Abstract
Background
Pulse oximetry is a standart of anesthesia for perioperative monitoring. Due to the principles of Hb oxygen dissociation curve, peripheral oxygen saturation has an approximate sensitivity and specificity of 90% for the detection of hypoxemia.
Objectives
The primary outcome of the study was to evaluate ORiⓇ as an early parameter to determine hypoxia in morbidly obese patients. The secondary outcome was to compare the effectiveness of ORiⓇ with SpO2 in non-obese patients.
Design
Prospective, observational study.
Setting
Department of elective operating room at tertiary hospital.
Patients and methods
Observational study included written informed consent from 51 patients with 19 < BMI < 25 kg/m2 and 51 patients with BMI > 40 kg/m2 undergoing an elective surgery requiring tracheal intubation. In addition to standard monitors, an ORi sensor was placed and baseline values were recorded. The patients were preoxygenated until end tidal expiratory oxygen concentration is reached to 90%. After anesthesia induction and tracheal intubation, the breathing circuit was not connected tracheal tube until the SpO2 decreased to 95%. Shapiro-Wilk, Pearson Chi-square, t-test, and Mann Whitney U test were used for the study.
Main outcome measures
Times of tolerable apnea, ORiⓇ and SpO2 values at the end of preoxygenation, beginning of intubation, beginning of the ORi alarm, when SpO2 reached 95%, and when ORi reaches a plateau.
Sample size
102 patients.
Results
The alert period: time to reach ORiⓇ from 0.24 to a value of 95% SpO2 was observed as 32 s in morbidly obese patients and 94 s in patients with a normal body mass index. The SpO2 alert period was determined as time difference between 97% and 95% SpO2. The data were recorded as 15 s and 36 s, respectively. It was observed that tolerable apnea, ORiⓇ, SpO2 and added alert times were longer in patients with normal BMI compared to morbidly obese patients.
Conclusions
As a result, ORiⓇ can provide an early warning to prevent unexpected hypoxia before saturation begins to decrease in morbidly obese patients.
Limitations
Inability to perform arterial blood gas sampling in the time periods when we looked at the parameters to determine the relationship between ORiⓇ and PaO2.
Clinical trials. gov identifier
NCT05480748 registered 2022-07-29.
Keywords: Oxygen reserve index, Hypoxemia, Tolerable apnea time, Oxygen reserve index warning time, Percutaneous oxygen saturation warning time, Morbidly obese patients
Introduction
Background/rationale
Obesity is a chronic complex disease with a significant increasing rate globally. According to World Health Organization (WHO), 1 in 8 people were defined as obese in 2022 [1]. Obesity related respiratory changes include increased work of breathing and decreased chest wall compliance [2]. An additional decrease becomes remarkable in morbidly obese patients regarding the Total Lung Capacity, Vital Capacity, Forced Expiratory Volume in 1 s (FEV1) and Forced Vital Capacity (FVC) [3]. Moreover, they have high oxygen consumption rate due to their increased metabolism. Under general anesthesia they are prone to restrictive ventilation pattern that impairs diaphragmatic descent.
Pulse oximetry is a standart of anesthesia for perioperative monitoring. Due to the principles of Hb oxygen dissociation curve, peripheral oxygen saturation has an approximate sensitivity and specificity of 90% for the detection of hypoxemia [4].
Oxygen Reserve Index (ORiⓇ) (Masimo Corp., Irvine, CA, USA) is a novel continuous and noninvasive parameter that serves as a relative indicator of the PaO2. Regression analyzes report a correlation between ORiⓇ and PaO2, especially at PaO2 ≤ 240 mmHg (r2 = 0.536), and ORiⓇ> 0.24 indicates PaO2 ≥ 100 mmHg [5–7]. ORiⓇ ranges from 0 to 1 as PaO2 increases from 80 mmHg to 200 mmHg [8]. On the other hand, a key limitation of SpO2 is its inability to reflect values above 100%, even when PaO2 surpasses 100 mmHg. The patient’s oxygen status can be evaluated more precisely when used in conjunction with SpO2 to prevent hypoxemia [9].
While the ORiⓇ cannot show us the actual, direct PaO2 value, it can warn of impending PaO2 decline even without a change in SpO2. In particular, ORiⓇ monitoring may be useful in patients at risk for insufficient preoxygenation, such as difficult mask ventilation [10], aspirated hypoxemic patients [11], rapid sequence induction [12], obese patients [13], intubations in intensive care unit [14], intubation of hypoxic patients requiring non-invasive ventilation [15]. It has also been shown that in some patient groups, ORiⓇ provides an early warning of desaturation, providing additional time to improve patient safety [16, 17]. However the number of comparative studies in morbid obese patients are quite limited. Therefore we aimed to compare ORiⓇ and SpO2 in this unique population.
Objectives
The primary outcome of this prospective observational study was to evaluate ORiⓇ as an early warning parameter in predicting hypoxemia. The secondary outcome was to compare the effectiveness of ORiⓇwith SpO2 in non-obese patients.
Materials and methods
Study design
This single-center prospective, observational study was approved by Institutional Ethics Committee (Decision No: 2022/514/222/9, Date:30/03/2022), was registered at ClinicalTrials.gov (NCT05480748) on 29th of July 2022, and was performed in accordance with the Declaration of Helsinki.
