Abstract
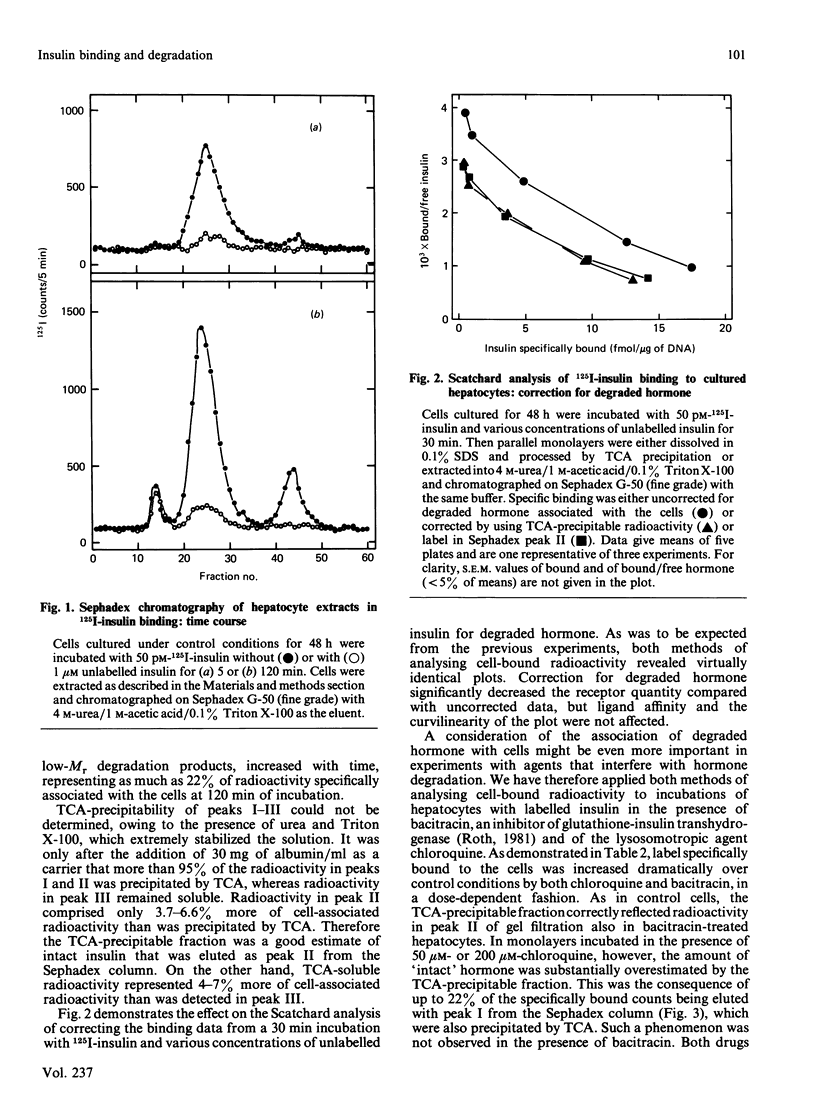
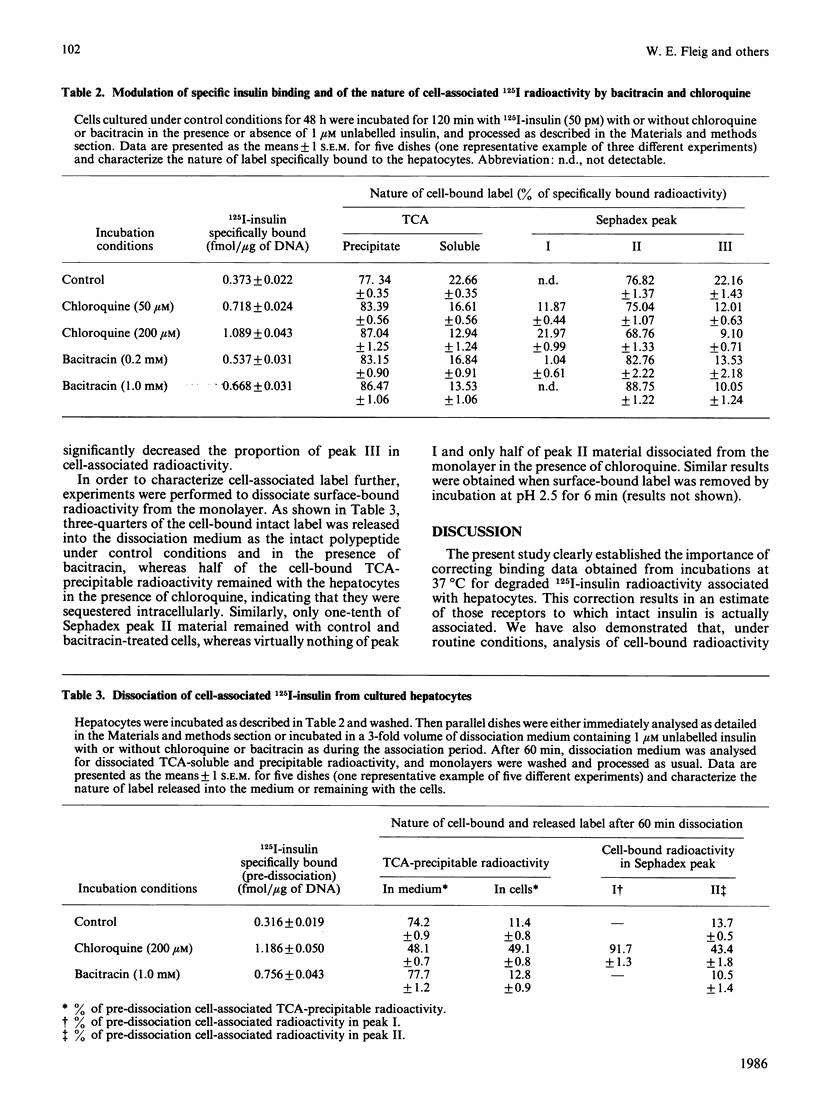
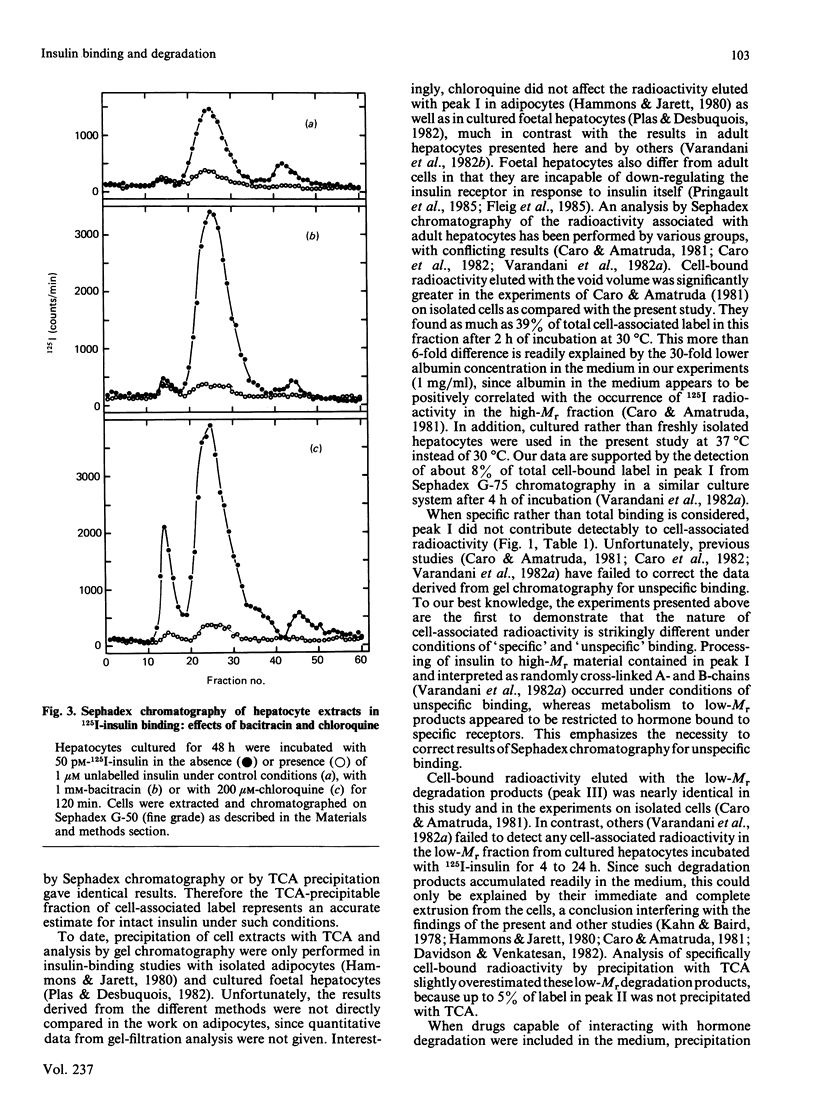
Sephadex (G-50 fine grade)-gel chromatography and trichloroacetic acid (TCA) precipitation were used to investigate the effects of chloroquine and bacitracin on the nature of cell-associated radioactivity in studies on the binding and degradation of 125I-insulin in cultured rat hepatocytes. Sephadex peak I, eluted with the void volume, increased with hepatocyte incubation time and comprised 6% of total cell-bound radioactivity at 120 min. However, all radioactivity in this peak was due to unspecific binding. Peak II, corresponding to intact insulin, represented 95% of specifically cell-associated label at 5 min and decreased to 77% at 120 min. Peak III, containing the final low-Mr degradation products, increased with incubation time (22% of specifically bound label at 120 min). The TCA-precipitable and TCA-soluble fractions of hepatocytes extracted with 0.1% SDS were within 4-7% of the proportions of radioactivity in peaks II and III respectively. Scatchard plots based on insulin-binding data from Sephadex chromatography or TCA precipitation were identical. Dissociation studies revealed that at least 75% of the intact insulin associated with the hepatocytes was bound to receptors at the cell surface. Bacitracin increased the proportion of cell-associated intact hormone and decreased that of ligand degraded when analysed by either Sephadex chromatography or TCA precipitation. The proportion of surface-bound to internalized intact hormone remained unaltered, indicating that bacitracin acted predominantly at the cell surface. In the presence of chloroquine, which dramatically increased the contribution of peak I to specific binding, 'intact' insulin was substantially overestimated when determined as the TCA-precipitable fraction. In addition, all peak I material and 50% of cell-associated label in peak II was trapped intracellularly, thereby pointing to the lysosomal or prelysosomal site of action of this drug.