmayer KS, Krummen D, Ho G, et al. Meta-analysis of effect of modest (≥10%) weight loss in management of overweight and obese patients with atrial fibrillation. Am J Cardiol. 2019;124(10):1568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stone JA, editor. Canadian guidelines for cardiac rehabilitation and cardiovascular disease prevention: translating knowledge into action. 3rd ed. Winnipeg, MB: Canadian Association of Cardiac Rehabilitation; 2009.
- 20.Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the american heart association. Circulation. 2011;124(25):2951–60. [DOI] [PubMed] [Google Scholar]
- 21.Taylor RS, Dalal HM, McDonagh STJ. The role of cardiac rehabilitation in improving cardiovascular outcomes. Vol. 19, Nature Reviews Cardiology. Nature Research; 2022. p. 180–94. [DOI] [PMC free article] [PubMed]
- 22.Oesterle A, Giancaterino S, Van Noord MG, Pellegrini CN, Fan D, Srivatsa UN, et al. effects of supervised exercise training on atrial fibrillation: a meta-analysis of randomized controlled trials. J Cardiopulm Rehabil Prev. 2022;42(4):258–65. [DOI] [PubMed] [Google Scholar]
- 23.Smart NA, King N, Lambert JD, Pearson MJ, Campbell JL, Risom SS, et al. Exercise-based cardiac rehabilitation improves exercise capacity and health-related quality of life in people with atrial fibrillation: a systematic review and meta-analysis of randomised and non-randomised trials. Open Heart. 2018;5(2):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malmo V, Nes BM, Amundsen BH, Tjonna AE, Stoylen A, Rossvoll O, et al. Aerobic interval training reduces the burden of Atrial fibrillation in the short term: a randomized trial. Circulation. 2016;133(5):466–73. [DOI] [PubMed] [Google Scholar]
- 25.Osbak PS, Mourier M, Kjaer A, Henriksen JH, Kofoed KF, Jensen GB. A randomized study of the effects of exercise training on patients with atrial fibrillation. Am Heart J. 2011;162(6):1080–7. [DOI] [PubMed] [Google Scholar]
- 26.Ades PA, Savage PD. The treatment of obesity in cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2021;41(5):295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ades PA, Savage PD. Obesity in coronary heart disease: an unaddressed behavioral risk factor. Vol. 104, Preventive Medicine. Academic Press Inc.; 2017. p. 117–9. Available from: https://pubmed.ncbi.nlm.nih.gov/28414064/. [cited 2020 Sep 20]. [DOI] [PMC free article] [PubMed]
- 28.Wharton S, Lau DCW, Vallis M, Sharma AM, Biertho L, Campbell-Scherer D, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):E875–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143–64. [DOI] [PubMed] [Google Scholar]
- 30.Ntoumanis N, Ng JYY, Prestwich A, Quested E, Hancox JE, Thøgersen-Ntoumani C, et al. A meta-analysis of self-determination theory-informed intervention studies in the health domain: effects on motivation, health behavior, physical, and psychological health. Health Psychol Rev. 2020; Available from: https://www.tandfonline.com/doi/abs/10.1080/17437199.2020.1718529. [cited 2020 Oct 16]. [DOI] [PubMed]
- 31.Lutes LD, Winett RA, Barger SD, Wojcik JR, Herbert WG, Nickols-Richardson SM, et al. Small changes in nutrition and physical activity promote weight loss and maintenance: 3-month evidence from the ASPIRE randomized trial. Annals of Behavioral Medicine. 2008 Jun 21;35(3):351–7. Available from: https://academic.oup.com/abm/article/35/3/351-357/4561496. [cited 2018 Nov 22]. [DOI] [PubMed]
- 32.Lutes LD, DiNatale E, Goodrich DE, Ronis DL, Gillon L, Kirsh S, et al. A randomized trial of a small changes approach for weight loss in veterans: design, rationale, and baseline characteristics of the ASPIRE-VA trial. Contemp Clin Trials. 2013 Jan;34(1):161–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23041618. [cited 2018 Nov 22]. [DOI] [PubMed]
- 33.Lutes LD, Daiss SR, Barger SD, Read M, Steinbaugh E, Winett RA. Small changes approach promotes initial and continued weight loss with a phone-based followup: nine-month outcomes from ASPIRES II. American Journal of Health Promotion. 2012 Mar;26(4):235–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22375574. [cited 2018 Nov 13]. [DOI] [PubMed]
