Abstract
Biomaterials are defined as “engineered materials” and include a range of natural and synthetic products, designed for their introduction into and interaction with living tissues. Biomaterials are considered prominent tools in regenerative medicine that support the restoration of tissue defects and retain physiologic functionality. Although commonly used in the medical field, these constructs are inherently foreign toward the host and induce an immune response at the material–tissue interface, defined as the foreign body response (FBR). A strong connection between the foreign body response and tissue regeneration is suggested, in which an appropriate amount of immune response and macrophage polarization is necessary to trigger autologous tissue formation. Recent developments in this field have led to the characterization of immunomodulatory traits that optimizes bioactivity, the integration of biomaterials and determines the fate of tissue regeneration. This review addresses a variety of aspects that are involved in steering the inflammatory response, including immune cell interactions, physical characteristics, biochemical cues, and metabolomics. Harnessing the advancing knowledge of the FBR allows for the optimization of biomaterial‐based implants, aiming to prevent damage of the implant, improve natural regeneration, and provide the tools for an efficient and successful in vivo implantation.
Keywords: biofabrication, biomaterials, foreign body response, immune response
Biomaterials are prominent tools in regenerative medicine, but can be held back by strong inflammatory responses at the material–tissue interface. Here, the authors address multiple aspects involved in steering the foreign body response post‐implantation. Harnessing the advancing knowledge of the cell–biomaterial interface progresses current implants toward a bioinstructive generation, where spatiotemporal interactions serve as guiding cues toward successful implant resolution.
1. Introduction
The concept of regenerative medicine encompasses the broader scope of tissue regeneration and utilizes a multitude of strategies including modulation of biochemical signals, stimulation of cellular processes and the introduction of 3D scaffolds to promote the hosts’ natural regeneration; hereby structurally or functionally stimulating the restoration of tissues.[ 1 , 2 ] The fundamental principle of tissue engineering, conversely, is to rebuild functional tissues and organs by combining scaffolds and factors such as cells, biochemical and physicochemical signals.[ 3 ] Tissue engineering is a regenerative medicine strategy that focuses on the engineering and manufacturing aspects of tissue replacement, utilizing foreign biological materials to exploit host cells for tissue and organ regeneration with promising results in several applications.[ 4 , 5 ]
These materials have been termed “biomaterials,” and are developed using principles from material science, biology, and medicine.[ 6 ] In general, biomaterials are described as “typically engineered materials, which have been adapted to the conditions prevailing in the human body.”[ 7 ] This includes any form of natural or synthetic materials that are intended to be introduced into living tissues, hereby stimulating the process of regeneration, support or replace the function of tissues, or aid in the controlled delivery of medication.[ 8 ] From a clinical perspective, a biomaterial includes any substance, which is utilized in a therapeutic or diagnostic treatment and interacts with biological material or fluids, with the exception of food or drugs.[ 9 ]
Although the use of foreign materials as implants has existed for centuries (e.g., prostheses), the use of materials related to the concepts of regenerative medicine has a more recent origin. Some of the earliest applications of biomaterial‐based approaches appeared in the mid‐twentieth century with the introduction of angiogenesis inhibitors, where polymer pellets mixed in organic solvents were used to deliver tumor angiogenesis factors and became among the first controlled delivery macrovesicles.[ 10 ] Since then, the introduction of innovative biomaterial‐based techniques has greatly expanded, including the use of organic host materials (e.g., decellularized extracellular matrices) as scaffolds, controllable delivery systems on micro‐ and nanoscale, and the fabrication of complex architectures in a 3D environment.[ 11 , 12 , 13 ] The introduction of these foreign materials, which were officially referred to as biomaterials, interacted with the host biological system as templates to support cellular growth, or are utilized as vehicles for controlled delivery of therapeutics.[ 14 ] The overall aim within this field is to achieve a stable equilibrium between a biomaterial‐based construct and the host.[ 6 ]
As biomaterials evolved and increased in complexity, a specific focus was laid on the interactions between the properties of a material and the host response. Originally, biomaterials were designed to exert specific physical functions that supported the clinical state of the patient whilst minimizing any deleterious responses from the surrounding cells, effectively aiming toward a bioinert state.[ 15 ] As scientific advances were made over decades, biomaterials evolved to create an increased interactive network, being able to actively interact with the environment and the surrounding cells to optimize healing and minimize adverse effects.[ 16 ] Modulation of these characteristics introduced the first classes of “self‐healing biomaterials” that aimed toward halting damage, stimulated healing, and induced full recovery of tissue function.[ 17 ]
As new applications moved from the laboratory to a practical setting, biomaterials became clinically applied within a wide range of ailments. The clinical application of current biomaterials is often defined as the third generation. The first generation entails the inventions during the 1960–1970s, focusing on a physical/mechanical balance with aqueous and corrosive resistance and minimal toxicity to the host. The second generation includes the addition of bioactivity within a construct. Examples include mixtures of materials that undergo phase changes, display thermoregulation or are resorbable. The third generation is defined by a materials’ ability to invoke a desired cellular response, essentially serving a bioinstructive role to guide tissue regeneration. Porous nanoscale scaffolds and localized delivery of therapeutics attempts to augment the environment and activate genes that stimulate natural tissue regeneration.[ 8 , 18 ]
This review aims at giving a comprehensive overview of the current state of biomaterials and their effects on the immune response upon integration. Whether a biomaterial‐based implant is successfully accepted by the host, is determined by a multitude of factors, originating from both the intrinsic properties of the material as well as the proximate environment. An in‐depth overview is given on various topics involved in this process, starting with the mechanisms upon implantation of a biomaterial. Next, various major cell types are discussed that play a role in the progression of the inflammatory response. The interplay between a biomaterial and the physiological environment is then discussed through both physical and biochemical interactions. Finally, the role of metabolomics is introduced as a potentially novel way of optimizing the biocompatibility of biomaterials. Taken together, this review attempts to elucidate some of the complex, multidisciplinary processes that take place upon the implantation of a biomaterial, and offers suggestions to regulate the inflammatory response and optimize the biocompatibility of these materials.
2. Biomaterial Integration, the Foreign Body Response, and Wound Healing
Regardless of whether biomaterials are used as scaffolds or drug delivery devices, the injection, insertion or surgical implantation of these materials in an organism triggers the host defense system to respond with some form of tissue–material interactions, resulting in a foreign body response (FBR) in an attempt to clear any potential threats from the host.[ 19 ] The reaction scheme of the host tissue after implantation of a foreign material includes the initial injury, followed by blood–material interactions, the formation of a matrix around the implant, acute inflammation, prolonged or chronic inflammation, development of granulation tissue, foreign body reaction, and finally the formation of fibrosis or a tissue capsule.[ 20 ] These reactions are initiated through the original implantation and further stimulated through the continuous presence of the biomaterial, medical, or tissue‐engineered device. Whilst the initiation of an inflammatory response is considered beneficial due to the protection it provides against infection and pathogens, failure to reside back to a homeostatic balance can cause dysregulation, resulting in toxic effects and severe damage to the tissue and host.[ 21 ] Improper integration of the implant will result in fibrosis, formation of a fibrous cap infiltrating the implant, or result in cellular immunity against the biological components in the implanted device (Figure 1 ).[ 22 , 23 , 24 ]
Figure 1.
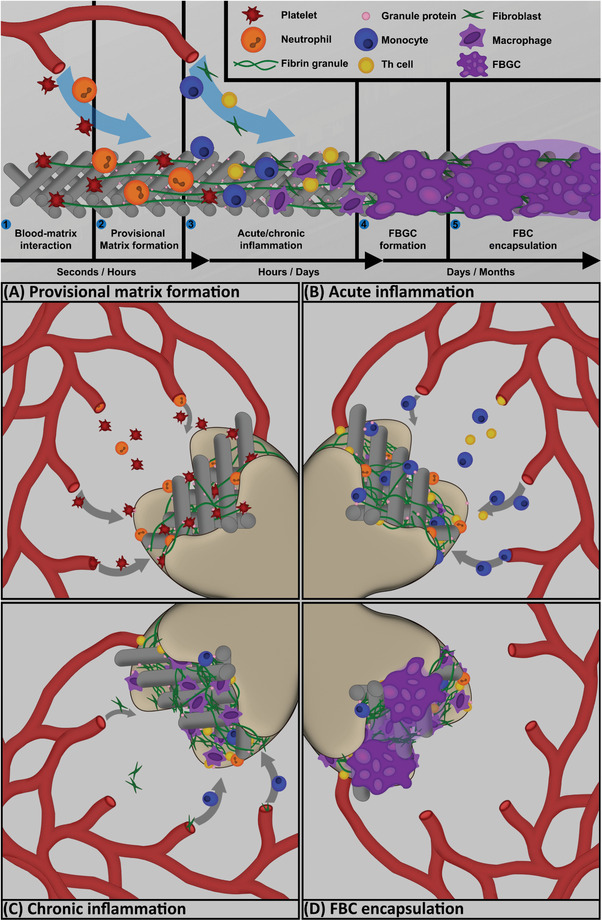
A progression chart of the foreign body response induced by a biomaterial, displaying the major cell types and effects over time. 1) Upon implantation, blood–matrix interactions recruit platelets in order to initiate site sterilization and wound closure. 2) Neutrophils and platelets excrete products to form a provisional matrix, which aids in further recruitment of inflammatory cells (A). 3) A collection of inflammatory cells, including monocytes, Th cells, and fibroblasts migrate to the wound site and initiate the inflammatory response to phagocytose pathogens and remodel the microenvironment (B). Over time, monocytes differentiate to macrophages and take on either a proinflammatory (M1) or anti‐inflammatory (M2) phenotype. Chronic inflammation of the wound site is often caused by the foreign nature of the biomaterial resulting in further macrophage polarization and recruitment of fibroblasts (C). 4) Persisting inflammation leads to the fusion of macrophages, forming a foreign body giant cell. FBGCs attempt to engulf the biomaterial in order to isolate the implant from the environment through frustrated phagocytosis (D). The combination of FBGCs and continuous collagen output by fibroblasts forms a fibrous foreign body capsule around the implant, indicating a chronically inflamed state.
After the initial injury post‐implantation, the disruption of the local homeostatic mechanisms sets off the cascade to induce regeneration. The severity of the initial response to surgical injury depends on a variety of factors, including the extent of injury, affected tissues and organs, loss of structural integrity through basement membrane disruption and cellular necrosis. These factors further influence both the extent and severity of further events.[ 23 , 24 , 25 ] Furthermore, the effects of the human immune system may vary between individuals due to genetic variations, symbiotic interactions with bacteria or other external effectors. Whilst within the individual this remains relatively stable over time, factors such as age, sex, circadian rhythm, host–commensal interactions, seasonal changes, and fitness have shown to substantially affect interindividual variation of the immune response.[ 26 ] In particular, aging appears to display high variability to the FBR on implants, specifically affecting the innate immune system, macrophage behavior, and local tissue microenvironment, because of a dysregulated progression and chronic inflammation.[ 27 , 28 ] Since interindividual variation of the FBR is still a relatively understudied topic, future research could benefit by including patient specific tissue and disease basis into account when optimizing a material for biocompatibility.
The first interactions between the implant and the host material includes blood–matrix interactions and the formation of a provisional matrix (Figure 1A). The blood–matrix interaction occurs after injury to vascularized connective tissue and is the initial activator of the FBR. The formation of thrombus and blood clots initiates various responses to sterilize the implanted material and curtail bleeding, which is initiated by the absorption of proteins such as albumin, fibrinogen, and fibronectin. This includes intrinsic and extrinsic coagulation, the complement system, the fibrinolytic system, the kinin‐generating system, and platelets. Vascular delivery of inflammatory cells referred to as monocytes and neutrophils coordinates acute inflammation through chemical mediators, sterilizing the wound site and initiating the formation of a provisional matrix. Pathogens that may have entered the wound site are recognized by the innate immune response through toll‐like receptors (TLRs) or NOD‐like receptors and are subsequently removed. Of note is the short deactivation state of these chemicals, suggesting that the effects are mainly restricted to the site of injury.[ 21 , 23 , 29 , 30 ]
Following blood–matrix interactions is the formation of a fibrin protein matrix, which aids blood clotting and provides a foundation for cellular infiltration (Figure 1B). The formation of a provisional matrix occurs within minutes to hours post‐implantation and consists of an accumulation of adhesive molecules bound to fibrin and platelet granule components. The matrix is stabilized through factor XIIIa fibrin crosslinking and serves as a complex 3D network of proteins that facilitates cell adhesion and migration. Circulating platelets adhere to the matrix to prevent further bleeding while neutrophils and monocytes are recruited to the site, releasing enzymes and reactive radicals that result in the phagocytosis of bacteria and debris. A variety of mitogens, growth factors, chemoattractants, and cytokines present in the provisional matrix are responsible for coordinating the next stages of the inflammatory response.[ 23 ]
The physicochemical properties of the implanted biomaterial largely determine the composition of the provisional matrix. Of special note is the rate of continuous protein adsorption on the material surface, referred to as the Vroman effect. This effect states that proteins with a high mobility (unbound to the material surface) are increasingly adsorbed and replaced, while proteins that have a higher molecular weight or affinity for the material last within the microenvironment and accumulate.[ 31 ] This effect is found most prominently on hydrophilic surfaces, where a more spacious binding allows for a faster replacement. The final composition of the provisional matrix depends on protein concentrations and interactions with the biomaterials’ surface characteristics.[ 32 , 33 ]
Following the formation of the provisional matrix, the immune response recruits inflammatory cells to the local injury site, with the intent to contain or remove the cause of injury whilst also providing regenerative patterns that aim to replace the lost tissue. It does so through recruiting either regenerating native parenchymal cells or the formation of fibrotic tissue (Figure 1C).[ 20 ] The recruitment is mediated by mast cells, which secrete granular products that attract and promote the adhesion of monocytes and macrophages to the implant.[ 34 ] The inflammatory process of the FBR can be subdivided in an acute and chronic phase.
Acute inflammation is initiated by the migration of leukocytes to the implantation site and lasts between minutes to days depending on the severity of the injury. Leukocytes are responsible for the recognition of the cause of injury and the phagocytosis of potential pathogens adhering to the implant surface. During this initial phase, neutrophils are the dominant cell type regulating the inflammatory response in the first days post‐implantation. Due to their relatively short life (24–48 h), and decreased migratory chemotactic factors, neutrophils are eventually replaced by monocytes, which can continue accumulating at the implant site for weeks, eventually differentiating into macrophages.[ 24 , 35 ]
Macrophages that are exposed to foreign biomaterials take part in various processes, including fibrous encapsulation, remodeling, and angiogenesis.[ 36 ] Macrophages provide further help in engulfing the foreign devices and chemotactically signal additional neutrophils and monocytes to participate in the inflammatory response. Whether macrophages induce proinflammatory or anti‐inflammatory behavior is highly dependent on their phenotypic profile.
The classical model distinguishes two major polarization phenotypes, which represent a continuum, providing a balance between the pro‐ and anti‐inflammatory responses by alternating between classically (M1) or alternatively (M2) activated macrophages.[ 37 , 38 ] The M1/M2 polarization determines the effect of macrophages to either stimulate further tissue damaging and inflammation, or constructive remodeling and new tissue regeneration. Classically during the initial stage of host defense, migration of M1 macrophages to the target site is necessary to stimulate inflammation, as they release proteolytic enzymes, initiate phagocytosis, and support the recruitment of other inflammatory cells. Persistent foreign material degradation can continuously activate M1 macrophages.[ 39 ]
A more recent model also hypothesizes the existence of a macrophage spectrum between M1 and M2, regulated through three major homeostatic processes involving the host defense, wound healing, and immune modulation.[ 40 ] The balance between M1 and M2 macrophage types is crucial in streamlining pathogen clearance and tissue renewal, where the quantity and shift from M1 to M2 macrophages over time is an important factor in the phagocytosis of bacteria, clearance of dead tissue, and environmental remodeling.[ 41 ] Collectively, these processes rely on a continuous shift of functional phenotype balance between the M1 and M2 polarization state.[ 42 ]
The switch to the regenerative stage of a wound site is usually initiated within 24 h post‐injury, modulated by the secreted products of monocytes and macrophages as well as fibroblast proliferation and angiogenesis. Over time, the immune response aims to enclose the body from the external environment or object through the recruitment of fibroblasts adjacent to the site of injury. Macrophages secrete signals to provoke migrated fibroblasts to proliferate, secrete proteins such as collagen, differentiate into myofibroblasts, or attract other cell types such as endothelial cells (ECs). The accumulation of these products causes the formation of granulation tissue, consisting of macrophages, fibroblasts, newly formed blood vessels, and extracellular matrix (ECM) that indicate the switch from an inflammatory to regenerative state. Biomaterial implants generally do not run the risk of being phagocytosed by either neutrophils or macrophages due to the size of the material being significantly larger than the cells. However, receptor‐mediated interaction of macrophages with the implant may still result in a response mechanism against a potential pathogen and induce a process known as “frustrated phagocytosis”, in which large amounts of toxic leukocyte products (oxygen‐free radicals and lysosomal proteases) are released extracellularly in an attempt to degrade larger particles. The extent of this process is dependent on the size and makeup of the implant. Acute inflammation can therefore result in a toxic environment that may damage or destroy the functionality of an implant.[ 22 , 43 , 44 ]
Over time, the acute inflammatory response may evolve to a chronic state, indicated by the continuous presence of monocytes and lymphocytes, as well as early development of vasculature and connective tissue. Chronic inflammation can be found around most synthetic materials postimplantation.[ 29 , 45 , 46 ] Various causative factors may influence this switch to a chronic state, be it persisting inflammatory signals, physical or chemical composition of the implant, or displacement of the material in the inflamed site. Chronic inflammation is usually limited to the implant site, with implants that express a higher level of biocompatibility generally having a shorter chronic response.
The last stage for both wound healing and the FBR is remodeling of the surrounding tissue and environment, which defines the level of resolution of the wound and formation of a fibrocellular capsule (FBC) around the implanted device. The presence of a biomaterial initiates the FBC formation, creating an environment consisting of various components of granulation tissue, as well as the formation of foreign body giant cells (FBGCs); macrophages that fuse together to form multinucleated cells in an attempt to encapsulate the material.[ 47 ] The degree of encapsulation and composition of the FBC is a major determinant of whether the appropriate response is achieved.[ 48 ] The exact composition of an FBC is defined by the size and topography of the implant and may persist throughout the entire lifetime of the implant. Although FBGCs play a role in the biodegradation of polymeric implants, it is not known whether this process continues throughout the entire lifespan of the FBGC or deactivates over time.[ 22 , 24 , 46 , 49 , 50 ]
The final stages of resolution involve two major processes: regeneration of the lost tissue through multiplication of parenchymal cells, or replacement of the lost tissue by connective tissue, hereby effectively creating scar tissue. Whether resolution results in full regeneration or fibrosis is dependent on the type of tissue, the proliferation rate (labile, stable or permanent), retention of the original environmental framework, and the extent of provisional matrix formation.[ 29 ] Albeit the general mechanisms in the immune response to biomaterials are relatively well known, a tremendous number of additional events involving various cell types, biochemical interactions and mechanical properties influence the process of regeneration.
3. Response of Inflammatory Cell Types to Biomaterials
The implantation of a biomaterial in a host always leads to some form of cellular response that determines the severity of the FBR, and hence the integration success of the implant. The modulation of the hosts’ response toward an implant is divided between the innate and the adaptive immune system, where the innate system acts as a first responder to any form of invasion whilst the adaptive system provides direct support to the innate immune system through two main forms of adaptive responses: antibody and cell‐mediated responses.[ 51 , 52 ] A plethora of cells interacts with a foreign material upon implantation, leading to a cascade of immune responses that progress the FBR. In the following sections, the most influential cell types are discussed that coordinate the inflammatory response, including the overall effect of the biomaterials’ properties on the activation of the cells, responses toward and secretion of biochemical cues, and eventual influence toward a pro‐ or anti‐inflammatory state.
3.1. Neutrophils, Monocytes, and Macrophages
The first type of inflammatory cells to arrive at the site of injury following surgical implantation are neutrophils, which mainly determine the effects of the inflammatory response and the severity of the body's defenses.[ 51 ] Neutrophils are recruited from the peripheral blood toward the wound site through a variety of chemoattractant markers released by the surrounding tissue, endothelial cells, and platelets. Once at the implant site, attachment to the material occurs via β2‐integrins, followed by removal of pathogens, formation of a provisional matrix and the initiation of material degradation through phagocytosis, proteolytic enzymes, and ROS formation.[ 23 , 53 ] A network of granule proteins and DNA (in particular chromatin) is also formed, referred to as the neutrophil extracellular trap (NET), which is actively made to immobilize bacteria, degrade virulence factors, and allow neutrophils to phagocytize pathogens.[ 54 ] NET control is of particular interest for inflammatory quantification due to its early formation in the FB and many immunomodulatory mechanisms; as well as recent advances in quantification methods through fluorescence analysis and ELISA.[ 55 ]
Additionally, the adhesion of neutrophils is the first modulatory factor by the implant, as neutrophil survival is influenced by surface topography and roughness. On polystyrene surfaces in both in vitro and in vivo conditions, neutrophils that were exposed to rougher surfaces showed considerably less viability compared to smooth surfaces whilst cells that managed to adhere to the rougher surface excreted more reactive oxygen intermediates, further invoking nonapoptotic cell death.[ 56 ] Aside from NETs, further neutrophil mediators such as myeloperoxidase (MPO) and neutrophil elastase (NE) all have a mediatory effect on cytokine deposition and macrophage polarization. A decrease in MPO levels directly relates to an increase in anti‐inflammatory cyto‐ and chemokines (IL‐1β, IL‐6, TNF‐α), whilst NE seems to be more prominent in the resolution phase of the FBR.[ 57 ] In particular, NET persistence within the FBR is critical as this significantly contributes to chronic inflammation and collateral tissue damage. For biomaterials, NET resolution can be controlled by targeting the neutrophil sensing of the biomaterial, where the initial contact with the microenvironment as well as surgical stress and localized hypoxia are responsible for the degree of NET formation.[ 58 ]
The increase of chemokines within the implant microenvironment eventually triggers the migration of monocytes from the bone marrow, blood, and spleen, causing neutrophils to disappear.[ 20 , 59 ] Monocytes exert functions crucial in the progression of the immune response: the modulation of signals between the innate and adaptive immune system, removal of pathogens, promoting regeneration of the surrounding tissue and the eventual differentiation to macrophages and dendritic cells (DCs).[ 60 ] Monocytes are subdivided depending on their characterized expression markers and classified as either classical inflammatory monocytes (IMs; CD14+CD16−), intermediate (CD14++CD16+), and anti‐inflammatory monocytes (AMs; CD14+CD16++).[ 59 , 60 ] In humans, this subdivision can also be modulated through material properties. Since monocytes eventually differentiate into macrophages, early polarization of monocytes toward their anti‐inflammatory state allows for modifications that promote a regenerative environment, including matrix remodeling, vascularization, and prevention of fibrosis.
Manipulation of monocytes toward an anti‐inflammatory state has been performed through specified dexamethasone delivery with microparticles by means of phagocytosis.[ 61 ] Wofford et al. used this exact methodology by loading poly(lactic‐co‐glycolic) acid microparticles (0.98–2.05 µm) with dexamethasone and administering them to primary human monocytes. Interestingly, after differentiation the resulting macrophages displayed a decrease of inflammatory factors for up to 7 days, and continued displaying an anti‐inflammatory polarization state even when exposed to the proinflammatory lipopolysaccharide (LPS) and interferon gamma (IFNγ).[ 62 ] The surface potential of the implant surface also seems to play an important role in the control of adhesion‐related genes of monocytes such as PI3K, Akt, and the mTOR pathways. A lower surface potential through, e.g., polydopamine coating creates a slight repulsive force, which favors the upregulation of integrin β1 and β3, in turn resulting in an anti‐inflammatory phenotype.[ 63 ] An early anti‐inflammatory state postimplantation can therefore potentially already be stimulated through the modification of surface potential and the addition of controlled drug release from the biomaterial.
