Abstract
Question:
The opioid epidemic causes massive morbidity, and males have substantially greater overdose mortality rates than females. It is unclear whether there are sex-related disparities at different stages in the trajectory of opioid use disorders, in “real world” settings.
Goal:
To determine sex disparities in non-medical opioid use (NMOU) at the end of outpatient medication-assisted treatment (MAT), using nationally representative data.
Design:
Observational epidemiological study of publicly funded outpatient MAT programs in the national “Treatment episode data set-discharges” (TEDS-D) for 2019.
Participants:
Persons aged ≥18 in their first treatment episode, in outpatient MAT for use of heroin or other opioids (N=11,549). The binary outcome was presence/absence of NMOU.
Results:
In univariate analyses, males had significantly higher odds of NMOU, compared to females (odds ratio=1.27; Chi2 [df:1]=39.08; uncorrected p<0.0001; p=0.0041 after Bonferroni correction). A multivariable logistic regression detected a male>female odds ratio of 1.19 (95%CI=1.09–1.29; p<0.0001), adjusting for socio-demographic/clinical variables. Several specific conditions were revealed in which males had greater odds of NMOU compared to females (e.g., at ages 18–29 and 30–39; corrected p=0.012, or if they used opioids by inhalation; corrected p=0.0041).
Conclusions:
This nationally representative study indicates that males have greater odds of NMOU in their first episode of MAT, indicating more unfavorable outcomes. The study reveals specific socio-demographic and clinical variables under which this sex disparity is most prominent.
Keywords: opioid, methadone, buprenorphine, fentanyl, medication-assisted treatment, medications for opioid use disorders, real world data
1. Introduction
The opioid epidemic causes massive health consequences in the U.S. (Butelman et al., 2023; Wilson et al., 2020), and we recently reported approximately 2.5-fold greater opioid-induced overdose mortality rates in males versus females, even after adjusting for sex-specific frequency of opioid use (Butelman et al., 2023). Therefore, this sex disparity was not simply due to differing levels of overall opioid use, and may reflect multiple biological, socio-demographic and clinical factors, as well as response to treatment (Apsley et al., 2024; Little and Kosten, 2023; McEvoy et al., 2023; Towers et al., 2023). Understanding these sex differences could inform the development of personalized sex or gender-specific interventions, and decrease morbidity and mortality (Apsley et al., 2024; Kreek et al., 2019; Marsh et al., 2021; Strang et al., 2020).
Research studies have compared different stages in the trajectory of opioid use disorders (OUD) in males and females, as well as response to medication-assisted treatment (MAT) (Bawor et al., 2015; Marsh et al., 2021; Zheng et al., 2024). However, it is difficult to draw overall conclusions across studies on MAT outcomes, based on differences across time, cohort, study methodology and environment (Bawor et al., 2015; Huhn et al., 2019; Zheng et al., 2024).
It has been noted that some research studies may not be representative of “real world” MAT settings, due to relative lack of inclusion based on socio-demographic factors, which can also be related to social determinants of health (Braveman and Gottlieb, 2014; Eghaneyan et al., 2020; Rudolph et al., 2022). Intriguingly, a “real world” study (using the 2017 Treatment Episode Data Set-Discharges; TEDS-D) detected that females in MAT had 1.1 greater odds of staying in treatment at least 6 months, compared to males (Krawczyk et al., 2021). However, duration of treatment in MAT can differ because of environmental and program-level features, in addition to client-level factors (Durand et al., 2021; Krawczyk et al., 2021). Other outcomes such as “treatment completion” may also be influenced by program-level features, racial/ethnic disparities, and socioeconomic disadvantages and social determinants of health (Askari et al., 2020; Franz et al., 2023; Mennis et al., 2019). A major favorable clinical outcome of MAT is decreased NMOU (Goldstein, 2022; Kreek et al., 2019; Strang et al., 2020). Therefore, this study examined whether there are sex differences in the frequency of NMOU in the month prior to discharge from MAT (Burgess-Hull et al., 2022; Kowalczyk et al., 2015), in a nationally representative “real world” outpatient sample, adjusting for length of stay in treatment, and major socio-demographic and clinical variables (Fischer et al., 2023; Stahler and Mennis, 2018; Surratt et al., 2011).
2. Methods
2.1. Data Source:
This study used the Treatment Episode Data Set-Discharges (TEDS-D), from 2019 (https://www.datafiles.samhsa.gov/), the newest data available prior to the disruptions of the COVID-19 pandemic (Volkow, 2020). The TEDS-D is composed of individual episodes from substance use treatment facilities that receive public funding; data includes the continental US, excluding Oregon, Washington state and West Virginia. Data extraction and variable coding are described below.
2.2. Outcome variable:
The outcome variable was NMOU in the month prior to discharge (TEDS-D variable FREQ1_D), recoded as a binary variable, combining “some use” and “daily use”, versus “no use”.
2.3. Inclusion Criteria:
A) First episodes of treatment (variable NOPRIOR=0). This criterion avoids confounding due to potentially examining several episodes in the same person, or due to prior treatment history (Huhn et al., 2018; Kitsantas et al., 2023). B) Episodes in which an opioid is the primary substance (i.e., heroin, non-prescription methadone, or “other opiates and synthetics”) at admission and discharge (i.e., variables SUB1 and SUB1_D=5,6, or 7, respectively). C) Episodes with MAT in the treatment plan (variable METHUSE=1; principally methadone and buprenorphine, but also naltrexone) (Ross et al., 2024; Strang et al., 2020; Wakeman et al., 2020). The “METHUSE” variable pools these medications, not allowing further stratification. D) Service in an ambulatory (outpatient) setting (variable SERVICES=6 or 7) (Kreek et al., 2019; Strang et al., 2020; Wakeman et al., 2020). E) Episodes with a length of stay (variable LOS) of ≥30 days, to focus on those who had at least initial treatment engagement (Blanco and Volkow, 2019; Krawczyk et al., 2021). F) Episodes in census divisions 1–9 (i.e., continental U.S).
2.4. Exclusion criteria:
A) Age <18, due to low prevalence. B) Episodes terminated because the person had died (cause of death is unavailable; variable REASON=5), or became incarcerated (variable REASON=6). C) Episodes in which the route of non-medical opioid use was “other” (i.e., variable ROUTE1=5), due to low prevalence. D) Missing data: If there were missing data in any of these variables, the episode was excluded from analysis (episode-wise deletion).
2.5. Demographic variables:
In addition to sex, several variables were examined: Length of stay in treatment (variable LOS), coded in bins denoting increasing duration (31–45, 46–60, 61–90, 91–180, 181–365, ≥366 days). Route of NMOU (variable ROUTE1) was coded as four categories: oral, smoking, inhalation or injection. Age at admission was recoded as 18–29, 30–39, 40–49, ≥50 age bins. Housing at discharge was coded as: independent housing, dependent (e.g., supportive) housing, and homeless (unhoused). Employment at discharge was coded as: employed full time, employed part-time, “not in the labor force” and unemployed. Racial background was studied in three categories (African-American/black, white, and all remaining recoded as “other”). Psychiatric comorbidity was coded as a binary variable (present/absent). Hispanic/latino ethnicity was re-coded as a binary variable (any hispanic/latino versus non-hispanic/latino). Referral source was recoded as a binary variable, with court/criminal justice referrals versus all others. Census divisions were also examined.
2.6. Data analyses:
Analyses were carried out with GraphPad Prism (V. 10) and TIBCO Statistica Data Science Workbench. The alpha-level of significance was p=0.05.
2.6.1. Univariate Analyses
Demographics were examined with Chi2 analyses across sex. The relative frequency of the outcome (use vs no use of non-medical opioids) in males and females was also examined in Chi2 2X2 contingency analyses. The p-values for these contingency analyses were Bonferroni-corrected, and male/female odds ratios are shown for those that survive correction. 95%CI for the proportions of males and females with the outcome were calculated with the Wilson-Brown method (Brown et al., 2001). Data are also presented as % of males and females with non-medical opioid use, and male-female difference.
