Abstract
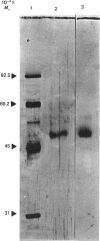
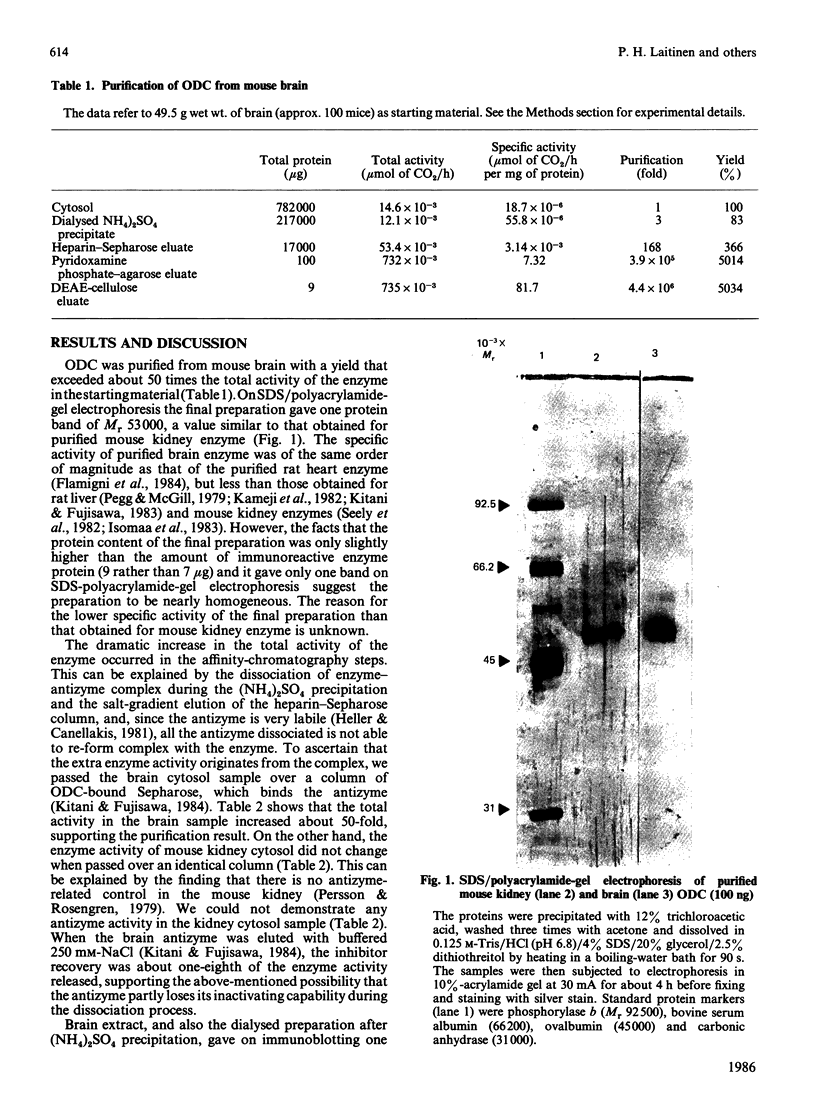
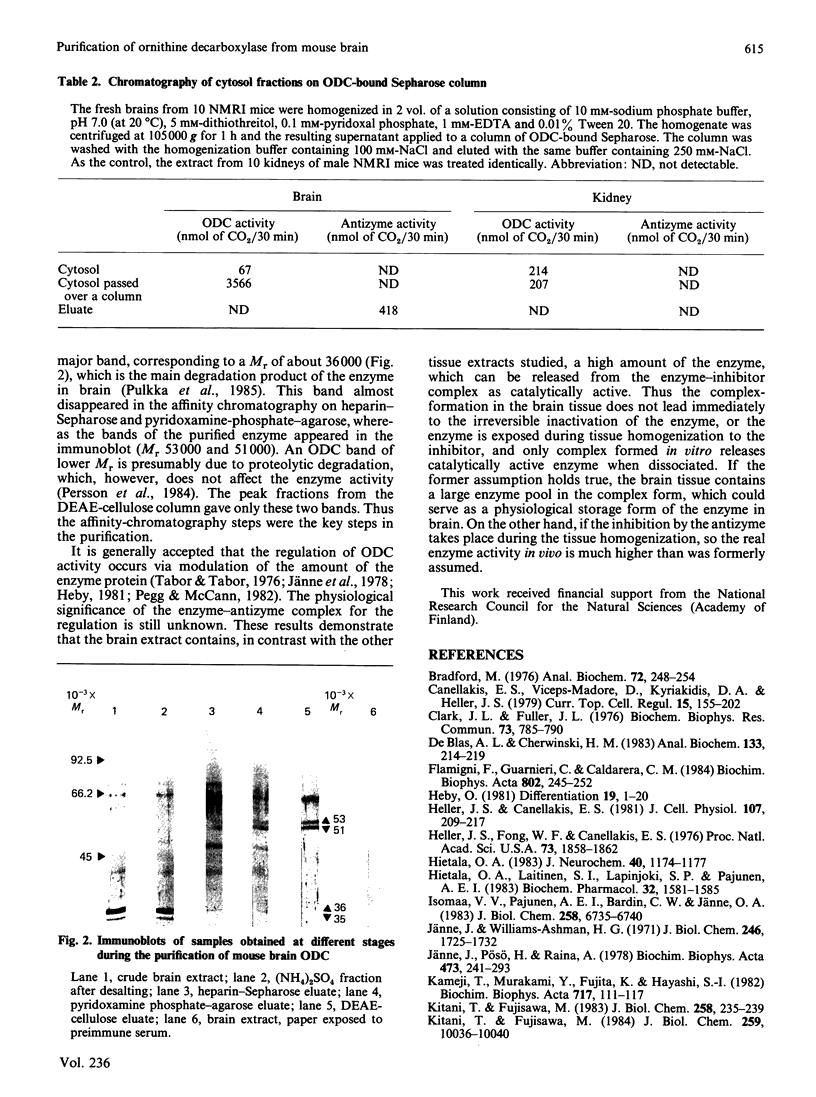
Mouse brain ornithine decarboxylase (ODC) was purified to near-homogeneity by using (NH4)2SO4 precipitation and chromatography on heparin-Sepharose, pyridoxamine phosphate-agarose and DEAE-cellulose. On SDS/polyacrylamide-gel electrophoresis, the final preparation gave one protein band similar to that obtained for purified mouse kidney enzyme, corresponding to an Mr of 53.000. The overall yield of the purification exceeded about 50-fold the total activity of the enzyme in the starting material. By affinity chromatography on ODC-bound Sepharose, the extra enzyme activity was shown to originate, at least partly, from the enzyme-antizyme complex. These results demonstrate that ODC in mouse brain occurs mainly in an inactive form and is activated during purification.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Canellakis E. S., Viceps-Madore D., Kyriakidis D. A., Heller J. S. The regulation and function of ornithine decarboxylase and of the polyamines. Curr Top Cell Regul. 1979;15:155–202. [PubMed] [Google Scholar]
- Clark J. L., Fuller J. L. Protein inhibitor of ornithine decarboxylase does not account for effect of putrescine on 3T3 cells. Biochem Biophys Res Commun. 1976 Dec 6;73(3):785–790. doi: 10.1016/0006-291x(76)90878-0. [DOI] [PubMed] [Google Scholar]
- De Blas A. L., Cherwinski H. M. Detection of antigens on nitrocellulose paper immunoblots with monoclonal antibodies. Anal Biochem. 1983 Aug;133(1):214–219. doi: 10.1016/0003-2697(83)90245-2. [DOI] [PubMed] [Google Scholar]
- Flamigni F., Guarnieri C., Caldarera C. M. Characterization of highly purified ornithine decarboxylase from rat heart. Biochim Biophys Acta. 1984 Nov 28;802(2):245–252. doi: 10.1016/0304-4165(84)90168-5. [DOI] [PubMed] [Google Scholar]
- Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19(1):1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Canellakis E. S. Cellular control of ornithine decarboxylase activity by its antizyme. J Cell Physiol. 1981 May;107(2):209–217. doi: 10.1002/jcp.1041070206. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietala O. A. Detection of ornithine decarboxylase antizyme in mouse brain. J Neurochem. 1983 Apr;40(4):1174–1177. doi: 10.1111/j.1471-4159.1983.tb08110.x. [DOI] [PubMed] [Google Scholar]
- Hietala O. A., Laitinen S. I., Laitinen P. H., Lapinjoki S. P., Pajunen A. E. The inverse changes of mouse brain ornithine and S-adenosylmethionine decarboxylase activities by chlorpromazine and imipramine. Dependence of ornithine decarboxylase induction on beta-adrenoceptors. Biochem Pharmacol. 1983 May 15;32(10):1581–1585. doi: 10.1016/0006-2952(83)90331-3. [DOI] [PubMed] [Google Scholar]
- Isomaa V. V., Pajunen A. E., Bardin C. W., Jänne O. A. Ornithine decarboxylase in mouse kidney. Purification, characterization, and radioimmunological determination of the enzyme protein. J Biol Chem. 1983 Jun 10;258(11):6735–6740. [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- Kameji T., Murakami Y., Fujita K., Hayashi S. Purification and some properties of ornithine decarboxylase from rat liver. Biochim Biophys Acta. 1982 Jul 16;717(1):111–117. doi: 10.1016/0304-4165(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Kitani T., Fujisawa H. Purification and properties of ornithine decarboxylase from rat liver. J Biol Chem. 1983 Jan 10;258(1):235–239. [PubMed] [Google Scholar]
- Kitani T., Fujisawa H. Purification and some properties of a protein inhibitor (antizyme) of ornithine decarboxylase from rat liver. J Biol Chem. 1984 Aug 25;259(16):10036–10040. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laitinen P. H., Huhtinen R. L., Hietala O. A., Pajunen A. E. Ornithine decarboxylase activity in brain regulated by a specific macromolecule, the antizyme. J Neurochem. 1985 Jun;44(6):1885–1891. doi: 10.1111/j.1471-4159.1985.tb07184.x. [DOI] [PubMed] [Google Scholar]