Within the implant site, monocytes eventually maturate and differentiate into macrophages. Macrophages belong to the group of active innate immune cells and provide some of the most important responses following the interaction with a biomaterial. These responses include a number of proteins, interleukins, chemokines, and immunomodulatory factors that steer the environment toward either a pro‐ or anti‐inflammatory state.[ 64 ] In addition, macrophages have a critical role in early vascularization of the tissue surrounding the biomaterial, and determine cascades of the FBR events by differentiating between two major phenotypes.[ 47 ] The phenotypical division of macrophages depends on the function of the cell: classically activated macrophages appear in initial inflammation and are involved in microbicidal activity; regenerative macrophages are involved in tissue repair whilst regulatory macrophages modulate anti‐inflammatory activity.[ 41 , 65 , 66 ]
The classical activation of proinflammatory M1 macrophages is induced by inflammatory signals, such as secreted Th1 cell cytokines, and triggers secretion of inflammatory cytokines, free radicals, and phagocytosis. The collection of secreted cytokines creates an anti‐proliferative environment, destroys local matrix, and induces tissue reorganization, which allows for an increased migratory process and improved pathogen clearance.[ 36 ] The alternative activation that triggers M2 polarization can occur through varying stimuli and may trigger the emergence of three different M2 profiles. As a collective, the spectrum of M2 macrophages secretes a number of anti‐inflammatory cytokines, promotes remodeling of the environment, aids in angiogenesis, and inhibits fibrous tissue formation.[ 67 ]
M1 macrophages are typically identified by the expression of nitric oxide synthase (iNOS), chemokines, macrophage chemotactic protein 1 (MCP‐1), macrophage inflammatory proteins alpha (MIP‐α), and other proinflammatory cytokines such as TNF‐α, IL‐1β, IL‐6, and IL‐12. Macrophages that polarized toward to the M2 phenotype are identified by high expression levels of IL‐10, CD163, and amplification of the Arginase 1 (Arg1) enzyme, hereby switching the focus to releasing signals for constructive tissue remodeling.[ 68 ] The M1/M2 polarization paradigm consists of an overlap as both phenotypes have been shown to stimulate fibroblast migration and differentiation, as well as collagen production (Figure 1D).[ 69 , 70 , 71 ]
Recently, M2 macrophages have also been further subcategorized in M2a, M2b, and M2c, depending on their different roles within tissue regeneration. M2a is considered the original M2 phenotype and is triggered by the presence of IL‐4 or IL‐13 secreted by Th2 responses to inflammation. The M2b phenotype is polarized by various immune complexes, TLRs, Fc receptors, and IL‐1. Its presence is identified by elevated levels of IL‐10 but reduced IL‐12. M2c phenotype is stimulated by IL‐10 or glucocorticoids, secreting high levels of IL‐10, transforming growth factor beta‐1 (TGFβ‐1), vascular endothelial growth factor (VEGF), and other growth factors leading to immune suppression and FBC remodeling.[ 72 ] Furthermore, a novel macrophage phenotype was found in response to abundance of phospholipids. The Mox macrophage phenotype is activated through oxidized lipids and regulated through Nrf2. It appears as a response to oxidative damage and displays a differing phenotype compared to either M1 or M2 macrophages, displaying decreased phagocytic and chemotactic capacities.[ 73 ]
The factors that determine the polarization state of macrophages can roughly be divided in two categories: inherent cues that are directly provided by the biomaterial, and consequent cues that are the effects following the implantation within the host. Direct cues that modulate macrophage phenotypes are substrate stiffness, topography, 3D cues, dynamic loading, and environmental triggers such as the presentation of cytokines or disruptive states such as hypoxia.[ 67 ] The biochemical composition of the microenvironment is especially important for the determination of macrophage phenotypes. Common examples of cytokines that polarize macrophages are IFN‐γ and LPS, which skew macrophages toward the M1 phenotype, whereas cytokines such as IL‐4 and IL‐13 promote a regenerative M2 phenotype (Figure 2 ).[ 40 ]
Figure 2.
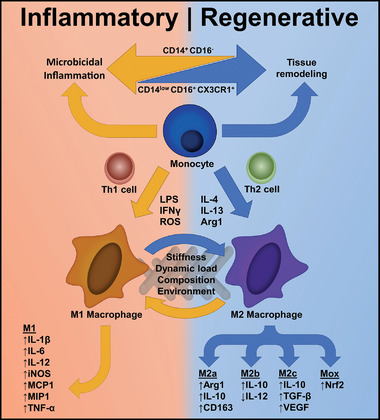
The macrophage phenotype spectrum is directly related to the stimulation of either inflammation (orange) or regeneration (blue) within the implant site. A combination of biochemical compounds and adaptive cell types affect monocyte behavior and the subsequent differentiation to macrophages. The differentiation to varying polarization states, either proinflammatory (M1) or anti‐inflammatory (M2), is defined by a unique mix of cytokines and chemokines present in the microenvironment. Additionally, the physical traits of the implant are able to steer polarized macrophages toward expressing inflammatory or regenerative effects.
Macrophages are also able to respond to the presence of surface proteins on biomaterials. An example was provided by Kim et al. who used the immunoregulatory protein CD200 to induce immunosuppression. CD200 can bind to the CD200 receptor (CD200R) on the surface membrane of macrophages. Exposure to the CD200 coated surfaces resulted in a decrease in tumor necrosis factor‐α (TNF‐α) and IL‐6 concentrations, promoting the switch to an M2 phenotype.[ 74 ] This suggests that surface interaction of macrophages with biomaterials is a contributing factor to the overall phenotype and inflammatory state. Regulating polarization of M1/M2 phenotypes and their ratio is made possible by tuning physicochemical properties, including surface chemistry and topography, degradation profile and stiffness, or using it as a delivery system to release inflammatory mediators for macrophage polarization, as depicted in Figure 2.[ 75 ]
3.2. T‐Helper Lymphocytes
T‐helper lymphocytes (Th cells) are the main driver of most responses of the adaptive immune response and therefore greatly contribute to the overall regulation of the inflammatory response. Th cells support the activation of B cells and cytotoxic T cells, further promoting antibody secretion, macrophage accumulation, and the removal of infected cells.[ 52 ] However, a Th cell in its natural state is passive, requiring triggers from the innate immune response through macrophages or DCs to develop into an effector cell (CD4+).[ 51 ] The composition of these triggers furthermore determines the phenotype of the Th cell. Th1 and Th17 cells are generally considered proinflammatory and secrete various factors including IFN‐γ, TNF‐α, T‐bet, RORγt, and IL‐17; resulting in enhanced phagocytic activity whilst simultaneously recruiting monocytes and neutrophils from the local environment, the bone marrow and circulatory system.[ 76 , 77 ]
Th2 and T‐regulatory (Treg) cells promote an anti‐inflammatory profile and are responsible for the immune‐mediated regeneration of the surrounding tissue.[ 78 ] Th2 cells secrete a number of interleukins (IL‐4, IL‐5, IL‐10, IL‐13) and promote the secretion of antibodies by B cells that collectively stimulate a macrophage switch to an M2 phenotype.[ 52 ] Treg cells suppress potentially damaging effects of the immune response through direct interaction with effector cells.[ 79 ] Treg cells also downregulate Th1 cells through the release of IL‐10 and prevent autoimmunity through the suppression of local effector cells and prevention of T cell activation (located around dendritic cells).[ 51 , 80 , 81 ]
Concerning biomaterial influence, the surface roughness, and wettability of an implant seems to affect the polarization state of Th cells. Hotchkiss et al. recorded changes in Th cell populations in vivo through upon implantation of titanium disks in mice over a period of 7 days.[ 51 ] Noticeable changes in the Th profile were apparent after 3 days postimplantation, with rougher hydrophilic surfaces showing a significant increase in genes that are associated with Th2 and Treg polarization (Gata3, IL‐4, IL‐13, FoxP3, and IL‐10).[ 82 ] Furthermore, systemic changes in the Th population were found in both the spleen and bone marrow. Contralateral leg bone marrow displayed increased Th2 and decreased Th1, Th17, and Treg, while the spleen contained decreased Th1 and Th17 and upregulated levels of Treg. Altogether, this research indicates that surface and wettability have a profound effect on Th profiles in both local and systemic environments, with rough‐hydrophilic implants being beneficial in stimulating a Th2 skewed anti‐inflammatory state.[ 51 ] The exact optimal roughness was not determined in this study, since only two average surface roughness (Sa) compositions were tested (smooth Sa = 0.59 µm, rough Sa = 3.58 µm, and hydrophilic surface Sa = 3.55 µm). Although a study on increasing roughness that used osteoblasts has shown that the gradual increase in surface roughness did lead to enhanced cell integration and viability, it is not yet confirmed whether a continuous increase in roughness has the same effect on Th cells.[ 83 ]
Th cell profiles also seem to be largely affected by surface profiles. In a study form Tian et al., nanoscale poly(ε‐caprolactone)/hyaluronic acid (PCL/HA) scaffolds were coated with ε‐poly‐l‐lysine (EPL), which has previously been shown to increase antibacterial and osteogenic properties of the implant.[ 84 ] In a further in vivo study this coating has also presented increased tissue regeneration in the muscle (≈Δ50%, 8 weeks post‐implantation), as well as a doubling in Th2 cell population 2 weeks post‐implantation. Whilst both coated and uncoated scaffolds presented anti‐inflammatory M2 and Th2 cell groups in the implant site at 8 weeks, it is during earlier time points (week 1–2) where the EPL coating controlled inflammation through a reduced Th1 and Th17 and M1 population (T‐bet−, RORγt−, CD68−, CD206+).[ 85 ]
The extent of Th cell responses on biomaterials was further explored by Sadtler et al., who used tissue‐derived extracellular matrix scaffold implants in vivo to assess the regenerative microenvironment.[ 78 ] Tissue‐derived ECM scaffolds from bone and cardiac muscle were used to replace the defect on C57BL wild type (WT) mice and RAG1 −/− mice that were genetically modified to lack mature T and B cells. The implanted scaffolds induced a Th2 mediated profile, with enhanced IL‐4 and decreased Ifng and Tbx21 expression levels (indicating that a Th1 immune profile is suppressed), and elevated levels of Jag2 that represents a differentiation shift to Th2. Seeding the scaffolds with either regular effective Th cells (CD4+) or cells subduing Th2 polarization (Rictor −/− or Il4ra −/−) also showed a lack of CD206 in the microenvironment, a marker for M2 macrophage polarization. This study showed that Th2 polarization is a requirement for M2 polarization of macrophages, and that this shift is actively promoted through the use of ECM‐derived scaffolds.
3.3. Dendritic Cells
Dendritic cells (DCs) are antigen‐presenting cells present throughout the entirety of the body. They originate from bone marrow and play a role in the immunomodulatory system through the control of both exogenous and endogenous antigens, as well as the activation of T lymphocytes including Th1, Th2, Treg, and Th17.[ 86 ] During initial inflammation, interaction with antigen presenting cells from the innate immune response causes DC maturation, bridging the innate and adaptive immune activation through subsequent Th cell activation.[ 87 ] A mature DC is capable of taking on either a Th stimulatory state through pathogen associated molecular patterns (PAMPs), a Th inhibitory state through self‐antigens (referred to as a tolerogenic state), or an alternatively activated state that controls Treg expansion.[ 42 ] DCs also play a major role in the initial wound healing process, being associated with early cell proliferation, the formation of granulation tissue and increased levels of transforming growth factor β1 (TGF‐β1) and CD31+ vasculature formation.[ 88 ] DC activation is shortly followed by apoptosis in both in vitro and in vivo models, since prolonged existence of these cells can lead to lasting lymphocyte activation and subsequent damage through chronic inflammation.[ 89 ]
Since the exact profile of DCs can be steered through a multitude of factors, including location, type (cDC, pCD or mDC), maturity and external triggers (LPS, anti CD40 or TNF‐α), this allows for a high plasticity in DC polarization and excreted factors, which may be modulated through physical and chemical properties of biomaterials.[ 86 ] DCs respond to biomaterials by means of pattern recognition receptors (PRRs) such as TLRs functioning as PAMPs to recognize characteristics like hydrophilicity, surface roughness, and material type.[ 90 , 91 , 92 ] Eventually, the control of PRRs may allow to steer the DCs response to biomaterials, hereby controlling the release of triggers in the microenvironment, the profile of immunogenic cells and an overall inhibition of the inflammatory response.[ 93 ]
Direct interactions with the implanted biomaterial and its degraded products also seems to directly influence the state of a mature DC. Park and Babensee have tested the variations of DC profiles exposed to several biomaterial types, including alginate, agarose, chitosan, HA, and poly(lactic‐co‐glycolic acid) (PLGA).[ 87 ] PLGA and chitosan films stimulated DC maturation, proinflammatory marker release (CD80, CD86, CD83, HLA‐DQ, and CD44), decreased endocytic ability and displayed enhanced allostimulatory effects, indicating further implications for immunoregulation.[ 94 ] Alginate films caused a switch toward a proinflammatory environment, with a significant increase in CD83, CD86, and HLA‐DQ when compared to immature DC cytokine excretion. HA‐treated DCs displayed a lower expression of the aforementioned proinflammatory cytokines, whilst also having decreased endocytic ability and CD44 expression compared to immature DCs. CD44 has previously been found to be secreted by DCs in hypoxic environments to stimulate Th2 polarization.[ 95 ] Agarose caused no significant differences in phenotype compared to immature DCs other than lowered levels of CD44. These results indicate that biomaterial type modulates the activation of DCs, where PLGA, alginate, and chitosan are able to induce DC maturation and invoke a shift toward a Th1 proinflammatory state, while HA and agarose create a more bioinert‐like state in DCs, suppressing both Th1 and Th2 activation.
Roughness of a material may furthermore steer DCs toward either a pro‐ or anti‐inflammatory state, where increased roughness on polystyrene (PS) and polyester imide (PEI) scaffolds displayed elevated secretion of chemokines CCL2, CCL3, and CCL4, as well as the anti‐inflammatory cytokine IL‐10, indicating a pro‐regenerative Th2 mediated profile. Although roughness in these materials provides evidence for the steering of the inflammatory response, both PS and PEI displayed significant IL‐17 cytokine secretion, indicating that both material types induce a Th17 skewed cytokine profile, indicative of a proinflammatory environment. Additionally, CCL3 levels seemed to only increase in PS‐based inserts, suggesting that the intrinsic properties of these materials may also exert a nuanced effect on the inflammatory profile.[ 92 ] The method of creating roughness also seems to affect the DC response as seen on a variety of modified titanium surfaces by Kou et al. Whereas pretreating, sandblasting, and acid etching all invoked maturation of DCs, modified acid etching supported a more immature DC phenotype, which was attributed to nonstimulating properties of modified acid etching, as well as high hydrophilicity, and oxygen and titanium concentrations.[ 96 ] The surface composition properties were also highlighted by Shankar et al. on self‐assembling monolayers that displayed varying chemicals including ─OH, ─COOH, ─CH3, and ─NH2 groups.[ 97 ] ─OH, ─COOH, and ─NH2 groups displayed modest DC maturation. ─CH3 groups displayed the least amount of maturation, but also contained highly elevated levels of TNF‐α, IL‐6, and several apoptotic markers, indicating increased inflammation and cytotoxic T cell activation. Being able to control TLR pathways through biomaterials also allows control over the behavior of DCs and subsequently the T lymphocyte activation cascade. Of note are TLR2, TLR4, and TLR6, whose interactions involve the release of activation markers, proinflammatory cytokines, and activates antigen specific T‐cells.[ 98 ]
3.4. (Myo)Fibroblasts
Fibroblasts are a supportive cell type that are mainly active during the resolution phase of the FBR. These highly dynamic cells are naturally present in connective tissue and provide nonrigid ECM, which is rich in type I and III collagen.[ 52 ] In the FBR, fibroblasts are recruited through biochemical signals (cytokines and chemokines) and mechanical changes in the microenvironment. Upon migration, fibroblasts change to their active proto‐myofibroblast state and settle in the inflamed microenvironment. Proto‐myofibroblasts differentiate to myofibroblasts upon exposure to both chemical (reactive oxygen species or excreted cytokines) and mechanical (tensile strength) environmental stressors.[ 99 ] Myofibroblasts create highly contractile alpha‐smooth muscle actin (αSMA) incorporated stress fibers and produce high levels of collagenous matrix to replace damaged tissue in the surrounding environment of an implanted biomaterial.[ 100 ] The accumulation of αSMA by mature myofibroblasts is of importance as the cyclic stress contractility stimulates the contractile closure of wound tissue and the deposition of ECM matrix in the inflamed microenvironment.[ 101 ] Furthermore, the arrangements of these collagen networks, including thicker fibers, larger pore sizes, and higher shear moduli directly enhances the differentiation toward myofibroblasts.[ 102 ] Adjusting these physical factors within biomaterial structures may therefore directly impact the newly formed matrix, which can either integrate with the material and promote tissue regeneration or create a pathological feedback loop, accumulating ECM until it forms a thick collagenous capsule that isolates the implant from the environment.[ 103 ]
The successful resolution of the FBR is, like the transient mechanisms of previously discussed cell types, dependent on the quantity and duration of active myofibroblasts. As resolution progresses and tissue integrity is restored, the lack of stressors causes cessation of myofibroblast activity and the removal of excessive cells through programmed apoptosis.[ 104 ] However, persistent activity results in tissue deformation by continuous contracture, accumulating fibrosis, and the overexcretion of ECM, forming a collagen capsule around the implant.[ 105 ] Aside from the FBC and parallel to macrophages, in vitro research has also shown that fibroblasts possess the ability to form FBGCs.[ 106 ] The collective outcome of these mechanisms is undesirable and may result in the impairment of the implants’ function and integrity.[ 53 ]
The eventual morphology and fate of a fibroblast is largely defined by the inherent structure of the implanted biomaterials. As the encapsulating FBC makeup includes large numbers of (myo)fibroblasts, these cells are influenced by the surface properties through direct interaction.[ 20 ] Factors such as the surface roughness, wettability, and oxygen content have been found to directly influence the morphology and behavior of fibroblasts. Higher levels of hydrophilicity, roughness, and oxygen content induced enhanced homogenous spread and formation of focal adhesion points. Furthermore, material pore size also affected fibroblast elongation, where condensed focal adhesion areas were found in larger pores while smaller pores resulted in increased elongation.[ 107 ] These surface property dependent findings were also confirmed in macrophage/fibroblast cocultures, where increased hydrophilicity has a positive effect on the biocompatibility of the material.[ 108 ]
Within implantable structures, in particular the formation of vascular phenotypes, fibroblasts have also shown to contribute to the restoration of tissue by further differentiating in vascular smooth muscle cells (VSMCs). A study by Rothuizen et al. created a tissue‐engineered blood vessel through in vivo subcutaneous implantation of chloroform etched poly(ethylene oxide terephthalate)/poly(butylene terephthalate) (PEOT/PBT) rods in a porcine model.[ 109 ] Within four weeks postimplantation, tissue capsules were formed that mainly expressed fibroblast cell types with minimal FBGC formation. Using these models as in vivo vascular grafts for four weeks resulted in a change to thick, homogenous grafts with αSMA+ Desmin+ VSMC‐like cells. Tissue capsule changes in mRNA profiles and Desmin/αSMA protein upregulation indicated that continuous flow and cyclic stress directly stimulated elastogenic cell differentiation, which may also result in the formation of VSMCs from fibroblasts within the granulation tissue.[ 110 ] These studies indicate that fibroblasts are directly affected by both the micro‐ and macroenvironment, where external stressors could influence the differentiation to native cell types that contribute to tissue regeneration.
To correctly identify the effects of a biomaterial on all involved cell types, in vitro research introduced coculture systems that may combine several of the previously mentioned cell types to mimic various stages of the inflammatory response. For example, coculture models using mesenchymal stem cells (MSCs) could be useful to study FBR in vitro due to its multipotent ability and its recruitment during the FBR.[ 111 ] Vallés et al. examined whether 3D porous scaffold topography features can modulate paracrine signaling in MSC–macrophage cocultures.[ 112 ] Compared to the 2D culture settings, the 3D coculture provided a shift to an M2 phenotype, stimulating the production of the anti‐inflammatory proteins Prostaglandin E2 (PGE2) and TSG‐6; and a decrease in the proinflammatory proteins IL‐6 and MCP‐1. Using neutralizing antibodies, coculture studies identified that the interplay between PGE2, IL‐6, TSG‐6, and MCP‐1 is strongly influenced by the microarchitecture that supports MSCs. Furthermore with the use of transwells, the FBR mimicking model triggered by 3D‐arranged MSCs in cocultures, decreased monocyte migration when compared to monolayer cells. In the FBR process, macrophage chemotactically attracts ECs to the FBR area and engages in a close interaction to modulate vascular formation. The most recent coculture model that includes ECs and macrophages has been reported by Liu et al., where vascular smooth muscle cells, ECs and macrophages were combined to study the effects of shear stress and inflammatory cues in atherosclerosis.[ 113 ] Albeit effective to mimic and quantify the immune response in nascent and intermediate plaques (LDLox, MCP‐1, IL‐1β, IL‐6, Cathepsin L, MMP‐1 variations under shear flow) this model lacks the introduction of biomaterials, leaving potential for future studies.
All in all, the implantation of a biomaterial is followed by a large, interactive network of cells that collectively determine the progression of the FBR. The implantation of a biomaterial has both inherent and consequent effects on the FBR of the host in which the direct properties of the implant, the surrounding environment and the follow‐up responses all contribute to the inflammatory state.
A multitude of physical and chemical factors can guide the involved cellular processes toward an anti‐inflammatory, regenerative state. Examples such as the engagement of TLRs in the alteration of adhesive properties toward biomaterials also indicates that these physicochemical regulatory networks are intertwined, rather than secluded effectors toward the immune response.[ 98 ] The key toward designing biomaterials is taking into consideration that the crosstalk between cells at various stages in the inflammatory response are connected and affected by the intrinsic properties of a biomaterial (Figure 3 ).
Figure 3.
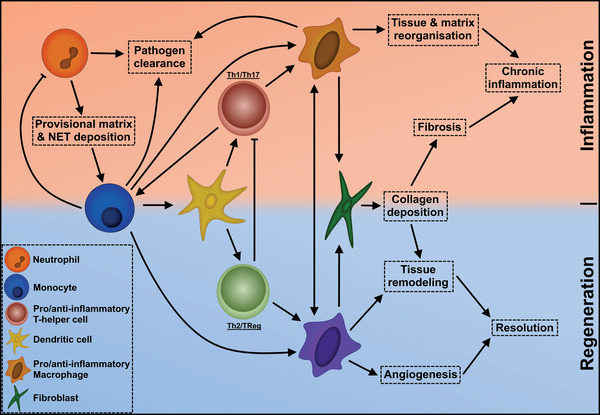
An overview of the crosstalk network between key cell types and inflammatory responses of the FBR. Each feature is categorized according to either inflammatory (orange) or regenerative (blue) effects it exerts on the progression of the FBR. Cellular interaction occurs through either cytokine crosstalk or direct differentiation into the directed cell type.
3.5. Influence of Physical Properties of Biomaterials on the FBR
The creation of suitable biomaterials is defined by a number of physical and chemical properties, where the combination of various physical and biochemical features makes them suitable for in vivo applications. Both inherent material properties as well as fabrication techniques are used to optimize the functionality of biomaterials whilst minimizing the negative inflammatory responses that occur upon implantation (Figure 4).
Different fabrication techniques include, but are not limited to additive manufacturing, electrospinning, layer‐by‐layer assembly, and freeze‐drying. Usage of varying fabrication techniques provides the possibility to construct organized structures and versatile functionalities.[ 114 ] This led researchers to extensively study various forms of biomaterials, such as hydrogels, scaffolds, membranes, tubular grafts, and micro‐ to nanospheres.[ 115 ] These physical characteristics are intended to interact with the direct microenvironment of the host, where spatiotemporal signals are able to modulate cellular migration, adhesion, proliferation, differentiation, and apoptosis.[ 116 ] Biophysical contact cues that invoke a desired cellular response are defined as durotaxis or mechanotaxis and include many characteristics that can invoke a directed FBR. These physicochemical characteristics directly contribute to the optimization of the FBR, hereby influencing the success of a biomaterial in a living host. Successful biomaterial‐based scaffolds demand a number of mechanical properties to be adapted to the microenvironment of the host, including an optimized size, roughness, and porosity of the material.[ 117 , 118 , 119 ]
3.6. Dimension and Geometry
The size of the biomaterial implant is a crucial factor in the FBR. Dimension input can determine whether macrophages are required to fuse and form the FBGCs to engulf, digest, and break down the biomaterial.[ 19 , 20 ] Zandstra et al. observed cellular and macrophage influx, phagocytosis, and collagen deposition mainly on smaller PLGA 5 µm microspheres, while larger 29.8 µm microspheres induced occasional FBGCs.[ 120 ] Implementing dimensional features in in vitro models can provide consistency when translating results to in vivo implantation. Kusaka et al. noted lysosomal destabilization, higher cell apoptosis and IL‐1β secretion on smaller 30–1000 nm silica particles, compared to larger particles of 3000–10 000 nm in vitro. This is consistent with its in vivo study that observed higher expression of inflammatory cytokines and neutrophil infiltration in 30 than 3000 nm size particles.[ 121 ]
Veiseh et al. supported this dimension effect, as spheres with a small diameter (≤0.5 mm) induced severe FBR, while large spheres (1.5 mm) scarcely provoked FBR.[ 122 ] This extensive study showed observation in size effect to be persistent in in vivo mouse, rat, and monkey models, implanting in both subcutaneous and intraperitoneal space, irrespective to the range of materials used, including alginate, stainless steel, glass, PCL, and polystyrene (PS). However, these size observations are restricted to spherical shapes, as changing the geometry and increasing the biomaterial size provoked in contrast fibrosis or implant rejection. For instance, single polypropylene fibers with a larger diameter of 26.7 µm provided a thicker FBC formation compared to smaller fibers of 6.5 µm in size.[ 123 ] In macroscale, a similar response was observed for cylindrical polyurethane substrates provoking a larger FBR and thicker FBC in large diameter cylinders of 2 mm compared to 0.3 mm implants. This suggests that fibers with smaller diameters provided a thicker FBC formation.[ 124 ] Nevertheless, further investigation on size of different implant architecture is still necessary, even more so in 3D complex structures.
