Abstract
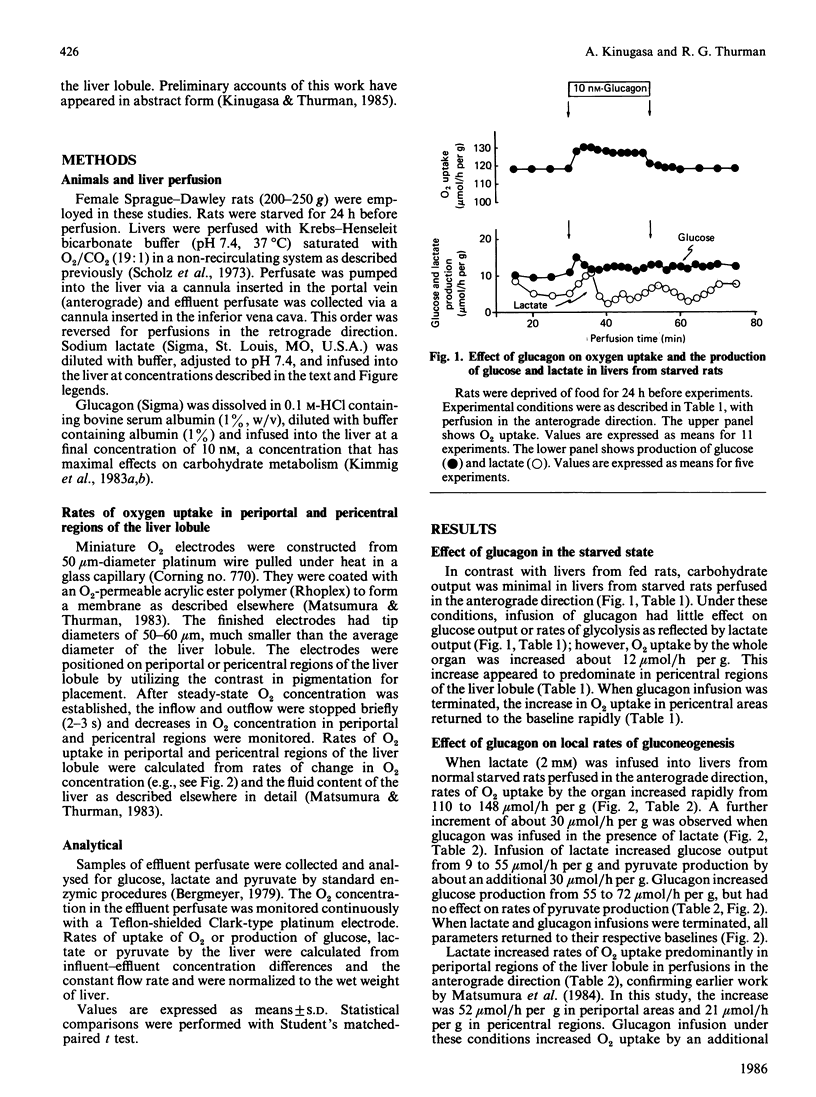
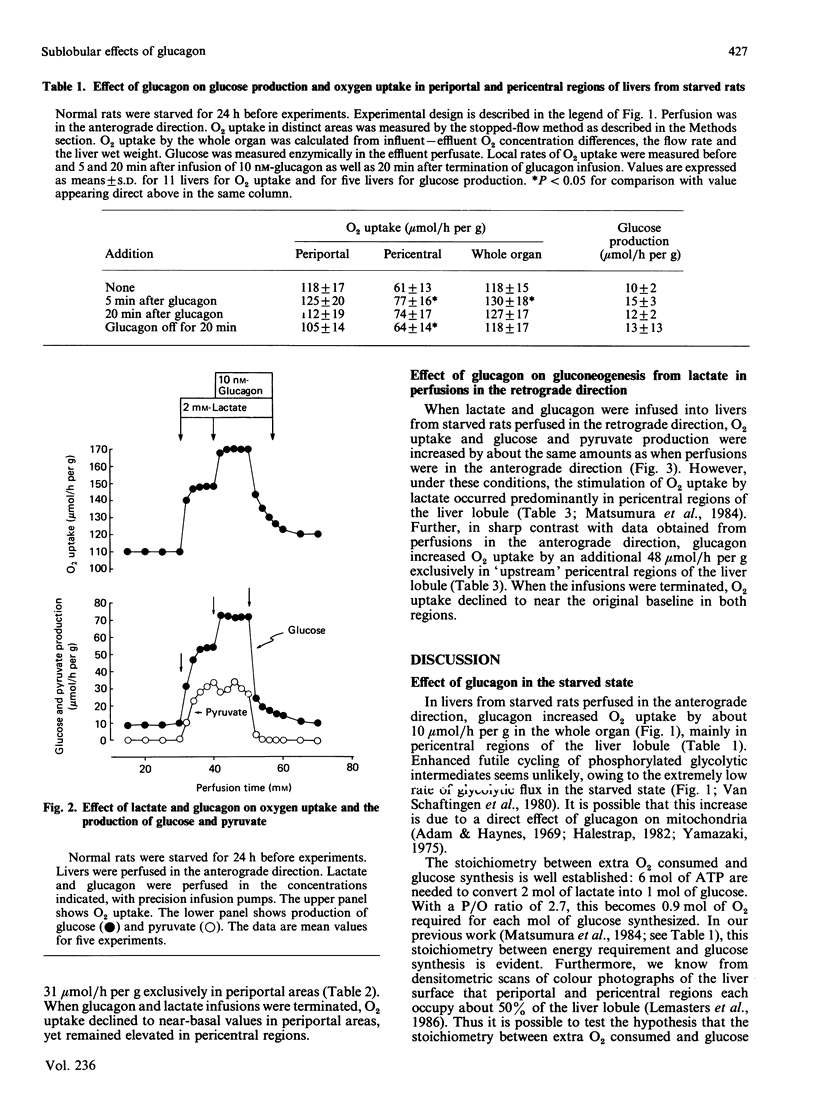
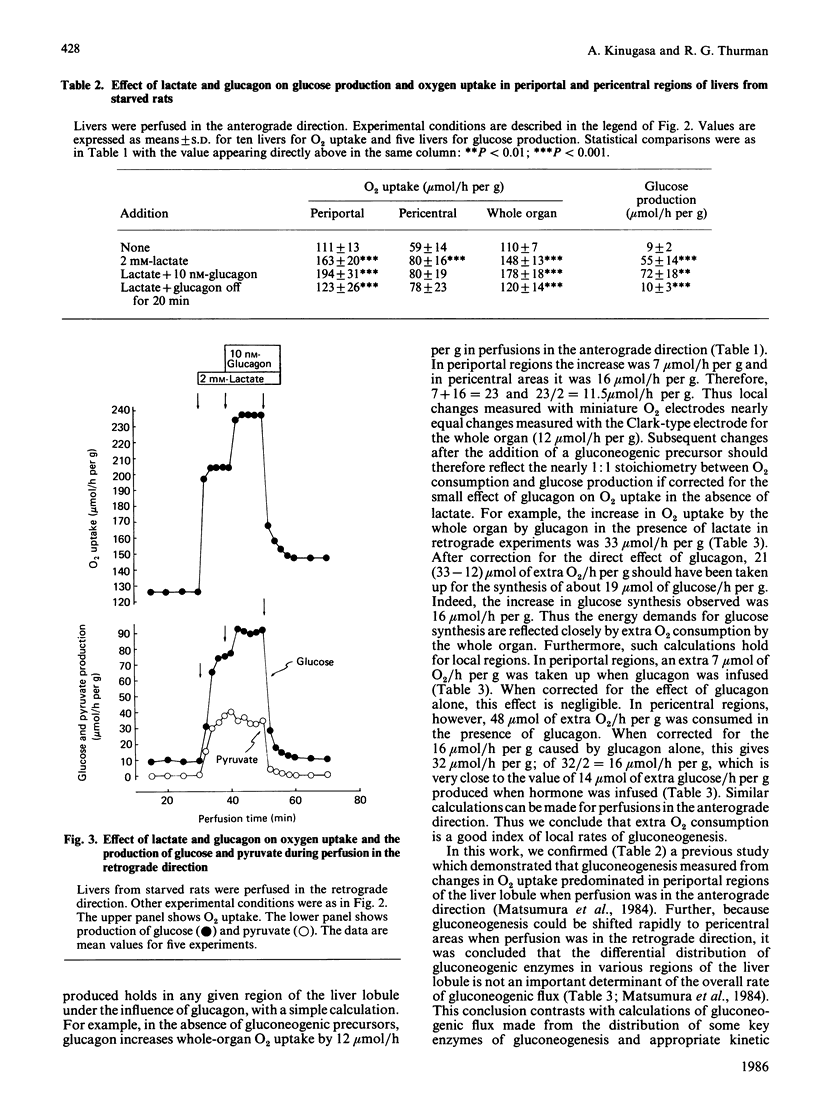
The effect of glucagon on gluconeogenesis was measured in periportal and pericentral regions of the liver lobule by monitoring changes in rates of O2 uptake on the surface of the perfused liver with miniature O2 electrodes after infusion of lactate. When lactate (2 mM) was infused into livers from starved rats perfused in the anterograde direction, O2 uptake was increased 2.5-fold more in periportal than in pericentral regions, reflecting increased energy demands for glucose synthesis. Under these conditions, glucagon infusion in the presence of lactate increased O2 uptake exclusively in periportal regions of the liver lobule. Thus, when perfusion is in the physiological anterograde direction, the metabolic actions of glucagon predominate in periportal regions of the liver lobule under gluconeogenic conditions in the starved state. When livers were perfused in the retrograde direction, however, glucagon stimulated O2 uptake exclusively in pericentral regions. Thus glucagon only stimulates gluconeogenesis in 'upstream' regions of the liver lobule irrespective of the direction of flow.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam P. A., Haynes R. C., Jr Control of hepatic mitochondrial CO2 fixation by glucagon, epinephrine, and cortisol. J Biol Chem. 1969 Dec 10;244(23):6444–6450. [PubMed] [Google Scholar]
