Abstract
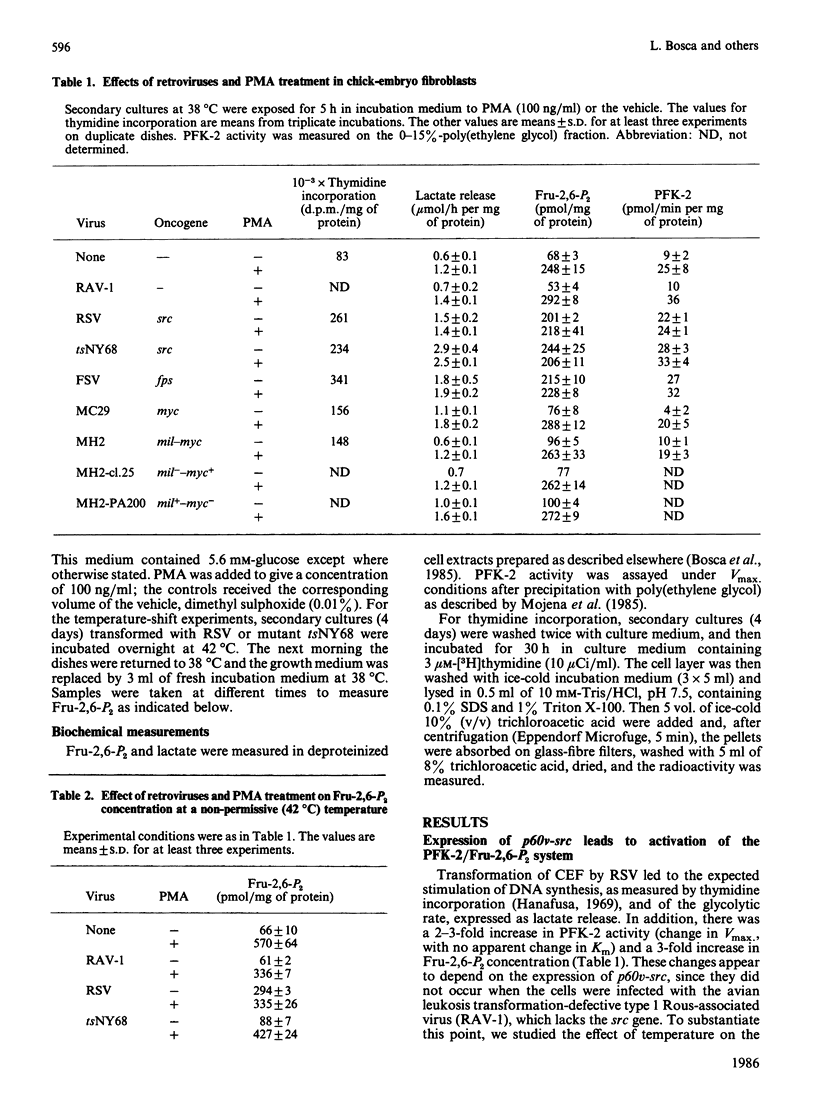
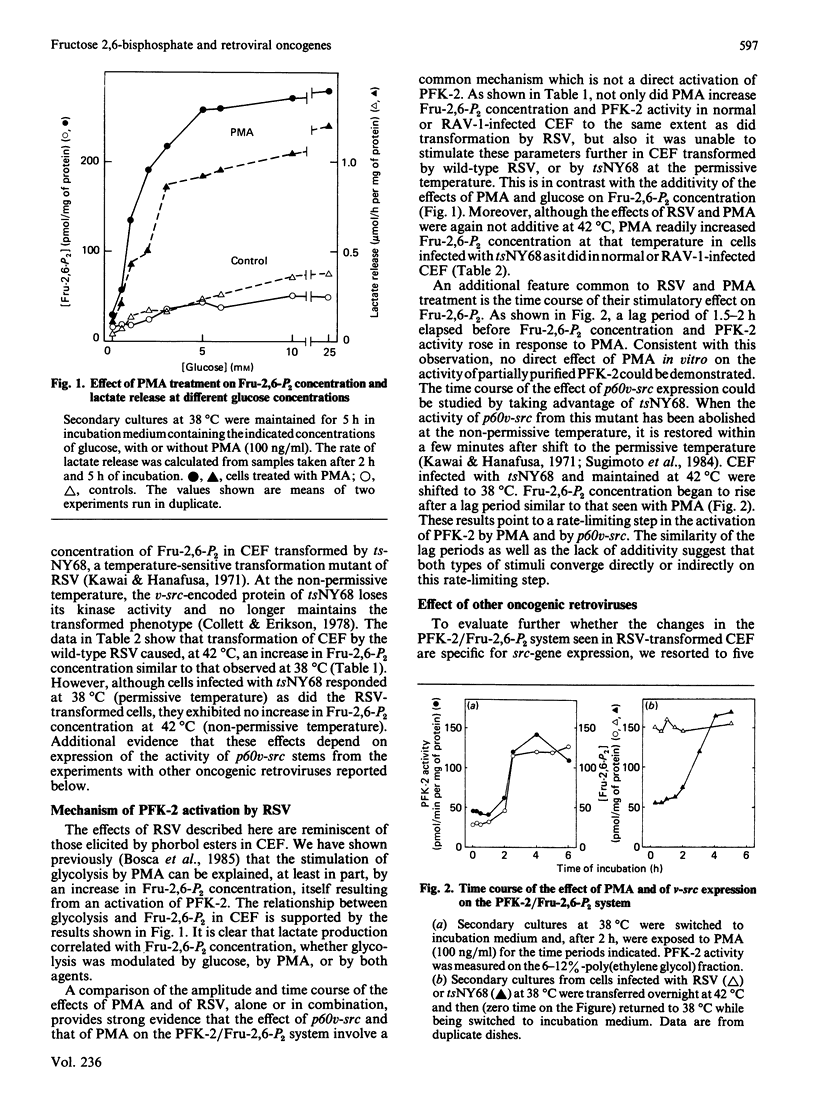
The concentration of fructose 2,6-bisphosphate and the activity of 6-phosphofructo-2-kinase are increased after infection of chick-embryo fibroblasts with the Rous sarcoma virus, or with a temperature-sensitive mutant of this virus at the permissive, but not at the non-permissive, temperature. This is observed after transformation by retroviruses carrying either the v-src or v-fps, but not the v-mil and/or v-myc, oncogenes. Comparison of the effects of the Rous sarcoma virus with those of phorbol myristate acetate on fructose 2,6-bisphosphate suggests that both result from the stimulation of a step which is rate-limiting for 6-phosphofructo-2-kinase activation and which is also controlled by protein kinase C.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bechade C., Calothy G., Pessac B., Martin P., Coll J., Denhez F., Saule S., Ghysdael J., Stéhelin D. Induction of proliferation or transformation of neuroretina cells by the mil and myc viral oncogenes. Nature. 1985 Aug 8;316(6028):559–562. doi: 10.1038/316559a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol lipids and cell proliferation. Biochem Soc Trans. 1985 Feb;13(1):67–71. doi: 10.1042/bst0130067. [DOI] [PubMed] [Google Scholar]
- Bosca L., Rousseau G. G., Hue L. Phorbol 12-myristate 13-acetate and insulin increase the concentration of fructose 2,6-bisphosphate and stimulate glycolysis in chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6440–6444. doi: 10.1073/pnas.82.19.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni P., Farnararo M., Vasta V., D'Alessandro A. Increase of the glycolytic rate in human resting fibroblasts following serum stimulation. The possible role of the fructose-2,6-bisphosphate. FEBS Lett. 1983 Aug 8;159(1-2):39–42. doi: 10.1016/0014-5793(83)80412-8. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Reiss N. A., Schwartz R. J., Hunter T. Three glycolytic enzymes are phosphorylated at tyrosine in cells transformed by Rous sarcoma virus. Nature. 1983 Mar 17;302(5905):218–223. doi: 10.1038/302218a0. [DOI] [PubMed] [Google Scholar]
- Diamond I., Legg A., Schneider J. A., Rozengurt E. Glycolysis in quiescent cultures of 3T3 cells. Stimulation by serum, epidermal growth factor, and insulin in intact cells and persistence of the stimulation after cell homogenization. J Biol Chem. 1978 Feb 10;253(3):866–871. [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. The effect of phorbol diesters on chicken embryo fibroblasts. Cancer Res. 1977 Sep;37(9):3257–3265. [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Hunter E. Biological techniques for avian sarcoma viruses. Methods Enzymol. 1979;58:379–393. doi: 10.1016/s0076-6879(79)58153-1. [DOI] [PubMed] [Google Scholar]