3.7. Spatiotemporal Gradients
Spatiotemporal gradient variation is defined as a difference in stimuli concentration both in spatial position (spatial) as well as over time (temporal), meaning that it is not just the location of an affector, but simultaneously the gradient change in expression of biomarkers that controls the regulation of inflammatory cells within the FBR.[ 125 ] Examples of important spatiotemporal factors include chemoattractants, surface‐attached molecules, and biophysical contact cues. The use of varying gradients within a biomaterial may have significant effects on the cellular responses concerning adhesion, distribution, and alignment.[ 126 ] Controlling gradients in vivo is particularly important in the guidance of cell migration, where the control and positioning of cells like fibroblasts and macrophages is vital in steering the immune response. Physical gradients within a biomaterial include factors such as porosity, stiffness, and topology.[ 127 ] Polymer composition is an important determinant of inflammation as well, with characteristics such as inertness of a material having great impact on biocompatibility.[ 118 ]
3.8. Complex Structures: Fiber and Pore Size
The polarization state of macrophages also seems to be directly influenced by fiber diameter and pore size of scaffolds. When creating a scaffold, factors such as the nature of the material or the method of fabrication may result in varying thickness or pore size of the material. Macrophage cytokine secretion responds to the geometry or pore size of the construct it is interacting with, where sizes differing from nanometer to micrometer range can influence the expression of inflammatory cytokines, morphology, and polarization state.
Researchers have tried to mimic the ECM of natural tissues to provide a platform for cells to attach, proliferate, and form new tissue using techniques such as electrospinning or scaffold templating. Studies on synthetic polymers, PCL and poly(l‐lactic) (PLLA) showed reduced FBR in 3D electrospun scaffolds compared to 2D films.[ 41 , 128 ] Cao et al. seeded human blood monocytes on to PCL electrospun nanofibers, and showed less adhesion on aligned fibers (506 ± 24 nm) compared to random fibers (313 ± 5 nm).[ 128 ] However, this in vitro study lacked insight on the outcome of the in vivo implantation, which showed thinner FBC formation in random electrospun fibers having smaller diameter than aligned electrospun fibers.
A thorough in vitro study was done by Saino et al. where macrophage polarization was investigated through cytokines and chemokines secretion and the level of formed FBGCs, using a murine RAW 264.7 macrophage cell line.[ 41 ] The FBR increased when comparing PLLA microfibers (1.6 µm) to nanofibers (0.6 µm) indicated by the increase in TNF‐α and G‐SCF levels, which could suggest M1 polarization. Additionally, VEGF was increased in larger fibers showing potential in angiogenesis as well as M2 polarization. Garg et al. supported M2 polarization results when using mouse bone marrow‐derived macrophages (BMDMs), showing that larger fiber diameter of polydioxanone‐enhanced M2 macrophage polarization.[ 36 ] Increased fiber/pore size (0.35 ± 0.2 to 2.8 ± 0.5 µm fiber diameter; 0.96 ± 0.09 to 14.73 ± 0.63 µm pore size) increased expression of M2 marker Arg1 and angiogenic cytokines, VEGF, TGF‐β1, and basic fibroblast growth factor (bFGF), while decreasing the M1 marker iNOS.
In classical electrospinning, fiber diameter is correlated to pore size, where nonporous materials display a “prototypical FBR” by forming a collagenous FBC and avascular isolation of the implant. On the other hand, porous materials showed reduced fibrosis, improved cellular integration and angiogenesis.[ 129 ] The earliest findings of pore size contributing to macrophage behavior and angiogenesis was established in 1973, where smaller pore sizes (≤0.1 µm) increased in vivo fibrosis and FBC formation in mice.[ 130 ] Although the general consensus lies with porosity positively affecting the FBR, much controversy remains between the exact correlation of pore size and material biocompatibility.
Porous materials can be fabricated using a number of mechanisms, where classical methods include gas foaming, thermal phase separation, and electrospinning. Nowadays computerized methods such as 3D printing, sintering, stereolithography, and fusion deposition modeling are also used since ths allows for exact, highly accurate pore formation within polymer scaffolds.[ 131 ]
The pore size range has been shown to affect the FBR progression, where 34 µm pores were infiltrated by slightly fibrous cellular tissue while 160 µm pores displayed enhanced fibrous tissue infiltration.[ 129 ] Different physiological processes may also favor varying pore sizes: processes such as neovascularization and fibroblast ingrowth prefer smaller (5–15 µm) pores while the optimal regeneration of other mammalian cell types such as hepatocytes or skin requires larger (20–125 µm) diameters.[ 132 ]
The ideal pore size in creating an M2 skewed polarization was investigated by Tylek et al., and seems to reside in the 40 µm range. In this research, melt electrowriting was utilized to create polycaprolactone constructs with varying morphologies and pore sizes (40 to 100 µm). Smaller pore sizes seemed to stimulate macrophage elongation and induced a switch to an M2 phenotype in the 40–60 µm pore range indicated by upregulated CD163 and CD206 expression. In 40 µm scaffolds, an M2 switch was further confirmed by IL‐10 upregulation and IL‐6 downregulation. Most interestingly, scaffold structure also seemed to directly affect the morphology and behavior of macrophages. Box‐shaped structures hereby expressed the highest levels of M2 markers whilst also displaying the strongest downregulation of M1 markers IL‐β1 and IL‐8.[ 133 ] The 40 µm pore diameter was also confirmed by Hady et al., who compared 40 and 100 µm pore sizes and characterized the small extracellular vesicle (sEV) release of resident cells. Gene expression indicated that, particularly in 100 µm porous scaffolds, the sEV profile promoted a Th1 inflammatory phenotype whereas both pore sizes promoted upregulation of Treg markers.[ 134 ] Pore size within the implant may therefore have a beneficial effect on a multitude of inflammatory cell populations.
Further evidence of the influence of pore size on macrophage phenotype polarization was provided in an electrospun model by Garg et al., who showed this correlation by exposing bone marrow‐derived macrophages to polydioxanone scaffolds with varying concentrations (60, 100, and 140 mg mL−1).[ 36 ] The study used air force impedance to create a more porous structure to smaller fibers, and compression with hydraulic press to reduce pore size of larger fibers. Cells seeded on scaffolds with a higher fiber diameter and pore size showed a clear switch toward an M2 phenotype through the expression of the M2 marker Arginase 1, while displaying decreased levels of iNOS. Furthermore, VEGF, TGF‐β, and bFGF were also upregulated, indicating that a larger pore size may also promote angiogenesis.[ 36 ]
Additional research on the effect of pore size on macrophage polarization comes from a study by Bota et al. in which macrophage response to extended polytetrafluoroethylene materials was evaluated in vitro as well as in a subcutaneous mouse model.[ 135 ] These results revealed that the 3 µm porous structures provided an increase in levels of IL‐6, TNF‐α, MCP‐1, and MIP‐1β when compared to the nonporous substrate. Pore size solely increased IL‐1β expression, as low levels were produced by macrophages in nonporous structures, while a gradual increase in IL‐1β production was observed by macrophages in porous structures of 0.2, 1, and 3 µm.
These results provide support for previous studies by Saino et al. who observed that M1 polarization was triggered by larger fiber diameter, which was correlated to larger pore size.[ 41 ] Moreover, in vivo studies displayed a thinner, less dense, organized FBC formation in 3 µm porous structures compared to nonporous structures, suggesting that polarization of M1 macrophages in vitro may not be an indication of thicker FBC formation in vivo. The above fiber and porosity study seemed to support, as well as conflict each other in terms of M1 and M2 polarization. Yet, as none of these studies observed differences in secretion of the same protein, it might be possible that both population of M1 and M2 macrophages were present, distributed in different places of the biomaterial. This hypothesis is supported by Sussman et al. who observed M1 macrophages deep within the interconnected porous structure whereas M2 macrophage where mainly located in the surrounding tissue.[ 129 ]
Whereas previous research defined a specific range for macrophage polarization, the use of extremely small or large pore sizes may also offer unique characteristics that affect FBR progression. Nanoporous structures have been investigated in bulk nanostructured polymers. These materials mostly focus on creating ranges under <100 nm, since this approaches the first level of organizations in structures and allows for the modification of fundamental properties and functions of a material.[ 136 ] Inducing porosity in polymer materials at a nanoscale level is performed through techniques such as solvent‐based precipitation, lithography, and layer‐by‐layer assembly. Although nanoscale porosity is already being investigated through nanoparticles and nanofiber technology, nanostructured bulk polymers, and nanoporous membranes may also offer a multitude of benefits to a material, including biofiltration (<10 nm), immune‐isolation membranes, and controlled drug delivery.[ 137 ] In contrast to nanopores, macropore variations have also been used in polypropylene mesh implants, serving as clinical in vivo treatments of abdominal wall defects. Denser “heavyweight” meshes with a 0.8 mm pore size induced perifilamentous fibrosis and filament bridging while “lighter” 4.0 mm pore sized meshes resulted in decreased FBR and bridging fibrosis.[ 138 ]
Finally, the determination of an optimal pore size must be a combination between the characteristics exerted on the cells and the structural integrity of the scaffold. Whilst a larger pore size may influence macrophage function, creating these pores may also damage the structural integrity and overall mechanical strength of the construct.[ 131 ] Ratner described a porosity of 30–40 µm as well‐healing in various forms of tissue such as skin, heart, bone, sclera, and the vaginal wall.[ 139 ] However, this paper also addressed that two varying reactions on biomaterials were both considered biocompatible, leading to the question whether biocompatibility is not a single state, but rather a performance range defined by a number of characteristics that can be expressed quantitatively. Examples of these parameters include macrophage polarization, capsule collagen density, and angiogenesis.
3.9. Porosity in Angiogenesis
The composition of the implanted material is not solely crucial for the inflammatory response, but also affects angiogenesis within the surrounding issue, which could be crucial for tissue regeneration purposes. Various studies have identified that the porosity of the implanted biomaterial is an influential parameter to modify angiogenesis. Brauker et al. evaluated the role of surface porosity in promoting angiogenesis by comparing PTFE membranes with pore size of 5 µm against membranes with pore size of 0.02 µm.[ 140 ] Upon rat subdermal implantation, larger pore membranes provided 80–100 times more vascular structures. Furthermore, Madden et al. created porous poly(2‐hydroxyethyl methacrylate) (PHEMA)‐co‐methacrylic acid hydrogels (0–80 µm pore size) by microsphere templating.[ 141 ] Implanting these hydrogels in rats and mice demonstrated an even stronger angiogenic response with reduced encapsulation by tuning the pore sizes specifically to 30–40 µm. Immunohistochemistry staining explained the response was due to M2 phenotype activation, though identification was done by a single M1 and M2 marker, in which the M1 marker was also expressed in most of the M2 macrophages. However, in vitro studies using monoculture cardiomyocytes showed sustainable cell viability and proliferation, but did not provide any insight on the difference between the pore sizes. VEGF is an inducer of angiogenesis and stimulates EC proliferation, migration, and blood vessel formation.[ 142 ] Hence, predictable angiogenesis can be identified by upregulation and secretion of VEGF by macrophages as seen in the FBR in vitro models.[ 36 , 41 ] In vitro models using a 3D angiogenesis bead assay with EC‐coated microcarrier beads showed an enhancement in sprouting, confirming VEGF secretion from macrophages increased in larger pore size. However, this only provides a one way conditioned medium coculture, with no further interaction between macrophage and ECs.
3.10. Surface Topography: Roughness, Crystallinity, and Wettability
Another physical factor that may alter FBR is surface topography, which can be categorized simply as smooth or rough surfaces with either identified topographies or random structures. The surface roughness of biomaterials is directly capable of modulating the morphology of cells. In macrophages, the stiffness directly influences elasticity and phagocytic activity.[ 143 ]
Almeida et al. examined this on 3D printed chitosan with squared and triangular pores (orthogonal, ChO; diagonal, ChD).[ 144 ] ChO with larger pores and wider angles led to a higher proinflammatory cytokine secretion compared to ChD. Moreover, ChO‐squared pores promoted the presence of rounded FBGCs, while ChD triangular pores resulted in elongated macrophages. Furthermore, McWhorter et al. used micropatterned 20 and 50 µm width grooves to control macrophage cell shape, resulting in higher cell elongation in 20 µm grooves.[ 145 ] Cellular elongation enhanced M2‐inducing cytokines IL‐4 and IL‐13, and provided upregulation of Arg1, while protecting the cell from M1‐inducing stimuli LPS and IFN‐γ. This led Luu et al. to fabricate titanium surfaces containing micro‐ and nanogrooves ranging from 150 to 50 µm in order to promote macrophage elongation.[ 146 ] In vitro studies observed elongation peak on the 400–500 nm grooves, which showed the highest expression of IL‐10 and Arg1 positive cells, suggesting polarization toward M2 macrophages.
Chen et al. confirmed this topography‐induced polarization on PCL, poly(lactic acid) (PLA), and poly(dimethyl siloxane) imprinted with 250–2000 nm grooves.[ 147 ] Seeded RAW 264.7 established its longest elongation in 500 nm grooves. Upregulation of TNF‐α and VEGF provided a mixture of M1 and M2 phenotype in smaller gratings (higher surface roughness). The combined upregulation with TNF‐α might be beneficial for the healing process, as depletion of TNF‐α impaired healing in mice.[ 148 ] Similarly, Barth et al. stated that murine macrophages on rough porous sandblasted acid‐etched (SLA) surfaces, adopted elements of an M2‐like phenotype due to downregulation of M1‐associated chemokine IP‐10 when compared to smooth surfaces.[ 149 ] No expression of either NOS2 or Arg1 was upregulated, while upregulation MCP‐1 and MIP‐1α was observed, again suggesting an effect of surface topography on both M1 and M2 related genes in macrophages.
Surface topography is also affected by the level of crystallinity, which is of importance for biomaterials interacting with denser tissue. The structural arrangement, shape, and hardness of materials such as hydroxyapatite (HAp) directly influence the secretion of inflammatory cytokines by monocytes. The secretion of TNF‐α, IL‐1, IL‐8, IL‐9, IL‐10, and presence of inactive matrix metalloproteinases (MMPs): ProMMP‐2 and proMMP‐9, are direct indicators of an enhanced inflammatory response, which increases with the crystallinity of the material and further contributes to the overall integration and resorption of the material.[ 150 ] Crystallinity further plays a role in favoring the growth of different cell types. In poly(caprolactone)/poly(glycolic acid) (PCL/PGA) with varying levels of crystallinity the high rigidity of more crystalline surfaces supported fibroblast growth, whilst surfaces displaying amorphous properties favored osteoblast proliferation.[ 151 ] Crystallinity may therefore largely affect the adhesion and proliferation of different cells, and can be exploited to increase the frequency of anti‐inflammatory cell types in the FBR.
Interestingly, the stiffness of materials also seems to directly influence the phenotypic polarization of macrophages. Sridharan et al. have differentiated THP‐1 monocytes into macrophages whilst being exposed to collagen‐coated polyacrylamide gels with varying levels of stiffness. Gels that range between a softer (11 kPa) and medium (88 kPa) stiffness turned the cells into an anti‐inflammatory phenotype while a higher (323 kPa) stiffness invoked a proinflammatory response in macrophages combined with impaired phagocytosis. The cellular means of migration is also affected by stiffness, with a Rho‐A kinase dependent fast amoeboid migration on gels with a low to medium stiffness and a podosome dependent slow mesenchymal migration on stiffer gels.[ 152 ] This stiffness‐induced inflammation was also found in polyethylene glycol (PEG) hydrogels, where increased crosslinking resulted in increased macrophage spreading along the surface of the construct.[ 153 ] Further effects of porosity on immunomodulation was presented in dual‐porosity scaffolds with varying degrees of porosity and topologies. Here, a liquid–liquid phase separation technique was performed to introduce porosity within the fibers of P(l)LCL and P(l,d)LCL polymer scaffolds, creating stiffness variations between 4 and 40 kPa. Both in vitro and in vivo the lower stiffness scaffolds (<5 kPa) resulted in an M1 phenotype with elevated IL10, TGF‐β, and fibrous encapsulation of the implant in vivo; whilst higher values (>40 kPa) promoted a resolution phenotype with and proper integration of the scaffolds.[ 154 ]
The stiffness of the material mainly affects mechanical protein complexes such as YAP/TAZ within macrophages. Here, the degree of YAP activation is dependent on the substrate stiffness of the microenvironment, which directly relates to macrophage polarization. Meli et al. have found that in both in vitro and in vivo situations, softer microenvironments (≈1 kPa) resulted in the depletion of YAP localization and promote M2 polarization, while stiffer materials (≈140 kPa) increased inflammatory markers such as TNF‐α and IL10.[ 155 ] Interestingly, due to the complexity of the regulatory signaling pathways no uniform consensus on the optimal stiffness ranges exist that promotes an immediate shift to the resolution state. A review by Li et al. has compiled a number of articles related to the effects of substrate stiffness on macrophage polarization, which accurately captures both the complexity and dichotomous nature of this modulatory aspect.[ 154 ]
Another surface property that steers the FBR upon biomaterial implantation, which is correlated to surface roughness, is wettability. Defined as the level of surface hydrophobicity or hydrophilicity, wettability defines surface interaction throughout the entire FBR. Albeit it is most prominently active in the earlier stages where it affects platelet accumulation, coagulation, protein adsorption, and cell adhesion,[ 156 ] the level of wettability has been shown to be adjustable through gas plasma treatment, which additionally affects the roughness of the material.[ 107 ] Argon plasma treatment was used to topographically etch the surface of PEOT/PBT cylindrical rods. Varying levels of surface wettability were achieved with hydrophilic surfaces being created through treatment with argon (Ar), oxygen (O2), and sodium hydroxide (NaOH). Hydrophobic surfaces were made through treating the rods with fluoroform (CHF3) and chloroform (CHCl3).
Interestingly, the increased wettability of NaOH treated rods seemed to influence macrophage migration and cytokine excretion. Smooth hydrophilic surfaces created through the NaOH treatment of polymeric rods (PCL + PEOT/PBT) showed decreased branching, but a higher spread of filopodia. Furthermore, a secretory increase of TGF‐β1 and IL‐6 was also observed, indicating a macrophage polarization shift toward the M2c state, an increase of collagen production and increased tissue remodeling. Changes in surface topography also appear to exert modulatory effects on other cell types involved in the FBR, where exposed fibroblast cultures created two morphologically distinct populations of either low spread with little focal adhesion sites or a polygonal morphology with more defined adhesion sites. Ar and O2 treated surfaces (increased roughness) displayed a higher homogenous spread of fibroblasts.[ 107 ]
Further evidence on effects of surface roughness on the polarization shift of macrophages was found on nanorough titanium films, where a roughness of 4.76 ± 0.01 nm provided increased hydrophilicity over flat titanium surfaces. This resulted in a higher protein absorption and M2 polarization of murine macrophages, identified by downregulation of iNOS, TNF‐α, and IL‐1β.[ 157 ]
Hydrophilic polymer surfaces have been shown to reduce the FBR by minimizing the amount of nonspecific protein absorbed. Similarly, hydrophilic‐modified SLA titanium surfaces resulted in downregulation of M1 cytokines TNF‐α, IL‐1β and IL‐6, and chemokine CCL‐2.[ 158 ] The investigation of various drug eluting stent wettability suggested hydrophobic, not hydrophilic, surfaces support activated monocytes adhesion while inducing an inflammatory response.[ 159 ] Moreover, blood serum proteins could be absorbed via hydrophobic interaction, resulting in alteration to protein conformation.[ 160 ]
3.11. Scaffold Dimensionality
A final physical characteristic that has seen recent advances in the biomaterials field is the development of 3D scaffolds. In regular cell cultures, a change to a 3D model results in drastic changes in cellular behavior over 2D situations, including cell morphology, polarity, and proliferation. The introduction to a 3D environment allows cells to morphologically change to a natural spheroid structure, in which the spatial interactions with the microenvironment mimics the natural situation in the host.[ 161 ] In the field of biomaterials, 3D microarchitectures were introduced to investigate the inflammatory responses in an environment representing the host. Almeida et al. have hypothesized that both the chemical composition and the architecture of a 3D scaffold is able to affect the behavior of macrophages.[ 162 ] In this research, human monocytes were cultured on four different scaffolds with either PLA or chitosan, and produced in two different geometries with either orthogonal or diagonal/orthogonal struts. All 3D scaffolds were able to support the integration of macrophages, monocytes and stimulate cytokine production. Interestingly, major differences were found between the material types, with chitosan displaying upregulated levels of TNF‐α while PLA consistently induced higher levels of IL‐6, IL‐12, IL‐23, and IL‐10. In this case, IL‐12, IL‐23, and IL‐6 indicate a proinflammatory state toward M1 macrophages, whereas Th1 and Th17, and IL‐10 are indicators for an anti‐inflammatory state. Significantly varying cytokine secretion levels were also found when comparing the 3D scaffolds to 2D cultures, indicating that the 3D structure does seem to influence the inflammatory response. Differences in structural morphology (orthogonal/diagonal) also displayed slightly higher levels of TNF‐α, IL‐12, and IL‐23 in the orthogonal structure. The lack of increased levels in IL‐10 and TGF‐β suggests an increase in redox metabolic activity over cell proliferation, which may contribute to macrophage polarization. An elongated cell morphology was observed in the diagonal structure, which was attributed to tighter angles and smaller pores, and may attribute to cellular behavior and macrophage polarization. Interestingly, secretion profiling of all scaffolds resulted in profiles that could not be defined as a classic M1 or M2 phenotype, suggesting that macrophages in a 3D environment take on a nuanced, intermediate state in which they have the plasticity to transdifferentiate toward either state.[ 162 , 163 , 164 ]
The effects of a scaffolds’ microstructure on the immune system was further highlighted by Indolfi et al. who implanted matrix‐embedded endothelial cells (MEECs) in both 2D and 3D collagen matrices to study the immunomodulatory behavior of these cells.[ 165 ] MEECs have the ability to produce a large number of soluble factors that control arterial homeostasis and immunobiology, guiding the vascular response to injury and controlling arteriovenous anastomoses.[ 166 ] After culturing, the soluble factors derived from both the 2D and 3D culture of MEECs were applied to an endothelial monolayer, which was stimulated with TNF‐α to induce inflammation. A fivefold decrease in monocyte adhesion within the 3D MEEC treated monolayer was observed, indicating that in a 3D environment MEECs exert a much higher immunosuppressive activity. MCP‐1 levels were found to be eight times higher in 3D‐MEEC treated cultures, hereby reducing the migration and adhesion of monocytes.[ 165 ] All in all the 3D architecture seems to play a vital role in suppressing the initial inflammation toward a wound site.
Within the upcoming years, advances in tissue engineering and scaffold printing are expected to take the scaffolds dimensionality of a biomaterial toward an additional dimension. 4D printing is a newly proposed technique that adds a fourth modifiable dimension over a given period (“3D printing + time”) through the introduction of smart, shape‐shifting materials.[ 167 ]
4D printing applies a wide variety of “smart material” components in either solid or gel state, where materials can be in a single‐ or multicomponent setup. These materials are responsive toward predetermined stimuli such as water, light, temperature, magnetism or pH. They respond to triggers with self‐shifting behaviors like folding (inhomogenous expansion), (dis)assembly, expansion or shrinkage (affine transformations). It does so through the utilization of, e.g., the thermoresponsivity of polymers or the shape memory effect (SME) retention of metallic smart materials like titanium nickel (Ti50.6 Ni49.4) alloys.[ 167 , 168 , 169 ] Of additional importance is the longevity of the SME in materials. Where one‐way SME creates an irreversible change to the material, two‐way SME creates a temporary change in the material, which remains until it is no longer exposed to stimuli, whilst a three‐way SME includes three possible forms typically with a linear, intermediate step when exposed to varying stimuli. Materials that possess multiple intermediary steps are referred to as a “multiple shape memory effect.”[ 168 ]
Future opportunities for 4D materials lie in their adjustable form and nature upon implantation. Self‐modifiable materials could reduce the invasiveness of surgical procedures, act as target drug delivery systems and mimic physiologically relevant environments.[ 169 ] While the development and use of 4D printing in the biomedical field is still in its infancy, current techniques are promising and widely applicable in a number of fields. Advances in creating an implantable environment capable of shape‐shifting over time and deliver the required stimuli could greatly benefit the progression of the inflammatory response, which requires a multitude of highly organized changes over a prolonged period of time in order to minimize the FBR.