- Laitinen S. I., Laitinen P. H., Hietala O. A., Pajunen A. E., Piha R. S. Developmental changes in mouse brain polyamine metabolism. Neurochem Res. 1982 Dec;7(12):1477–1485. doi: 10.1007/BF00965090. [DOI] [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Duchesne M. C., Mamont P. S. Effect of alpha-methyl ornithine on ornithine decarboxylase activity of rat hepatoma cells in culture. Biochem Biophys Res Commun. 1977 Jun 6;76(3):893–899. doi: 10.1016/0006-291x(77)91585-6. [DOI] [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Hornsperger J. M., Böhlen P. Two distinct mechanisms for ornithine decarboxylase regulation by polyamines in rat hepatoma cells. J Cell Physiol. 1979 May;99(2):183–190. doi: 10.1002/jcp.1040990204. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McGill S. Decarboxylation of ornithine and lysine in rat tissues. Biochim Biophys Acta. 1979 Jun 6;568(2):416–427. doi: 10.1016/0005-2744(79)90310-3. [DOI] [PubMed] [Google Scholar]
- Persson L., Seely J. E., Pegg A. E. Investigation of structure and rate of synthesis of ornithine decarboxylase protein in mouse kidney. Biochemistry. 1984 Jul 31;23(16):3777–3783. doi: 10.1021/bi00311a033. [DOI] [PubMed] [Google Scholar]
- Pulkka A., Taskinen T., Aaltonen H., Ramberg J., Pajunen A. E. Studies on the degradation of ornithine decarboxylase by the immunoblotting technique. Biochem Int. 1985 Dec;11(6):845–851. [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Effect of 1,3-diaminopropane on ornithine decarboxylase enzyme protein in thioacetamide-treated rat liver. Biochem J. 1983 Dec 15;216(3):701–707. doi: 10.1042/bj2160701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Purification of ornithine decarboxylase from kidneys of androgen-treated mice. Biochemistry. 1982 Jul 6;21(14):3394–3399. doi: 10.1021/bi00257a023. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]