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Tager H. S. Peptide intermediates in the cellular metabolism of insulin. J Biol Chem. 1982 Aug 10;257(15):9078–9085. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Caro J. F., Amatruda J. M. Evidence for modulation of insulin action and degradation independently of insulin binding. Am J Physiol. 1981 Mar;240(3):E325–E332. doi: 10.1152/ajpendo.1981.240.3.E325. [DOI] [PubMed] [Google Scholar]
- Caro J. F., Amatruda J. M. Functional relationships between insulin binding, action, and degradation. A reassessment. J Biol Chem. 1980 Nov 10;255(21):10052–10055. [PubMed] [Google Scholar]
- Caro J. F., Muller G., Glennon J. A. Insulin processing by the liver. J Biol Chem. 1982 Jul 25;257(14):8459–8466. [PubMed] [Google Scholar]
- Davidson M. B., Venkatesan N. Persistence of a curvilinear Scatchard plot for insulin binding despite correcting for degradation. Metabolism. 1982 Dec;31(12):1206–1209. doi: 10.1016/0026-0495(82)90005-1. [DOI] [PubMed] [Google Scholar]
- Donner D. B. Receptor- and non-receptor-mediated uptake and degradation of insulin by hepatocytes. Biochem J. 1982 Oct 15;208(1):211–219. doi: 10.1042/bj2080211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleig W. E., Noether-Fleig G., Fussgaenger R., Ditschuneit H. Modulation by a sulfonylurea of insulin-dependent glycogenesis, but not of insulin binding, in cultured rat hepatocytes. Evidence for a postreceptor mechanism of action. Diabetes. 1984 Mar;33(3):285–290. doi: 10.2337/diab.33.3.285. [DOI] [PubMed] [Google Scholar]
- Fleig W. E., Noether-Fleig G., Roeben H., Ditschuneit H. Hormonal regulation of key gluconeogenic enzymes and glucose release in cultured hepatocytes: effects of dexamethasone and gastrointestinal hormones on glucagon action. Arch Biochem Biophys. 1984 Feb 15;229(1):368–378. doi: 10.1016/0003-9861(84)90164-4. [DOI] [PubMed] [Google Scholar]