Since the 3D environment offers a multitude of factors regarding the initiation and modulation of the immune response, it is important that a paradigm shift occurs when observing the immune response in an in vivo setting. Specifically, with regard to the M1/M2 phenotype, 3D and 4D environments display macrophages in a heterogeneous state, possessing a certain level of plasticity which is affected by the entire environment, including structure, materials, and secreted cytokines. It is the combination of all these factors that orchestrates a unique spectrum of secretory profiles, resulting in a subsequent inflammatory state (Figure 4 ).
Figure 4.
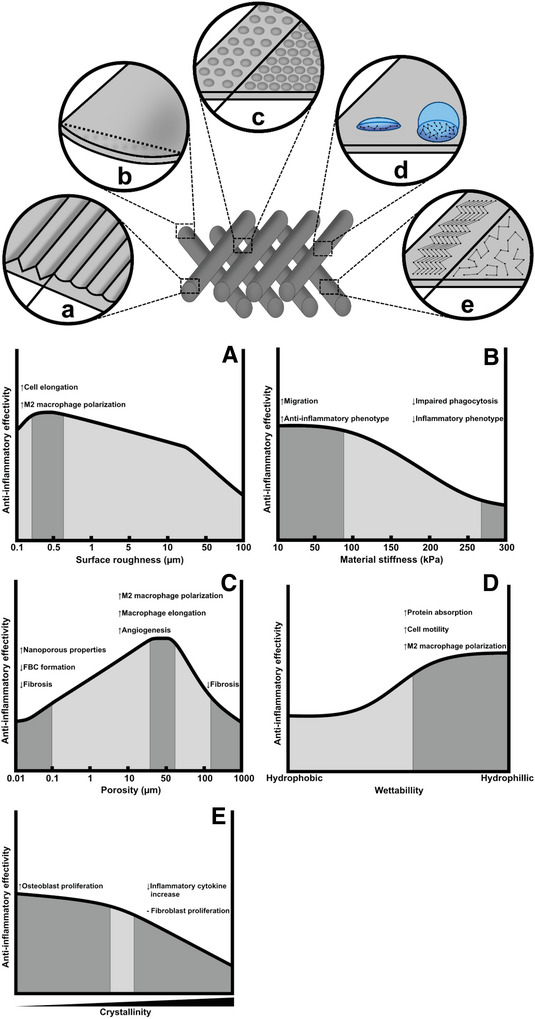
A qualitative analysis on the effect of physical biomaterial properties on the inflammatory response. Upon implantation, varying features can display a multitude of positive (↑) or negative (↓) effects on inflammatory cell types and the FBR progression. Preferred anti‐inflammatory effects are usually defined by an optimal range. A) Surface roughness is defined in µm where a roughness between 0.2 and 20 µm results in macrophage elongation and M2 polarization. Optimal polarization occurred between 0.4 and 0.5 µm. Generally, rougher surfaces are always more effective over smooth material surfaces. B) Material stiffness is expressed in kPa and concerns both the flexibility and resistance to deformation. A lower stiffness (<88 kPa) causes increased cell migration and promotes an overall anti‐inflammatory environment whilst higher stiffnesses (>300 kPa) impairs phagocytosis and stimulates inflammation. C) Porosity measures the empty space between a material that allows for cellular integration and migration. Pore ranges between 5 and 60 µm have been found to support an anti‐inflammatory environment, where pore ranges between 30 and 60 µm stimulate angiogenesis, macrophage elongation, and M2 polarization. Of note is that different cell types may prefer different pore sizes. Extremely small (<0.1 µm) or large (>160 µm) are generally unfavorable since this may stimulate fibrosis and FBC formation. Nanoporous materials could also exert positive effects on the FBR by modulating the fundamental properties of the material. D) Wettability is defined by the level of hydrophilicity of a material. Increasingly hydrophilic materials create a more anti‐inflammatory environment through providing enhanced protein absorption, cell motility, and M2 polarization. E) Crystallinity indicates the structural arrangement of atoms in a material and becomes increasingly important in stiffer components (e.g., bone tissue). Amorphous materials supported the growth and proliferation of stem cells like osteoblasts, whilst crystalline materials resulted in increased fibroblast proliferation. Of note is that a higher crystallinity may negatively affect the FBR, since persistent fibroblast activity can result in chronic inflammation through increased levels of inflammatory cytokines and a buildup of fibrous matrix.
3.12. Biomaterial Types
A wide variety of material types are currently utilized in the creation of biomaterials, of which the most commonly used biomaterials are categorized in three categories: synthetic, biological, or semisynthetic/hybrid biomaterials.[ 8 ] Synthetic materials are created through the composition or manipulation of various chemical substances to form polymers, metals and ceramics.[ 170 ] Advantages include a tightly controlled manufacturing process, accurate reproducibility, adjustable biodegradability, and a wide variety of both chemical and physical properties.[ 171 ] On the other hand, biological biomaterials are composed of materials that naturally occur within the cellular environment. They can be synthesized from natural products or derived from donor sources such as ECM derived from native tissues of autogenic, allogenic or xenogenic sources. Most biological scaffolds contain a complex mix of natural composites including proteins, proteoglycans, and other cellular products, with varying compositions depending on the type of donor tissue. Although the increased complexity of biological materials makes their properties harder to modulate, in vivo integration within multiple tissue types have resulted in improved scaffold integration and regeneration.[ 45 ]
Unfortunately, most commonly used synthetic materials are prone to degradation and may invoke severe immune responses when implanted, while natural polymers are prone to enzymatic degradation and generally possess poor mechanical properties.[ 172 ] This facilitates the need for new types of biomaterials that aim to overcome these challenges within their respective category. In the following subsections, a number of synthetic and natural materials are highlighted that display characteristics, which may influence the inflammatory response by inflammatory cell recruitment, modulation of the polarization state of cells, or generally improve the biocompatibility through unique features of these components.
3.13. Natural Biomaterials
In medical applications, a classical preference in the choice of biomaterials has traditionally been for “bioactive” materials. Natural polymers, such as HA, alginate, chitosan, and collagen, are generally considered “bioactive” due to their biological macromolecular origin. Collagen and HA are main components of the ECM, and hence have been used as coatings for their biomimicking ability.[ 173 ] As realistically no exogenous material is bioinert, introducing these polymers into the host will always trigger an FBR, though to a limited extend, as illustrated by studies on collagen coating of silicone rubber prostheses that triggered an FBR, of lesser intensity when compared to uncoated prostheses.[ 174 ]
Recently, the ECM has gained popularity as a mostly bioactive material. The removal of cells from a natural piece of ECM allows for the isolation of these structures from various sources and for their use as a natural scaffold, creating a 3D environment capable of regulating proliferation, differentiation, and overall survival of cells. Decellularized ECM is composed of natural products such as collagens, HA, and elastin, which have shown their advantages over cellular scaffolds. Through mimicking the original environment of a wound site, decellularized ECM inhibits the severity of the hosts’ immune response and displays less potential for pathogenic transfer.[ 175 , 176 ]
Natural scaffolds, such as the aforementioned decellularized ECM have also been shown to shift the macrophage polarization from M1 to M2 due to degradation of remaining biological products.[ 39 ] Decellularized ECM has been shown to release cytokines, growth factors such as VEGF, TGF‐β, and bFGF, as well as cryptic peptides upon degradation.[ 177 ] Incorporation of biological factors into biomaterials with tunable release profiles would be highly beneficial to directly modulate the FBR for appropriate tissue reconstruction.
In practice, the in vitro seeding of kidney‐derived pig cells on decellularized porcine kidney scaffolds displayed a positive proliferative ability, with cell numbers increasing between day 3 and day 7 postseeding. Most seeded cells also displayed Ki‐67, a staining marker that detects the proliferative ability of cells. Subcutaneous implantation of the constructs in rats indicated a lesser extent of cellular infiltration compared to in vitro testing, although there was a continued integration of the host cells into the scaffold. A mild host immune response appeared up to 7 days postimplantation, with complete resolution occurring 21 days postimplantation. No significant changes in macrophages were found although the decellularized scaffolds did present a significant decrease in neutrophils and lymphocytes compared to regular tissue.[ 178 ] The collective results of this study confirm that decellularized ECM can be used as a viable scaffold in both in vitro and in vivo experiments. Although integration and proliferation of cells was found in both situations, decellularized ECM does seem to induce a slight inflammatory response in vivo.
In an in vivo study on mouse models by Sadtler et al., mice that were treated with synthetic polyethylene (PE) or PEG scaffolds showed a more proinflammatory wound site over biological ECM implants, with an increased scaffold‐derived macrophage population and a collection of nonmyeloid cells including T cells, B cells, and NK cells within the microenvironments.[ 45 ] Interestingly, the stiffness of the material also affected granulocyte accumulation. When 3 PEG–diacrylate hydrogels with varying crosslinking densities were compared (PEG concentrations of 5%, 10%, and 15%; with 100, 900, and 2500 Pa of stiffness, respectively), the two stiffer concentrations created persisting ly6G+ neutrophil coronas that lasted over 3 weeks, while a 100 Pa stiffness resulted in a lower persistence of 1–3 weeks. Neutrophil density inversely correlated with the accumulation of fibrotic collagen within the materials’ microenvironment, displaying lower levels of collagen because of cellular activity. Increased PEG stiffness also decreased MHCII and CD206 expression, displaying involvement in the macrophage phenotype and CD4+ T cell recruitment.[ 45 ]
The bioactivity of decellularized ECM was also confirmed in an in vivo primate model, where decellularized scaffolds did not induce significant severe immune response compared to Xenograft cellular scaffolds.[ 179 ] Similarly, Brown et al. investigated the potential to trigger macrophage polarization using acellular allografts, cellular autografts and, cellular and acellular xenografts in a rat study.[ 180 ] Immunohistochemical and gene expression analysis using CCR7 and iNOS for M1 and CD163 and Arg for M2 macrophages revealed that cellular scaffolds had a majority of M1 phenotype and provided a dense, poorly organized collagenous FBC, while decellularized scaffolds favored the M2 phenotype, and displayed both neomatrix formation and early angiogenesis. As the mechanism of cell‐mediated FBR on decellularized scaffolds is yet to be fully understood, Fishman et al. investigated this by correlating in vitro and in vivo xenotransplantation results.[ 181 ] A splenocyte coculture model displayed diminished T cell activity, reduced levels of M1 cytokines IL‐2 and IFN‐γ, and increased levels of M2 cytokines when comparing decellularized scaffolds to cellular xenografts. This explained the attenuated T‐cell response and M2 polarization, indicated by an increase in CD163+ and Arg1+ cells and decrease of CD86+ and CCR7+ cells in decellularized scaffolds in vivo.
Aside from the biological components that naturally occur in the ECM, recent research has also looked into polymer‐like structures that are produced by organisms. One particular type of polymer that has gained attention over the years is chitosan. Chitosan is a natural deacetylated cationic polysaccharide consisting of glucosamine and N‐acetyl‐glucosamine, derived from chitin extracted from the exoskeleton of crustaceans. It displays antimicrobial and antioxidant effects and has gained recent popularity in tissue engineering through its low immunogenicity and nontoxic effects.[ 182 ] Since these materials reside in or are produced by the organisms, a high level of biocompatibility is expected from these materials. The main drawback of chitosan lies in its poor solubility in physiological solvents, limiting its use in the creation of biocompatible scaffolds.[ 183 ]
Recent research has tried to overcome this limitation by crosslinking chitosan and synthesizing various forms of hybridized hydrogels. An example is the combination of HA and chitosan, which has been investigated by Deng et al. as a regenerative application for abdominal wounds.[ 184 ] HA is a major component in the natural ECM and displays both excellent biodegradability and biocompatibility, although the rapid degradation time has limited its effectiveness in tissue engineering.[ 185 ]
The cytocompatibility of chitosan/HA (1:2 ratio) was assessed both in vitro on fibroblasts and in vivo in rat models, where the degradation rate was estimated at 60% weight loss over 7 days. In vitro cell viability was measured at over 90% survivability 48 h postseeding. In vivo histological analysis showed increased fibroblast and endothelial cell numbers compared to a polypropylene control. CD31, vimentin, Col1a2, and α‐SMA immunofluorescence displayed enhanced capillary density, gradual collagen deposition, and fiber formation, indicating the regeneration of a natural ECM and vascular network. Cytotoxicity of chitosan/HA gels were assessed with pan (CD68), M1 (CD86), and M2 (CD206) macrophages, where these gels displayed a significant increase in the M2 phenotype, indicating a resolution in the FBR. Gene analysis displayed significant upregulation of anti‐inflammatory factors IL‐10 and IL‐4. Upregulation of MCP‐1, bFGF, and TGF‐β indicated fibroplasia, while a decrease of signal transducer and activator of transcription (STAT) protein STAT1 and increase in STAT6 phosphorylation provides further evidence toward a shift to M2 polarization.[ 184 ]
Hydrogel forms of chitosan have also been used in situ to address general biocompatibility and degradation. Moura et al. tested the inflammatory responses on chitosan–genipin hydrogels prepared through either physical or physico/chemical crosslinking. After subcutaneous implantation in adult Wistar rats, degradation and structural rigidity was maintained for over four weeks, with a pore size range between 400 and 0.006 µm, and resulting in enhanced cell infiltration. Histological analysis displayed mild to moderate inflammation as a result of implantation, but no signs of fibrous encapsulation or FBGCs were found in either implants over a period of 30 days, proving that chitosan does not invoke a negative immune response in rats.[ 186 , 187 ]
3.14. Synthetic Polymers
Synthetic polymers have gained popularity over the years as a cheaper, uniformly reproducible alternative to natural materials, with improved structural integrity and functionality. Their usefulness is further expanded through modifiable properties such as porosity, degradation time, and mechanical characteristics.[ 188 ] However, synthetic polymers are limited in clinical effectiveness as the composition of these materials is recognized as a foreign body upon implantation, hereby always initiating an FBR. Additionally, the hydrolytic degradation that characterizes these materials releases CO2, which increases acidity in the local environment and may result in cell damage and tissue necrosis.[ 53 ]
The most commonly used synthetic polymers for the creation of scaffolds belong to the poly(α‐esters) group and include PLA, PGA, PCL, and PLGA. These polymers are often used in combination with natural polymers to improve hydrophilicity and biocompatibility, as they all possess a hydrolysable backbone and are susceptible to biodegradation once implanted.[ 189 , 190 ] The common drawback of poly(α‐ester) groups is the rapid degradation in vivo, causing accumulation of lactic and glycolic acid metabolites that induce inflammatory responses, halt cell growth, and prevent differentiation.[ 191 ]
PCL displays a high level of elasticity and drug permeability, and creates fewer toxic byproducts during degradation. However, PCL is less favorable due to its slow degradation time of 2–4 years and has extremely low cellular adhesive properties, requiring additional surface functionalization to be used in vivo.[ 192 ] Furthermore, PCL has shown to induce massive foreign body reactions when implanted in vivo. In a rat model by Duda et al., sheathing a chitosan‐based scaffold with a PCL shell was implanted to induce autologous nerve regeneration. Two weeks post‐implantation, the scaffold displayed severe FBGC formation in the entire PCL shell and peripheral chitosan layer with ingested material debris, indicating an advanced stage of fibrotic FBR.[ 193 ] Although not optimal, the biocompatibility of PCL can be slightly improved through electrospinning. In in vivo adipose flap expansions, nanofibrous PCL meshes expressed reduced inflammatory cytokine levels of (IL‐1β, IL‐6, TGF‐β) whilst also reducing FBC formation and collagen deposition.[ 194 ]
PLA is a popular biodegradable polymer that induces minimal inflammation in vivo due to its organic sources such as starch, and appears in various grades (PLLA, PDLA, PDLLA) and compositions depending on optically active enantiomers (α, β, γ).[ 195 ] The main drawbacks of PLA are its slow degradation rate when compared to other polymers and a high level of crystallinity, resulting in brittleness and enhanced inflammation.[ 196 ] Crystallinity can be improved through the addition of d‐lactic and d,l‐lactic acid monomers, but results in an accelerated degradation time.[ 195 ] PLA has been used in combination with PGA as effective clinical scaffolds since the 1960s, with varying FBR responses depending on the type of host, material concentrations, and implantation site.[ 197 ] Again, the morphology of PLA implants changes the biocompatibility, as shown in in vivo intramuscular implantation of PLA‐based membranes and electrospun meshes. Interestingly, PLA meshes accumulated a significantly higher amount of inflammatory CD68+ macrophages and FBGCs, even in early (7 days) postimplantation.[ 198 ]
PGA is desirable as a biomaterial component due to its excellent biodegradation, mainly because of the abundance of functional groups present in the hydrolysable backbone. It is also bioinert toward the host tissue and possesses relatively high mechanical strength. Surprisingly, the high degradation is also what creates undesirable effects, since in vivo PGA may degrade faster than the required time needed for regeneration. Further insolubility of PGA in most solvents also makes it difficult to study in vitro.[ 199 ] The PGA metabolite glycolide has also shown to induce FBR through the recruitment of monocytes and complement activation, in particular C5a upon the hydrolysis of PGA that activates both the classical and alternative inflammatory pathways.[ 200 ] As previously mentioned, PGA is most commonly used in combination with either PLA or PLLA, defined as PLGA.[ 197 ]
PLGA is made through the copolymerization of PGA and PLLA and considered the optimal biomaterial with regard to drug delivery performance. It allows for adjustable degradation rates and hydrophilicity by changing the PGA:PLA ratio, where higher PLA concentrations increase hydrophobicity, degradation time, and consequently incorporated drug release.[ 201 ] In biomaterials, PLGA has been proposed to be effective in bone regeneration when combined with hydroxyapatite (HAp) for their load‐bearing applications, osteoconductive, and osteoinductive properties.[ 202 ] As with PLA and PGA, the metabolite accumulation of lactic acid and glycolic acid are also what hinders this material from widespread use in vivo, since their highly acidic character induces an inflammatory immune response.[ 189 ]
The gold standard in many biomedical applications is PEG, due to its protein‐resistance abilities and active monocyte detachment by MMPs production.[ 203 ] However, immunogenicity of PEG depends on the degree of PEGylation to which molecule it is coupled with and have been shown to induce unpredictable complement activation.[ 204 ] For instance, PEG hydrogels triggered a robust inflammatory reaction characterized by a thick layer of macrophages, with higher secretion of M1 cytokines, TNF‐α, and IL‐1β, when compared to medical grade silicone, though still lower than tissue culture polystyrene (TCP).[ 205 ]
Overall, synthetic polymers display promising use in the field of biomaterials, but are hindered by several discussed drawbacks including possible toxicity, lower cell–material interactions, and an increased inflammatory response. Of note is that a lack of immediate cytotoxicity does not necessarily relate to a high biocompatibility, as a material that displays minimal toxicity in vitro, may behave entirely different once implanted in the host.[ 206 ] Optimization of implants is done through either the introduction of various new materials that display improved physicochemical characteristics, or the addition of bioactive compounds to already preexisting materials in order to functionalize them or reduce undesirable traits.
Recently, zwitterionic materials have emerged as a rising platform of biocompatible materials, due to its unique moieties with both cationic and anionic groups. The exertion of high hydrophilicity upon implantation shows potential in reducing the FBR and enhancing its performance.
Hydrogels loaded with ultralow‐fouling zwitterionic ions have already shown to completely negate the in vivo capsule formation of the FBR for a period of at least 3 months while also promoting angiogenesis in the surrounding microenvironment.[ 207 ] Jansen et al. further explored this potential by using PEG‐based hydrogels and loading them with different concentrations of zwitterion phosphorylcholine (PC).[ 208 ] PEG in its natural form invokes chronic inflammation in vivo, likely caused because of its susceptibility to degradation by macrophages and adherence of inflammatory proteins to the surface of the material.[ 209 , 210 ] PEG–PC hydrogels were tested both in vitro and in vivo with varying PEG/PC concentrations and stiffness gradients (3 ± 1 to 174 ± 34 kPa modulus). Hydrogel swelling inversely correlated with increasing stiffness. Results displayed variation in FBR formation depending on both hydrogel stiffness and zwitterionic concentration. Both anti‐inflammatory (IL‐10) and proinflammatory (TNF‐α) cytokines were observed in all hydrogels, with hydrogels containing the highest modulus and PC concentration invoking the strongest inflammatory phenotype in macrophages. The invoked FBR and capsule formation was furthermore modifiable by changing either the PC concentration or stiffness of the hydrogel. Overall, the exact use of zwitterionic ions in biomaterials remains to be further explored, as current evidence seems to contradict the exact usefulness of the material. Although Jansen et al. found proinflammatory markers when combining zwitterion PC with hydrogels, another study has found that a zwitterionic chitosan derivative creates protective effects, decreasing the risk of sepsis in mice and being an active suppressor of LPS‐mediated inflammation.[ 211 ] It is the combination of these findings that displays that, aside from the zwitterionic characteristics, the origin of the material as well as the combination with other components determines either a pro‐ or anti‐inflammatory state.
Zhang et al. have also investigated a long‐term in vivo mice model comparison between ultralow‐fouling pure carboxybetaine zwitterionic hydrogels (PCBMA) compared to conventional PHEMA hydrogels.[ 207 ] During the first week PCBMA attracted fewer inflammatory cells and after three months displayed minimum FBC formation with increased blood‐vessel density around the implant compared to PHEMA implants.
So far, the current optimal use of zwitterions seems to be in the nonfouling prevention of nonspecific protein adhesion, where the protein adhesion resistance of pSBMA was found to be up to 96% and 76% when exposed to fibronectin and bovine serum albumin, respectively.[ 212 ] What can be concluded is that further research must include both chemical and physical properties of the synthesized material, as evidence has been provided that material characteristics, concentration gradient, and stiffness affects the FBR.[ 208 ]
Nonfouling materials have also been introduced to materials through other densely grafted hydrophilic polymeric brushes functioning as a shield to minimize the absorption of serum proteins. Though Yu et al. mainly highlighted neutral polymeric brushes, both charged and neutral polymeric brushes have been investigated to modulate the FBR.[ 213 ] Charged polymers can be hydrophilic resulting in low protein absorption. Ma et al. developed a vast library of 216 cationic polymers, poly(β‐amino alcohols) via step‐growth polymerization, providing tunable brushes to modulate the FBR to one's biomedical purpose.[ 214 ] In vitro screening of monocytes/macrophages morphological response and proinflammatory cytokine secretion provided a prediction of FBR tunability, in which ex vivo histology confirmed the tunability of the cationic brushes.
3.15. Ceramic Biomaterials
The class of ceramic‐based material implants includes nonorganic materials that are defined by a crystalline makeup and supportive functionality in the regeneration of tissues that display a higher mechanical stiffness. In bone regeneration, resorbable grafts such as those based on calcium sulphate and hydroxyapatite (HAp) have gained popularity because of their structural functionality supporting weight, osteoconductive properties, and adjustable resorption rates to prevent fibrous tissue formation. Furthermore, upon resorption the release of ionic components (e.g., calcium or phosphate) in the microenvironment can stimulate skeletal cell proliferation and bone formation.[ 215 ]
Bone tissue is the second highest transplanted tissue and therefore a valuable target in biomaterial engineering. Although this tissue possesses a high capacity for natural regeneration, critical‐sized tissue defects require surgical intervention with autologous transplants or biomaterial‐based implants.[ 216 ] As the M2 polarization stage of macrophages is believed to be critical in osteogenesis, many ceramic biomaterial‐based implants have focused on immunomodulatory properties to tune the microenvironment toward the required inflammatory state. While M1 polarization through LPS, TNF‐α, IL‐6, IL‐1, M‐CSF, and iNOS is a necessity for early osteogenic repair, late phase remodeling of bone tissue requires an IL‐4, IL‐13, and efferocytosis stimulated anti‐inflammatory environment in order to initiate the excretion of M2‐derived products (IL‐10, TGF‐β, and osteopontin) and bone repair.[ 217 ] Two important types of ceramic biomaterials have been investigated for their immunomodulatory and bone neoformation properties: calcium phosphate (CaP) and brushite.
CaP cements were discovered in the 1980s and have been used as treatment for bone defects and deformities ever since. Today, a large variety of CaP‐based materials exist that differ in setting time, cohesion, fatigue, resorption rates, and potential drug delivery.[ 218 ] When utilized in combination with MSCs, CaP biomaterials induce ectopic and orthotopic bone formation, modulated by both the innate and adaptive immune system.
Macrophage populations are affected by the CaP–MSC interaction where MSCs caused macrophage polarization, which in turn induced osteogenic differentiation of MSCs. Whether it is the M1 or M2 polarization state that supports the initial osteogenesis is still under debate, as both states seem to affect this in differing methods (M1: early osteogenic differentiation, M2: matrix mineralization).[ 219 ] Humbert et al. also proposed that the optimal chance of successful bone regeneration with a minimal FBR is achieved through combining CaP biomaterials with MSCs, which in turn steers the immunomodulatory properties in the wound site toward promoting osteoclastogenesis and a controlled resolution state.[ 219 ]
Yuan et al. showed the capability of CaP biomaterials to induce higher biocompatibility and enhanced MSC migration through the addition of microporosity.[ 220 ] Through varying material composition (HAp, TCP or BCP) and sintering temperatures (BCP1150 °C, BCP1300 °C) micropore numbers varied with BCP1150 and TCP scaffolds displaying smaller grain sizes, larger surface areas (1.2 m2 g−1 for TCP as opposed to 0.1 m2 g−1 for HA) and increased protein adsorption. Furthermore, in vivo implementation in sheep models displayed that CaP scaffolds with increased porosity resulted in higher bone apposition (TCP: 28.7% ± 4.8%, BCP1150: 17.7% ± 5%, BCP1300: 11% ± 7.5%). TCP scaffolds also performed of similar quality to autologous bone in critical defects with new bone formation (33.9% ± 6.8%), scaffold resorption (56% to 21% within 12 weeks), and bone bonding without fibrous tissue formation. This data proposes microporous TCP biomaterials as biocompatible scaffolds for in vivo bone regeneration.
