Abstract
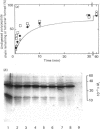
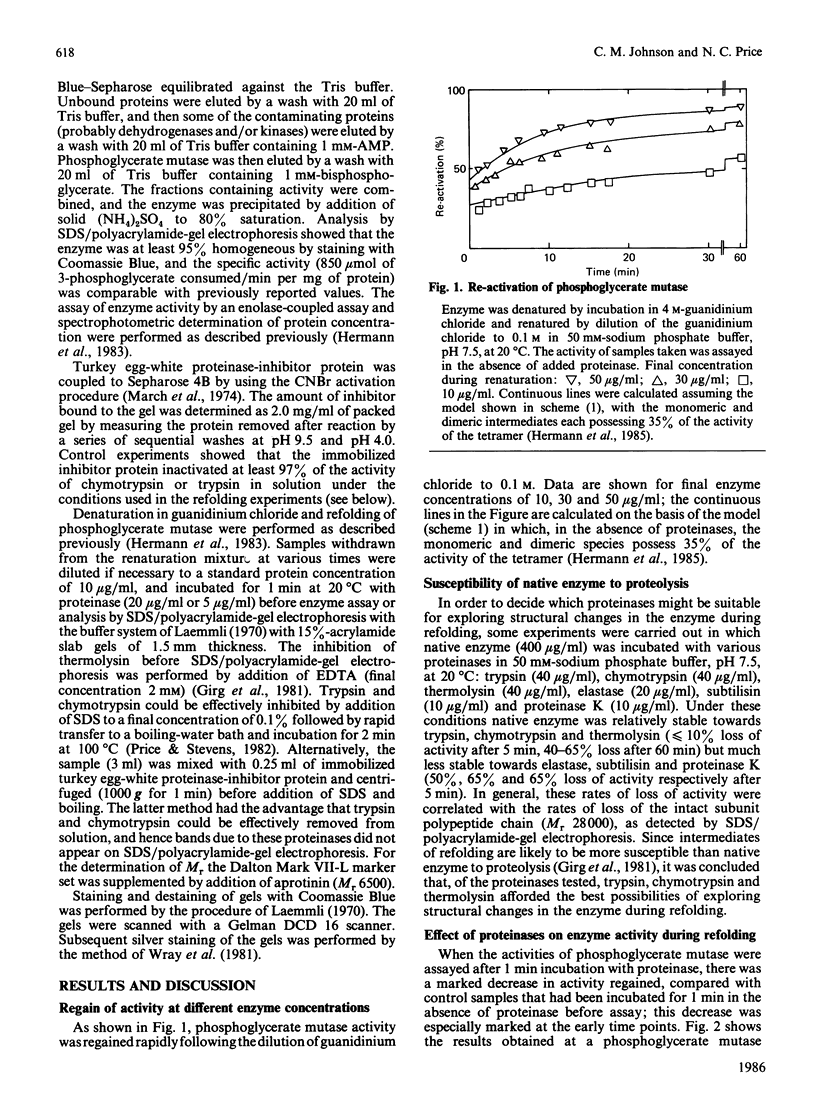
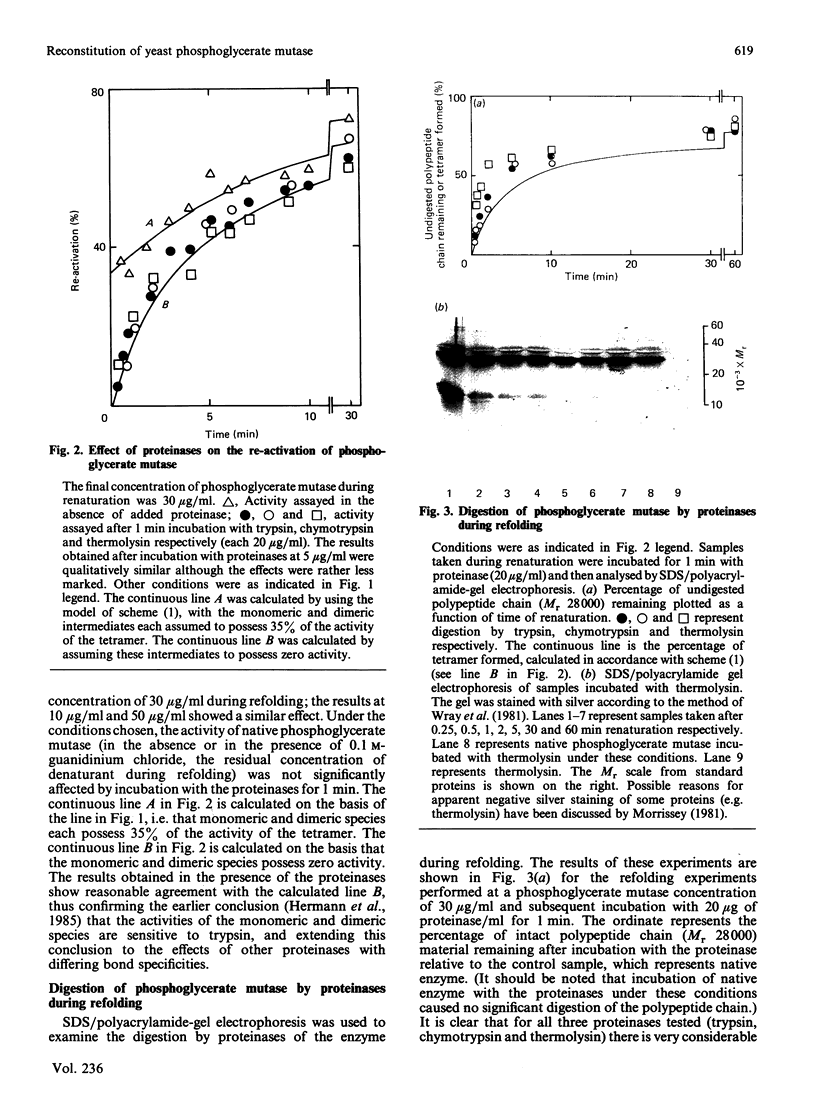
The renaturation of the tetrameric enzyme phosphoglycerate mutase from baker's yeast after denaturation in guanidinium chloride was studied. Three proteinases (trypsin, chymotrypsin and thermolysin) cause extensive loss of activity of samples taken during the early stages of refolding. As judged by SDS/polyacrylamide-gel electrophoresis, the proteinases cause substantial degradation of the polypeptide chain with no evidence for large quantities of fragments of Mr greater than 6500. These data suggest that the early intermediates in the refolding, especially the folded monomer, possess a number of sites that are susceptible to proteolysis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan W. W., Mort J. S., Chong D. K., Macdonald P. D. Studies on protein subunits. 3. Kinetic evidence for the presence of active subunits during the renaturation of muscle aldolase. J Biol Chem. 1973 Apr 25;248(8):2778–2784. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- De la Morena E., Santos I., Grisolia S. Homogenous crystalline phosphoglycerate phosphomutase of high activity. A simple method for lysis of yeast. Biochim Biophys Acta. 1968 Feb 5;151(2):526–528. doi: 10.1016/0005-2744(68)90121-6. [DOI] [PubMed] [Google Scholar]
- Fothergill L. A., Harkins R. N. The amino acid sequence of yeast phosphoglycerate mutase. Proc R Soc Lond B Biol Sci. 1982 Apr 22;215(1198):19–44. doi: 10.1098/rspb.1982.0026. [DOI] [PubMed] [Google Scholar]
- Girg R., Rudolph R., Jaenicke R. Limited proteolysis of porcine-muscle lactic dehydrogenase by thermolysin during reconstitution yields dimers. Eur J Biochem. 1981 Oct;119(2):301–305. doi: 10.1111/j.1432-1033.1981.tb05608.x. [DOI] [PubMed] [Google Scholar]
- Grossman S. H., Pyle J., Steiner R. J. Kinetic evidence for active monomers during the reassembly of denatured creatine kinase. Biochemistry. 1981 Oct 13;20(21):6122–6128. doi: 10.1021/bi00524a032. [DOI] [PubMed] [Google Scholar]
- Hermann R., Jaenicke R., Price N. C. Evidence for active intermediates during the reconstitution of yeast phosphoglycerate mutase. Biochemistry. 1985 Apr 9;24(8):1817–1821. doi: 10.1021/bi00329a002. [DOI] [PubMed] [Google Scholar]