2.6.2. Multivariable analysis
A multivariable logistic regression (Hosmer and Lemeshow, 2004; Osborne, 2014) using maximum likelihood estimation was carried out for the binary outcome of NMOU, versus no use. Sex and all the variables above were entered in the regression. Adjusted Beta-parameters and odds ratios of the outcome are reported. Regression performance was examined with receiver operating characteristic (ROC) analysis, Nagelkerke R2 and likelihood ratio test (Meurer and Tolles, 2017).
2.7. Sensitivity Analyses:
a) Exploration of interaction terms between sex and other relevant variables in the multivariable regression model, b) Examination of the subset whose primary drug was heroin (as opposed the combined group with heroin, non-medical methadone and “other opiates and synthetics”), and c) Examination of major poly-drug use patterns, with the subset who had an opioid as primary substance and either cocaine or methamphetamine as a secondary substance (using variable SUB2).
3. Results:
After the inclusion and exclusion criteria above were satisfied, and deletion of episodes with missing data in any of the studied variables, n=11,549 episodes available for analysis (see Supplement Flowchart).
3.1. Sample demographics by sex:
The number of males and females for each condition is in Supplementary Table S1, examined with Chi2 contingency analyses. Among sex disparities observed, there was a smaller relative frequency of males versus females with a psychiatric comorbidity.
3.2.1. Univariate association of sex with the outcome (NMOU):
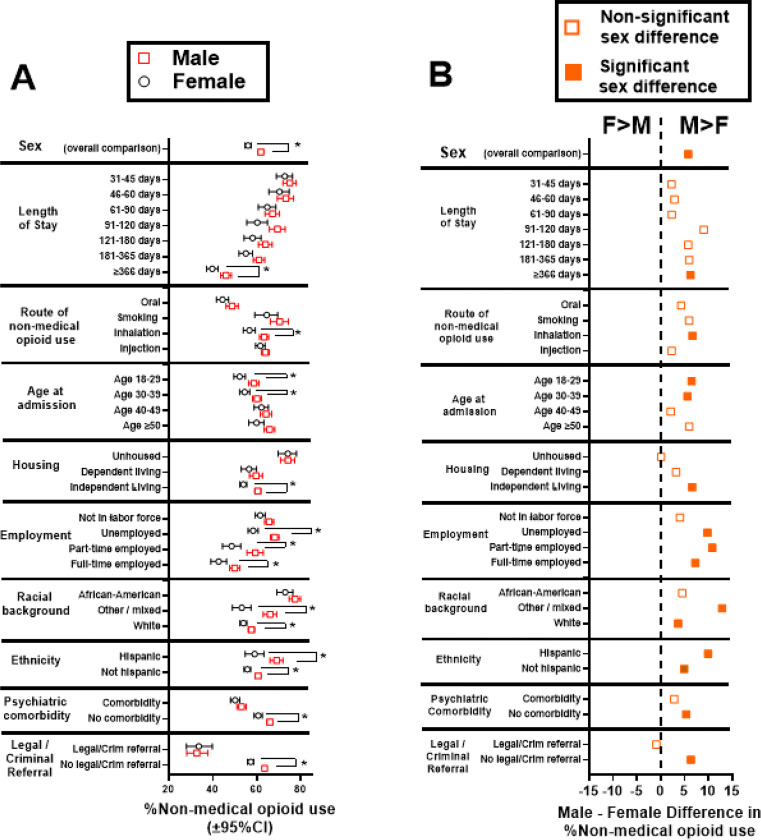
Overall, 61.8% of males and 56.0% of females had NMOU. The 2X2 contingency analysis (male/female X use/no use) was significant (Chi2[1]=39.08; uncorrected p<0.0001; Bonferroni-corrected p=0.0041) with a male/female unadjusted odds ratio of =1.27 (95%CI: 1.18–1.38) (Table 1; Figure 1).
Table 1:
Sex-related association with the outcome: NMOU in the month prior to discharge (2X2 contingency analyses; univariate)
| Variable | Condition | Outcome: Non-medical opioid use (NMOU) | Number of Episodes | Chi2 analysis; P-value uncorrected | Survives Bonferroni correction?* | Male/Female Odds Ratio of NMOU (±95%CI) | |
|---|---|---|---|---|---|---|---|
| Male (%) | Female (%) | ||||||
| Sex | Used | 4,131 (61.8%) | 2,724 (56%) | Chi2 [1] =39.2; p<0.0001 | YES p=0.0041 | =1.27 (1.18 to 1.38) | |
| Not Used | 2,554 (38.2%) | 2,140 (44.0%) | |||||
| Length of stay: (days) | 31–45 | Used | 591 (75.00%) | 410 (72.70%) | Chi2 [1]=0.91; p=0.34; NS | NO | N/A |
| Not Used | 197 (25.00%) | 154 (27.30%) | |||||
| 46–60 | Used | 396 (73.20%) | 265 (70.29%) | Chi2 [1]=0.93; p=0.34; NS | NO | N/A | |
| Not Used | 145 (26.80%) | 112 (29.71%) | |||||
| 61–90 | Used | 520 (67.10%) | 360 (64.75%) | Chi2 [1]=0.80; p=0.37; NS | NO | N/A | |
| Not Used | 255 (32.90%) | 196 (35.25%) | |||||
| 91–120 | Used | 440 (69.40%) | 251 (60.19%) | Chi2 [1] 9.47; p=0.0021 | NO | N/A | |
| Not Used | 194 (30.60%) | 166 (39.81%) | |||||
| 121–180 | Used | 584 (63.90%) | 359 (59.09%) | Chi2 [1]=5.25; p=0.022 | NO | N/A | |
| Not Used | 330 (36.10%) | 259 (41.91%) | |||||
| 181–365 | Used | 834 (61.01%) | 549 (55.01%) | Chi2 [1] 8.55; p=0.0035 | NO | N/A | |
| Not Used | 533 (38.99%) | 449 (44.99%) | |||||
| ≥366 | Used | 766 (45.98%) | 530 (39.73%) | Chi2 [1]=11.79; p=0.0006 | YES p=0.025 | 1.29 (1.12 to 1.49) | |
| Not Used | 900 (54.02%) | 804 (60.27%) | |||||
| Route of non-medical opioid use | Oral | Used | 611 (48.72%) | 559 (44.40%) | Chi2 [1]= 4.80 p=0.028 | NO | N/A |
| Not Used | 642 (51.28%) | 700 (55.60%) | |||||
| Smoking | Used | 333 (70.40%) | 212 (64.44%) | Chi2 [1]=3.17; p=0.075; NS | NO | N/A | |
| Not Used | 140 (29.60%) | 117 (35.56%) | |||||
| Inhalation | Used | 1372 (63.34%) | 730 (56.68%) | Chi2 [1] 15.47; p<0.0001 | YES p=0.0041 | 1.33 (1.15 to 1.53) | |
| Not Used | 794 (36.66%) | 560 (43.32%) | |||||
| Injection | Used | 1784 (63.90%) | 1223 (61.58%) | Chi2 [1]=2.62; p=0.11; NS | NO | N/A | |
| Not Used | 1009 (36.10%) | 763 (38.42%) | |||||
| Age | 18–29 | Used | 942 (58.66%) | 727 (52.15%) | Chi2 [1]=12.79; p=0.0003 | YES p=0.012 | 1.30 (1.13 to 1.50) |
| Not Used | 664 (41.34%) | 667 (47.85) | |||||
| 30–39 | Used | 1396 (60.12%) | 997 (54.51%) | Chi2 [1]=13.35 p=0.0003 | YES p=0.012 | 1.26 (1.11 to 1.43) | |
| Not Used | 927 (39.88%) | 834 (45.49%) | |||||
| 40–49 | Used | 814 (64.20%) | 535 (62.06%) | Chi2 [1]=1.0; p=0.32 | NO | N/A | |
| Not Used | 454 (35.80%) | 327 (37.94%) | |||||
| 50+ | Used | 979 (65.84%) | 465 (59.85%) | Chi2 [1]=7.81 p=0.0052 | NO | N/A | |
| Not Used | 509 (34.16%) | 312 (40.15%) | |||||
| Housing at Discharge | Unhoused | Used | 525 (74.15%) | 311 (74.05%) | Chi2 [1]=00.15; p=0.97; NS | NO | N/A |
| Not Used | 183 (25.85%) | 109 (25.95%) | |||||
| Dependent living | Used | 621 (59.71%) | 442 (56.45%) | Chi2 [1]=1.89; p=0.17; NS | NO | N/A | |
| Not Used | 420 (40.35%) | 341 (43.55%) | |||||
| Independent living | Used | 2985 (60.49%) | 1971 53.87% | Chi2 [1]=37.92; p<0.0001 | YES p=0.0041 | 1.31 (1.20 to 1.43) | |
| Not Used | 1951 (39.51%) | 1690 46.13% | |||||