Setting and participants
Written, informed consent was obtained from 51 patients with BMI > 40 kg/m2 (morbidly obesity) and 51 patients with 19 < BMI < 25 kg/m2 (non-obese), 18–80 years aged, American Society of Anesthesiologists (ASA) physical status I-III, scheduled for an elective surgical procedure requiring general anesthesia with tracheal intubation. Patients with significant history of cardiopulmonary disease, difficult intubation, pregnancy, hemoglobinopathies and preoperative hemoglobin of less than 10.0 mg/dL were defined as exclusion criteria. Our University Hospital’s surgical profile was consist of gynecologic procedures including hysterectomy, myomectomy and ovarian cystectomy with acute care surgery procedures including cholecystectomy, appendectomy and hernia repair. The data collection period was between August 1, 2022 and December 31, 2022.
Data sources and measurement
Standard monitors were routinely established for each patient, including heart rate (HR), noninvasive blood pressure measurements. In addition, an ORiⓇ and SpO2 were measured simultaneously at 1-s interval with a pulse oximetry sensor (Rainbow sensor, R2-25) applied to the finger and connected to a Masimo Root with Radical-7 pulse oximeter (Masimo Corp.). Data for analysis were downloaded from the Root monitor. Rainbow® technology uses Pulse CO-Oximetry sensors connected to rainbow®-enabled devices. By this way the operators can monitor the oxygen content noninvasively. ORi is a part of this package. Light absorption is performed to increase the resolution of changes in oxygenation via a Pulse CO-Oximeter sensor. This utilizes multiple wavelengths of light. ORi is trended together with SpO2 as a real-time, continuous, unitless index between 0.00 and 1.00. This allows monitoring of patients’ oxygenation. Pulse oximeter uses spectrophotometry as its operating principle to determine oxyhemoglobin in peripheral arterial blood. The two wavelengths of light originating from oxygenated and deoxygenated hemoglobin in the blood are compared to each other.
Patients were admitted to the operating theatre without any premedication. A 20G cannula was used to establish intravenous access. Following the placement of monitors, baseline values were recorded. Patients were then preoxygenated with spontaneous ventilation and 100% FiO2 at a flow rate of 8 L/min via a tight-fitting face mask until end tidal expiratory oxygen concentration (etO2) reached to 90%. Anesthesia was induced by titrating intravenous propofol 2–3 mg/kg, fentanyl 1 mcg/kg, and rocuronium 0.6 mg/kg. The trachea was intubated after 3–4 min under direct visualization using a videolaryngoscope to confirm placement. The tracheal tube was not connected to the breathing circuit, and the patients remained apneic. The World Health Organization defines intraoperative SpO2 ≥ 95% as normal in its training materials, and treatment steps are mentioned for SpO2 ≤ 94% [18]. However, since we included morbidly obese patients with limited functional residual capacities in our study and wanted to stay within the safe range, we allowed SpO2 to decrease to 95%. At the same time, the alarm point for SpO2 was applied as 95% in our clinic’s protocol for morbidly obese patients. When SpO2 reached to 95%, an oral airway was placed. Afterwards the patients were ventilated by face mask with two hands technique. As a resque method, proper sizes of second generation supraglottic airway devices were kept ready.
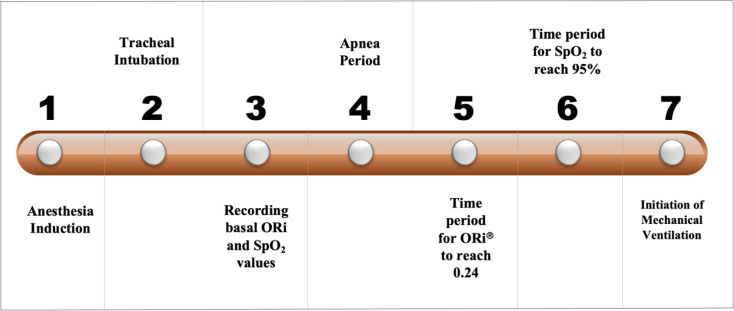
ORiⓇ and SpO2 values were recorded continuously. Subsequently, the anesthesia circuit was connected and etCO2 was confirmed. Patients were ventilated with 100% FiO2, tidal volume targeted 6–8 mL/kg and 5 cmH2O of positive end-expiratory pressure until ORiⓇ plateaued. In order to keep etCO2 between 30 and 35 mmHg, the respiratory rate was adjusted. Thereafter, anesthesia maintenance was achieved by 2% inhaled sevoflurane with 1 MAC in 50% oxtgen and 50% air mixture. The steps are summarized in Timeline Diagram (Fig. 1).
Fig. 1.
The Timeline Diagram of the clinical trial
ORiⓇ and SpO2 data were compared at five specific time points: (1) baseline; (2) at the end of pre-oxygenation (when the EtO2 reaches to 90%); (3) at the beginning of intubation; (4) when SpO2 reaches 95%; and (5) when the ORi reaches a plateau with 100% FiO2.