- Babcock M. B., Cardell R. R., Jr Hepatic glycogen patterns in fasted and fed rats. Am J Anat. 1974 Jul;140(3):299–337. doi: 10.1002/aja.1001400302. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Robison G. A., Sutherland E. W., Park C. R. Studies on the role of adenosine 3',5'-monophosphate in the hepatic actions of glucagon and catecholamines. J Biol Chem. 1971 Oct 25;246(20):6166–6177. [PubMed] [Google Scholar]
- Guder W. G., Schmidt U. Liver cell heterogeneity. The distribution of pyruvate kinase and phosphoenolpyruvate carboxykinase (GTP) in the liver lobule of fed and starved rats. Hoppe Seylers Z Physiol Chem. 1976 Dec;357(12):1793–1800. doi: 10.1515/bchm2.1976.357.2.1793. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. The nature of the stimulation of the respiratory chain of rat liver mitochondria by glucagon pretreatment of animals. Biochem J. 1982 Apr 15;204(1):37–47. doi: 10.1042/bj2040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers H. G., Hue L. Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem. 1983;52:617–653. doi: 10.1146/annurev.bi.52.070183.003153. [DOI] [PubMed] [Google Scholar]
- Hildebrand R. Nuclear volume and cellular metabolism. Adv Anat Embryol Cell Biol. 1980;60:1–54. [PubMed] [Google Scholar]
- Jones D. P., Mason H. S. Gradients of O2 concentration in hepatocytes. J Biol Chem. 1978 Jul 25;253(14):4874–4880. [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Jungermann K., Katz N. Functional hepatocellular heterogeneity. Hepatology. 1982 May-Jun;2(3):385–395. doi: 10.1002/hep.1840020316. [DOI] [PubMed] [Google Scholar]