- Fleig W. E., Nöther-Fleig G., Steudter S., Enderle D., Ditschuneit H. Regulation of insulin binding and glycogenesis by insulin and dexamethasone in cultured rat hepatocytes. Biochim Biophys Acta. 1985 Dec 12;847(3):352–361. doi: 10.1016/0167-4889(85)90041-2. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Hammons G. T., Jarett L. Lysosomal degradation of receptor-bound 125I-labeled insulin by rat adipocytes: its characterization and dissociation from the short-term biologic effects of insulin. Diabetes. 1980 Jun;29(6):475–486. doi: 10.2337/diab.29.6.475. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. The fate of insulin bound to adipocytes. Evidence for compartmentalization and processing. J Biol Chem. 1978 Jul 25;253(14):4900–4906. [PubMed] [Google Scholar]
- Plas C., Desbuquois B. Receptor-mediated insulin degradation and insulin-stimulated glycogenesis in cultured foetal hepatocytes. Biochem J. 1982 Feb 15;202(2):333–341. doi: 10.1042/bj2020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringault E., Plas C., Desbuquois B., Clauser H. Reutilization of insulin receptor and hormonal response in cultured foetal hepatocytes: the effects of chloroquine and vinblastine. Biol Cell. 1985;53(1):13–21. doi: 10.1111/j.1768-322x.1985.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Roth R. A. Bacitracin: an inhibitor of the insulin degrading activity of glutathione-insulin transhydrogenase. Biochem Biophys Res Commun. 1981 Jan 30;98(2):431–438. doi: 10.1016/0006-291x(81)90858-5. [DOI] [PubMed] [Google Scholar]
- Varandani P. T., Darrow R. M., Nafz M. A. Cellular processing of insulin. Effects of lectin, lysosomotropic and other agents. Am J Physiol. 1982 Aug;243(2):E140–E151. doi: 10.1152/ajpendo.1982.243.2.E140. [DOI] [PubMed] [Google Scholar]
- Varandani P. T., Darrow R. M., Nafz M. A., Norris G. L. Binding, degradation, and bioactivity of insulin in primary cultures of rat hepatocytes. Am J Physiol. 1982 Aug;243(2):E132–E139. doi: 10.1152/ajpendo.1982.243.2.E132. [DOI] [PubMed] [Google Scholar]