We also recorded the tolerable apnea time defined as the time from the beginning of apnea until SpO2 reached 95% and ventilation was reinstated. The ORiⓇ alert period time was defined as the time between the onset of the ORiⓇ alarm (the time at which the ORiⓇ alarm would have started was also calculated using the manufacturer’s proprietary algorithm and the ORiⓇ alarm was set to ORi = 0.24 to stay within the safe range) and the SpO2 reaching 95%. We defined the SpO2 alert period as the time for SpO2 to decrease from 97 to 95%. The added warning time provided by ORiⓇ was defined as the difference between ORiⓇ warning time and SpO2 warning time.
Statistical analysis
SPSS version 25 statistical package program was used for statistical analysis. The data were summarized by using descriptive statistical methods (mean, median, frequency, percentile, minimum, maximum). Shapiro-Wilk test was used for normality tests of continuous variables. Pearson Chi-square Test of independence tests were performed for independence tests between two categorical variables. To investigate the differences between the two groups, t-test was used for continuous variables with normal distribution, and Mann Whitney U Test was used to compare data that did not. In addition, 95% confidence intervals were obtained with the Hodges Lehman median estimation in order to see the median changes.
The relationships between the classified variables forming the 2 × 2 crosstabs were investigated with Fisher’s Exact tests. The significance level was taken as 0.05 for all tests performed.
Study size
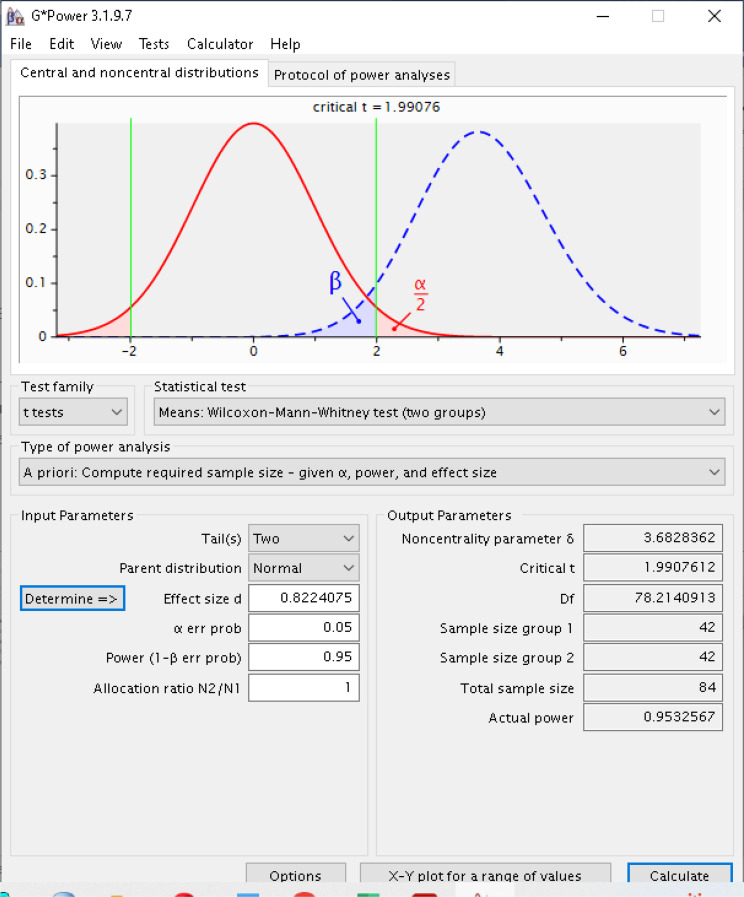
Tsymbal et al. [19] found the mean and standard deviation of obese group for Oxygen Reserve Index Values 0.41 and 0.09 respectively, and the mean and standard deviation of normal group 0.57 and 0.26. When this information was given into Gpower program, d parameter which is effect size was calculated as 0.822 seen in window at the below with alpha 0.05 and power of 0.95. Tsymbal et al. [19] also mentioned that they had used sampling information given by Szmuk P et al. [20], with power of 0.80 and alpha 0.05.
Gpower found that 42 patients for both groups, totally 84 patients with d effect size 0,822. Closely, we have calculated 80 patients by using Medcalc program. According to calculation of Gpower, non-centrality parameter of t test is 3.68 which was calculated by using sampling sizes of two groups and effect size. For this research it was planned to include 51 patients for each group in case of data collection errors, thus 102 patients were totally enrolled in the research. In summary, we have used sampling information from Tsymbal et al. [19] and calculated the parameter of effect size and used it with alpha 0.05 and power of 0.95 as seen in Gpower window. The calculation screen shot of Gpower program can be seen in Fig. 2.
Fig. 2.
The calculation of samole size using Gpower program
Results
Descriptive data
The total number of patients included in the study was 102, and the power of the test was calculated as 85.10% according to this number. No patient was excluded from the study. Fifty one of the 102 patients were of normal weight according to their BMI values, and 51 were morbidly obese. Twenty-eight of the patients were male and 74 were female, and their ages ranged from 18 to 78 years.
Table 1 shows the differences in mean age, weight, height, BMI, frequency and percentage ratios, as well as gender and ASA values between patients with normal BMI and those with morbidly obese patients. According to the p values obtained as a result of the statistical tests, there was no difference between non-obese and morbidly obese patients in terms of age and ASA values distribution (p > 0.05). None of the patients showed desaturation with SpO2 level fall below 95%.
Table 1.