Finally, Alhamdi et al. have explored the use of a bioactive coating to create an appropriately timed M1/M2 macrophage phenotype.[ 221 ] A biomimetic calcium phosphate coating (bCaP) was used to serve as a localized delivery system for IFNγ and simastavin (SIMV). bCaP was chosen due to its high resemblance to natural bone mineral and ability to stimulate various regenerative processes such as enhanced cell adhesion and proliferation, and improved fixation to the host tissue.[ 222 ] IFNγ stimulated a proper M1 macrophage activation and initiates vascularization, whilst SIMV promoted an anti‐inflammatory M2 phenotype. This experiment aimed to use controlled release of each stimulant to induce a properly regulated inflammatory response. Timing of release is crucial where, especially in older organisms, the balance between M1 and M2 polarization may be dysregulated and impairs wound healing.[ 223 ] Polystyrene disks were first coated with SIMV incorporated into bCaP, followed by a coverage of IFNγ to create multilayered disks capable of controlled, sequential release. In vitro testing on bone marrow macrophages from elderly mice showed a release of 40 pg mL−1 IFNγ one day post‐implantation, with a continuous release of 7 pg mL−1 over the following 5 days. Gene expression of human THC‐1 monocytes and mouse bone marrow‐derived macrophages showed an upregulation in three M1 biomarkers (ILβ1, Nos2, CXCL11) on day 1, indicating an M1 phenotype. Delayed delivery of SIMV caused a significant increase in two M2 biomarkers (Ccl17, Arg1) on day 6, while all M1 biomarkers were decreased in expression. This change over time indicates a successful transition from an M1 to M2 macrophage phenotype over a period of 6 days and shows tremendous potential in biomaterial‐based drug delivery therapy.
The second popular type of ceramics, brushite‐based biomaterials, is of particular interest in replacing harder tissues in the human body. The combination of brushite with collagen is a new concept that combines the excellent solubility and resorption rate of brushite with the structural collagen framework sharing a high similarity with the makeup of bones. The low pH solubility of collagen as well as the setting of brushite, which occurs at similar pH values, creates an easily applicable, self‐settling cement, which is biocompatible, osteoconductive, and bioresorbable.[ 224 ]
Regular brushite, as well as eggshell‐derived brushite composites have been tested for their potential immune responses and biocompatibility in vivo. In mice, both materials showed integration of newly woven bone, with active osteoblasts present on the scaffolds, although eggshell derived brushite showed a significantly higher level of bone formation. During the 12‐week period, regular brushite displayed continuous construct degradation with surrounding inflammatory cells, while eggshell‐derived brushite scaffolds induced minimal inflammation. Interestingly, these effects between regular and eggshell derived brushite composites were not found during in vitro testing, giving rise to the question whether additional reactions by the hosts’ immune system influence the integration of the scaffold.[ 225 ] However, brushite also appears to stimulate the initial inflammation, as Fine et al. showed polymorphonuclear leukocyte activation upon interacting with brushite, indicated by elevated levels of CD11b, CD18, CD55, and CD66a. Furthermore, monocytes responded to brushite coating by elevated levels of CD63 and CD55.[ 226 ] While these cells mostly represent the initial inflammatory response, a brushite‐based coating might be beneficial in regulating proper FBR progression. Further research must be performed to validate the degradation rates in vivo and to assess the risks of brushite inducing elongated inflammation.
3.16. Concluding Remarks
A large number of biophysical properties seem to affect the behavior of the hosts’ immune response toward a biomaterial, where a variety of spatiotemporal signals are able to modulate cellular migration, adhesion, proliferation, differentiation, and apoptosis.[ 116 ] A variety of cell–material interactions can be regulated through the physical characteristics of a biomaterial. Cell motility and integration seems to be the most prominently affected feature, with material dimensionality, stiffness, wettability, and pore size all contributing to the migration around and within a construct. Furthermore, cell adherence to a material is defined by material composition, structure, and surface properties, which can be improved through the addition of a bioactive coating. Lastly, the structure, contact cues, wettability and stiffness of a material may also contribute to the polarization of immune cells, stimulating either a pro‐ or anti‐inflammatory phenotype.
Aside from physical interactions, the type and overall composition of a biomaterial is vital in determining the level of biocompatibility and severity of the FBR. Commonly used materials are often defined by having either a synthetic or natural origin. Both types induce positive and negative effects. Natural polymers are highly bioactive, while only creating a low level of inflammation, but are prone to enzymatic degradation and display poor mechanical properties. Synthetic polymers are customizable, have adjustable degradation rates, are cheap and reliable, but have low levels of cell–material interaction and may invoke severe immune responses when implanted.[ 172 ] It is often the combination of natural and synthetic polymers that creates optimal bioactivity, whilst also limiting the severity of the FBR (Table 1 ).
Table 1.
A comparison between synthetic and natural polymers, displaying the advantages and disadvantages of each type.
| Synthetic polymers | Natural polymers | |
|---|---|---|
| Advantages | Customizable | Bioactive |
| Adjustable degradation rates | Minimal FBR response | |
| Affordable | High cell–material interaction | |
| Minimal variability | ||
| Disadvantages | High FBR response | High variability |
| May induce metabolite toxicity | Limited mechanical properties | |
| Low cell–material interaction | Unwanted enzymatic degradation |
To fully optimize a material, all physical properties must be adjusted to match the intended use, and mimic the natural environment in situ. Although each characteristic displays its own effect on the surrounding cells and the environment, it is the collective properties of all these characteristics that determine the severity of the FBR post‐implantation.
3.17. Biochemical Responses to Biomaterials
Aside from the direct physical interaction between a biomaterial and its surrounding environment, a large number of biochemical cues also play a role in the cellular and molecular events that occur during the FBR. These factors, loaded within the implant or produced directly as a result of implantation, create an intricate network that steers the response of cells from both the innate and adaptive immune system. Many of these factors therefore determine the formation of a pro‐ or anti‐inflammatory environment that eventually decides cell fate and progression of the FBR. In order to optimally integrate a biomaterial it is vital that the interplay between biochemical cues and the immune response is steered toward a regenerative phenotype at the correct time.
3.18. Cytokine and Chemokine Triggers in Biomaterials
The chemomechanical signaling within the FBR is a highly modulatory factor in the determination of cellular behavior, and coordinates migration, proliferation, differentiation, and secretion.[ 227 ] Directly after implantation there is a significant upregulation of proinflammatory cytokines (IL‐1β, IL‐6, TNF‐α), which initiate acute inflammation and progress the FBR.[ 228 ] Additionally, chemokines are released, which are defined as a subcategory of cytokines and attract motile cells toward the environment through the activation of G‐coupled receptors.[ 229 ] The release of cytokines and chemokines regulates the blood flow to the area, controls the recruitment of lymphocytes and macrophages, is involved in collagen deposition and eventually contributes to angiogenesis and the promotion of vessel maturation.[ 75 , 230 ]
The importance of biochemical interplay between a biomaterial and the local environment is what has driven researchers to search for various methods to exploit these reactions. The developed methods have been introduced among a spectrum of complexity and include single cytokine targeting, polyclonal antibody responses, integration of poly‐amino acids, and the creation of full biomimicking microenvironments.[ 231 ] The increase in complexity in each method is attributed to the increasing number of biochemical compounds that are integrated and may further invoke multiple follow‐up reactions once introduced to the environment.
Besides cytokine‐mediated activation, macrophages are also responsible for the secretion of various biochemical substances. For M1 macrophages the major secretory chemicals are VEGF and several proinflammatory cytokines such as TNF‐α and IL‐1β, resulting in a positive feedback loop that creates a shift toward a proinflammatory environment. M2 macrophages mainly secrete chemicals responsible for ECM and tissue remodeling, including mitogens, platelet‐derived growth factors, and profibrotic ligands CCL17 and CCL18.[ 103 ]
Some of the more common cytokines used in biomaterial‐based research involves the proinflammatory IFN‐γ or IL‐4, which are important effectors in steering the macrophage phenotypes.[ 103 , 232 , 233 , 234 ] These compounds can be loaded in scaffolds, as demonstrated by Reeves et al., to successfully control the differentiation of monocytes toward either a pro‐ or anti‐inflammatory polarization state. In this study, silk‐based biopolymer films were loaded with either IFN‐γ or IL‐4 through which the release profile was adjusted depending on the film solubility and chemical conjugation of disulfide bonds, for a release up to 10 days.[ 235 ] Interestingly, while specific macrophage polarization stages can be induced in vitro through stimulation with cytokines, this remains difficult in vivo.
Spiller et al. physically absorbed IFN‐γ to decellularized bone scaffold while IL‐4 was immobilized by biotin–steptavidin binding to provide a rapid release of IFN‐γ and sustainable release of IL‐4.[ 236 ] Separate exposure of IFN‐γ and IL‐4 was conducted in vitro and resulted in M1 polarization from gene expression, and M2 polarization in gene and protein expression. However, no specific polarization was seen in vivo due to IFN‐γ and IL‐4 single releases. Moreover, in vitro and in vivo analysis on sequential release provided no skewing of M1 phenotype at early time points and M2 at later time points. Reasons for the lack of influence in sequential release results could be due to the poor sustainable release of IL‐4, while conflicting in vitro and in vivo results on single release could be due to time point differences, as well as influence of other cell types in vivo, stressing the need for in vitro coculture studies.
This was demonstrated by Mokarram et al. who confirmed the in vitro effects of IFN‐γ and IL‐4 in a coculture study using macrophage and Schwann cells (SCs). Results showed an increase in SC migration when exposed to IFN‐γ and IL‐4, while a decrease in SC proliferation occurred after IFN‐γ exposure.[ 237 ] IL‐4 exposed macrophages stimulated a higher SC migration and proliferation compared to IFN‐γ macrophages. In vivo, these compounds were used with loaded polysulfone tubes as biomaterial conduits across a gap defect showed that release of IL‐4 led to a significant increase in M2 macrophage accumulation and enhanced SC migration to the middle of the scaffold compared to the tubes releasing IFN‐γ, supporting the in vitro coculture prediction. Moreover, the IL‐4 releasing tube stimulated axon regeneration 20 times greater than the IFN‐γ releasing tube, and interestingly the ratio of M2/M1 markers was linearly correlated with axon regeneration.
So far, we have highlighted the benefits of shifting macrophage polarization to an M2 phenotype, although a combination of mixed M1 and M2 population in the early onset of the FBR is also observed to be beneficial in implant acceptance, especially in regard to angiogenesis and vascularization.
Spiller et al. in vitro studies showed that M1 macrophages produced the highest levels of potent angiogenic mediators including VEGF; M2a macrophages secreted the highest levels of a chemoattractant stabilizing pericytes, platelet‐derived growth ββ (PDGF)‐ββ, and promoted endothelial sprouting; and M2c macrophages expressed the highest levels of MMP9, which is crucial for vascular remodeling.[ 75 ] Murine subcutaneous implantation of unmodified, LPS‐coated, and glutaraldehyde‐crosslinked porous collagen scaffold displayed its influence on macrophage polarization. Immunohistochemical analysis revealed that the unmodified scaffolds were surrounded by the highest numbers of M2 macrophages, followed by a dense tissue capsule. LPS‐coated scaffolds, as expected, stimulated high amounts of M1 macrophages, but with no visible fibrous encapsulation. Interestingly, glutaraldehyde crosslinked scaffolds triggered a tissue capsule infiltrated by many newly formed blood vessels and exhibited a mixed quantity of M1/M2. This explained the controversy over which macrophage phenotype triggers angiogenesis and suggests the need to pertain presence of both M1 and M2 macrophages for necessary angiogenesis and scaffold vascularization.
As angiogenesis in FBR involves various growth factors at different stages of blood vessel development, sequential delivery of proangiogenic growth factors could provide a more stable vascular network. Though VEGF have been shown to trigger ECs migration, proliferation, sprouting, and lumen formation, PDGF may attract required mural cells and prevent vessel regression. Awada et al. developed a sequential delivery system using VEGF directly embedded into the fibrin gel and PDGF‐ββ indirectly embedded via a heparin‐based coacervate, to provide rapid release of VEGF and slower sustainable release of PDGF‐ββ.[ 238 ] PDGF‐ββ coacervates provided an increase in SMC migration and proliferation, and sequential delivery enhanced endothelial proliferation and sprouting in vitro. In vivo studies were performed in a rat myocardial infarction (MI) model, and showed enhanced preservation of cardiac function and reduced cardiac fibrosis. Most importantly, sequential delivery provided persistent angiogenesis and cardiac functionality. Though this study confirmed enhancement in blood vessel stability by sequential delivery, comparison was only done with the absence of growth factors or with uncontrolled delivery of growth factors. Cao et al. provided a more extensive study, which compared angiogenesis of pellets releasing VEGF, PDGF‐ββ or FGF‐2 alone, and dual release of VEGF and PDGF‐ββ, VEGF, and FGF‐2, as well as PDGF‐ββ and FGF‐2 (PDGF‐ββ/FGF‐2) in a mouse corneal micropocket and matrigel assay, as well as rat and rabbit hind limb ischemic model.[ 239 ] All types of dual release provided a substantial increase in angiogenesis, with PDGF‐ββ/FGF‐2 providing the highest number of microvessel formation. Over time PDGF‐ββ/FGF‐2 provided awell‐defined vascular tree‐like structure with increased mural cell association, while other releasing pellets induced vessels regression. Moreover, only PDGF‐ββ/FGF‐2 provided vascular stability despite depletion of angiogenic factors at day 6. Furthermore, the stable vessels stimulated the highest collateral growth and blood perfusion. As presence of mural cells are necessary to maintain stability of ECs forming vessel, an in vitro coculture model using endothelial and mural cells could be beneficial to understand the mechanism underlining the differences in these proangiogenic growth factors.
With regard to the commonly used synthetically produced polymers, cytokines and growth factors have been analyzed to assess whether a material classifies toward the promotion of either a pro‐inflammatory or anti‐inflammatory environment. Generally, cytokine, chemokine, and growth factor levels slowly decrease for up to 8 weeks postimplantation. Commonly elevated cytokines in polymers such as PE and polyurethane can be pro‐regenerative (VEGF, IL‐1β, IL‐4), anti‐regenerative (IL‐10, IL‐2, IL‐6, MCP‐1, MIP‐α, TNF‐α), anti‐inflammatory (IL‐4, IL10, VEGF) or pro‐inflammatory (IL‐1β, IL‐2, IL‐6, MCP‐1, MIP‐1α, TNF‐α).[ 240 ] Furthermore, macrophage fusion cytokines (IL‐4, IL‐13) and Th cell regulatory cytokines (IL‐2) can be expressed depending on the nature of the implant. Interestingly, material types that do not promote macrophage fusion, such as Elasthane 80A (PUE) or silicone rubber (SR), display a significant upregulation of IL‐6 and TNF‐α and promote a proinflammatory environment.[ 241 ]
TGF‐β and PDGF have been considered the main modulators of the FBR, providing the chemotactic signals for monocytes, macrophages, and fibroblasts. TGF‐β activates these cells and enhances formation of ECM proteins. Re'em et al. displayed sustainable release of TGF‐β1 from affinity‐binding alginate scaffolds, providing a significant increase in in vitro and in vivo collagen deposition and cartilage matrix deposition.[ 242 ] Additionally, to provide a closer prediction of the in vivo implantation outcome of TGF‐β‐loaded microcapsule for cartilage regeneration, Guo et al. fabricated a bilayered hydrogel composites mimicking the structure and function of osteochondral tissue, using MSCs and osteogenic cells.[ 243 ] A versatile drug delivery system of using self‐assembled peptide nanofibers (NFs) provided the capability of conjugating proangiogenic mediator, PDGF‐ββ, VEGF‐A, bFGF, and angiopoietin‐1 (ANGPT1).[ 244 ] However, despite of PDGF‐ββ capabilities in regulating all three stages of FBR, including angiogenesis, no enhancement of neovascularization was seen, possibly due to the weak nonspecific binding with NFs peptide. Moreover, direct modulation of angiogenesis using VEGF alone has been extensively investigated and provided successful triggers for angiogenesis.
Another complicating factor is the integration of multiple cell types within the design of a biomaterial. An excellent example is provided by Parks et al., who elucidated the vital effect of monocultures and 3D cocultures of fibroblasts and monocytes on inflammation.[ 245 ] When quantifying the inflammatory cytokine protein expression levels of human IL‐1β, IL‐6, IL‐8 GM‐CSF, and TNF‐α, there was a significant elevation in cocultures compared to monocultures. This was repeated with the addition of PLGA particles to both cultures to represent the addition of a biomaterial. Again, important differences were seen in the different layouts, where monocultures with monocytes did not trigger an immune response at all whilst fibroblasts expressed significant levels of IL‐1β and IL‐6. The combination of monocytes and fibroblasts produced a full expression of all five previously mentioned cytokines with a significantly heightened expression. This experiment indicates that individual cell colonies may present an entirely different biochemical profile compared to cocultures. Additionally, the integration of a biomaterial, as well as the environment in which cells reside may all contribute to secretory variation and the consecutive progression of the inflammatory response.
The external delivery of various cytokines in order to trigger desirable effects has also been tested in vitro. The chemokine MCP‐1, used for mural cell recruitment, has been used in a dual delivery setup with VEGF, which improves survival of transplanted EC. Jay et al. fabricated alginate microparticles to deliver VEGF and MCP‐1, possessing distinct release kinetics that can be integrated into a collagen/fibronectin gel construct for EC transplantation support.[ 246 ] Dual release of VEGF and MCP‐1 increased functional vessel formation and SMC‐invested vessels and a majority of M2 macrophage polarization indicated by the Arg1/iNOS ratio. Sequential delivery of chemokines could also stimulate M2 polarization.
Kim et al. used an MSC chemokine, stromal‐derived factor‐1 (SDF‐1), and sphingosine‐1 phosphate agonist (SEW2871) were incorporated in micelles and embedded into gelatin hydrogels to provide release in a controlled manner.[ 247 ] In vitro monoculture studies displayed an increase in MSC migration with SDF‐1 alone, while macrophages required a dual release for increased migration. Coculture studies of macrophages and MSCs showed increased levels in TNF‐α, IL‐10, and PGE2, and an increased population of M2 macrophages. The addition of fibroblasts in either monoculture macrophages or MSCs showed decreased expression of angiogenic ANGPT1 and VEGF genes from both, with higher expression levels in MSC conditioned medium. Coculture systems provided a closer indication of the in vivo results, in regard to cytokine secretion and macrophage polarization, in which single release of SEW2871 induced the recruitment of M1 phenotype, and dual release of SDF‐1 and SEW2871 stimulated M2 macrophage polarization.
3.19. Coculture Studies
The combination of proangiogenic growth factors and other macrophage‐recruiting factors release from biomaterials have been shown to be crucial in proper vascularization of the implant site. Macrophages have been highlighted to be a crucial mediator of angiogenesis in the FBR and provided recruitment and stabilization of pericytes. Hsu et al. investigated the correlation PDGF‐ββ/FGF‐2 synergy to pericyte and macrophage recruitment.[ 248 ] When implanting polyethylene glycol diacrylate hydrogels in vivo, live imaging found PDGF‐ββ/FGF‐2 to stimulate macrophage infiltration several days before robust vessel formation and pericyte recruitment, with involvement of both M1 and M2 macrophages. Additionally, the role of macrophages on VEGF‐induced vessels was investigated using colony stimulating factor 1 (CSF1). Macrophage‐mediated CSF1 recruitment improved angiogenic response of VEGF, providing a robust and stable angiogenic response similar to PDGF‐ββ/FGF‐2 induced vessels. However, long‐term investigation should be done to confirm the stability of VEGF/CSF1 induced vessels.
The effects of angiogenic growth factors (VEGF, FGF‐2) and hepatocyte growth factors (HGFs) on macrophage polarization have also been demonstrated in albumin–alginate microcapsules HGF/FGF‐2 delivery provided an increase in macrophage accumulation compared to VEGF‐A delivery, with a majority of M2 macrophage, while VEGF‐A delivery stimulated both M1 and M2 macrophages.[ 249 ] These results indicate that in vivo therapeutic regulation of the FBR can benefit from coupling the release kinetics of multiple growth factors within biomaterials.
The success of most tissue‐engineered constructs involves the recruitment of ECs and formation of new blood vessels, mediated by macrophages. Bone marrow mononuclear cells (BMCs) seeded onto biodegradable scaffolds from a PGA/[P(CL/LA)] polymer combination, remodeled to a functional blood vessel in vivo with EC lining by SMCs, provided a clinically useable tissue‐engineered vascular graft.[ 250 ] Roh et al. investigated the remodeling process by implanting the human BMC seeded scaffolds into an immunodeficient mouse recipient, and showed that seeded cells were no longer detected a few days after implantation.[ 250 ] Rather, the process of inflammation‐mediated vascularization was observed, potentially driven by infiltration of mouse monocytes, followed by accumulation of mouse ECs and SMCs and gradually transformed the implant into functional neovasculature over a period of 6 months.
In vitro studies suggested that seeded BMCs secreted significant amounts of MCP‐1 to provide an increase of early monocyte recruitment, leading to the mediated vascularization. As macrophage polarization is responsible for tissue remodeling and neovascularization, cell transplantation of polarized macrophage could provide many therapeutic benefits. Jetten et al. transplanted M1‐induced IFN‐γ, M2a‐induced IL‐4, or M2c‐induced IL‐10 eGFP labeled murine BMDMs for its effect on reperfusion recovery and collateral remodeling in vivo.[ 251 ] All transplanted polarized macrophages improved reperfusion recovery and were preserved, despite of clodronate liposome treatment, causing depletion of endogenous circulating monocytes and macrophages. This again suggests the importance of coexistence of both macrophage populations, and its importance in mediating tissue regeneration.
Although there is quite a lot of understanding with regard to the biochemical components that reside in the microenvironment, it remains difficult to integrate this in synthetically made biomaterials due to the complexity and adaptive responses depending on the implant site. For this reason, research has also laid the focus on the utility of native scaffolds, with in particular natural components derived from the ECM.
The ECM is composed of many natural components, including fibers, proteoglycans, glycoproteins, and polysaccharides.[ 252 ] The specific composition creates a 3D architecture, which exerts both structural and functional characteristics that are crucial in steering the initial FBR. Together with surrounding cells and growth factors, the ECM regulates cell proliferation, differentiation, and provides a supportive network for the creation of vascular, lymphatic, and nervous branches.[ 177 , 253 ] The biomimetic nature of the components residing in the ECM are beneficial for cellular homeostasis and may provide potential therapeutic uses when integrated in biomaterials.
Within the ECM, matrix metalloproteases (MMPs) are important enzymes that are capable of hydrolyzing components, hereby playing a critical role in ECM maintenance and various other physiological processes.[ 254 ] In the inflammatory response, MMPs are secreted by fibroblasts and leukocytes, which determine the rate of ECM degradation, leukocyte infiltration, and consequent inflammation. Varying stages of the inflammatory response are controlled by different MMPs, including overall inflammation, cell morphogenesis, tissue remodeling, wound healing, and cell apoptosis.[ 252 , 255 ] MMPs have also been shown to play a role in the promotion of TNF‐α, IL‐1β, and TGF‐β by cleaving environmental network components that would have otherwise restricted the dispersion of these molecules.[ 256 ] The immense amount of interactions have made MMPs a valuable target for increasing degradation in biomaterials. Specifically, MMP‐1 and MMP‐8 are important, as they have been found to be active during wound healing and remodeling. Both are used in the cleavage of collagen, with MMP‐1 being more proficient in the cleavage of collagen type III, while MMP‐8 is more efficient in degrading collagen type I and is the main collagenase active in regular healing.[ 257 , 258 ]
Another major component of the ECM is HA, a glycosaminoglycan (GAG) that regulates lubrication through the binding of water, and is responsible for various cellular processes related to movement and maturation.[ 259 ] Within a wound site HA accumulates and is responsible for the recruitment of leukocytes, suppresses the inflammatory response in its complete form, and depolymerizes when exposed to an inflamed environment, which activates macrophages and DCs.[ 260 ] Interestingly, HA fragments have also shown to induce forms of anti‐inflammatory effects through a toll‐like receptor (TLR‐4) in colonic tissue, revealing the tissue microenvironment as a contributive factor to the effects of depolymerized HA fragments.[ 261 ] Beldman et al. tested the potentially therapeutic effects of HA‐functionalized NPs in vivo on atherosclerotic rabbits where, compared to a control, immune cell interactions significantly decreased in all HA‐treated animals.[ 262 ] HA–NP treatment resulted in a 30% macrophage decrease when compared to either free HA insertion or the control. This study indicates that HA can display a therapeutic effect, and specifically reacts to proinflammatory macrophages depending on disease progression. HA‐based biomaterials may also act as atherosclerotic therapeutics through their reduction in macrophages (30%) and an increase in collagen content within plaques (30–40%) that is critical in improving stability.