| Employment at Discharge | Full time employed | Used | 862 (49.91%) | 315 42.63% | Chi2 [1]=11.02; p=0.0009 | YES 0.037 | 1.34 (1.13 to 1.60) |
| Not Used | 865 (50.09%) | 424 57.37% | |||||
| Part-time employed | Used | 398 (59.40%) | 269 48.56% | Chi2 [1]=14.16; p=0.0002 | YES p=0.0082 | 1.55 (1.23 to 1.94) | |
| Not Used | 273 (40.60%) | 285 51.44% | |||||
| Unemployed | Used | 1554 (68.16%) | 1045 58.32% | Chi2 [1]=42.11; p<0.0001 | YES p=0.0041 | 1.53 (1.35 to 1.74) | |
| Not Used | 726 (31.84%) | 747 41.68% | |||||
| Not in labor force | Used | 1317 (65.62%) | 1095 61.55% | Chi2 [1]=6.75; p<0.0094 | NO | N/A | |
| Not Used | 690 (34.38%) | 684 34.45% | |||||
| Race / Background | Black / African-American | Used | 796 (77.36%) | 414 (72.89%) | Chi2 [1]=3.98 p=0.046 | NO | N/A |
| Not Used | 233 (22.64%) | 154 (27.11%) | |||||
| White | Used | 2713 (57.56%) | 2022 (53.89%) | Chi2 [1]=11.54 p=0.0007 | YES p=0.029 | 1.16 (1.07 to 1.27) | |
| Not Used | 2001 (42.44%) | 1732 (46.11%) | |||||
| Other | Used | 622 (66.03%) | 288 (53.14%) | Chi2 [1]=24.11 p<0.0001 | YES p=0.0041 | 1.71 (1.38 to 2.12) | |
| Not Used | 320 (33.97%) | 254 (46.86%) | |||||
| Ethnicity | Hispanic | Used | 652 (68.99%) | 301 (59.02%) | Chi2 [1]=14.58 p<0.0001 | YES p=0.0041 | 1.55 (1.24 to 1.93) |
| Not Used | 293 (31.01%) | 209 (40.98%) | |||||
| Non-hispanic | Used | 3479 (60.61%) | 2423 (55.68%) | Chi2 [1]=24.79 p<0.0001 | YES p=0.0041 | 1.23 (1.13 to 1.33) | |
| Not Used | 2261 (39.39%) | 1931 (44.32%) | |||||
| Psychiatric comorbidity | Yes | Used | 1140 (53.02%) | 1074 (50.10%) | Chi2 [1]=3.57 p=0.06 NS | NO | N/A |
| Not Used | 1010 (46.98%) | 1068 (49.90%) | |||||
| No | Used | 2991 (66.00%) | 1650 (60.62%) | Chi2 [1]=21.01 p<0.0001 | YES p=0.0041 | 1.26 (1.14 to 1.39) | |
| Not Used | 1544 (34.00%) | 1072 (39.38%) | |||||
| Court or criminal justice referral | Yes | Used | 123 (32.71%) | 82 (33.61%) | Chi2 [1]=0.05; p=0.82; NS | NO | N/A |
| Not Used | 253 (67.29%) | 162 (66.39%) | |||||
| No | Used | 4008 (63.53%) | 2642 (57.19%) | Chi2 [1]=45.03 p<0.0001 | YES p=0.0041 | 1.30 (1.21 to 1.41) | |
| Not Used | 2301 (36.47%) | 1978 (42.81%) | |||||
| Census Divisions | New England | Used | 328 (35.85%) | 186 (31.69%) | Chi2 [1]=2.75; p=0.10; NS | NO | N/A |
| Not used | 587 (64.15%) | 401 (68.31%) | |||||
| Middle Atlantic | Used | 1013 (63.75%) | 599 (58.84%) | Chi2 [1]=6.34; p=0.012 | NO | N/A | |
| Not Used | 576 (36.25%) | 419 (41.16%) | |||||
| East North Central | Used | 340 (48.85%) | 272 (41.34%) | Chi2 [1]=7.71; p=0.0055 | NO | N/A | |
| Not Used | 356 (51.15%) | 386 (58.66%) | |||||
| West North Central | Used | 33 (59%) | 29 (57%) | Chi2 [1]=0.05; p=0.83; NS | NO | N/A | |
| Not Used | 23 (41%) | 22 (43%) | |||||
| South Atlantic | Used | 1033 (75.46%) | 654 (68.55%) | Chi2 [1]=13.47; p=0.0002 | YES p=0.0082 | 1.41 (1.17 to 1.70) | |
| Not Used | 336 (24.54%) | 300 (31.45%) | |||||
| East South Central | Used | 113 (49.13%) | 122 (47.84%) | Chi2 [1]=0.08; p=0.78; NS | NO | N/A | |
| Not Used | 117 (50.87%) | 133 (52.16%) | |||||
| West South Central | Used | 29 (64%) | 24 (69%) | Chi2 [1]=0.15; p=0.71; NS | NO | N/A | |
| Not Used | 16 (36%) | 11 (31%) | |||||
| Mountain | Used | 89 (40%) | 97 (43%) | Chi2 [1]=0.37; p=0.54; NS | NO | N/A | |
| Not Used | 131 (60%) | 127 (57%) | |||||
| Pacific | Used | 1153 (73.67%) | 741 68.48% | Chi2 [1]=8.47; p=0.0036 | NO | N/A | |
| Not Used | 412 (26.33%) | 341 31.52% | |||||
Correction for multiple comparisons with the Bonferroni method, reaching 0.05 significance with 41 comparisons (i.e., with an uncorrected threshold of p=0.0012).
Fig. 1:
Fig 1A: Percent of males and females with NMOU; *Conditions with significant 2X2 contingency analyses by sex, after Bonferroni correction (see Table 1, which includes data for census divisions). Fig 1B: Male-Female differences in %NMOU (data from Fig. 1A). Positive values indicate greater %NMOU in males vs females (labeled “M>F” at the top), and negative values indicate greater %NMOU in females vs males (labeled “F>M”). Filled symbols indicate sex comparisons that survive Bonferroni corrections (Table 1); open symbols indicate comparisons that did not reach significance.
3.2.2. Univariate association of demographic and clinical variables with NMOU, by sex:
Contingency analyses examined sex-associated outcomes (Table 1). Figure 1A shows %non-medical opioid use in males and females; Figure 1B shows the Male-Female difference. After correction for multiple comparisons, males had greater odds of NMOU compared to females in the following conditions: a) at the longest lengths of stay (i.e., ≥366 days), b) if NMOU was by inhalation, c) in the younger age bins (i.e., 18–29 and 30–39), d) in independent housing, e) employed full-time, part-time or unemployed, f) white or “mixed” racial categories g) whether they were of hispanic/latino ethnicity or not, h) if they did not have a psychiatric comorbidity, i) if they did not have a court/criminal referral to treatment and j) in the South Atlantic census division. Importantly, females did not have greater odds of NMOU than males in any of the conditions under study, after multiple comparison correction (Figure 1A and 1B).