Patient characteristics
| Variables | Normal BMI (18 < BMI < 25 kg/m2) (n = 51) | Morbidly obese (BMI > 40 kg/m2) (n = 51) | p values |
|---|---|---|---|
| Age, years, mean (min.-max) | 49.86 (18–78) | 49.78 (29–77) | 0.975* |
| Sex, n, % | |||
| Male | 24 (47.10%) | 4 (7.80%) | 0.000 ** |
| Female | 27 (52.90%) | 47 (92.20%) | |
|
Weight, kg, mean (min.-max) |
65.29 (48–83) | 104.77 (85–126) | 0.000 *** |
|
Height, cm, mean (min.-max) |
168.49 (150–190) | 158.08 (146–153) | 0.000 *** |
| BMI, mean | 22.88 (18–25) | 41.91 (40-51.20) | 0.000 *** |
| ASA, n 1/2/3 | 8/37/6 | 2/40/9 | 0.116**** |
*ttest **Fisher’s ExactTest ***Mann-Whitney Test **** Chi-square Test of independence
BMI; body mass index, n; number, ASA; American Society of Anesthesiologists, cm; centimeters, kg; kilogram
There was a statistically significant difference between patients with normal BMI and morbid obesity in terms of gender, weight, height and BMI variables (p <0.05). Although we observed that the gender frequency and percentage encountered in patients with normal BMI are close to each other, there were more morbidly obese cases in women than in men. Considering the average height, patients with normal BMI were approximately 10 cm taller than morbidly obese patients.
Outcome data
Table 2 shows the median, 25–75 percentiles and averages of the tolerable apnea, ORiⓇ alert period, SpO2 alert period and added warning times of normal weight and morbidly obese patients. Since the p values obtained were less than 0.05, the difference between the groups was statistically significant for all periods. The Hodges Lehman median estimation and 95% confidence intervals also show that patients with normal weight parallel to the tests have a higher median.
Table 2.
Median, 25–75 percentiles, mean, mean difference test results, and Hodges Lehman median estimation and 95% confidence intervals of defined time periods in patients with normal BMI and morbidly obese
| Time (seconds) | Normal BMI (18 < BMI < 25 kg/m2) (n = 51) |
Morbidly obese (BMI > 40 kg/m2) (n = 51) | Difference 95%CI | P values | Hodges Lehman Median difference | Hodges Lehman Median difference %95 CI |