- Hunter T. The proteins of oncogenes. Sci Am. 1984 Aug;251(2):70–79. doi: 10.1038/scientificamerican0884-70. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Moelling K., Heimann B., Beimling P., Rapp U. R., Sander T. Serine- and threonine-specific protein kinase activities of purified gag-mil and gag-raf proteins. Nature. 1984 Dec 6;312(5994):558–561. doi: 10.1038/312558a0. [DOI] [PubMed] [Google Scholar]
- Mojena M., Bosca L., Hue L. Effect of glutamine on fructose 2,6-bisphosphate and on glucose metabolism in HeLa cells and in chick-embryo fibroblasts. Biochem J. 1985 Dec 1;232(2):521–527. doi: 10.1042/bj2320521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Brien T. G., Saladik D., Diamond L. The tumor promoter 12-O-tetradecanoylphorbol-13-acetate stimulates lactate production in BALB/c 3T3 preadipose cells. Biochem Biophys Res Commun. 1979 May 14;88(1):103–110. doi: 10.1016/0006-291x(79)91702-9. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Franchi A., Silvestre P. Relationship between increased aerobic glycolysis and DNA synthesis initiation studied using glycolytic mutant fibroblasts. Nature. 1980 Oct 2;287(5781):445–447. doi: 10.1038/287445a0. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Beug H., Claviez M., Winkhardt H. J., Friis R. R., Graf T. Transformation parameters in chicken fibroblasts transformed by AEV and MC29 avian leukemia viruses. Cell. 1978 Apr;13(4):751–760. doi: 10.1016/0092-8674(78)90225-8. [DOI] [PubMed] [Google Scholar]
- Singh V. N., Singh M., August J. T., Horecker B. L. Alterations in glucose metabolism in chick-embryo cells transformed by Rous sarcoma virus: intracellular levels of glycolytic intermediates. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4129–4132. doi: 10.1073/pnas.71.10.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Whitman M., Cantley L. C., Erikson R. L. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2117–2121. doi: 10.1073/pnas.81.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H. G. Fructose 2,6-bisphosphate, the probably structure of the glucose- and glucagon-sensitive stimulator of phosphofructokinase. Biochem J. 1980 Dec 15;192(3):897–901. doi: 10.1042/bj1920897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. J., Nakamura K. D., Salter D. W. Molecular events leading to enhanced glucose transport in Rous sarcoma virus-transformed cells. Fed Proc. 1984 May 15;43(8):2246–2250. [PubMed] [Google Scholar]
- Weinhouse S. The Warburg hypothesis fifty years later. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1976;87(2):115–126. doi: 10.1007/BF00284370. [DOI] [PubMed] [Google Scholar]