- Kimmig R., Mauch T. J., Kerzl W., Schwabe U., Scholz R. Actions of glucagon on flux rates in perfused rat liver. 1. Kinetics of the inhibitory effect on glycolysis and the stimulatory effect on glycogenolysis. Eur J Biochem. 1983 Nov 15;136(3):609–616. doi: 10.1111/j.1432-1033.1983.tb07784.x. [DOI] [PubMed] [Google Scholar]
- Kimmig R., Mauch T. J., Scholz R. Actions of glucagon on flux rates in perfused rat liver. 2. Relationship between inhibition of glycolysis and stimulation of respiration by glucagon. Eur J Biochem. 1983 Nov 15;136(3):617–620. doi: 10.1111/j.1432-1033.1983.tb07785.x. [DOI] [PubMed] [Google Scholar]
- Matsumura T., Kashiwagi T., Meren H., Thurman R. G. Gluconeogenesis predominates in periportal regions of the liver lobule. Eur J Biochem. 1984 Nov 2;144(3):409–415. doi: 10.1111/j.1432-1033.1984.tb08480.x. [DOI] [PubMed] [Google Scholar]
- Matsumura T., Thurman R. G. Measuring rates of O2 uptake in periportal and pericentral regions of liver lobule: stop-flow experiments with perfused liver. Am J Physiol. 1983 Jun;244(6):G656–G659. doi: 10.1152/ajpgi.1983.244.6.G656. [DOI] [PubMed] [Google Scholar]
- Matsumura T., Thurman R. G. Predominance of glycolysis in pericentral regions of the liver lobule. Eur J Biochem. 1984 Apr 16;140(2):229–234. doi: 10.1111/j.1432-1033.1984.tb08091.x. [DOI] [PubMed] [Google Scholar]
- Rappaport A. M. The microcirculatory acinar concept of normal and pathological hepatic structure. Beitr Pathol. 1976 May;157(3):215–243. doi: 10.1016/s0005-8165(76)80083-2. [DOI] [PubMed] [Google Scholar]