|---|---|---|---|---|---|---|
| Tolerable apnea time Mean, Median 25–75 percentiles | 383 (364.61) 286–469 | 194 (195.43) 135–236 | 169.18 (126.83; 211.52) | 0.000* | -173 | (-218; -129) |
| ORiⓇ warning time Mean, Median 25–75 percentiles | 94 (97.25) 55–116 | 32 (38.80) 24–45 | 58.45 (41.20; 75.70) | 0.000** | -51 | (-67; -34) |
| SpO2 warning time Mean, Median 25–75 percentiles | 36 (49.53) 18–59 | 15 (27.06) 13–32 | 22.47 (6.54; 38.41) | 0.007** | -11 | (-22; -3) |
| Added warning time Mean, Median 25–75 percentiles | 38 (54.08) 29–68 | 17 (21.82) 11–28 | 32.26 (18.01; 46.51) | 0.000** | -24 | (-33; -17) |
*ttest **Mann-Whitney Test
BMI; body mass index, ORiⓇ; oxygen reserve index, SpO2;percutaneous oxygen saturation
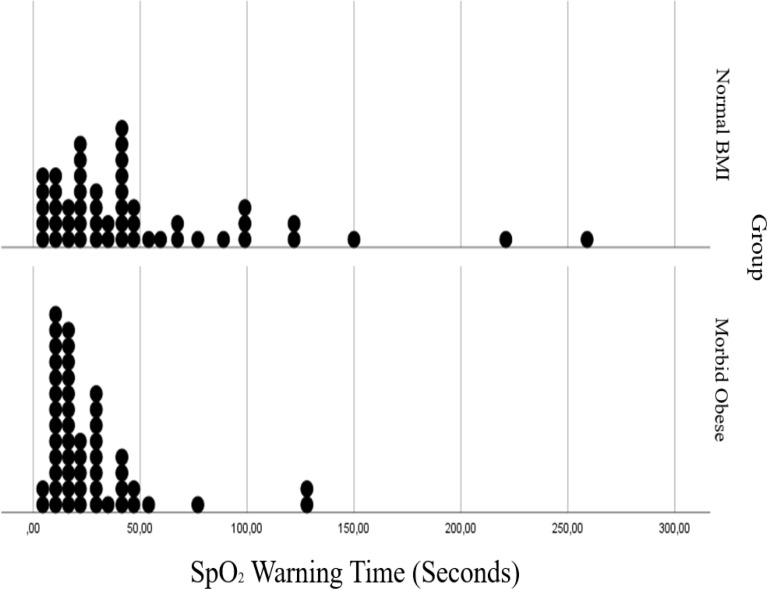
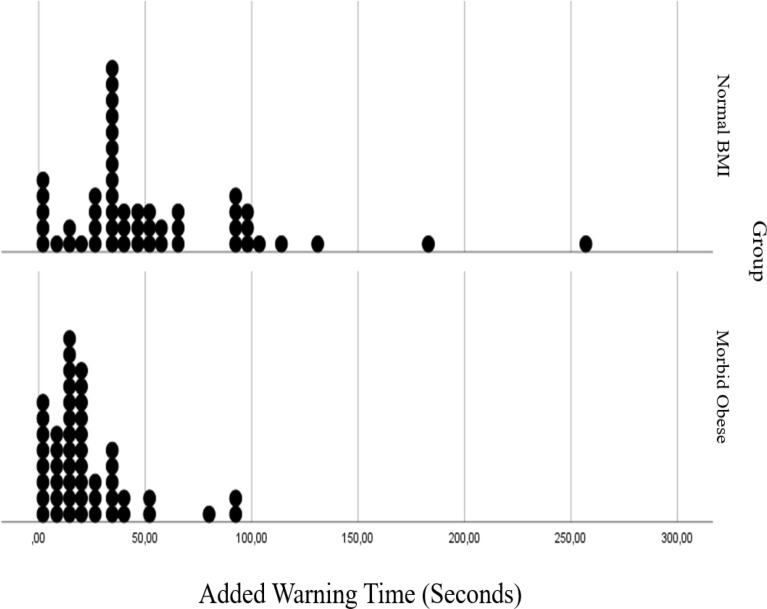
In both groups, the ORiⓇ alarm started before SpO2 reached 97%, so the ORiⓇ alert period was longer than the SpO2 alert period. While ORiⓇ alert period was observed as 32 s in morbidly obese patients and 94 s in patients with normal BMI, SpO2 alert period was determined as 15 s and 36 s in these patients, respectively (Table 2). It was observed that non-obese patients have longer tolereble apnea (p < 0.000), ORiⓇ (p < 0.000), SpO2 (p = 0.007), and added warning times (p < 0.000) than morbidly obese subjects (Table 2).
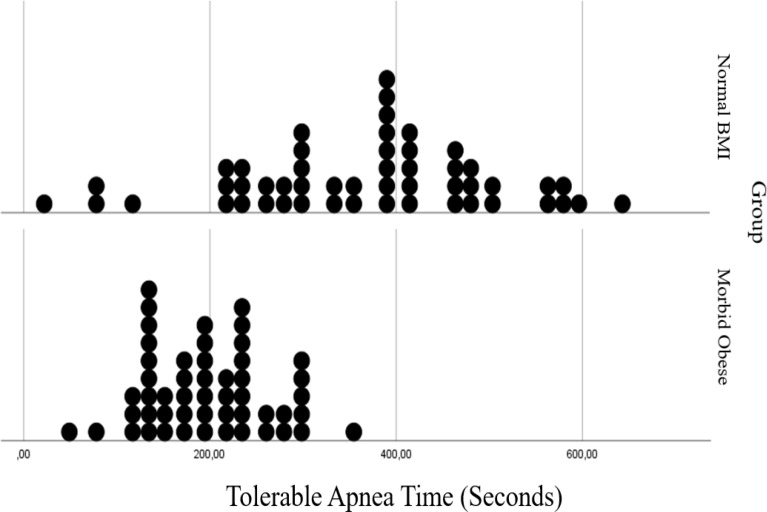
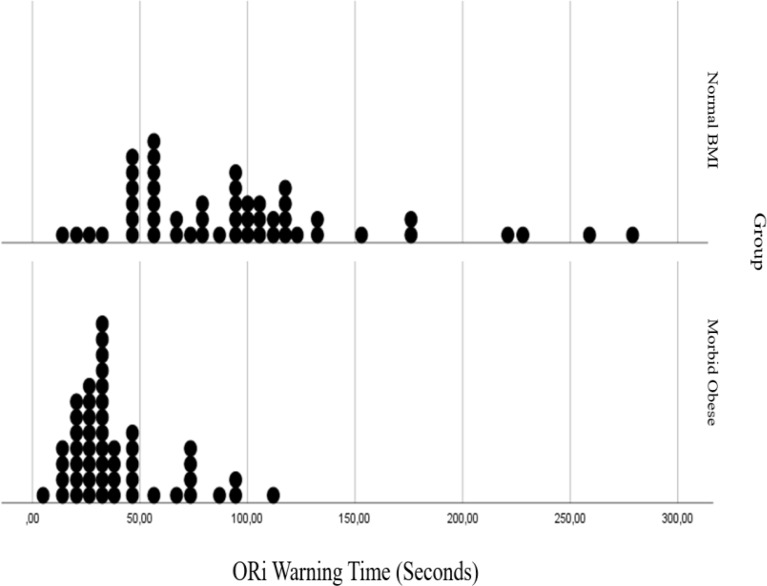
In Figs. 3, 4, 5 and 6 distributions of tolerable apnea, ORiⓇ, SpO2 and added warning time for normal weight and morbidly obese patients according to BMI values are given. Morbidly obese patients for all time shift to the left in the graph, took shorter times, and their distribution fitted into a narrower area than non-obese patients.
Fig. 3.
Comparision of tolerable apnea time in normal BMI and morbidly obese patients
Fig. 4.
Comparision of oxygen reserve index (ORi) warning time in normal BMI and morbidly obese patients
Fig. 5.
Comparision of percutaneous oxygen saturation (SpO2) warning time in normal BMI and morbidly obese patients
Fig. 6.