The potential of GAGs has been incorporated within biomaterials as bioactive surface proteins, which can modulate the biochemical profile within the surrounding environment of an implant. GAG implementation on materials is often performed through covalent surface crosslinking, where natural polymers such as HA and Heparin are added to biomaterial surfaces through a layer‐by‐layer technique, creating a more hydrophilic, negatively charged surface. The addition of GAGs as surface proteins reduces the levels of IL‐1β and NF‐κB, as well as an overall reduction of FBGC formation compared to a NH2 control.[ 263 ] The effective natural coating also reduces the overall opsonization, likely caused due to the increased levels of hydrophilicity.[ 264 ] The addition of multilayered bioactive coatings derived from ECM substances seems to interact with THP‐1 derived macrophages and creates an overall immunosuppressive effect, where a coating combining Hep and chitosan (Chi) seems to result in the lowest expression of mononucleated giant cells, indicating the lowest level of frustrated phagocytosis.[ 263 ] These findings were in correspondence to Zhou et al., who also found a decrease in IL‐1β and overall FBGC formation, with a Hep–Chi coating creating a significantly lower FBR.[ 265 ] Overall this displays a promising role of GAG‐integrated coatings for increasing biocompatibility. However, all studies mentioned were only performed in vitro with THP‐1 derived macrophages. To confirm the potential of GAG coatings, particularly Hep–Chi multilayers, in vivo assessment of other possible interactions must be performed.
3.20. Concluding Remarks
The biochemical interplay between cells and biomaterials is perhaps one of the most critical factors in regulating the progression of the FBR. As the cell–implant interactions create a mixture of signaling molecules, it is the collective microenvironment that affects cellular behavior and directly coordinates migration, proliferation, differentiation, and secretion.[ 227 ] The network is further complicated by a ripple effect, where one signaling molecule may be an effector for multiple cell types. This not only creates the coordinated progression of the FBR, but also has a direct effect on the inflammatory phenotype of macrophages, Th cells, DCs, and FBGCs.[ 103 , 266 ] A collection of all biochemical components that were discussed in this review can be found in Table 2 .
Table 2.
A categorization of discussed biochemical compounds that have been used in biomaterial‐related studies to influence the FBR. Compounds are structured according to their active molecule/receptor, the used biomaterial, cell targets (excreted and responding), in vitro or in vivo studies and their observed responses with regard to a pro‐inflammatory or anti‐inflammatory effect.
| Molecule/effector | Biomaterial | Target cells | In vitro/in vivo | Observed results | Refs. |
|---|---|---|---|---|---|
| Heparin, chitosan | Polyelectrolite multilayers on glass surfaces | Macrophages, FBGCs | In vitro | Decrease of macrophage spreading, IL‐1β expression, and FBGC formation. Significantly reduced FBR. | [265] |
| Hyaluronic acid | HA‐functionalized nanoparticles | Macrophages | In vivo | 30% decrease in macrophage population within wound site. 30–40% increase in collagen content. Beneficial effect in stability for atherosclrerotic lesions. | [262] |
| Hyaluronic acid, heparin | Layer‐by‐layer chitosan scaffolds with functionalized coatings | Macrophages, FBGCs | In vivo | Lowered IL‐1β and NF‐κB levels. Reduced inflammation & reduction of FBGC formation. | [263] |
| IFN‐γ, IL‐4 | Loaded polysulfone tubes. | Schwann cells, macrophages | In vitro/in vivo | Sc accumulation and control over early macrophage polarization | [235] |
| IFN‐γ, IL‐4 | Silk‐based biopolymer | Schwann cells, macrophages | In vitro/in vivo | Controlled release of cytokines steering the M1/M2 polarization of macrophages | [236] |
| IL‐1β, IL‐6, IL‐8 GM‐CSF, TNF‐α | PLGA particles | Fibroblasts, monocytes, macrophages | In vitro | Varying expression profiles between individual cell colonies and cocultures. Enhanced secretion of all cytokines when exposed to biomaterials but variation found in monocultures. | [245] |
| IL‐6, TNF‐α, TGF‐β, IL10 | PUE (Elasthane 80A), SR (silicone rubber), PET (polyethylene terephthalate) | Monocytes, macrophages, FBGCs | In vivo | Varying levels of both pro‐ and anti‐inflammatory cytokines at wound sites over 14‐day period. Continuous IL‐6 and TNF‐α expression in PUE/SR. | [241] |
| MCP‐1 | Polyglycolic acid (PGA) grafts fitted with cellular cocultures | BMCs, monocytes, macrophages | In vitro/in vivo | Early monocyte recruitment through BMC mediated increase of MCP‐1. Full resorption of BMCs days after implantation and induction of inflammation‐mediated vessel formation. | [250] |
| MCP‐1, VEGF | Alginate microparticles integrated in collagen/fibronectin gel | SMCs, macrophage polarization | In vitro | Controlled dual release of VEGF and MCP‐1. SMC‐induced vessel formation and M2 polarization of macrophages through Arg1/iNOS ratio. | [246] |
| PDGF‐ββ, VEGF‐A, bFGF, ANGPT1 | Injectable peptide nanofibers (NFs) | ECs, SMCs | In vitro/in vivo | Increased angiogenesis but no major increase in neovascularization. Interaction with in vivo cardiomyocytes through PDGF‐ββ regulation. | [244] |
| PDGF‐ββ/FGF‐2, CSF1, VEGF | PEG–diacrylate hydrogels | Pericytes, macrophages | In vivo | Early recruitment of macrophages and pericytes. Robust vessel formation. Macrophage polarization of both M1 and M2 observed. Stable angiogenic responses in vivo. | [248] |
| SDF‐1, SEW2871 | Micelle incorporation in alginate hydrogels | MSCs, macrophages | In vitro/in vivo | Increased migration of MSCs in SDF‐1 with increased macrophage migration in dual‐release conditions. Increased release of TNF‐α, IL‐10, and PGE2. | [247] |
| TGF‐β1 | Affinity‐binding alginate scaffolds, bilayered cell‐laden hydrogels | MSCs, macrophages | In vitro/in vivo | Sustained TGF‐β1 release. Enhanced collagen production and cartilage matrix deposition. | [242, 243] |
| VEGF, PDGF‐ββ | Heparin‐coacervated fibrin gel | SMCs, ECs | In vivo | Sequential delivery of VEGF followed by PDGF‐ββ. Promoted SMC migration, EC proliferation, and angiogenesis | [244] |
| VEGF, PDGF–ββ, MMP9 | Collagen/glutaraldehyde scaffolds | Macrophages | In vivo | Promoted angiogenesis and vascularization in mixed M1/M2 models | [75] |
| VEGF, PDGF‐ββ or FGF‐2 | Matrigel micropellets | ECs | In vivo | Angiogenesis and controllable vessel formation in vivo | [239] |
| VEGF‐A, FGF‐2, HGF | Albumin–alginate microcapsules in matrigel plugs | Macrophage polarization | In vivo | Combination therapy resulted in enhanced M2 macrophage infiltration whilst single compound treatments with VEGF‐A mainly recruited M!‐type macrophages | [249] |
Future research should take into account the highly dynamic nature of the biochemical microenvironment, where slight changes can be introduced to steer the FBR progression toward a resolution state. Of particular interest is the use of biological products already present in the ECM, where components such as MMPs and GAGs can be exploited to steer cell–cell interactions, recruitment, cell–matrix interactions, biochemical profile modulation, binding of proteins, and interactions with cells through cell surface receptor and proteins.[ 259 , 267 ] In particular, bioactive surfaces of GAGs or Mg have resulted in lower secretory levels of proinflammatory cytokines, decreased FBGCs, less frustrated phagocytosis, and increased tissue restoration.[ 263 , 268 ]
Overall, the implants’ microenvironment already offers a multitude of cues that regulates the progression of the immune response. Currently, in vitro studies are already exploiting these mechanisms through the addition of functional groups to a biomaterial in order to optimize cellular responses. The next generation of biomaterials may greatly benefit from the incorporation of these biochemical cues, being either integrated within the scaffold or as an interactive coating, functioning as an important contributor to changing the inherent properties, and may overall help in making the material more biocompatible.
3.21. The Effects of Biomaterials on Metabolomics
The high dynamism of the human body suggests that a variety of metabolic mechanisms are involved in the process of inflammation. The variety of molecular mechanisms and cell–cell interactions creates a complex network of signaling pathways and metabolites that are involved in the modulation of both pro‐ and anti‐inflammatory processes. Here, molecular pathways are discussed that are involved in the regulation of inflammation and could potentially play a role in improving the effects of the FBR. A select number of metabolites derived from biomaterials have also been shown to affect the inflammatory response. Obtaining further insight in the molecular makeup and function of these molecules may potentially serve a role in the creation of a next generation biomaterials.
The metabolic behavior of macrophages has been considered a key factor in energy regulation and biosynthesis, but recent evidence has also established metabolic signaling to be involved in functional behavior and polarization. Environmental metabolites including proteins, lipopolysaccharides, and interleukins have been found to play a plethora of roles in the M1 to M2 transition, creating a bidirectional network between stromal cells and macrophages that modulate local inflammation.[ 269 ] The engagement of metabolites in both intra‐ and extracellular signaling pathways can drastically change the phenotype of the cell, since these interactions often involve changes in pathways required for general cellular homeostasis. Examples are the glycolysis and the pentose phosphate pathways, facilitating the cells energy in the form of ATP, NADPH, and ribonucleotide precursors. Stimuli may furthermore react in either a synergistic or antagonistic manner, with multiple external sources creating a cascade that may ultimately trigger either a pro‐ or anti‐inflammatory effect in the macrophage (Figure 5 ).[ 270 ]
Figure 5.
A simplified overview of metabolomic products influencing the FBR. Natural metabolic products may originate from the degradation of the biomaterial implant, be excreted by cells, or are present in the ECM. These products include signaling molecules (cytokines and interleukins), fats (lipopolysaccharides), proteins or metabolic products created through the breakdown of larger substances. Within the microenvironment of the FBR, the mixture of these products bidirectionally influences the interaction between stromal and inflammatory cells and may directly contribute toward skewing the FBR toward an inflammatory or resolutory state.
Meiser et al. presented an example in which macrophages attain a proinflammatory state through metabolic changes after exposure to external stimuli. Cytokines secreted by Th1 cells or bacterial lipopolysaccharides result in an immediate shift to a pro‐inflammatory state. Within these processes Th1 cells actively promote the M1 phenotype of macrophages through excreted cytokine interactions including IFN‐γ and TNF‐α; whilst bacillary LPS triggers both monocytes and macrophages to take on a pro‐inflammatory state by TLR4, mediated through serine/threonine kinase Akt Pathway (SHIP1).[ 271 , 272 ] To compensate for sudden changes in cellular activity, changes in HIF1 gene expression control the flux of downstream metabolites such as the inhibition of pyruvate dehydrogenase (PDH). With PDH concentrations being an important trigger for a switch to an inflammatory state, external influence on this metabolite might be a future target for the prevention of an LPS‐activated inflammatory switch.[ 273 ]
The process of glycolysis has been defined by Soto‐Heredero et al. as a prominent pathway in the homeostasis and modulation of immune cells.[ 274 ] Glycolysis is considered one of the major processes in providing energy for the cells, involving several enzymatic reactions that take part in converting glucose molecules to pyruvate and is fully metabolized to produce energy. Although the net energy production is considered to be less effective than oxidative phosphorylation (OXPHOS), glycolysis is preferred by cells that undergo high levels of proliferation. During inflammation, the route of energy acquisition strongly defines the fate and impact of immune cells, with highly inflamed cells preferring glycolysis whilst the OXPHOS metabolic pathway is favored during resolution.[ 275 ] The metabolites of glycolysis are furthermore responsible for the activation of the pentose phosphatase pathway, the hexosamine pathway and glutaminolysis, subsequently promoting cellular growth and differentiation.[ 276 ]
Further evidence for the involvement of glycolysis in inflammation can be found in each phase of the inflammatory response. Different cell types can utilize the glycolytic pathway to initiate inflammation. After the identification of pathogens through various pattern recognition receptors, DCs may undergo a metabolic switch to glycolysis by expressing Nos2 and produce NO, hereby effectively blocking OXPHOS and enhancing T‐cell responses.[ 277 , 278 ] ECs are another type of cell that utilizes glycolysis to initiate inflammation. Albeit that ECs already rely on glycolysis for energy production and basic functionalities such as permeability and vasodilation, overexpression initiates expression of activation markers, pro‐inflammatory cytokines and chemokines that activate the inflammation process.[ 279 ]
Glycolysis further plays a role in the modulation of various immune cells. In neutrophils, glycolysis is directly involved in transmigration and microbial clearance. The metabolic switch between glycolysis and OXPHOS directly influences t‐cell migration. Finally, glycolysis is vital in M1 macrophage activation. Inhibiting glycolysis in M1 macrophages disrupts phagocytosis, ROS production, and cytokine secretion; effectively deactivating M1 functionality.[ 274 , 280 , 281 , 282 ]
Another group of molecules that may positively affect the inflammatory response through metabolic pathways are flavonoids. These compounds have shown to induce a shift from a pro‐ to anti‐inflammatory state within macrophages, hereby promoting localized resolution. Various mechanisms have been accredited to this shift, including inhibition of NF‐κB, PI3K/Akt, and mTORC1 signaling, regulation of the MAPK and arachidonic pathways, lowering of ROS and NO concentrations, and general downregulation of pro‐inflammatory gene expression.[ 283 ] Mendes et al. have tested several flavonoid types on human monocytic THP‐1 macrophages that were polarized to an M1 phenotype (LPS + IFN‐γ incubation).[ 284 ] Three flavonoids were tested (quercetin, naringenin, and naringin) and all significantly altered the endometabolome of M1 macrophages, each with overlapping and distinct impacts. Overall, all tested flavonoids significantly reduced TNF‐α levels. IL‐β1 and IL‐6 also showed reduced concentrations, although these levels were not significant. Furthermore, a metabolomics NMR assay revealed flavonoid‐modulated metabolic effects in various pathways, including the tricarboxylic acid (TCA) cycle, antioxidant makeup, and macrophage membrane composition. Collectively, the various effects of flavonoids may contribute to stimulate local resolution of inflammation.
Interestingly, knowledge on the involved metabolic pathways may also serve as a foundation for the development of new biomaterials. Korley et al. utilized dihydroxyacetone (DHA), a diol present in the eukaryotic glucose metabolic pathway, in the synthesis of a novel polyester family with the potential of being converted to a biomaterial.[ 266 ] Polymers based on DHA showed potential in biomedical devices, tissue engineering, and sustainable plastics due to their high biocompatibility and degradation rate, but are difficult to use in polymers due to their intrinsic reactivity and unstable C2 carbonyl group.[ 285 , 286 ] Korley et al. combined DHA with even‐carbon aliphatic diacids found in the TCA cycle metabolic pathway, hereby creating DHA‐based polyesters through one‐step synthesis. These polymers showed varying thermal responses based on molecular weight and diacid length, allowing for modifiable degradation rates. In vivo, degradation rates varied between ≈6% and ≈70% over a duration of 16 weeks depending on diacid length and molecular weight. Furthermore, a normal inflammatory response was observed with resolution of inflammation within 4 weeks. Based on these results, this polymer serves as an excellent example for future metabolite‐derived biomaterials.
The immense number of monomers derived from metabolomic processes in macrophages offers ample opportunity for the creation of novel biomaterials. Molecular engineering of these structures can give rise to polymers that have almost limitless structural formation, functionality, and performance characteristics. These polymers can be optimized further through the addition of various reactive/functional groups and stereochemical centers, effectively creating synthons. Furthermore, after completing their intended function, these synthons can be designed to degrade back to their original monomeric state and be eliminated through the natural metabolic pathways. Ricapito et al. have established a collection of natural metabolic monomers that can be utilized to create a new generation of metabolically derived biomaterials.[ 286 ]
The practical utility of metabolic processes in biomaterials has recently been rising as a characterization tool for NP, where metabolic sequencing has given insight in the direct effects of NPs on the molecular upregulation within macrophages. Research by Saborano et al. has indicated these changes in the metabolic profile and its related effects on the inflammatory response.[ 287 ] Since NPs have attained increased relevance as modulatory coatings on biomaterials, it is vital that the underlying molecular mechanisms are explored as thoroughly as possible. Three types of NPs were investigated and included silk, PLGA, and silica with a size diameter ranging from 100 to 125 nm. These types were highlighted due to their current use in clinical and/or pharmaceutical treatments or shown potential in future therapeutics. Introduction of all types to RAW 264.7 macrophages, followed by H‐NMR assessment revealed variations in ≈40 identifiable metabolites. Variance of the metabolic profile was also noted between differing exposure times and NP concentration. Macrophages that were exposed to low NP concentrations generally displayed a metabolic profile resembling control. Interestingly, 72 h of exposure created three distinct cluster profiles in a principle component analysis, related to the used NP type. The most significant metabolite changes were noted in glycolysis upregulation resulting in alterations of the TCA cycle and decreased ATP generation, indicating a switch to a proinflammatory phenotype. Further changes were found in the creatine kinase system, cluster dependent upregulation of various amino acids and an increase of osmolytes and antioxidants. Although this indicates potential proinflammatory effects after exposure to NPs, the exact magnitude, as well as effects in vivo is yet to be elucidated. Furthermore, this research has shown that the molecular makeup of NPs can invoke varying metabolic responses, further highlighting the importance of material type in NP research.[ 287 ]
The proinflammatory effects of NPs were also found in titanium dioxide (TiO2) NPs, which are already commonly used in foods, cosmetics, and medicine.[ 288 ] Chen et al. conducted a proteomic study in which TiO2 NPs with a diameter size of 10 nm were introduced to RAW macrophages.[ 289 ] After a 24 h exposure, macrophages expressed significant membrane alterations and inflammatory responses, causing heightened levels of phagocytic activity. Exposure to TiO2 NPs caused over 1000 proteins to be differentially expressed, affecting both cellular components and molecular functions within macrophages. Of notice are changes in phosphorylated proteins modulating the binding of tubulin, actin, and cytoskeletal proteins; indicating significant membrane reorganization within the organelle. Other relevant findings include reduced levels of cardiolipin, indicating a dysregulated mitochondrial electron transport chain; encapsulation of TiO2 NPs in multivesicular bodies; and alterations in the TCA cycle metabolism through decreased ATP production. These results indicate a clear FBR with enhanced pro‐inflammatory macrophage activity. The enhanced inflammation through metabolic changes in the TCA cycle seems to be a recurring effect in different NP types and, through comparison of expression levels, could potentially be utilized as a marker for macrophage biocompatibility. Furthermore, material types have shown to induce altering metabolic processes on both cellular components and molecular functions, showing that macrophages may respond differently to NPs with a varying molecular makeup. Further metabolic profiling of macrophages exposed to NPs may therefore help in the optimization of biocompatibility and effectiveness.
3.22. Concluding Remarks
Metabolic processes are natural phenomena in regular cellular homeostasis. However, a switch in specific processes may indicate that a cell underwent a change in behavior or phenotype. With regard to inflammation, a number of metabolic pathways change whenever an inflammatory cell interacts with a biomaterial. These pathways mainly involve energy regulation and biosynthesis which, upon change, can trigger the expression of proinflammatory cytokines and chemokines.[ 279 ] The glycolysis to OXPHOS energy transition is a major metabolic contributor involved in the M1/M2 macrophage transition.[ 274 ]
The increased knowledge about metabolic processes that are involved in inflammation has also given rise to a potential new form of biomaterial fabrication. Since metabolites are naturally degraded and excreted, these products may serve as the foundation for a new group of biocompatible materials. These monomeric units can be functionalized to optimize surface properties or cell–material interactions, create backbones for biomaterials through simple polymerization, and will naturally degrade over time through the natural metabolome (Figure 6 ). All in all, compounds derived from the metabolome can offer an exciting new insight toward developing biomaterials.[ 286 ]
Figure 6.
A predictive model depicting the creation of novel biomaterials based on products derived from the metabolome. First, naturally occurring metabolites can be isolated or synthesized as basic, biocompatible building blocks. Next, these monomers are functionalized through the addition of functional groups, creating synthons with the desired properties. These synthons are polymerized to create biomaterials, after which these can be implanted in vivo. As the material degrades over time, the compounds will revert to their original, biocompatible monomers, which are then processed and excreted through the metabolome.
4. Conclusion
The development of new biomaterials is a complex, multidisciplinary process that has been rapidly evolving over the years. The extent of biomaterial functionality ranges from plain bioinert to achieving full bioactive control through physicochemical modulation, delivery of biologically active molecules or genes and control over cellular function. Biomaterial development has achieved an incredible degree of sophistication over the years through increased understanding of physical, chemical, and bioactive components that interact with the living tissue.[ 327 ] Various scientific advances have led to the evolution of biomaterials among several generations, where current implants actively interact with the environment and the surrounding tissue to optimize healing.[ 16 ]
However, the insertion of a biomaterial in a living host always invokes a form of immune response. This naturally occurring reaction has evolved to protect the host from a variety of invading pathogens, toxins, allergens, and other exogenous threats.[ 291 ] Any form of biomaterials will induce a number of adverse responses that hamper the overall effectiveness of the implant, including chronic inflammation, destruction of the surrounding tissue, the inability to regenerate, and encapsulation, isolation or even rejection of the implant.[ 53 ] Although advances have been made in minimizing the FBR, no material currently exists that is biologically accepted and does not invoke adverse effects.[ 7 ] Current research is aimed toward optimizing material integration in the host whilst preventing the FBR, with the success of a biomaterial depending on a vast amount of both physical and chemical factors that collectively creates an intricate, interactive network of signals.
This review has collected some of the most recent research that covered the effects of biomaterials on the immune response. It highlighted the various properties that define biomaterial characteristics and affect the FBR, resulting in either resolution and adaptation to the implant, or fibrous cap formation leading to chronic immune responses and progressive damage to the implant and environment.[ 22 ] This review highlighted various design properties, including dimensions, geometry, topography, and incorporation of biochemical cues and biological factors. Providing insight toward the interactions between the immune system and biomaterial‐based constructs is crucial in the development of safe, biocompatible, and functional implants.[ 20 ] Moreover, it is crucial to characterize the physical and chemical properties, and degradation/release profile of the final biomaterial to provide the appropriate environment before progressing to complex in vitro culture setup or animal studies.
It is the combination of physical and chemical properties that are responsible for creating and regulating the inflammatory response of the host. Exciting advancements are prospected in the use of naturally derived biomaterials, functionalization on micro‐ or nanolevels and the steering of the secretory environment toward an anti‐inflammatory state. As the knowledge in this field grows, biomaterials are expected to become increasingly advanced with regard to biocompatibility and regenerative potential, aiming toward full integration of the construct and healing of the host.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the Dutch Province of Limburg. L.M. is grateful to the European Research Council Starting Grant “Cell Hybridge” for financial support under the Horizon 2020 framework program (Grant No. 637308). The authors would like to thank Marinus Leeuwerik and Joëlle van Wissen for critical discussions, textual and graphic revisions.
Biographies
Tim ten Brink is a Ph.D. candidate within the Complex Tissue Regeneration department at MERLN Institute for Technology‐Inspired Regenerative Medicine. After completing his Master's degree in biomedical sciences, a growing interest in regenerative medicine led him to pursue a Ph.D. that focuses on bridging the fundamental and translational interface. Currently, he is working on projects involving the development of optimized biomaterials for future in vivo implantation, utilizing naturally occurring biological design principles to fabricate scaffolds with tunable mechanical properties in order to control cell behavior and improve multilayered tissue regeneration. His areas of expertise include bioengineering in vascular and osteochondral tissue.

Febriyani Damanik received a Bachelor's degree in biomedical engineering from Swiss German University, and a Diplom‐Ingenieur in mechanical engineering from University of Applied Sciences Soest. She achieved a Double Master's in nanoscience and nanotechnology in KU Leuven and molecular bioengineering in TU Dresden. Expanding on her Ph.D. research in University of Twente of in vivo bioreactor for vascular tissue engineering, she explored the host response of various biomaterials for tissue regeneration in MERLN Institute for Technology‐Inspired Regenerative Medicine at Maastricht University for her postdoctoral research. In 2020, she became Senior Scientist and Manager of Biologics Development at Chondropeptix.