3.2.3. Multivariable logistic regression of NMOU as the outcome:
Adjusted odds ratios of NMOU are in Figure 2 and Supplement Table S2 (which also includes data for census divisions). Overall regression performance: The regression had an area under the receiver operating curve (AUROC)=73.8, Nagelkerke R2=0.22 and likelihood ratio G2=2,027 [df=31; p<0.0001).
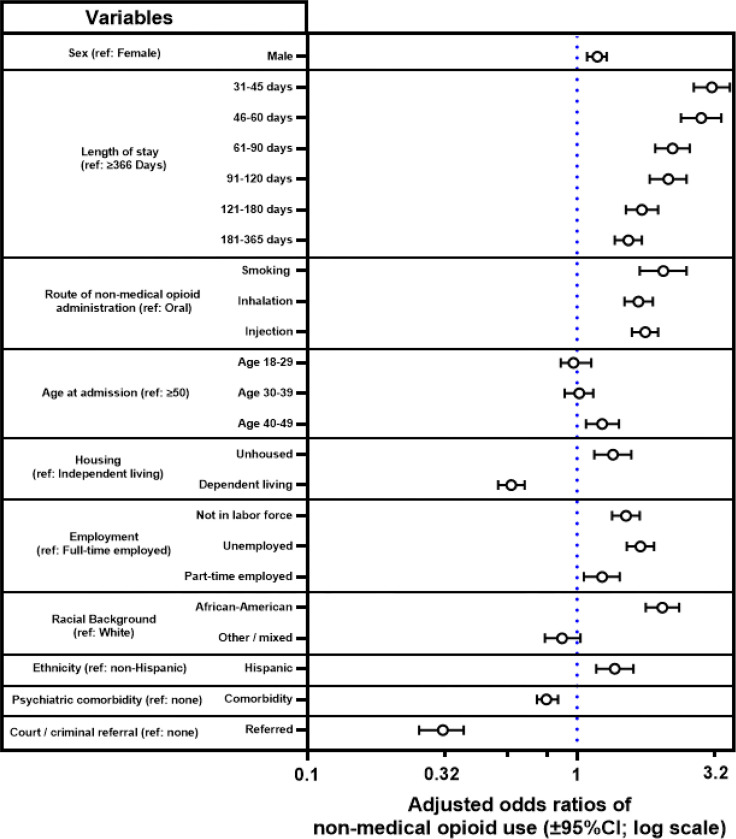
Fig. 2:
Adjusted odds ratios of NMOU, from multivariable logistic regression. Label “ref.” indicates the reference category for each variable. Data for census divisions and full regression parameters are in the Supplement (Table S2).
Sex: Males had an adjusted odds ratio =1.19 (95%CI: 1.089–1.291; p<0.0001) of NMOU, compared to females. Length of stay in treatment: All of the lengths of stay <366 days were associated with greater odds of NMOU, compared to the reference category (≥366 days). The greatest odds ratios were observed at the shorter lengths of stay (e.g., 31–45 and 46–60 days). Route of NMOU: Those with inhalation, smoking, or injection as route of administration had greater odds of NMOU, compared to the oral route. Age: Persons in the age range 40–49 had increased odds of NMOU, compared to the reference category (≥50). Housing: Persons who were unhoused had increased odds of NMOU, compared to those in independent living, whereas those in “dependent” living (e.g., supportive housing) had decreased odds. Employment: Those who were unemployed or not in the labor force had greater odds of NMOU, compared to being employed full-time. Racial background: African-American/black persons had greater odds of NMOU, compared to white persons. Ethnicity: Persons of hispanic/latino ethnicity also had increased odds of NMOU, compared to non-hispanic/latinos. Psychiatric comorbidity: Persons with psychiatric comorbidities had lower odds of NMOU, compared to those without such comorbidity. Court or criminal referral to treatment: Persons entering treatment through court/criminal referral had lower odds, compared to those without such a referral. Census divisions: Compared to the reference category (Middle Atlantic), those in the Pacific and South Atlantic divisions had greater odds of NMOU, whereas those in New England, East North Central, East South Central and Mountain had lower odds (see Table S2).
3.3. Sensitivity analyses:
a) Adding relevant interaction terms (e.g., sex by length of stay) to the multivariable regression did not result in an increased AUROC (not shown). b) In the main analysis above, we combined episodes with any opioid, by cumulating available categories (heroin, illicit methadone, and other opiates and synthetics). In a sensitivity analysis, we examined only episodes with heroin as the primary substance. The sex disparity in outcomes was also observable in this subset (not shown). c) Polysubstance use is an important feature of opioid use disorder(Butelman et al., 2019; Krawczyk et al., 2021; Luo et al., 2023). As a follow-up, we examined the subset with an opioid as primary substance and either cocaine or methamphetamine as secondary substance. In a univariate analysis, this subset also exhibited a similar sex disparity (not shown).
4. Discussion:
This is one of the first nationally representative “real world” studies on non-medical opioid use (considered an undesirable outcome) prior to discharge from MAT. Overall, males had greater adjusted odds of use, compared to females. More broadly, this study also identified socio-demographic and clinical conditions associated with this greater odds of this undesirable outcome, and specific conditions under which this sex disparity occurred.
4.1. Length of stay:
Shorter lengths of stay in treatment overall (i.e., analyzing males and females together) resulted in greater odds of NMOU. This is consistent with studies showing that longer duration of MAT is associated with other improved outcomes (Kimber et al., 2010; Wakeman et al., 2020) (e.g. decreased overdose incidence). Intriguingly, a sex difference emerged at longer treatment durations (i.e., ≥366 days), where males had greater odds of NMOU compared to females (Krawczyk et al., 2021; Wakeman et al., 2020; Williams et al., 2018).
4.2. Route of non-medical opioid administration:
Overall, persons for whom the main route of use was by inhalation (i.e., snorting), smoking (e.g., “chasing the dragon”) or injection had higher odds of NMOU compared to those using by the oral route. Increased frequency of opioid use by inhalation and smoking routes has recently been reported (Fischer et al., 2023; Huhn et al., 2018; Tanz et al., 2024), in parallel with increases in use of potent synthetic opioids (Ciccarone, 2017; Palamar et al., 2022; Rosenblum et al., 2020). Inhaled opioids cause less potent effects than after i.v. injection, likely due to pharmacokinetic mechanisms (Comer et al., 1999; Foster et al., 2008; Jones et al., 2014; Lofwall et al., 2012); less information is available for the smoking route (Hendriks et al., 2001; Jenkins et al., 1994). Importantly, these data show that inhalation (and smoking) result in outcomes comparable with injection. A sex disparity was observed in the inhalation route, in which males had greater odds of NMOU, compared to females. Overall, opioid use by inhalation and smoking routes (and relevant sex disparities) need to be investigated, given the relatively negative outcomes observed here.
4.3. Age at admission:
Overall, persons admitted to MAT in the age bin 40–49 had greater odds of NMOU, compared to the reference category (age ≥50). While some studies report that older patients in MAT have better outcomes (Carew and Comiskey, 2018; Rajaratnam et al., 2009), this study focused specifically on first episodes of treatment, and it is possible that persons who enter their first MAT in middle age have sub-optimal outcomes. Focusing specifically on sex disparities across the lifespan, males had greater odds of NMOU compared to females, at younger age categories (18–29 and 30–39).
4.4. Social determinants:
Social determinants, including housing and employment (as well as race and ethnicity) can affect outcomes of MAT, as well as other health interventions (Braveman and Gottlieb, 2014). Unhoused persons had greater odds of NMOU, compared to those in independent housing. Conversely, persons in “dependent” (e.g., supportive) housing had decreased odds of NMOU, compared to those in independent housing. Being unhoused is associated with diverse biopsychosocial stressors and difficulty in treatment adherence, which could underlie sub-optimal MAT outcomes (Fine et al., 2024; Gaeta Gazzola et al., 2023). Conversely, dependent housing could result in improved MAT outcomes, potentially due to biopsychosocial “wraparound” support (McLaughlin et al., 2021; Miller-Archie et al., 2019). Intriguingly, a sex disparity (greater NMOU in males vs females) was observed only in persons in independent housing, not those who are unhoused or in dependent housing.