- Sasse D., Katz N., Jungermann K. Functional heterogeneity of rat liver parenchyma and of isolated hepatocytes. FEBS Lett. 1975 Sep 1;57(1):83–88. doi: 10.1016/0014-5793(75)80157-8. [DOI] [PubMed] [Google Scholar]
- Scholz R., Hansen W., Thurman R. G. Interaction of mixed-function oxidation with biosynthetic processes. 1. Inhibition of gluconeogenesis by aminopyrine in perfused rat liver. Eur J Biochem. 1973 Sep 21;38(1):64–72. doi: 10.1111/j.1432-1033.1973.tb03034.x. [DOI] [PubMed] [Google Scholar]
- Thurman R. G., Kauffman F. C. Sublobular compartmentation of pharmacologic events (SCOPE): metabolic fluxes in periportal and pericentral regions of the liver lobule. Hepatology. 1985 Jan-Feb;5(1):144–151. doi: 10.1002/hep.1840050128. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H. G. Study of the fructose 6-phosphate/fructose 1,6-bi-phosphate cycle in the liver in vivo. Biochem J. 1980 Oct 15;192(1):263–271. doi: 10.1042/bj1920263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki R. K. Glucagon stimulation of mitochondrial respiration. J Biol Chem. 1975 Oct 10;250(19):7924–7930. [PubMed] [Google Scholar]
- Yamazaki R. K., Haynes R. C., Jr Dissociation of pyruvate dehydrogenase from the glucagon stimulation of pyruvate carboxylation in rat liver mitochondria. Arch Biochem Biophys. 1975 Feb;166(2):575–583. doi: 10.1016/0003-9861(75)90422-1. [DOI] [PubMed] [Google Scholar]