Joris I. Rotmans received his MD degree at the Free University in Amsterdam in 2000 and his Ph.D. degree at Amsterdam University in 2004. Since 2011, he has been internist‐nephrologist at the Department of Internal Medicine of the LUMC. He was the principle investigator of the DialysisXS consortium, in which a novel method to generate in vivo engineered blood vessels was developed. Prof. Rotmans is the chairman of the thematic working group on vascular tissue engineering at TERMIS and head of the subdepartment of nephrology at the LUMC.

Lorenzo Moroni received his Ph.D. cum laude in 2006 from Twente University on biofabrication technologies for tissue engineering and regenerative medicine. In 2014 he joined Maastricht University, as a founding member of the MERLN Institute for Technology‐Inspired Regenerative Medicine. In 2016, he became full professor in biofabrication for regenerative medicine, and has now been chair of the Complex Tissue Regeneration Department since 2019 and director of MERLN since 2022. His research group aims at developing biofabrication technologies to control cell fate, with applications spanning from skeletal to vascular, neural, and organ regeneration.

ten Brink T., Damanik F., Rotmans J. I., Moroni L., Unraveling and Harnessing the Immune Response at the Cell–Biomaterial Interface for Tissue Engineering Purposes. Adv. Healthcare Mater. 2024, 13, 2301939. 10.1002/adhm.202301939
References
- 1. Vacanti J., Vacanti C., 2000, Ch.1, pp. 3–7.
- 2. Bajaj P., Schweller R. M., Khademhosseini A., West J. L., Bashir R., Annu. Rev. Biomed. Eng. 2014, 16, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vacanti J., Vacanti C. A., 2007, Ch.1, pp. 3–6.
- 4. Salgado A. J., Oliveira J. M., Martins A., Teixeira F. G., Silva N. A., Neves N. M., Sousa N., Reis R. L., Int. Rev. Neurobiol. 2013, 108, 1. [DOI] [PubMed] [Google Scholar]
- 5. Katari R., Peloso A., Orlando G., Front Bioeng Biotechnol. 2014, 2, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parisi L., Toffoli A., Ghiacci G., Macaluso G. M., J. Funct. Biomater. 2018, 9, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Batchelor A. W., Chandrasekaran M., Service Characteristics of Biomedical Materials and Implants, Imperial College Press, London, 2004. [Google Scholar]
- 8. Bhat S., Kumar A., Biomatter 2013, 3, e24717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Langer R., Chem. Eng. Sci. 1995, 50, 4109. [Google Scholar]
- 10. Langer R., Folkman J., Nature 1976, 263, 797. [DOI] [PubMed] [Google Scholar]
- 11. Parenteau‐Bareil R., Gauvin R., Berthod F., Materials (Basel) 2010, 3, 1863. [Google Scholar]
- 12. Langer R., J. Biomed. Mater. Res., Part A 2013, 101A, 2449. [DOI] [PubMed] [Google Scholar]
- 13. Jakus A. E., Rutz A. L., Shah R. N., Biomed. Mater. 2016, 11, 014102. [DOI] [PubMed] [Google Scholar]
- 14. Boehler R. M., Graham J. G., Shea L. D., Biotechniques 2011, 51, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sadtler K., Singh A., Wolf M., Wang X., Pardoll D., Elisseeff J., Nat. Rev. Mater. 2016, 1, 16040. [Google Scholar]
- 16. Zakrzewski J., Brink M., Hubbell J., Nat. Biotechnol. 2014, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brochu A. B. W., Craig S. L., Reichert W. M., J. Biomed. Mater. Res., Part A 2011, 96A, 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hubbell J. A., Curr. Opin. Biotechnol. 1999, 10, 123. [DOI] [PubMed] [Google Scholar]
- 19. Anderson J. M., Biomater. Sci. 2013, 503.32482014 [Google Scholar]
- 20. Anderson J. M., Rodriguez A., Chang D. T., Semin. Immunol. 2008, 20, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Medzhitov R., Nature 2008, 454, 428. [DOI] [PubMed] [Google Scholar]
- 22. Bryers J. D., Giachelli C. M., Ratner B. D., Biotechnol. Bioeng. 2012, 109, 1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anderson J. M., in Principles of Regenerative Medicine, 3rd edn (Eds: Atala A., Lanza R., Mikos A. G., Nerem R.), Academic Press, Boston, 2019, pp. 675–694. [Google Scholar]
- 24. Anderson J. M., Annu. Rev. Mater. Res. 2001, 31, 81. [Google Scholar]
- 25. Broughton G. 2nd, Janis J. E., Attinger C. E., Plast Reconstr Surg. 2006, 117, 12s. [DOI] [PubMed] [Google Scholar]
- 26. Brodin P., Davis M. M., Nat. Rev. Immunol. 2017, 17, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hachim D., Wang N., Lopresti S. T., Stahl E. C., Umeda Y. U., Rege R. D., Carey S. T., Mani D., Brown B. N., J. Biomed. Mater. Res., Part A 2017, 105, 1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown B. N., Haschak M. J., ST Lopresti, Stahl E. C., Semin. Immunol. 2017, 29, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson J., Cramer S., in Host Response to Biomaterials (Ed.: Badylak S. F.), Academic Press, Oxford, 2015, pp. 13–36. [Google Scholar]
- 30. Barton G. M., J. Clin. Invest. 2008, 118, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vroman L., Adams A. L., Fischer G., Munoz P., Blood 1980, 55, 156. [PubMed] [Google Scholar]
- 32. Klopfleisch R., Jung F., J. Biomed. Mater. Res., A 2017, 105, 927. [DOI] [PubMed] [Google Scholar]
- 33. Wilson C., Clegg R., Leavesley D., Pearcy M., Tissue Eng. 2005, 11, 1. [DOI] [PubMed] [Google Scholar]
- 34. Tang L., Jennings T. A., Eaton J. W., Proc. Natl. Acad. Sci. USA 1998, 95, 8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anderson J. M., ASAIO Trans. 1988, 34, 101. [DOI] [PubMed] [Google Scholar]
- 36. Garg K., Pullen N. A., Oskeritzian C. A., Ryan J. J., Bowlin G. L., Biomaterials 2013, 34, 4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Novak M. L., Koh T. J., J. Leukoc. Biol. 2013, 93, 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sica A., Mantovani A., J. Clin. Invest. 2012, 122, 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brown B. N., Londono R., Tottey S., Zhang L., Kukla K. A., Wolf M. T., Daly K. A., Reing J. E., Badylak S. F., Acta Biomater. 2012, 8, 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mosser D. M., Edwards J. P., Nat. Rev. Immunol. 2008, 8, 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saino E., Focarete M. L., Gualandi C., Emanuele E., Cornaglia A. I., Imbriani M., Visai L., Biomacromolecules 2011, 12, 1900. [DOI] [PubMed] [Google Scholar]
- 42. Kou P. M., Babensee J. E., J. Biomed. Mater. Res., A 2011, 96, 239. [DOI] [PubMed] [Google Scholar]
- 43. Henson P. M., Am. J. Pathol. 1980, 101, 494. [PMC free article] [PubMed] [Google Scholar]
- 44. Mikos A. G., McIntire L. V., Anderson J. M., Babensee J. E., Adv. Drug Deliv. Rev. 1998, 33, 111. [DOI] [PubMed] [Google Scholar]
- 45. Sadtler K., Wolf M. T., Ganguly S., Moad C. A., Chung L., Majumdar S., Housseau F., Pardoll D. M., Elisseeff J. H., Biomaterials 2019, 192, 405. [DOI] [PubMed] [Google Scholar]
- 46. Browder T., Folkman J., Pirie‐Shepherd S., J. Biol. Chem. 2000, 275, 1521. [DOI] [PubMed] [Google Scholar]
- 47. Yu T., Tutwiler V. J., Spiller K., The Role of Macrophages in the Foreign Body Response to Implanted Biomaterials, 2015, pp. 17–34. [Google Scholar]
- 48. Rajesh P. S. M., Verma S., Verma V., Host Response of Implanted Biomaterials. Biosurfaces, A Materials Science and Engineering Perspective, John Wiley & Sons, Inc, 2014, pp. 106–125. [Google Scholar]
- 49. Yu T., Tutwiler V., Spiller K., The Role of Macrophages in the Foreign Body Response to Implanted Biomaterials. Biomaterials in Regenerative Medicine and the Immune System, Springer, Cham, 2015, pp. 17–34. [Google Scholar]
- 50. Brodbeck W. G., Anderson J. M., Curr. Opin. Hematol. 2009, 16, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hotchkiss K. M., Clark N. M., Olivares‐Navarrete R., Biomaterials 2018, 182, 202. [DOI] [PubMed] [Google Scholar]
- 52. Alberts B., Molecular Biology of the Cell. [Hauptbd.]. [Hauptbd.], Garland Science Taylor & Francis, New York, NY: 2008. [Google Scholar]
- 53. Mariani E., Lisignoli G., Borzì R. M., Pulsatelli L., Int. J. Mol. Sci. 2019, 20, 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., Zychlinsky A., Science 2004, 303, 1532. [DOI] [PubMed] [Google Scholar]
- 55. Fetz A. E., King W. E. 3rd, Minden‐Birkenmaier B. A., Bowlin G. L., Methods. Mol. Biol. 2022, 2394, 727. [DOI] [PubMed] [Google Scholar]
- 56. Chang S., Popowich Y., Greco R. S., Haimovich B., J. Vasc. Surg. 2003, 37, 1082. [DOI] [PubMed] [Google Scholar]
- 57. Morandini L., Avery D., Angeles B., Winston P., Martin R. K., Donahue H. J., Olivares‐Navarrete R., Acta Biomater. 2023, 166, 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fetz A. E., Bowlin G. L., Tissue Eng., Part B Rev. 2022, 28, 437. [DOI] [PubMed] [Google Scholar]
- 59. Shi C., Pamer E. G., Nat. Rev. Immunol. 2011, 11, 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ogle M. E., Segar C. E., Sridhar S., Botchwey E. A., Exp. Biol. Med. (Maywood). 2016, 241, 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Underhill D. M., Goodridge H. S., Nat. Rev. Immunol. 2012, 12, 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wofford K. L., Singh B. S., Cullen D. K., Spiller K. L., Acta Biomater. 2020, 101, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li M., Chu X., Wang D., Jian L., Liu L., Yao M., Zhang D., Zheng Y., Liu X., Zhang Y., Peng F., Biomaterials 2022, 282, 121408. [DOI] [PubMed] [Google Scholar]
- 64. Koh T. J., DiPietro L. A., Expert. Rev. Mol. Med. 2011, 13, e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ratner B., Biomater. Sci. 2012. [Google Scholar]
- 66. Ratner B. D., Hoffman A. S., Schoen F. J., Lemons J. E., in Biomaterials Science, 3rd edn (Eds: Ratner B. D., Hoffman A. S., Schoen F. J., Lemons J. E.), Academic Press, 2013. [Google Scholar]
- 67. Sridharan R., Cameron A. R., Kelly D. J., Kearney C. J., O'Brien F. J., Mater. Today 2015, 18, 313. [Google Scholar]
- 68. Badylak S. F., Valentin J. E., Ravindra A. K., McCabe G. P., Stewart‐Akers A. M., Tissue Eng., Part A 2008, 14, 1835. [DOI] [PubMed] [Google Scholar]
- 69. Nissinen L. M., Kahari V. M., J. Invest. Dermatol. 2015, 135, 2350. [DOI] [PubMed] [Google Scholar]
- 70. Levinson H., Adv. Wound Care (New Rochelle) 2013, 2, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Burgoyne R. D., Morgan A., Physiol. Rev. 2003, 83, 581. [DOI] [PubMed] [Google Scholar]
- 72. Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M., Trends Immunol. 2004, 25, 677. [DOI] [PubMed] [Google Scholar]
- 73. Kadl A., Meher A. K., Sharma P. R., Lee M. Y., Doran A. C., Johnstone S. R., Elliott M. R., Gruber F., Han J., Chen W., Kensler T., Ravichandran K. S., Isakson B. E., Wamhoff B. R., Leitinger N., Circ. Res. 2010, 107, 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim Y. K., Que R., Wang S.‐W., Liu W. F., Adv. Healthcare Mater. 2014, 3, 989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Spiller K. L., Anfang R. R., Spiller K. J., Ng J., Nakazawa K. R., Daulton J. W., Vunjak‐Novakovic G., Biomaterials 2014, 35, 4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lazarevic V., Glimcher L. H., Lord G. M., Nat. Rev. Immunol. 2013, 13, 777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. MO Li, Rudensky A. Y., Nat. Rev. Immunol. 2016, 16, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sadtler K., Estrellas K., Allen B. W., Wolf M. T., Fan H., Tam A. J., Patel C. H., Luber B. S., Wang H., Wagner K. R., Powell J. D., Housseau F., Pardoll D. M., Elisseeff J. H., Science 2016, 352, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Corthay A., Scand. J. Immunol. 2009, 70, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vojdani A., Erde J., Complement Altern. Med. 2006, 3, 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kondĕlková K., Vokurková D., Krejsek J., Borská L., Fiala Z., Ctirad A., Acta Med. (Hradec Kralove) 2010, 53, 73. [DOI] [PubMed] [Google Scholar]
- 82. Hirahara K., Nakayama T., Int. Immunol. 2016, 28, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zareidoost A., Yousefpour M., Ghaseme B., Amanzadeh A., J. Mater. Sci. Mater. Med. 2012, 23, 1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tian L., Zhang Z., Tian B., Zhang X., Wang N., RSC Adv. 2020, 10, 4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tian B., Wang N., Jiang Q., Tian L., Hu L., Zhang Z., J. Mater. Sci. Mater. Med. 2021, 32, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Castell‐Rodríguez A., Dendritic Cells: Location, Function, and Clinical Implications , 2017.
- 87. Park J., Babensee J. E., Acta biomater. 2012, 8, 3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vinish M., Cui W., Stafford E., Bae L., Hawkins H., Cox R., Toliver‐Kinsky T., Wound Repair Regener. 2016, 24, 6. [DOI] [PubMed] [Google Scholar]
- 89. Chen M., Wang Y.‐H., Wang Y., Huang L., Sandoval H., Liu Y.‐J., Wang J., Science (New York, NY) 2006, 311, 1160. [DOI] [PubMed] [Google Scholar]
- 90. Reddy S. T., Swartz M. A., Hubbell J. A., Trends Immunol. 2006, 27, 573. [DOI] [PubMed] [Google Scholar]
- 91. Aderem A., Ulevitch R. J., Nature 2000, 406, 782. [DOI] [PubMed] [Google Scholar]
- 92. Roch T., Kratz K., Ma N., Jung F., Lendlein A., Clin. Hemorheol. Microcirc. 2013, 55, 157. [DOI] [PubMed] [Google Scholar]
- 93. Keselowsky B. G., Lewis J. S., Semin. Immunol. 2017, 29, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kouwenberg M., Jacobs C. W., van der Vlag J., Hilbrands L. B., PLoS One 2016, 11, e0159986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yang M., Liu Y., Ren G., Shao Q., Gao W., Sun J., Wang H., Ji C., Li X., Zhang Y., Qu X., Sci. Rep. 2015, 5, 13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kou P. M., Schwartz Z., Boyan B. D., Babensee J. E., Acta Biomater. 2011, 7, 1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shankar S. P., Petrie T. A., García A. J., Babensee J. E., J. Biomed. Mater. Res., Part A 2010, 92, 1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shokouhi B., Coban C., Hasirci V., Aydin E., Dhanasingh A., Shi N., Koyama S., Akira S., Zenke M., Sechi A. S., Biomaterials 2010, 31, 5759. [DOI] [PubMed] [Google Scholar]
- 99. Kollmannsberger P., Bidan C. M., Dunlop J. W. C., Fratzl P., Vogel V., Sci. Adv. 2018, 4, eaao4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Desmoulière A., Geinoz A., Gabbiani F., Gabbiani G., J. Cell Biol. 1993, 122, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tomasek J. J., Gabbiani G., Hinz B., Chaponnier C., Brown R. A., Nat. Rev. Mol. Cell Biol. 2002, 3, 349. [DOI] [PubMed] [Google Scholar]
- 102. Seo B. R., Chen X., Ling L., Song Y. H., Shimpi A. A., Choi S., Gonzalez J., Sapudom J., Wang K., Andresen Eguiluz R. C., Gourdon D., Shenoy V. B., Fischbach C., Proc. Natl. Acad. Sci. USA 2020, 117, 11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Witherel C. E., Abebayehu D., Barker T. H., Spiller K. L., Adv. Healthcare Mater. 2019, 8, 1801451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Desmoulière A., Redard M., Darby I., Gabbiani G., Am. J. Pathol. 1995, 146, 56. [PMC free article] [PubMed] [Google Scholar]
- 105. Hinz B., Curr. Res. Transl. Med. 2016, 64, 171. [DOI] [PubMed] [Google Scholar]
- 106. Holt D. J., Grainger D. W., Biomaterials 2011, 32, 3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Damanik F. F. R., Rothuizen T. C., van Blitterswijk C., Rotmans J. I., Moroni L., Sci. Rep. 2014, 4, 6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhou G., Loppnow H., Groth T., Acta Biomater. 2015, 26, 54. [DOI] [PubMed] [Google Scholar]
- 109. Rothuizen T. C., Damanik F. F., Lavrijsen T., Visser M. J., Hamming J. F., Lalai R. A., Duijs J. M., van Zonneveld A. J., Hoefer I. E., van Blitterswijk C. A., Rabelink T. J., Moroni L., Rotmans J. I., Biomaterials 2016, 75, 82. [DOI] [PubMed] [Google Scholar]
- 110. Efendy J. L., Campbell G. R., Campbell J. H., J. Pathol. 2000, 192, 257. [DOI] [PubMed] [Google Scholar]
- 111. Trindade R., Albrektsson T., Tengvall P., Wennerberg A., Clin. Implant Dent. Relat. Res. 2014, 18, 192. [DOI] [PubMed] [Google Scholar]
- 112. Vallés G., Bensiamar F., Crespo L., Arruebo M., Vilaboa N., Saldaña L., Biomaterials 2015, 37, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liu M., Samant S., Vasa C. H., Pedrigi R. M., Oguz U. M., Ryu S., Wei T., Anderson D. R., Agrawal D. K., Chatzizisis Y. S., PLoS One 2023, 18, e0280385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Do A. V., Khorsand B., Geary S. M., Salem A. K., Adv. Healthcare Mater. 2015, 4, 1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Khan F., Tanaka M., Ahmad S. R., J. Mater. Chem. B 2015, 3, 8224. [DOI] [PubMed] [Google Scholar]
- 116. Jagur‐Grodzinski J., Polym. Adv. Technol. 2006, 17, 395. [Google Scholar]
- 117. Redd M. J., Kelly G., Dunn G., Way M., Martin P., Cell Motil. Cytoskeleton 2006, 63, 415. [DOI] [PubMed] [Google Scholar]
- 118. Jordan S. W., Fligor J. E., Janes L. E., Dumanian G. A., Plast. Reconstr. Surg. 2018, 141, 103e. [DOI] [PubMed] [Google Scholar]
- 119. Bružauskaitė I., Bironaitė D., Bagdonas E., Bernotienė E., Cytotechnology 2016, 68, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zandstra J., Hiemstra C., Petersen A. H., Zuidema J., van Beuge M. M., Rodriguez S., Lathuile A. A., Veldhuis G. J., Steendam R., Bank R. A., Popa E. R., Eur. Cell Mater. 2014, 28, 335. [DOI] [PubMed] [Google Scholar]
- 121. Kusaka T., Nakayama M., Nakamura K., Ishimiya M., Furusawa E., Ogasawara K., PLoS One 2014, 9, e92634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Veiseh O., Doloff J. C., Ma M., Vegas A. J., Tam H. H., Bader A. R., Li J., Langan E., Wyckoff J., Loo W. S., Jhunjhunwala S., Chiu A., Siebert S., Tang K., Hollister‐Lock J., Aresta‐Dasilva S., Bochenek M., Mendoza‐Elias J., Wang Y., Qi M., Lavin D. M., Chen M., Dholakia N., Thakrar R., Lacik I., Weir G. C., Oberholzer J., Greiner D. L., Langer R., Anderson D. G., Nat. Mater. 2015, 14, 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sanders J. E., Stiles C. E., Hayes C. L., J. Biomed. Mater. Res. 2000, 52, 231. [DOI] [PubMed] [Google Scholar]
- 124. Ward W. K., Slobodzian E. P., Tiekotter K. L., Wood M. D., Biomaterials 2002, 23, 4185. [DOI] [PubMed] [Google Scholar]
- 125. Patrick B., Lukas K., Kevin T., bioRxiv. 2022, 2022.03.17.484722.