Comparision of added warning time in normal BMI and morbidly obese patients
Values of SpO2 at baseline, the end of pre-oxygenation and the beginning of intubation, and SpO2, ORiⓇ during ventilation with 100% FiO2 differed according to the groups (p <0.05). The mean values of SpO2 at baseline, the end of pre-oxygenation, the beginning of intubation, and SpO2 and ORiⓇ during ventilation with 100% FiO2 were found to be higher in patients with normal BMI. Hodges Lehman median estimations and 95% confidence intervals of the median difference between groups are given in Table 3 in order to examine the median change of the investigated parameters of normal and morbidly obese patients. According to the median confidence intervals, changes can be seen in Mann-Whitney test results for values of SpO2 at the beginning of intubation, and SpO2 and ORiⓇ during ventilation with 100% FiO2. There was no location shift for values of SpO2 at the beginning of intubation, and the upper limit of the confidence interval was 0. There was a -1 median change for values of SpO2 during ventilation with 100% FiO2, obese patients had less values of SpO2 during ventilation with 100% FiO2, however the upper limit of the confidence interval was still 0. When the averages of ORiⓇ during ventilation with 100% FiO2 are examined, it is seen that normal weight patients had a higher mean value than morbidly obese patients, however the median difference was in favor of obese patients.
Table 3.
Means and p values of parameters, Hodges Lehman median estimation, and 95% confidence intervals of patients with normal BMI and morbidly obese
| Parameters | Normal BMI (18 < BMI < 25kg/m2) (n = 51) |
Morbidly obese (BMI > 40 kg/m2) (n = 51) |
p value | Hodges Lehman Median difference | Hodges Lehman Median difference %95 CI |
|---|---|---|---|---|---|
| SpO2# | 98,57 | 97,55 | 0,000 ** | -1 | (-2; -0.5) |
| ORi# | 0,0155 | 0,0159 | 0,344** | 0 | (0;0) |
| SpO2## | 99,98 | 99,88 | 0,051** | 0 | (0;0) |
| ORi## | 0,838 | 0,842 | 0,797** | 0 | (-0.01;0.05) |
| SpO2### | 99,88 | 99,49 | 0,003 * | 0 | (0.5;0) |
| ORi### | 0,69 | 0,66 | 0,373** | -0.04 | (-0.10;0.04) |
| SpO2#### | 95,00 | 95,00 | All values are 95 | 0 | (0;0) |
| ORi#### | 0,00 | 0,00 | All values are 0 | 0 | (0;0) |
| SpO2##### | 99,45 | 98,71 | 0,001 ** | -1 | (-1;0) |
| ORi##### | 0,485 | 0,349 | 0,000 * | -0.15 | (-0.22;0.07) |
*ttest **Mann-Whitney Test
#baseline, ##at the end of pre-oxygenation, ### at the beginning of intubation, ####when SpO2 reaches 95%, ##### when the ORi reaches a plateau
ORi; oxygen reserve index, SpO2; percutaneous oxygen saturation
Discussion
Key results
In this prospective observational study we compared the ORiⓇ and SpO2 values during apnea period in both morbidly obese and non-obese patients. While the ORiⓇ alert period was observed as 32 s in morbidly obese patients and 94 s in patients with a normal BMI, the SpO2 alert period was determined as 15 s and 36 s in patients, respectively.
Obesity may aggravate the morbid condition in patients by causing anatomical, functional and systemic changes. Therefore, morbidly obese patients are considered to be at high risk for anesthesia. In our study we found that ORiⓇ provided an earlier warning of severe oxygen desaturation in both groups compared to pulse oximetry. Despite effective preoxygenation until etO2 reaches 90%, we found that morbidly obese patients with limited functional residual capacities had a shorter tolerable apnea period compared to patients with normal BMI.
Interpretation
Although pulse oximetry is an indispensable monitoring tool for detecting hypoxemia, the decrease in SpO2 and PaO2 is not always linear in the event of impending hypoxia. SpO2 may not decrease before PaO2 drops below 70 mmHg [21, 22]. This is one of the factors limiting its use in cases where early hypoxia should be detected, such as morbid obesity. Although many studies reported that ORiⓇ, a new generation pulse oximeter, provides early warning in predicting hypoxia, to the best of our knowledge, no studies have focused on morbidly obese patients. ORiⓇ might have a significant difference in morbidly obese patients as they perform poorer gas exchange even at rest compared with normal-weight individuals [23].