Persons who were not in the labor force, were unemployed, or who worked part-time, had greater odds of NMOU, compared to those who were employed full-time, consistent with disparities based on economic disadvantage (Baird et al., 2022; Gaeta Gazzola et al., 2023; Han et al., 2022). Further “real world” research is needed to explore potential contributing factors within these groups, and related sex/gender differences.
4.4.1. Racial background and ethnicity:
African-American/black persons had greater odds of NMOU, compared to white persons. Also, hispanic/latinos had greater odds, compared to non-hispanic/latinos. Socioeconomic segregation can affect healthcare resources including MAT (Barnett et al., 2023), and racial/ethnic factors may affect the likelihood of being prescribed a specific medication (e.g., methadone versus buprenorphine) (Goedel et al., 2020; Hansen et al., 2013).
4.5. Psychiatric comorbidities:
Psychiatric comorbidities (e.g., depression, anxiety, PTSD) are relatively frequent in individuals with opioid use disorders (Butelman et al., 2017; Gelkopf et al., 2006), and there may be bidirectional causal links between psychiatric comorbidities and the trajectory of opioid use disorder (Martins et al., 2009; Rogers et al., 2021; Rosoff et al., 2020). Males in MAT had lower odds of psychiatric comorbidity compared to females, consistent with prior studies (Braciszewski et al., 2022; Huhn et al., 2019). Prior studies show complex findings on whether psychiatric comorbidities are associated with differential MAT outcomes, based on methodological differences (Choi et al., 2015; Gelkopf et al., 2006; Krawczyk et al., 2017; Rosic et al., 2017; Zhu et al., 2021). In this nationally representative sample, presence of a psychiatric comorbidity was associated with decreased odds of NMOU. It may be hypothesized that being in MAT is associated with broader biopsychosocial support and psychiatric care (Hammond et al., 2022), supporting improved outcomes (Martins et al., 2009). Mu-, kappa-, and delta-opioid receptor systems (at which methadone, buprenorphine or naltrexone have differential pharmacological profiles) are involved in depression, anxiety and stress adaptation mechanisms (Bidlack et al., 2018; Browne et al., 2018; Huang et al., 2016; Jacobson et al., 2020; Perrine et al., 2006). Future large-scale studies should examine whether specific comorbid diagnoses are associated with the outcomes for specific medications, in a sex-related manner (Huhn et al., 2019).
4.6. Court or criminal justice referral:
Persons with a court/criminal justice referral had decreased adjusted odds of NMOU, compared the larger group without such a referral. Some prior studies indicate that persons with court/criminal justice referrals have relatively improved clinical outcomes of MAT (Coviello et al., 2013; Lucabeche and Quinn, 2022; Stahler et al., 2022). Intriguingly, there was no sex disparity in outcome among those with a court/criminal justice referral, while there was a disparity (greater odds of use in males vs females) in those referred to treatment through other means (self-referral, medical, etc).
4.7. Geographic disparities:
We adjusted for census divisions, to account for major environmental variables (Conway et al., 2023; Grimm, 2020). As shown before, there were geographic differences in overall numbers of episodes, and outcomes of MAT (Table 1 and S1-S2) (Krawczyk et al., 2021; Stahler and Mennis, 2018).
4.8. Limitations:
Some limitations of the study should be considered: a) The “gender” variable in TEDS-D is coded as male/female (i.e., typical definitions of sex); further real world investigation of gender on MAT outcomes is needed (Bahji et al., 2023; Paschen-Wolff et al., 2023). b) There is potential selection bias, based on publicly-funded clinics in TEDS-D, compared to other MAT settings (Xu et al., 2024). c) Overall, cross-sectional studies cannot be used to clearly discern causality, although features such as sex, racial background and ethnicity can be linked to long-term health-related consequences, potentially through social determinants.
4.9. Conclusions and future studies:
Males had a 1.19 adjusted odds ratio of an unfavorable outcome (NMOU) in their first MAT episode, compared to females in this real world study. Recent nationally representative data also show that heroin and synthetic opioid overdose mortality rates in males are approximately 2.5-fold greater than in females, even adjusting for overall sex-specific NMOU (Butelman et al., 2023). Overall, it may be hypothesized that several mechanisms, including MAT outcomes and riskier patterns of NMOU (e.g., higher doses, using alone) could underlie sex disparities in opioid-induced morbidity and mortality (Gicquelais et al., 2022; Kariisa et al., 2019).
While females can have specific disadvantages in MAT access (Marsh et al., 2021), this study shows that once in treatment, their outcome can be relatively more favorable than that of males. Of note, females who are unhoused, have psychiatric comorbidity or have a legal/criminal referral have similar outcomes to males in those conditions. Therefore, these conditions can put females at greater relative risk for negative MAT outcomes. Strengthening biopsychosocial support in females with opioid use disorder, and transitioning from court/criminal settings into MAT, are therefore important intervention targets (Kreek et al., 2019; Strang et al., 2020; Wakeman et al., 2020). More broadly, large scale studies should examine multiple potential mechanisms for the observed disparity in MAT outcomes, such as differences in risk-taking behaviors and executive functions (de Wit, 2009; Weidacker et al., 2023), and gender-based stigma or social/family buffering (Jalali et al., 2020; Polenick et al., 2022; Smith et al., 2021; Williams and Latkin, 2007).
Supplementary Material
Highlights.
It is unclear if there are sex-related disparities in outcomes for outpatient opioid medication-assisted therapy (MAT), in large-scale “real world” settings.
In this nationally representative “real world” study, adult males had significantly greater odds of non-medical opioid use (NMOU) in the month prior to discharge from their first MAT episode compared to females, adjusting for socio-demographic and clinical variables. Males were at higher risk than females for this undesirable outcome under several conditions (e.g., in younger age categories, or if their route of NMOU was by inhalation.
Sex disparities in MAT outcomes occur under specific conditions that can be examined and potentially addressed, with the goal of improving personalized approaches for OUD.
Acknowledgements:
This work was supported by NIDA U01DA053625 (ERB), and NIDA 1RO1DA048301-01A1 (RZG), and NIDA 1RO1DA049547 (NAK), as well as NIDA Intramural funds, and from Samaritan Daytop Village.
Role of the funding source:
The funding sources did not have a role in designing or writing the study.
Abbreviations:
- 95%CI
95% confidence intervals
- AUROC
Area under the receiver-operating curve
- df
degrees of freedom
- LOS
Length of service (duration of treatment episode)
- MAT
medication-assisted therapy for opioid use disorders
- NMOU
non-medical opioid use in the month prior to discharge (binary outcome under study)
- NS
Not significant
- Ref
Reference category for multivariable logistic regression
- TEDS-D
Treatment episode data set - Discharges
- MOR
mu-opioid receptor
Footnotes
Author Disclosures
Conflict of interest: No conflict of interest declared.
Declaration of generative AI in scientific writing: The authors have not used generative AI for scientific writing.
Submission declaration and verification: These data have not been published or deposited in pre-print form.