- 34.Damschroder LJ, Lutes LD, Kirsh S, Kim HM, Gillon L, Holleman RG, et al. Small-changes obesity treatment among veterans: 12-month outcomes. Am J Prev Med. 2014;47(5):541–53. Available from: 10.1016/j.amepre.2014.06.016. [cited 2018 Nov 2]. [DOI] [PubMed]
- 35.Lutes LD, Damschroder LJ, Masheb R, Kim HM, Gillon L, Holleman RG, et al. Behavioral treatment for Veterans with obesity: 24-month weight outcomes from the ASPIRE-VA Small Changes randomized trial. J Gen Intern Med. 2017 Apr 7;32(S1):40–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28271430. [cited 2018 Nov 22]. [DOI] [PMC free article] [PubMed]
- 36.Ciszewski S, Lutes LD, Walsh Z, Tate D. Small-Change behavioural intervention approaches to weight loss: a systematic review. Ann Behav Med. 2018;52:S577. [Google Scholar]
- 37.Larky DA, Bagheri R, Abbasnezhad A, Tinsley GM, Alipour M, Wong A. Effects of gradual weight loss vs rapid weight loss on body composition and resting metabolic rate: a systematic review and meta-analysis. British Journal of Nutrition. Cambridge University Press; 2020. p. 1–12. Available from: /core/journals/british-journal-of-nutrition/article/effects-of-gradual-weight-loss-v-rapid-weight-loss-on-body-composition-and-rmr-a-systematic-review-and-metaanalysis/427E2A512D278FC053CEBB73995FEEFC. [cited 2020 Oct 1].
- 38.Bates RW, Bailey C, Topping AE. € Out of sync’: a qualitative investigation of patients’ experiences of atrial fibrillation and perceptions of weight management. BMJ Open. 2022 Nov 7;12(11). [DOI] [PMC free article] [PubMed]
- 39.Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, SPIRIT, et al. explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;2013:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2(64). [DOI] [PMC free article] [PubMed]
- 41.Martin BJ, Hauer T, Arena R, Austford LD, Galbraith PD, Lewin AM, et al. Cardiac rehabilitation attendance and outcomes in coronary artery disease patients. Circulation. 2012 Aug 7;126(6):677–87. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22777176. [cited 2017 Jul 31]. [DOI] [PubMed]
- 42.Armstrong MJ, Sigal RJ, Arena R, Hauer TL, Austford LD, Aggarwal S, et al. Cardiac rehabilitation completion is associated with reduced mortality in patients with diabetes and coronary artery disease. Diabetologia. 2015 Mar 6;58(4):691–8. Available from: www.icd9data.com/2007/Volume1. [cited 2020 Oct 7]. [DOI] [PubMed]
- 43.Thompson S, Wiebe N, Arena R, Rouleau C, Aggarwal S, Wilton SB, et al. Effectiveness and utilization of cardiac rehabilitation among people with CKD. Kidney Int Rep. 2021;6(6):1537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu H, Southern DA, Arena R, Sajobi T, Aggarwal S, James MT, et al. Cardiac rehabilitation and risk of incident atrial fibrillation in patients with coronary artery disease. Can J Cardiol. 2022 Oct 1;38(10):1621–8. Available from: https://pubmed.ncbi.nlm.nih.gov/35691566/. [cited 2023 Dec 5]. [DOI] [PubMed]
- 45.Williamson T, Moran C, Chirico D, Arena R, Ozemek C, Aggarwal S, et al. Cancer and cardiovascular disease: the impact of cardiac rehabilitation and cardiorespiratory fitness on survival. Int J Cardiol. 2021;15(343):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armstrong MJ, Mottershead TA, Ronksley PE, Sigal RJ, Campbell TS, Hemmelgarn BR. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obesity Reviews. 2011 Jul 1;12(9):709–23. Available from: http://doi.wiley.com/10.1111/j.1467-789X.2011.00892.x. [cited 2017 Jun 29]. [DOI] [PubMed]
- 47.Miller WR, Rollnick S. Motivational interviewing, preparing people to change addictive behavior. New York: The Guilford Press; 1991. [Google Scholar]
- 48.Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan R, et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. EP Europace. 2018 Jun 14; Available from: http://www.ncbi.nlm.nih.gov/pubmed/29912366. [cited 2018 Nov 13]. [DOI] [PubMed]
- 49.KardiaMobile. 2020. Available from: https://www.alivecor.com/kardiamobile