Our study involved tracking the alert period when ORi® falls below 0.24, as a lower ORi® unit signifies a decrease in PaO2. It is already known that when the ORiⓇ diminishes to 0.24, PaO2 is approximately below 100 mmHg [5]. Normal PaO2 is approximately 75–100 mmHg. When the ORiⓇ reaches 0.00, it is accepted that PaO2 is below 80 mmHg [20]. In other words, ORiⓇ is typically 0.00 when SpO2 is less than 98%. It is possible that a fall in ORiⓇ could indicate PaO2 decreases before SpO2 reduces. On the other hand, a value of SpO2 higher than 95% is considered normal by the American Lung Association. For this reason, we determined the period of peripheral oxygen saturation change from 97 to 95%. Due to the principle of Hemoglobin Oxygen Dissociation curve, the time for decrease of peripheral oxygen saturation from 97 to 95% is significantly rapid. Considering the relationship between arterial partial pressure of oxygen and SpO2 is not linear, SpO2 may be recorded as 98% even if the arterial partial pressure of oxygen is as low as 70 mmHg [5]. In our study, when ORiⓇ was monitored, the difference between the monitors in non-obese patients was 58 s. In this manner, the anesthesiologist can notice and have more time to take action when the ORiⓇ value reaches 0.24 which corresponds to 100 mmHg of PaO2. ORiⓇ provides real-time oxygenation monitoring. Highlighting the decline in PaO2 enables the anesthesiologist to be alerted early to potential changes in the patient’s oxygenation, providing extra time to respond. In this way, it can be intervened before the PaO2 reaches a critical teshold level and patient safety can be increased. Moreover, to the best of our knowledge this is the first comparative study in morbid obese patients. This difference decreases up to 17 s in this population. This finding emphasizes the crucial significance of using ORiⓇ in patients with restricted pulmonary oxygen reserve.
Fleming et al. [24], in patients undergoing cardiac surgery, determined the time of ORiⓇ, SpO2, added warning time and tolerable apnea time as 80.4 s, 29.0 s, 48.4 s, 9.6±2.2 min respectively. In the study of Cheng et al. [25], ORiⓇ alarm triggers were adjusted according to the ORiⓇ peak and ORiⓇ 0.55 values, and it was reported to provide 300 s and 145 s of significant added warning time compared to SpO2 (p < 0.0001). We are in the opinion that the reason why they found the added warning time so long, unlike us, may be due to the fact they performed the study in ASA I-II healthy individuals and that they had long (6 min) preoxygenation and ventilation times, which provide better oxygen reserve. Yoshida et al. [7] found that ORiⓇ could predict reduced levels of oxygen 30 s before the SpO2 in patients undergoing rapid sequence intubation. In 2021, Tsymbal et al. [19] in their study in normal and obese patients, they determined the ORiⓇ alert period was longer than the SpO2 alert period, and the added warning time provided by ORiⓇ in obese patients was shorter (46.5 s vs. 87 s). The same researchers also found the tolerable apnea time as 256 s in morbidly obese and 381 s in patients with normal BMI. In our study, we found these values to be shorter, unlike Tymbal et al. We concluded that the reason for this was accepting 95%, not 94%, as the endpoint of alarm time for SpO2 and included morbidly obese patients.
The ORiⓇ alarm times were declared as significantly shorter than SpO2 in studies conducted on one-lung ventilation and pediatric patients [16, 20]. In our study, we determined the ORiⓇ alert period to be longer than the SpO2 alert period in both groups, in line with the results of the researchers. At the same time, these values were observed to be shorter in morbidly obese patients.
Limitations
There are several limitations in our study. Morbidly obese patients were mostly women, so there were differences between the groups in terms of demographic characteristics. It would have added more meaning to our study if we had also been able to perform arterial blood gas sampling in the time periods when we compared our parameters to determine the correlation between ORiⓇ and PaO2. Since PaO2 has not been measured at the end point of study it did not objectively correlate with hypoxemia which depends upon PaO2 levels than oxygen saturation. In addition, although the ORiⓇ created an added warning time, the necessary interventions made during this period could not be recorded.
Conclusion
We observed that desaturation occurred more rapidly in morbidly obese patients than in non-obese patients, and ORiⓇ provided significant added warning time before impending desaturation compared to SpO2 during prolonged apnea in morbidly obese high-risk patients. We concluded that added warning time may be an important factor in our ability to better manage prolonged apnea periods due to difficult airway management, to perform constructive interventions, and thus to increase patient safety.
Acknowledgements
Not applicable.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [GA], [KTS], [AS], [ÖS].The first draft of the manuscript was written by [GA], [KTS] All authors commented on previous versions of the manuscript. Editing and revision of manuscript [PR], [TG]All authors read and approved the final manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data availability
All data are publicly available or listed in the results of the paper.
Declarations
Ethical approval
Ethical approval from our University Hospital Ethics Committee was obtained prior to initiation of the research work. This study was approved by the Ethics Committee decision no: 2022/514/222/9, Date:30/03/2022. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975 (in its most recently amended version).
Informed consent
Informed consent was obtained from all patients included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kemal Tolga Saraçoğlu, Email: kemaltolgasaracoglu@gmail.com.
Tomasz Gaszynski, Email: tomasz.gaszynski@umed.lodz.pl.