References
- Apsley H.B., Brant K., Brothers S., Harrison E., Skogseth E., Schwartz R.P., Jones A.A., 2024. Pregnancy- and parenting-related barriers to receiving medication for opioid use disorder: A multi-paneled qualitative study of women in treatment, women who terminated treatment, and the professionals who serve them. Womens. Health 20, 17455057231224181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari M.S., Martins S.S., Mauro P.M., 2020. Medication for opioid use disorder treatment and specialty outpatient substance use treatment outcomes: Differences in retention and completion among opioid-related discharges in 2016. J. Subst. Abuse Treat. 114, 108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahji A., Bastien G., Bach P., Choi J., Le Foll B., Lim R., Jutras-Aswad D., Socias M.E., 2023. The Association Between Self-Reported Anxiety and Retention in Opioid Agonist Therapy: Findings From a Canadian Pragmatic Trial. Can. J. Psychiatry 7067437231194385. [DOI] [PMC free article] [PubMed]
- Baird A., Cheng Y., Xia Y., 2022. Use of machine learning to examine disparities in completion of substance use disorder treatment. PLoS One 17, e0275054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett M.L., Meara E., Lewinson T., Hardy B., Chyn D., Onsando M., Huskamp H.A., Mehrotra A., Morden N.E., 2023. Racial Inequality in Receipt of Medications for Opioid Use Disorder. N. Engl. J. Med. 388, 1779–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawor M., Dennis B.B., Bhalerao A., Plater C., Worster A., Varenbut M., Daiter J., Marsh D.C., Desai D., Steiner M., Anglin R., Pare G., Thabane L., Samaan Z., 2015. Sex differences in outcomes of methadone maintenance treatment for opioid use disorder: a systematic review and meta-analysis. CMAJ Open 3, E344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlack J.M., Knapp B.I., Deaver D.R., Plotnikava M., Arnelle D., Wonsey A.M., Fern Toh M., Pin S.S., Namchuk M.N., 2018. In Vitro Pharmacological Characterization of Buprenorphine, Samidorphan, and Combinations Being Developed as an Adjunctive Treatment of Major Depressive Disorder. J. Pharmacol. Exp. Ther. 367, 267–281. [DOI] [PubMed] [Google Scholar]
- Blanco C., Volkow N.D., 2019. Management of opioid use disorder in the USA: present status and future directions. Lancet 393, 1760–1772. [DOI] [PubMed] [Google Scholar]
- Braciszewski J.M., Idu A.E., Yarborough B.J.H., Stumbo S.P., Bobb J.F., Bradley K.A., Rossom R.C., Murphy M.T., Binswanger I.A., Campbell C.I., Glass J.E., Matson T.E., Lapham G.T., Loree A.M., Barbosa-Leiker C., Hatch M.A., Tsui J.I., Arnsten J.H., Stotts A., Horigian V., Hutcheson R., Bart G., Saxon A.J., Thakral M., Ling Grant D., Pflugeisen C.M., Usaga I., Madziwa L.T., Silva A., Boudreau D.M., 2022. Sex Differences in Comorbid Mental and Substance Use Disorders Among Primary Care Patients With Opioid Use Disorder. Psychiatr. Serv. 73, 1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman P., Gottlieb L., 2014. The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep. 129 Suppl 2, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne C.A., Falcon E., Robinson S.A., Berton O., Lucki I., 2018. Reversal of Stress-Induced Social Interaction Deficits by Buprenorphine. Int. J. Neuropsychopharmacol. 21, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L.D., Tony Cai T., DasGupta A., 2001. Interval Estimation for a Binomial Proportion. SSO Schweiz. Monatsschr. Zahnheilkd. 16, 101–133. [Google Scholar]
- Burgess-Hull A.J., Panlilio L.V., Preston K.L., Epstein D.H., 2022. Trajectories of Craving During Medication-Assisted Treatment for Opioid-Use Disorder: Subtyping for Early Identification of Higher Risk. Drug Alcohol Depend. 109362. [DOI] [PMC free article] [PubMed]
- Butelman E.R., Bacciardi S., Maremmani A.G.I., Darst-Campbell M., Correa da Rosa J., Kreek M.J., 2017. Can a rapid measure of self-exposure to drugs of abuse provide dimensional information on depression comorbidity? Am. J. Addict. 26, 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman E.R., Chen C.Y., Brown K.G., Kreek M.J., 2019. Escalation of drug use in persons dually diagnosed with opioid and cocaine dependence: Gender comparison and dimensional predictors. Drug Alcohol Depend. 205, 107657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman E.R., Huang Y., Epstein D.H., Shaham Y., Goldstein R.Z., Volkow N.D., Alia-Klein N., 2023. Overdose mortality rates for opioids and stimulant drugs are substantially higher in men than in women: state-level analysis. Neuropsychopharmacology. 10.1038/s41386-023-01601-8 [DOI] [PMC free article] [PubMed]
- Carew A.M., Comiskey C., 2018. Treatment for opioid use and outcomes in older adults: a systematic literature review. Drug Alcohol Depend. 182, 48–57. [DOI] [PubMed] [Google Scholar]
- Choi S., Adams S.M., Morse S.A., MacMaster S., 2015. Gender differences in treatment retention among individuals with co-occurring substance abuse and mental health disorders. Subst. Use Misuse 50, 653–663. [DOI] [PubMed] [Google Scholar]
- Ciccarone D., 2017. Fentanyl in the US heroin supply: A rapidly changing risk environment. Int. J. Drug Policy 46, 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer S.D., Collins E.D., MacArthur R.B., Fischman M.W., 1999. Comparison of intravenous and intranasal heroin self-administration by morphine-maintained humans. Psychopharmacology 143, 327–338. [DOI] [PubMed] [Google Scholar]
- Conway A., Krawczyk N., McGaffey F., Doyle S., Baaklini V., Marshall A.D., Treloar C., Davis C.S., Colledge-Frisby S., Grebely J., Cerdá M., 2023. Typology of laws restricting access to methadone treatment in the United States: A latent class analysis. Int. J. Drug Policy 119, 104141. [DOI] [PubMed] [Google Scholar]
- Coviello D.M., Zanis D.A., Wesnoski S.A., Palman N., Gur A., Lynch K.G., McKay J.R., 2013. Does mandating offenders to treatment improve completion rates? J. Subst. Abuse Treat. 44, 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H., 2009. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict. Biol. 14, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand L., Boland F., O’Driscoll D., Bennett K., Barry J., Keenan E., Fahey T., Cousins G., 2021. Factors associated with early and later dropout from methadone maintenance treatment in specialist addiction clinics: a six-year cohort study using proportional hazards frailty models for recurrent treatment episodes. Drug Alcohol Depend. 219, 108466. [DOI] [PubMed] [Google Scholar]
- Eghaneyan B.H., Sanchez K., Haeny A.M., Montgomery L., Lopez-Castro T., Burlew A.K., Rezaeizadeh A., Killian M.O., 2020. Hispanic participants in the National Institute on Drug Abuse’s Clinical Trials Network: A scoping review of two decades of research. Addict Behav Rep 12, 100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D.R., Critchley N., Hart K., Joyce A., Sporn N., Gaeta J., Wright J., Baggett T.P., Kruse G., 2024. “I’m on the Right Path”: Exploring 1-Month Retention in a Homeless-Tailored Outpatient-Based Opioid Treatment Program. Subst Use Addctn J 29767342231218529. [DOI] [PMC free article] [PubMed]
- Fischer B., Robinson T., Jutras-Aswad D., 2023. Three noteworthy idiosyncrasies related to Canada’s opioid-death crisis, and implications for public health-oriented interventions. Drug Alcohol Rev. 10.1111/dar.13796 [DOI] [PubMed]
- Foster D., Upton R., Christrup L., Popper L., 2008. Pharmacokinetics and pharmacodynamics of intranasal versus intravenous fentanyl in patients with pain after oral surgery. Ann. Pharmacother. 42, 1380–1387. [DOI] [PubMed] [Google Scholar]
- Franz B., Cronin C.E., Lindenfeld Z., Pagan J.A., Lai A., Krawczyk N., Rivera B.D., Chang J.E., 2023. Rural-urban disparities in the availability of hospital-based screening, medications for opioid use disorder, and addiction consult services. J Subst Use Addict Treat 209280. [DOI] [PMC free article] [PubMed]