- 126. DeLong S. A., Gobin A. S., West J. L., J. Control. Release 2005, 109, 139. [DOI] [PubMed] [Google Scholar]
- 127. Wu J., Mao Z., Tan H., Han L., Ren T., Gao C., Interface Focus. 2012, 2, 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Cao H., McHugh K., Chew S. Y., Anderson J. M., J. Biomed. Mater. Res., A 2010, 93, 1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sussman E. M., Halpin M. C., Muster J., Moon R. T., Ratner B. D., Ann. Biomed. Eng. 2014, 42, 1508. [DOI] [PubMed] [Google Scholar]
- 130. Karp R. D., Johnson K. H., Buoen L. C., Ghobrial H. K., Brand I., Brand K. G., J. Natl. Cancer Inst. 1973, 51, 1275. [DOI] [PubMed] [Google Scholar]
- 131. Loh Q. L., Choong C., Tissue Eng., Part B Rev. 2013, 19, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Marshall A. J., Ratner B. D., AIChE J. 2005, 51, 1221. [Google Scholar]
- 133. Tylek T., Blum C., Hrynevich A., Schlegelmilch K., Schilling T., Dalton P. D., Groll J., Biofabrication 2020, 12, 025007. [DOI] [PubMed] [Google Scholar]
- 134. Hady T. F., Hwang B., Pusic A. D., Waworuntu R. L., Mulligan M., Ratner B., Bryers J. D., J. Tissue. Eng. Regen. Med. 2021, 15, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bota P. C., Collie A. M., Puolakkainen P., Vernon R. B., Sage E. H., Ratner B. D., Stayton P. S., J. Biomed. Mater. Res., A 2010, 95, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Roco M. C., Curr. Opin. Biotechnol. 2003, 14, 337. [DOI] [PubMed] [Google Scholar]
- 137. Bernards D. A., Desai T. A., Soft Matter 2010, 6, 1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Cobb W. S., Kercher K. W., Heniford B. T., Surg. Innov. 2005, 12, 63. [DOI] [PubMed] [Google Scholar]
- 139. Ratner B. D., Regen. Biomater. 2016, 3, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Brauker J. H., Carr‐Brendel V. E., Martinson L. A., Crudele J., Johnston W. D., Johnson R. C., J. Biomed. Mater. Res. 1995, 29, 1517. [DOI] [PubMed] [Google Scholar]
- 141. Madden L. R., Mortisen D. J., Sussman E. M., Dupras S. K., Fugate J. A., Cuy J. L., Hauch K. D., Laflamme M. A., Murry C. E., Ratner B. D., Proc. Natl. Acad. Sci. USA 2010, 107, 15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hoeben A., Landuyt B., Highley M. S., Wildiers H., Van Oosterom A. T., De Bruijn E. A., Pharmacol. Rev. 2004, 56, 549. [DOI] [PubMed] [Google Scholar]
- 143. Beningo K. A., Wang Y. L., J. Cell Sci. 2002, 115, 849. [DOI] [PubMed] [Google Scholar]
- 144. Almeida C. R., Serra T., Oliveira M. I., Planell J. A., Barbosa M. A., Navarro M., Acta Biomater. 2014, 10, 613. [DOI] [PubMed] [Google Scholar]
- 145. McWhorter F. Y., Wang T., Nguyen P., Chung T., Liu W. F., Proc. Natl. Acad. Sci. USA 2013, 110, 17253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Luu T. U., Gott S. C., Woo B. W., Rao M. P., Liu W. F., ACS Appl. Mater. Interfaces 2015, 7, 28665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chen S., Jones J. A., Xu Y., Low H. Y., Anderson J. M., Leong K. W., Biomaterials 2010, 31, 3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lee R. H., Efron D. T., Tantry U., Stuelten C., Moldawer L. L., Abouhamze A., Barbul A., Wound Repair Regener. 2000, 8, 547. [DOI] [PubMed] [Google Scholar]
- 149. Barth K. A., Waterfield J. D., Brunette D. M., J. Biomed. Mater. Res., A 2013, 101, 2679. [DOI] [PubMed] [Google Scholar]
- 150. Velard F., Schlaubitz S., Fricain J. C., Guillaume C., Laurent‐Maquin D., Möller‐Siegert J., Vidal L., Jallot E., Sayen S., Raissle O., Nedelec J. M., Vix‐Guterl C., Anselme K., Amédée J., Laquerrière P., Nanomedicine (Lond) 2015, 10, 785. [DOI] [PubMed] [Google Scholar]
- 151. Cui H., Sinko P. J., Front. Mater. Sci. 2012, 6, 47. [Google Scholar]
- 152. Sridharan R., Cavanagh B., Cameron A. R., Kelly D. J., O'Brien F. J., Acta Biomater. 2019, 89, 47. [DOI] [PubMed] [Google Scholar]
- 153. Blakney A. K., Swartzlander M. D., Bryant S. J., J. Biomed. Mater. Res., A 2012, 100, 1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Camarero‐Espinosa S., Carlos‐Oliveira M., Liu H., Mano J. F., Bouvy N., Moroni L., Adv. Healthcare Mater. 2022, 11, 2101415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Meli V. S., Atcha H., Veerasubramanian P. K., Nagalla R. R., Luu T. U., Chen E. Y., Guerrero‐Juarez C. F., Yamaga K., Pandori W., Hsieh J. Y., Downing T. L., Fruman D. A., Lodoen M. B., Plikus M. V., Wang W., Liu W. F., Sci. Adv. 6, eabb8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Xu L.‐C., Siedlecki C. A., Biomaterials 2007, 28, 3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lee S., Choi J., Shin S., Im Y. M., Song J., Kang S. S., Nam T. H., Webster T. J., Kim S. H., Khang D., Acta Biomater. 2011, 7, 2337. [DOI] [PubMed] [Google Scholar]
- 158. Hamlet S., Alfarsi M., George R., Ivanovski S., Clin. Oral Implants Res. 2012, 23, 584. [DOI] [PubMed] [Google Scholar]
- 159. Hezi‐Yamit A., Sullivan C., Wong J., David L., Chen M., Cheng P., Shumaker D., Wilcox J. N., Udipi K., J. Biomed. Mater. Res., A 2009, 90, 133. [DOI] [PubMed] [Google Scholar]
- 160. Tang L., Thevenot P., Hu W., Curr. Top. Med. Chem. 2008, 8, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kapalczynska M., Kolenda T., Przybyla W., Zajaczkowska M., Teresiak A., Filas V., Ibbs M., Blizniak R., Luczewski L., Lamperska K., Arch. Med. Sci. 2018, 14, 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Almeida C. R., Serra T., Oliveira M. I., Planell J. A., Barbosa M. A., Navarro M., Acta Biomater. 2014, 10, 613. [DOI] [PubMed] [Google Scholar]
- 163. Oliveira M. I., Santos S. G., Oliveira M. J., Torres A. L., Barbosa M. A., Eur. Cell. Mater. 2012, 24, 136. [DOI] [PubMed] [Google Scholar]
- 164. Mooney J. E., Rolfe B. E., Osborne G. W., Sester D. P., van Rooijen N., Campbell G. R., Hume D. A., Campbell J. H., Am. J. Pathol. 2010, 176, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Indolfi L., Baker A. B., Edelman E. R., Biomaterials 2012, 33, 7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Methe H., Hess S., Edelman E. R., Thromb. Haemost. 2007, 98, 278. [PubMed] [Google Scholar]
- 167. Kuang X., Roach D. J., Wu J., Hamel C. M., Ding Z., Wang T., Dunn M. L., Qi H. J., Adv. Funct. Mater. 2019, 29, 1805290. [Google Scholar]
- 168. Pei E., Loh G. H., Prog. Addit. Manuf. 2018, 3, 95. [Google Scholar]
- 169. Shakibania S., Ghazanfari L., Raeeszadeh‐Sarmazdeh M., Khakbiz M., Drug Dev. Ind. Pharm. 2020, 1. [DOI] [PubMed] [Google Scholar]
- 170. Lam M. T., Wu J. C., Expert Rev. Cardiovasc. Ther. 2012, 10, 1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Gunatillake P. A., Adhikari R., Eur. Cell Mater. 2003, 5, 1. [DOI] [PubMed] [Google Scholar]
- 172. Orenstein S. B., Qiao Y., Klueh U., Kreutzer D. L., Novitsky Y. W., Hernia 2010, 14, 401. [DOI] [PubMed] [Google Scholar]
- 173. Johansson J. A., Halthur T., Herranen M., Soderberg L., Elofsson U., Hilborn J., Biomacromolecules 2005, 6, 1353. [DOI] [PubMed] [Google Scholar]
- 174. Ksander G. A., Gray L., Ann. Plast. Surg. 1985, 14, 351. [DOI] [PubMed] [Google Scholar]
- 175. Watt F. M., Huck W. T. S., Nat. Rev. Mol. Cell Biol. 2013, 14, 467. [DOI] [PubMed] [Google Scholar]
- 176. Zhang W., Yang J., Zhu Y., Sun X., Guo W., Liu X., Jing X., Guo G., Guo Q., Peng J., Zhu X., Cell Tissue Banking 2019, 20, 351. [DOI] [PubMed] [Google Scholar]
- 177. Teodori L., Costa A., Marzio R., Perniconi B., Coletti D., Adamo S., Gupta B., Tarnok A., Front. Physiol. 2014, 5, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Hussein K. H., Saleh T., Ahmed E., Kwak H.‐H., Park K.‐M., Yang S.‐R., Kang B.‐J., Choi K.‐Y., Kang K.‐S., Woo H.‐M., J. Biomed. Mater. Res., Part A 2018, 106, 2034. [DOI] [PubMed] [Google Scholar]
- 179. Xu H., Wan H., Sandor M., Qi S., Ervin F., Harper J. R., Silverman R. P., McQuillan D. J., Tissue Eng., Part A 2008, 14, 2009. [DOI] [PubMed] [Google Scholar]
- 180. Brown B. N., Valentin J. E., Stewart‐Akers A. M., McCabe G. P., Badylak S. F., Biomaterials 2009, 30, 1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Fishman J. M., Lowdell M. W., Urbani L., Ansari T., Burns A. J., Turmaine M., North J., Sibbons P., Seifalian A. M., Wood K. J., Birchall M. A., De Coppi P., Proc. Natl. Acad. Sci. USA 2013, 110, 14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Yang E.‐J., Kim J.‐G., Kim J.‐Y., Kim S., Lee H., Hyun C.‐G., Cent. Eur. J. Biol. 2010, 5, 95. [Google Scholar]
- 183. Dragostin O. M., Samal S. K., Dash M., Lupascu F., Pânzariu A., Tuchilus C., Ghetu N., Danciu M., Dubruel P., Pieptu D., Vasile C., Tatia R., Profire L., Carbohydr. Polym. 2016, 141, 28. [DOI] [PubMed] [Google Scholar]
- 184. Deng Y., Ren J., Chen G., Li G., Wu X., Wang G., Gu G., Li J., Sci. Rep. 2017, 7, 2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Leach J. B., Bivens K. A., Collins C. N., Schmidt C. E., J. Biomed. Mater. Res., A 2004, 70, 74. [DOI] [PubMed] [Google Scholar]
- 186. Moura M. J., Faneca H., Lima M. P., Gil M. H., Figueiredo M. M., Biomacromolecules 2011, 12, 3275. [DOI] [PubMed] [Google Scholar]
- 187. Moura M. J., Brochado J., Gil M. H., Figueiredo M. M., Mater. Sci. Eng., C 2017, 75, 279. [DOI] [PubMed] [Google Scholar]
- 188. Dhandayuthapani B., Yoshida Y., Maekawa T., Kumar D. S., Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar]
- 189. Stratton S., Shelke N. B., Hoshino K., Rudraiah S., Kumbar S. G., Bioact. Mater. 2016, 1, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Vroman I., Tighzert L., Biodegradable Polymers. Materials (Basel). 2009, 2, 307. [Google Scholar]
- 191. Nöth U., Rackwitz L., Steinert A. F., Tuan R. S., Adv. Drug Delivery Rev. 2010, 62, 765. [DOI] [PubMed] [Google Scholar]
- 192. del Ángel‐Sánchez K., Ulloa‐Castillo N. A., Segura‐Cárdenas E., Martinez‐Romero O., Elías‐Zuñiga A., MRS Commun. 2019, 9, 390. [Google Scholar]
- 193. Duda S., Dreyer L., Behrens P., Wienecke S., Chakradeo T., Glasmacher B., Haastert‐Talini K., Biomed. Res. Int. 2014, 2014, 835269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Luo L., He Y., Chang Q., Xie G., Zhan W., Wang X., Zhou T., Xing M., Lu F., Int. J. Nanomed. 2016, 11, 6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Davachi S. M., Kaffashi B., Polym.‐Plast. Technol. Eng. 2015, 54, 944. [Google Scholar]
- 196. Lasprilla A. J. R., Martinez G. A. R., Lunelli B. H., Jardini A. L., Filho R. M., Biotechnol. Adv. 2012, 30, 321. [DOI] [PubMed] [Google Scholar]
- 197. Athanasiou K. A., Niederauer G. G., Agrawal C. M., Biomaterials 1996, 17, 93. [DOI] [PubMed] [Google Scholar]
- 198. Lucke S., Walschus U., Hoene A., Schnabelrauch M., Nebe J. B., Finke B., Schlosser M., J. Biomed. Mater. Res., Part A 2018, 106, 2726. [DOI] [PubMed] [Google Scholar]
- 199. Göktürk E., Erdal H., Sakarya Üniversitesi Fen Bilimleri Enstitüsü Dergisi 2017, 21. [Google Scholar]
- 200. Ceonzo K., Gaynor A., Shaffer L., Kojima K., Vacanti C. A., Stahl G. L., Tissue Eng. 2006, 12, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Makadia H. K., Siegel S. J., Polymers (Basel). 2011, 3, 1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Gentile P., Chiono V., Carmagnola I., Hatton P. V., Int. J. Mol. Sci. 2014, 15, 3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Waldeck H., Wang X., Joyce E., Kao W. J., Biomaterials 2012, 33, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Knop K., Hoogenboom R., Fischer D., Schubert U. S., Angew. Chem. Int. Ed. Engl. 2010, 49, 6288. [DOI] [PubMed] [Google Scholar]
- 205. Lynn A. D., Kyriakides T. R., Bryant S. J., J. Biomed. Mater. Res., A 2010, 93, 941. [DOI] [PubMed] [Google Scholar]
- 206. Kohane D. S., Langer R., Pediatr. Res. 2008, 63, 487. [DOI] [PubMed] [Google Scholar]
- 207. Zhang L., Cao Z., Bai T., Carr L., Ella‐Menye J. R., Irvin C., Ratner B. D., Jiang S., Nat. Biotechnol. 2013, 31, 553. [DOI] [PubMed] [Google Scholar]
- 208. Jansen L. E., Amer L. D., Chen E. Y. T., Nguyen T. V., Saleh L. S., Emrick T., Liu W. F., Bryant S. J., Peyton S. R., Biomacromolecules 2018, 19, 2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Swartzlander M. D., Barnes C. A., Blakney A. K., Kaar J. L., Kyriakides T. R., Bryant S. J., Biomaterials 2015, 41, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Luk Y.‐Y., Kato M., Mrksich M., Langmuir 2000, 16, 9604. [Google Scholar]
- 211. Cho E. J., Doh K.‐O., Park J., Hyun H., Wilson E. M., Snyder P. W., Tsifansky M. D., Yeo Y., Sci. Rep. 2016, 6, 29739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Beltrán‐Osuna Á. A., Ródenas‐Rochina J., Gómez Ribelles J. L., Perilla J. E., Polym. Adv. Technol. 2019, 30, 688. [Google Scholar]
- 213. Yu K., Mei Y., Hadjesfandiari N., Kizhakkedathu J. N., Colloids Surf., B Biointer. 2014, 124, 69. [DOI] [PubMed] [Google Scholar]
- 214. Ma M., Liu W. F., Hill P. S., Bratlie K. M., Siegwart D. J., Chin J., Park M., Guerreiro J., Anderson D. G., Adv. Mater. 2011, 23, H189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Habibovic P., Barralet J. E., Acta Biomater. 2011, 7, 3013. [DOI] [PubMed] [Google Scholar]
- 216. Thompson E. M., Matsiko A., Farrell E., Kelly D. J., O'Brien F. J., J. Tissue Eng. Regen. Med. 2015, 9, 889. [DOI] [PubMed] [Google Scholar]
- 217. Jamalpoor Z., Asgari A., Lashkari M. H., Mirshafiey A., Mohsenzadegan M., Iran. J. Allergy. Asthma Immunol. 2018, 17, 398. [PubMed] [Google Scholar]
- 218. Bohner M., Gbureck U., Barralet J. E., Biomaterials 2005, 26, 6423. [DOI] [PubMed] [Google Scholar]
- 219. Humbert P., Brennan M. Á., Davison N., Rosset P., Trichet V., Blanchard F., Layrolle P., Front. Immunol. 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Yuan H., Fernandes H., Habibovic P., de Boer J., Barradas A. M. C., de Ruiter A., Walsh W. R., van Blitterswijk C. A., de Bruijn J. D., Proc. Natl. Acad. Sci. USA 2010, 107, 13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Alhamdi J. R., Peng T., Al‐Naggar I. M., Hawley K. L., Spiller K. L., Kuhn L. T., Biomaterials 2019, 196, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Oliveira A. L., Alves C. M., Reis R. L., J. Mater. Sci.: Mater. Med. 2002, 13, 1181. [DOI] [PubMed] [Google Scholar]
- 223. Jetten N., Roumans N., Gijbels M. J., Romano A., Post M. J., de Winther M. P., van der Hulst R. R., Xanthoulea S., PLoS One 2014, 9, e102994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Tamimi F., Kumarasami B., Doillon C., Gbureck U., Nihouannen D. L., Cabarcos E. L., Barralet J. E., Acta Biomater. 2008, 4, 1315. [DOI] [PubMed] [Google Scholar]
- 225. Jayasree R., Kumar T. S. S., Venkateswari R., Nankar R. P., Doble M., J. Mater. Sci. Mater. Med. 2019, 30, 113. [DOI] [PubMed] [Google Scholar]
- 226. Fine N., Sheikh Z., Al‐Jaf F., Oveisi M., Borenstein A., Hu Y., Pilliar R., Grynpas M., Glogauer M., J. Biomed. Mater. Res., Part B 2020, 108, 253. [DOI] [PubMed] [Google Scholar]
- 227. Hannan R. T., Peirce S. M., Barker T. H., ACS Biomater. Sci. Eng. 2018, 4, 1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Xia Z., Triffitt J. T., Biomed. Mater. 2006, 1, R1. [DOI] [PubMed] [Google Scholar]
- 229. Fernandez E. J., Lolis E., Annu. Rev. Pharmacol. Toxicol. 2002, 42, 469. [DOI] [PubMed] [Google Scholar]
- 230. Falanga V., Faria K., in Principles of Tissue Engineering, 3rd edn (Eds: Lanza R., Langer R, Vacanti J.), Academic Press, Burlington, 2007, pp. 1167–1185. [Google Scholar]
- 231. Kelly S. H., Shores L. S., Votaw N. L., Collier J. H., Adv. Drug Delivery Rev. 2017, 114, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Asai A., Nakamura K., Kobayashi M., Herndon D. N., Suzuki F., J. Leukocyte Biol. 2012, 92, 859. [DOI] [PubMed] [Google Scholar]
- 233. Martinez F. O., Gordon S., F1000Prime Rep. 2014, 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Fadok V. A., Bratton D. L., Konowal A., Freed P. W., Westcott J. Y., Henson P. M., J. Clin. Invest. 1998, 101, 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Reeves A. R. D., Spiller K. L., Freytes D. O., Vunjak‐Novakovic G., Kaplan D. L., Biomaterials 2015, 73, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Spiller K. L., Nassiri S., Witherel C. E., Anfang R. R., Ng J., Nakazawa K. R., Yu T., Vunjak‐Novakovic G., Biomaterials 2015, 37, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Mokarram N., Merchant A., Mukhatyar V., Patel G., Bellamkonda R. V., Biomaterials 2012, 33, 8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Awada H. K., Johnson N. R., Wang Y., J. Control. Release. 2015, 207, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Cao R., Brakenhielm E., Pawliuk R., Wariaro D., Post M. J., Wahlberg E., Leboulch P., Cao Y., Nat. Med. 2003, 9, 604. [DOI] [PubMed] [Google Scholar]
- 240. Schutte R. J., Xie L., Klitzman B., Reichert W. M., Biomaterials 2009, 30, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Rodriguez A., Meyerson H., Anderson J. M., J. Biomed. Mater. Res., Part A 2009, 89A, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Re'em T., Kaminer‐Israeli Y., Ruvinov E., Cohen S., Biomaterials 2012, 33, 751. [DOI] [PubMed] [Google Scholar]
- 243. Guo X., Park H., Liu G., Liu W., Cao Y., Tabata Y., Kasper F. K., Mikos A. G., Biomaterials 2009, 30, 2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Hsieh P. C., Davis M. E., Gannon J., MacGillivray C., Lee R. T., J. Clin. Invest. 2006, 116, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Parks A. C., Sung K., Wu B. M., Acta Biomater. 2014, 10, 4742. [DOI] [PubMed] [Google Scholar]
- 246. Jay S. M., Shepherd B. R., Andrejecsk J. W., Kyriakides T. R., Pober J. S., Saltzman W. M., Biomaterials 2010, 31, 3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Kim Y. H., Tabata Y., J. Biomed. Mater. Res., A 2015. [Google Scholar]
- 248. Hsu C. W., Poche R. A., Saik J. E., Ali S., Wang S., Yosef N., Calderon G. A., Scott L. Jr., Vadakkan T. J., Larina I. V., West J. L., Dickinson M. E., PLoS One 2015, 10, e0131643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Barbay V., Houssari M., Mekki M., Banquet S., Edwards‐Lévy F., Henry J. P., Dumesnil A., Adriouch S., Thuillez C., Richard V., Brakenhielm E., Angiogenesis 2015, 18, 191. [DOI] [PubMed] [Google Scholar]
- 250. Roh J. D., Sawh‐Martinez R., Brennan M. P., Jay S. M., Devine L., Rao D. A., Yi T., Mirensky T. L., Nalbandian A., Udelsman B., Hibino N., Shinoka T., Saltzman W. M., Snyder E., Kyriakides T. R., Pober J. S., Breuer C. K., Proc. Natl. Acad. Sci. USA 2010, 107, 4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Jetten N., Donners M. M., Wagenaar A., Cleutjens J. P., van Rooijen N., de Winther M. P., Post M. J., PLoS One 2013, 8, e68811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Laronha H., Caldeira J., Cells 2020, 9, 1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Crupi A., Costa A., Tarnok A., Melzer S., Teodori L., Eur. J. Immunol. 2015, 45, 3222. [DOI] [PubMed] [Google Scholar]
- 254. Visse R., Nagase H., Circ. Res. 2003, 92, 827. [DOI] [PubMed] [Google Scholar]
- 255. Cui N., Hu M., Khalil R. A., Prog. Mol. Biol. Transl. Sci. 2017, 147, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Jones J. A., McNally A. K., Chang D. T., Qin L. A., Meyerson H., Colton E., Kwon I. L. K., Matsuda T., Anderson J. M., J. Biomed. Mater. Res., Part A 2008, 84A, 158. [DOI] [PubMed] [Google Scholar]
- 257. Nwomeh B. C., Liang H. X., Cohen I. K., Yager D. R., J. Surg. Res. 1999, 81, 189. [DOI] [PubMed] [Google Scholar]
- 258. Helling A. L., Tsekoura E. K., Biggs M., Bayon Y., Pandit A., Zeugolis D. I., ACS Biomater. Sci. Eng. 2017, 3, 1922. [DOI] [PubMed] [Google Scholar]
- 259. Toole B. P., Nat. Rev. Cancer 2004, 4, 528. [DOI] [PubMed] [Google Scholar]
- 260. Petrey A. C., de la Motte C. A., Front. Immunol. 2014, 5, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Zheng L., Riehl T. E., Stenson W. F., Gastroenterology 2009, 137, 2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Beldman T. J., Senders M. L., Alaarg A., Pérez‐Medina C., Tang J., Zhao Y., Fay F., Deichmöller J., Born B., Desclos E., van der Wel N. N., Hoebe R. A., Kohen F., Kartvelishvily E., Neeman M., Reiner T., Calcagno C., Fayad Z. A., de Winther M. P. J., Lutgens E., Mulder W. J. M., Kluza E., ACS Nano 2017, 11, 5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Alkhoury H., Hautmann A., Fuhrmann B., Syrowatka F., Erdmann F., Zhou G., Stojanović S., Najman S., Groth T., Int. J. Mol. Sci. 2020, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. AlKhoury H., Hautmann A., Erdmann F., Zhou G., Stojanović S., Najman S., Groth T., J. Biomed. Mater. Res., Part A 2020, 108, 1099. [DOI] [PubMed] [Google Scholar]
- 265. Zhou G., Niepel M. S., Saretia S., Groth T., J. Biomed. Mater. Res., Part A 2016, 104, 493. [DOI] [PubMed] [Google Scholar]
- 266. Hernandez‐Pando R., Bornstein Q. L., Aguilar Leon D., Orozco E. H., Madrigal V. K., Martinez Cordero E., Immunology 2000, 100, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Sternlicht M. D., Werb Z., Annu. Rev. Cell Dev. Biol. 2001, 17, 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Adhikari U., An X., Rijal N., Hopkins T., Khanal S., Chavez T., Tatu R., Sankar J., Little K. J., Hom D. B., Bhattarai N., Pixley S. K., Acta Biomater. 2019, 98, 215. [DOI] [PubMed] [Google Scholar]
- 269. El Kasmi K. C., Stenmark K. R., Semin. Immunol. 2015, 27, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Rattigan K. M., Pountain A. W., Regnault C., Achcar F., Vincent I. M., Goodyear C. S., Barrett M. P., PLoS One 2018, 13, e0194126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Fang H., Pengal R. A., Cao X., Ganesan L. P., Wewers M. D., Marsh C. B., Tridandapani S., J. Immunol. 2004, 173, 360. [DOI] [PubMed] [Google Scholar]
- 272. Zhu F., Wang S., Zhu X., Pang C., Cui P., Yang F., Li R., Zhan Q., Xin H., Biomater. Sci. 2023, 11, 6977. [DOI] [PubMed] [Google Scholar]
- 273. Meiser J., Krämer L., Sapcariu S. C., Battello N., Ghelfi J., D'Herouel A. F., Skupin A., Hiller K., J. Biol. Chem. 2016, 291, 3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Soto‐Heredero G., Gómez de las Heras M. M., Gabandé‐Rodríguez E., Oller J., Mittelbrunn M., The FEBS J. 2020, 287, 3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Puleston D. J., Villa M., Pearce E. L., Cell Metab. 2017, 26, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. O'Neill L. A., Immunity 2015, 42, 393. [DOI] [PubMed] [Google Scholar]
- 277. Everts B., Amiel E., van der Windt G. J., Freitas T. C., Chott R., Yarasheski K. E., Pearce E. L., Pearce E. J., Blood 2012, 120, 1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Lawless S. J., Kedia‐Mehta N., Walls J. F., McGarrigle R., Convery O., Sinclair L. V., Navarro M. N., Murray J., Finlay D. K., Nat. Commun. 2017, 8, 15620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Bogdan C., Nat. Immunol. 2001, 2, 907. [DOI] [PubMed] [Google Scholar]
- 280. Branzk N., Lubojemska A., Hardison S. E., Wang Q., Gutierrez M. G., Brown G. D., Papayannopoulos V., Nat. Immunol. 2014, 15, 1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Kishore M., Cheung K. C. P., Fu H., Bonacina F., Wang G., Coe D., Ward E. J., Colamatteo A., Jangani M., Baragetti A., Matarese G., Smith D. M., Haas R., Mauro C., Wraith D. C., Okkenhaug K., Catapano A. L., De Rosa V., Norata G. D., Marelli‐Berg F. M., Immunity 2017, 47, 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Huang S. C., Smith A. M., Everts B., Colonna M., Pearce E. L., Schilling J. D., Pearce E. J., Immunity 2016, 45, 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Yahfoufi N., Alsadi N., Jambi M., Matar C., Nutrients 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Mendes L. F., Gaspar V. M., Conde T. A., Mano J. F., Duarte I. F., Sci. Rep. 2019, 9, 14906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Korley J. N., Yazdi S., McHugh K., Kirk J., Anderson J., Putnam D., Biomaterials 2016, 98, 41. [DOI] [PubMed] [Google Scholar]
- 286. Ricapito N. G., Ghobril C., Zhang H., Grinstaff M. W., Putnam D., Chem. Rev. 2016, 116, 2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Saborano R., Wongpinyochit T., Totten J. D., Johnston B. F., Seib F. P., Duarte I. F., Adv. Healthcare Mater. 2017, 6, 1601240. [DOI] [PubMed] [Google Scholar]
- 288. Martirosyan A., Schneider Y. J., Int. J. Environ. Res. Public Health 2014, 11, 5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Chen Q., Wang N., Zhu M., Lu J., Zhong H., Xue X., Guo S., Li M., Wei X., Tao Y., Yin H., Redox Biol. 2018, 15, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Huebsch N., Mooney D. J., Nature 2009, 462, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Chaplin D. D., J. Allergy Clin. Immunol. 2010, 125, S3. [DOI] [PMC free article] [PubMed] [Google Scholar]