References
- 1.http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 2.Farid AM, Taman HI. The impact of Sevoflurane and Propofol Anesthetic induction on Bag Mask Ventilation in Surgical patients with high body Mass Index. Anesth Essays Res 2020 Oct-Dec;14(4):594–9. 10.4103/aer.AER_20_21 [DOI] [PMC free article] [PubMed]
- 3.Ortiz VE, Kwo J. Obesity: physiologic changes and implications for preoperative management. BMC Anesthesiol. 2015;15:97. 10.1186/s12871-015-0079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hafen BB, Sharma S. Oxygen Saturation. [Updated 2022 Nov 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. https://www.ncbi.nlm.nih.gov/books/NBK525974/ [PubMed]
- 5.Dorotta RL, Wells IL, Juma B, Applegate D. The relationship between oxygen reserve index and arterial partial pressure of oxygen during surgery. Anesth Analg. 2016;123(3):626–33. 10.1213/ANE.0000000000001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vos JJ, Willems CH, van Amsterdam K, van den Berg JP, Spanjersberg R, Struys MMRF, et al. Oxygen reserve index: validation of a new variable. Anesth Analg. 2019;129:409–15. 10.1213/ANE.0000000000003706. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida K, Isosu T, Noji Y, Ebana H, Honda J, Sanbe N, et al. Adjustment of oxygen reserve index (ORi™) to avoid excessive hyperoxia during general anesthesia. J Clin Monit Comput. 2020;34:509–14. 10.1007/s10877-019-00341-9. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal BD, Karnik PP, Dave NM. Oxygen reserve index - a new paradigm in patient safety. J Anaesthesiol Clin Pharmacol 2020 Jan-Mar;36(1):125–6. 10.4103/joacp.JOACP_76_19 [DOI] [PMC free article] [PubMed]
- 9.Saracoglu A, Zengin SU, Ozturk N, Niftaliyev S, Harman F, Aykac Z. The outcomes of using high oxygen concentration in pediatric patients. J Clin Monit Comput. 2022;36(5):1341–6. 10.1007/s10877-021-00765-2. [DOI] [PubMed] [Google Scholar]
- 10.Bouroche G, Bourgain JL. Pre-oxygenation and general anesthesia: a review. Minerva Anestesiol. 2015;81(8):910–20. [PubMed] [Google Scholar]
- 11.Day T, Farnell S, Wilson-Barnett J, Suctioning. A review of current research recommendations. Intensiv Crit Care Nurs. 2002;18(2):79–89. 10.1016/s0964-3397(02)00004-6. [DOI] [PubMed] [Google Scholar]
- 12.Gebremedhn EG, Mesele D, Aemero D, Alemu E. The incidence of oxygen desaturation during rapid sequence induction and intubation. World J Emerg Med. 2014;5(4):279–85. 10.5847/wjem.j.issn.1920-8642.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy C, Wong DT. Airway management and oxygenation in obese patients. Can J Anaesth. 2013;60(9):929–45. 10.1007/s12630-013-9991-x. [DOI] [PubMed] [Google Scholar]
- 14.Lapinsky SE. Endotracheal intubation in the ICU. Crit Care. 2015;19(1):258. 10.1186/s13054-015-0964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baillard C, Fosse JP, Sebbane M, Chanques G, Vincent F, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med. 2006;174(2):171–7. 10.1164/rccm.200509-1507OC. [DOI] [PubMed] [Google Scholar]
- 16.Koishi W, Kumagai M, Ogawa S, Hongo S, Suzuki K. Monitoring the Oxygen Reserve Index can contribute to the early detection of deterioration in blood oxygenation during one-lung ventilation. Minerva Anestesiol. 2018;84(9):1063–9. 10.23736/S0375-9393.18.12622-8. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida K, Isosu T, Noji Y, Hasegawa M, Iseki Y, Oishi R, et al. Usefulness of oxygen reserve index (ORi), a new parameter of oxygenation reserve potential, for rapid sequence induction of general anesthesia. J Clin Monit Comput. 2018;32(4):687–91. 10.1007/s10877-017-0068-1. [DOI] [PubMed] [Google Scholar]
- 18.Wilson IH. Hypoxia. Update on anesthesia. J World Federation Soc Anesthesiol. 2009;25(2):21–5. [Google Scholar]
- 19.Tsymbal E, Ayala S, Singh A, Applegate RL, Fleming NW, et al. Study of early warning for desaturation provided by Oxygen Reserve Index in obese patients. J Clin Monit Comput. 2020;35(4):749–56. 10.1007/s10877-020-00531-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szmuk P, Steiner JW, Olomu PN, Ploski RP, Sessler DI, Ezri T. Oxygen Reserve Index: a novel noninvasive measure of oxygen reserve—a pilot study. Anesthesiology. 2016;124(4):779–84. [DOI] [PubMed] [Google Scholar]
- 21.Scheeren TWL, Belda FJ, Perel A. The oxygen reserve index (ORi): a new tool to monitor oxygen therapy. J Clin Monit Comput. 2018;32(3):379–89. 10.1007/s10877-017-0049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jubran A. Pulse oximetry. Crit Care. 2015;19(1):272. 10.1186/s13054-015-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carron M, Ieppariello G, Martelli G, Gabellini G, Foletto M, Perissinotto E, Ori C. Dual effects of leptin in perioperative gas exchange of morbidly obese patients. PLoS ONE. 2018;13(7):e0199610. 10.1371/journal.pone.0199610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleming NW, Singh A, Lee L, Applegate R. Oxygen Reserve Index: utility as an early warning for desaturation in high-risk surgical patients. Anesth Analg. 2021;132(3):770–6. 10.1213/ANE.0000000000005109. [DOI] [PubMed] [Google Scholar]
- 25.Cheng HW, Yeh CY, Chang MY, Ting CK, Chang PL. How early warning with the Oxygen Reserve Index (ORiTM) can improve the detection of desaturation during induction of general anesthesia? J Clin Monit Comput. 2022;36(1):379–1385. 10.1007/s10877-021-00776-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are publicly available or listed in the results of the paper.