- Gaeta Gazzola M., Carmichael I.D., Christian N.J., Zheng X., Madden L.M., Barry D.T., 2023. A National Study of Homelessness, Social Determinants of Health, and Treatment Engagement Among Outpatient Medication for Opioid Use Disorder-Seeking Individuals in the United States. Subst. Abus. 44, 62–72. [DOI] [PubMed] [Google Scholar]
- Gelkopf M., Weizman T., Melamed Y., Adelson M., Bleich A., 2006. Does psychiatric comorbidity affect drug abuse treatment outcome? A prospective assessment of drug abuse, treatment tenure and infectious diseases in an Israeli methadone maintenance clinic. Isr. J. Psychiatry Relat. Sci. 43, 126–136. [PubMed] [Google Scholar]
- Gicquelais R.E., Genberg B.L., Maksut J.L., Bohnert A.S.B., Fernandez A.C., 2022. Prevalence and correlates of using opioids alone among individuals in a residential treatment program in Michigan: implications for overdose mortality prevention. Harm Reduct. J. 19, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedel W.C., Shapiro A., Cerdá M., Tsai J.W., Hadland S.E., Marshall B.D.L., 2020. Association of Racial/Ethnic Segregation With Treatment Capacity for Opioid Use Disorder in Counties in the United States. JAMA Netw Open 3, e203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R.Z., 2022. Neuropsychoimaging Measures as Alternatives to Drug Use Outcomes in Clinical Trials for Addiction. JAMA Psychiatry. 10.1001/jamapsychiatry.2022.1970 [DOI] [PubMed]
- Grimm C.A., 2020. Geographic Disparities Affect Access to Buprenorphine Services for Opioid Use Disorder [WWW Document]. U.S. Office of the Inspector General, DHHS. URL https://oig.hhs.gov/oei/reports/oei-12-17-00240.asp [Google Scholar]
- Hammond C.J., Park G., Kady A., Rathod K., Rahman N., Vidal C., Wenzel K., Fishman M., 2022. Sex-based differences in psychiatric symptoms and opioid abstinence during buprenorphine/naloxone treatment in adolescents with opioid use disorders. J. Subst. Abuse Treat. 133, 108495. [DOI] [PubMed] [Google Scholar]
- Han B.H., Doran K.M., Krawczyk N., 2022. National trends in substance use treatment admissions for opioid use disorder among adults experiencing homelessness. J. Subst. Abuse Treat. 132, 108504. [DOI] [PubMed] [Google Scholar]
- Hansen H.B., Siegel C.E., Case B.G., Bertollo D.N., DiRocco D., Galanter M., 2013. Variation in use of buprenorphine and methadone treatment by racial, ethnic, and income characteristics of residential social areas in New York City. J. Behav. Health Serv. Res. 40, 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks V.M., van den Brink W., Blanken P., Bosman I.J., van Ree J.M., 2001. Heroin self-administration by means of “chasing the dragon”: pharmacodynamics and bioavailability of inhaled heroin. Eur. Neuropsychopharmacol. 11, 241–252. [DOI] [PubMed] [Google Scholar]
- Hosmer D.W. Jr, Lemeshow S., 2004. Applied Logistic Regression. John Wiley & Sons. [Google Scholar]
- Huang P., Tunis J., Parry C., Tallarida R., Liu-Chen L.Y., 2016. Synergistic antidepressant-like effects between a kappa opioid antagonist (LY2444296) and a delta opioid agonist (ADL5859) in the mouse forced swim test. Eur. J. Pharmacol. 781, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn A.S., Berry M.S., Dunn K.E., 2019. Review: Sex-Based Differences in Treatment Outcomes for Persons With Opioid Use Disorder. Am. J. Addict. 28, 246–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn A.S., Strain E.C., Tompkins D.A., Dunn K.E., 2018. A hidden aspect of the U.S. opioid crisis: Rise in first-time treatment admissions for older adults with opioid use disorder. Drug Alcohol Depend. 193, 142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M.L., Wulf H.A., Browne C.A., Lucki I., 2020. The kappa opioid receptor antagonist aticaprant reverses behavioral effects from unpredictable chronic mild stress in male mice. Psychopharmacology. 10.1007/s00213-020-05649-y [DOI] [PMC free article] [PubMed]
- Jalali M.S., Botticelli M., Hwang R.C., Koh H.K., McHugh R.K., 2020. The opioid crisis: a contextual, social-ecological framework. Health Res. Policy Syst. 18, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A.J., Keenan R.M., Henningfield J.E., Cone E.J., 1994. Pharmacokinetics and pharmacodynamics of smoked heroin. J. Anal. Toxicol. 18, 317–330. [DOI] [PubMed] [Google Scholar]
- Jones J.D., Madera G., Comer S.D., 2014. The reinforcing and subjective effects of intravenous and intranasal buprenorphine in heroin users. Pharmacol. Biochem. Behav. 122, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariisa M., Scholl L., Wilson N., Seth P., Hoots B., 2019. Drug Overdose Deaths Involving Cocaine and Psychostimulants with Abuse Potential - United States, 2003–2017. MMWR Morb. Mortal. Wkly. Rep. 68, 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimber J., Copeland L., Hickman M., Macleod J., McKenzie J., De Angelis D., Robertson J.R., 2010. Survival and cessation in injecting drug users: prospective observational study of outcomes and effect of opiate substitution treatment. BMJ 341, c3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsantas P., Gimm G., Aljoudi S.M., 2023. Treatment outcomes among pregnant women with cannabis use disorder. Addict. Behav. 144, 107723. [DOI] [PubMed] [Google Scholar]
- Kowalczyk W.J., Phillips K.A., Jobes M.L., Kennedy A.P., Ghitza U.E., Agage D.A., Schmittner J.P., Epstein D.H., Preston K.L., 2015. Clonidine Maintenance Prolongs Opioid Abstinence and Decouples Stress From Craving in Daily Life: A Randomized Controlled Trial With Ecological Momentary Assessment. Am. J. Psychiatry 172, 760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk N., Feder K.A., Saloner B., Crum R.M., Kealhofer M., Mojtabai R., 2017. The association of psychiatric comorbidity with treatment completion among clients admitted to substance use treatment programs in a U.S. national sample. Drug Alcohol Depend. 175, 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk N., Williams A.R., Saloner B., Cerdá M., 2021. Who stays in medication treatment for opioid use disorder? A national study of outpatient specialty treatment settings. J. Subst. Abuse Treat. 126, 108329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek M.J., Reed B., Butelman E.R., 2019. Current status of opioid addiction treatment and related preclinical research. Sci Adv 5, eaax9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little K.M., Kosten T.A., 2023. Focus on Fentanyl in Females: Sex and Gender Differences in the Physiological and Behavioral Effects of Fentanyl. Front. Neuroendocrinol. 101096. [DOI] [PubMed]
- Lofwall M.R., Moody D.E., Fang W.B., Nuzzo P.A., Walsh S.L., 2012. Pharmacokinetics of intranasal crushed OxyContin and intravenous oxycodone in nondependent prescription opioid abusers. J. Clin. Pharmacol. 52, 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucabeche V.X., Quinn P.V., 2022. Court-Mandated Treatment Outcomes for Prescribed Opioid Use Disorder: A Gender Based Study. J. Drug Issues 52, 47–66. [Google Scholar]
- Luo S.X., Feaster D.J., Liu Y., Balise R.R., Hu M.-C., Bouzoubaa L., Odom G.J., Brandt L., Pan Y., Hser Y.-I., VanVeldhuisen P., Castillo F., Calderon A.R., Rotrosen J., Saxon A.J., Weiss R.D., Wall M., Nunes E.V., 2023. Individual-Level Risk Prediction of Return to Use During Opioid Use Disorder Treatment. JAMA Psychiatry. 10.1001/jamapsychiatry.2023.3596 [DOI] [PMC free article] [PubMed]
- Marsh J.C., Amaro H., Kong Y., Khachikian T., Guerrero E., 2021. Gender disparities in access and retention in outpatient methadone treatment for opioid use disorder in low-income urban communities. J. Subst. Abuse Treat. 127, 108399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins S.S., Keyes K.M., Storr C.L., Zhu H., Chilcoat H.D., 2009. Pathways between nonmedical opioid use/dependence and psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 103, 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy A., Chawar C., Lamri A., Hudson J., Minuzzi L., Marsh D.C., Thabane L., Paterson A.D., Samaan Z., 2023. A genome-wide association, polygenic risk score and sex study on opioid use disorder treatment outcomes. Sci. Rep. 13, 22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin M.F., Li R., Carrero N.D., Bain P.A., Chatterjee A., 2021. Opioid use disorder treatment for people experiencing homelessness: A scoping review. Drug Alcohol Depend. 224, 108717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennis J., Stahler G.J., El Magd S.A., Baron D.A., 2019. How long does it take to complete outpatient substance use disorder treatment? Disparities among Blacks, Hispanics, and Whites in the US. Addict. Behav. 93, 158–165. [DOI] [PubMed] [Google Scholar]