- 50.Wegner FK, Kochhäuser S, Ellermann C, Lange PS, Frommeyer G, Leitz P, et al. Prospective blinded Evaluation of the smartphone-based AliveCor Kardia ECG monitor for Atrial Fibrillation detection: the PEAK-AF study. Eur J Intern Med. 2020 Mar 1;73:72–5. Available from: https://pubmed.ncbi.nlm.nih.gov/31806411/. [cited 2020 Sep 20] [DOI] [PubMed]
- 51.William AD, Kanbour M, Callahan T, Bhargava M, Varma N, Rickard J, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: The iREAD Study. Heart Rhythm. 2018 Oct 1;15(10):1561–5. Available from: http://www.heartrhythmjournal.com/article/S1547527118306611/fulltext. [cited 2020 Oct 6]. [DOI] [PubMed]
- 52.Brasier N, Raichle CJ, Dörr M, Becke A, Nohturfft V, Weber S, et al. Detection of atrial fibrillation with a smartphone camera: first prospective, international, two-centre, clinical validation study (DETECT AF PRO). Europace. 2019 Jan 1;21(1):41–7. Available from: https://clinicaltrials.gov/ct2/show/NCT02949180. [cited 2020 Oct 6]. [DOI] [PMC free article] [PubMed]
- 53.Parker K, Stone JA, Arena R, Lundberg D, Aggarwal S, Goodhart D, et al. An early cardiac access clinic significantly improves cardiac rehabilitation participation and completion rates in low-risk ST-elevation myocardial infarction patients. Can J Cardiol. 2011;27(5):619–27. [DOI] [PubMed] [Google Scholar]
- 54.Lv N, Azar KMJ, Rosas LG, Wulfovich S, Xiao L, Ma J. Behavioral lifestyle interventions for moderate and severe obesity: a systematic review. Vol. 100, Preventive Medicine. Academic Press Inc.; 2017 . p. 180–93. Available from: https://pubmed.ncbi.nlm.nih.gov/28450123/. [cited 2020 Oct 1]. [DOI] [PMC free article] [PubMed]
- 55.Vallis M, Macklin D, Russell-Mayhew S. Effective psychological and behavioural interventions in obesity management. Canadian Adult Obesity Clinical Practice Guidelines. 2020; Available from: https://obesitycanada.ca/guidelines/behavioural
- 56.Ecker AH, O’Leary K, Fletcher TL, Hundt NE, York-Ward KM, Kauth MR, et al. Training and supporting mental health providers to implement evidence-based psychotherapies in frontline practice. Vol. 12, Translational Behavioral Medicine. Oxford University Press; 2022. p. 63–9. [DOI] [PubMed]
- 57.McCay E, Carter C, Aiello A, Quesnel S, Howes C, Beanlands H, et al. Training frontline community agency staff in dialectical behaviour therapy: building capacity to meet the mental health needs of street-involved youth. Journal of Mental Health Training, Education and Practice. 2017;12(2):121–32. [Google Scholar]
- 58.Patel D, Smith A. Patient initiation and maintenance of GLP-1 RAs for treatment of obesity. 10.1080/1751243320211947796 . 2021. Available from: https://www.tandfonline.com/doi/abs/10.1080/17512433.2021.1947796. [cited 2021 Sep 2]. [DOI] [PubMed]
- 59.Dorian P, Paquette M, Newman D, Green M, Connolly SJ, Talajic M, et al. Quality of life improves with treatment in the Canadian trial of Atrial Fibrillation. Am Heart J. 2002;143(6):984–90. [DOI] [PubMed] [Google Scholar]
- 60.Singh SN, Tang XC, Singh BN, Dorian P, Reda DJ, Harris CL, et al. Quality of life and exercise performance in patients in sinus rhythm versus persistent atrial fibrillation a Veterans Affairs cooperative studies program substudy. J Am Coll Cardiol. 2006;48(4):721–30. [DOI] [PubMed] [Google Scholar]
- 61.Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MR, et al. Development and validation of the Atrial Fibrillation Effect on QualiTy-of-life (AFEQT) questionnaire in patients with Atrial Fibrillation. Circ Arrhythm Electrophysiol. 2011;4(1):15–25. [DOI] [PubMed] [Google Scholar]
- 62.Ware J, Sherbourne C. The MOS 36-Item Short-Form Health Survey (SF-36): conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 63.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983 Jun ;67(6):361–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6880820. [cited 2019 Jul 5]. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. SPIRIT Checklist for Trials.
Data Availability Statement
The intervention materials that will be used for the described protocol are available from the corresponding author on reasonable request. No data is associated with the present manuscript.