- Hermann R., Jaenicke R., Rudolph R. Analysis of the reconstitution of oligomeric enzymes by cross-linking with glutaraldehyde: kinetics of reassociation of lactic dehydrogenase. Biochemistry. 1981 Sep 1;20(18):5195–5201. doi: 10.1021/bi00521a015. [DOI] [PubMed] [Google Scholar]
- Hermann R., Rudolph R., Jaenicke R., Price N. C., Scobbie A. The reconstitution of denatured phosphoglycerate mutase. J Biol Chem. 1983 Sep 25;258(18):11014–11019. [PubMed] [Google Scholar]
- Jaenicke R. Folding and association of proteins. Biophys Struct Mech. 1982;8(4):231–256. doi: 10.1007/BF00537204. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Price N. C., Jaenicke R. The quaternary structure of phosphoglycerate mutase from yeast: evidence against dissociation of the tetrameric enzyme at low concentrations. FEBS Lett. 1982 Jul 5;143(2):283–286. doi: 10.1016/0014-5793(82)80117-8. [DOI] [PubMed] [Google Scholar]
- Price N. C., Stevens E. Distinction between cofactor-dependent and -independent phosphoglycerate mutases by chromatography on Cibacron Blue-Sepharose. Biosci Rep. 1983 Sep;3(9):857–861. doi: 10.1007/BF01133784. [DOI] [PubMed] [Google Scholar]
- Price N. C., Stevens E. The refolding of denatured rabbit muscle creatine kinase. Search for intermediates in the refolding process and effect of modification at the reactive thiol group on refolding. Biochem J. 1982 Jan 1;201(1):171–177. doi: 10.1042/bj2010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price N. C., Stevens E. The refolding of denatured rabbit muscle pyruvate kinase. Biochem J. 1983 Mar 1;209(3):763–770. doi: 10.1042/bj2090763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph R., Westhof E., Jaenicke R. Kinetic analysis of the reactivation of rabbit muscle aldolase after denaturation with guanidine-HCL. FEBS Lett. 1977 Feb 1;73(2):204–206. doi: 10.1016/0014-5793(77)80981-2. [DOI] [PubMed] [Google Scholar]
- Schmid F., Blaschek H. An early intermediate in the folding of ribonuclease A is protected against cleavage by pepsin. Biochemistry. 1984 May 8;23(10):2128–2133. doi: 10.1021/bi00305a004. [DOI] [PubMed] [Google Scholar]
- Winn S. I., Watson H. C., Harkins R. N., Fothergill L. A. Structure and activity of phosphoglycerate mutase. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):121–130. doi: 10.1098/rstb.1981.0066. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]