- Meurer W.J., Tolles J., 2017. Logistic regression diagnostics: Understanding how well a model predicts outcomes. JAMA 317, 1068–1069. [DOI] [PubMed] [Google Scholar]
- Miller-Archie S.A., Walters S.C., Singh T.P., Lim S., 2019. Impact of supportive housing on substance use–related health care utilization among homeless persons who are active substance users. Ann. Epidemiol. 32, 1–6.e1. [DOI] [PubMed] [Google Scholar]
- Osborne J.W., 2014. Best Practices in Logistic Regression. SAGE Publications. [Google Scholar]
- Palamar J.J., Cottler L.B., Goldberger B.A., Severtson S.G., Grundy D.J., Iwanicki J.L., Ciccarone D., 2022. Trends in characteristics of fentanyl-related poisonings in the United States, 2015–2021. Am. J. Drug Alcohol Abuse 48, 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen-Wolff M.M., Kidd J.D., Paine E.A., 2023. The State of the Research on Opioid Outcomes Among Lesbian, Gay, Bisexual, Transgender, Queer, and Other Sexuality- and Gender-Diverse Populations: A Scoping Review. LGBT Health 10, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine S.A., Hoshaw B.A., Unterwald E.M., 2006. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br. J. Pharmacol. 147, 864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polenick C.A., Han B.H., Meyers S.N., Arnold T.D., Cotton B.P., 2022. Associations between relationship quality and treatment-related stress among couples receiving methadone for opioid use disorder. J. Subst. Abuse Treat. 132, 108580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaratnam R., Sivesind D., Todman M., Roane D., Seewald R., 2009. The aging methadone maintenance patient: treatment adjustment, long-term success, and quality of life. J. Opioid Manag. 5, 27–37. [DOI] [PubMed] [Google Scholar]
- Rogers A.H., Zvolensky M.J., Ditre J.W., Buckner J.D., Asmundson G.J.G., 2021. Association of opioid misuse with anxiety and depression: A systematic review of the literature. Clin. Psychol. Rev. 84, 101978. [DOI] [PubMed] [Google Scholar]
- Rosenblum D., Unick J., Ciccarone D., 2020. The Rapidly Changing US Illicit Drug Market and the Potential for an Improved Early Warning System: Evidence from Ohio Drug Crime Labs. Drug Alcohol Depend. 208, 107779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosic T., Naji L., Bawor M., Dennis B.B., Plater C., Marsh D.C., Thabane L., Samaan Z., 2017. The impact of comorbid psychiatric disorders on methadone maintenance treatment in opioid use disorder: a prospective cohort study. Neuropsychiatr. Dis. Treat. 13, 1399–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff D.B., Smith G.D., Lohoff F.W., 2020. Prescription Opioid Use and Risk for Major Depressive Disorder and Anxiety and Stress-Related Disorders: A Multivariable Mendelian Randomization Analysis. JAMA Psychiatry. 10.1001/jamapsychiatry.2020.3554 [DOI] [PMC free article] [PubMed]
- Ross R.K., Nunes E.V., Olfson M., Shulman M., Krawczyk N., Stuart E.A., Rudolph K.E., 2024. Comparative effectiveness of extended release naltrexone and sublingual buprenorphine for treatment of opioid use disorder among Medicaid patients. medRxiv. 10.1101/2024.01.24.24301555 [DOI] [PMC free article] [PubMed]
- Rudolph K.E., Russell M., Luo S.X., Rotrosen J., Nunes E.V., 2022. Under-representation of key demographic groups in opioid use disorder trials. Drug Alcohol Depend Rep 4. 10.1016/j.dadr.2022.100084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.C., Alderman L., Attell B.K., Avila Rodriguez W., Covington J., Manteuffel B., DiGirolamo A.M., Snyder S.M., Minyard K., 2021. Dynamics of Parental Opioid Use and Children’s Health and Well-Being: An Integrative Systems Mapping Approach. Front. Psychol. 12, 687641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahler G.J., Mennis J., 2018. Treatment outcome disparities for opioid users: Are there racial and ethnic differences in treatment completion across large US metropolitan areas? Drug Alcohol Depend. 190, 170–178. [DOI] [PubMed] [Google Scholar]
- Stahler G.J., Mennis J., Stein L.A.R., Belenko S., Rohsenow D.J., Grunwald H.E., Brinkley-Rubinstein L., Martin R.A., 2022. Treatment outcomes associated with medications for opioid use disorder (MOUD) among criminal justice-referred admissions to residential treatment in the U.S., 2015–2018. Drug Alcohol Depend. 236, 109498. [DOI] [PubMed] [Google Scholar]
- Strang J., Volkow N.D., Degenhardt L., Hickman M., Johnson K., Koob G.F., Marshall B.D.L., Tyndall M., Walsh S.L., 2020. Opioid use disorder. Nat Rev Dis Primers 6, 3. [DOI] [PubMed] [Google Scholar]
- Surratt H., Kurtz S.P., Cicero T.J., 2011. Alternate routes of administration and risk for HIV among prescription opioid abusers. J. Addict. Dis. 30, 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanz L.J., Gladden R.M., Dinwiddie A.T., Miller K.D., Broz D., Spector E., O’Donnell J., 2024. Routes of Drug Use Among Drug Overdose Deaths - United States, 2020–2022. MMWR Morb. Mortal. Wkly. Rep. 73, 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers E.B., Williams I.L., Qillawala E.I., Rissman E.F., Lynch W.J., 2023. Sex/Gender Differences in the Time-Course for the Development of Substance Use Disorder: A Focus on the Telescoping Effect. Pharmacol. Rev. 75, 217–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., 2020. Collision of the COVID-19 and Addiction Epidemics. Ann. Intern. Med. 173, 61–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman S.E., Larochelle M.R., Ameli O., Chaisson C.E., McPheeters J.T., Crown W.H., Azocar F., Sanghavi D.M., 2020. Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder. JAMA Netw Open 3, e1920622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidacker K., Zhao Y., Zhang Y., Whiteford S., Ren Q., Zhang C., Voon V., 2023. Methadone maintenance treatment and impulsivity: premature responding. J. Clin. Exp. Neuropsychol. 45, 606–617. [DOI] [PubMed] [Google Scholar]
- Williams A.R., Nunes E.V., Bisaga A., Pincus H.A., Johnson K.A., Campbell A.N., Remien R.H., Crystal S., Friedmann P.D., Levin F.R., Olfson M., 2018. Developing an opioid use disorder treatment cascade: A review of quality measures. J. Subst. Abuse Treat. 91, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C.T., Latkin C.A., 2007. Neighborhood socioeconomic status, personal network attributes, and use of heroin and cocaine. Am. J. Prev. Med. 32, S203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N., Kariisa M., Seth P., Smith H. 4th, Davis N.L., 2020. Drug and Opioid-Involved Overdose Deaths - United States, 2017–2018. MMWR Morb. Mortal. Wkly. Rep. 69, 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K.Y., Gertner A.K., Greenfield S.F., Williams A.R., Grucza R.A., 2024. Treatment setting and buprenorphine discontinuation: an analysis of multi-state insurance claims. Addict. Sci. Clin. Pract. 19, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Cavrak M., Bowles H., Deng Y., Wen S., Gao S., Lander L., Berry J., Winstanley E.L., 2024. 10-year retention of a comprehensive treatment model of buprenorphine for opioid use disorder. J. Addict. Dis. 1–8. [DOI] [PMC free article] [PubMed]
- Zhu Y., Mooney L.J., Yoo C., Evans E.A., Kelleghan A., Saxon A.J., Curtis M.E., Hser Y.-I., 2021. Psychiatric comorbidity and treatment outcomes in patients with opioid use disorder: Results from a multisite trial of buprenorphine-naloxone and methadone. Drug Alcohol Depend. 228, 108996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.